Abstract
Background
EphA2 is a tyrosine kinase receptor in the ephrin family that is implicated in oncogenesis and angiogenesis. Our goal was to study the role of EphA2 in endometrial cancer and its relation to steroid hormone receptor expression.
Methods
EphA2, estrogen receptor (ER), progesterone receptor (PR) and Ki-67 expression were evaluated using immunohistochemistry in 139 endometrioid endometrial carcinomas (EEC) and in 10 benign endometrial samples. Samples were scored by 2 investigators blinded to clinical outcome. Results were correlated with clinicopathologic characteristics using univariate and multivariate analysis. A p value of <0.05 was considered statistically significant.
Results
High expression of EphA2 was detected in 48% of EEC samples vs. 10% of benign samples. EphA2 overexpression was significantly associated with high-stage (p=0.04), high-grade (p=0.003), increased depth of myometrial invasion (p=0.05), low ER (p=0.01), low PR (p=0.006) and high Ki-67 expression (p=0.04). Low ER and PR expression were both associated with high-grade, positive lymph nodes, high Ki-67 expression and high EphA2 expression. On univariate analysis of all patients, high EphA2 expression was significantly associated with shorter disease-specific survival (DSS, p<0.001). On multivariate analysis, age (p<0.001), high-stage (p=0.002) and high EphA2 expression (p=0.04) were independent predictors of poor DSS.
Conclusions
EphA2 overexpression is associated with aggressive phenotypic features in endometrioid endometrial carcinomas, and is inversely associated with ER and PR expression. Thus, EphA2 may be an important therapeutic target, especially in patients with hormone-receptor negative endometrial carcinoma.
Keywords: Endometrial cancer, EphA2, estrogen receptor, progesterone receptor, Ki-67
Introduction
Endometrial cancer is the most common cancer of the female genital tract and will account for 40,100 new cases in 2008 (1). Eighty percent of endometrial cancers are of endometrioid histology (2). While majority of patients (70-80%) present with early-stage disease and have a 5-year survival rate of approximately 80%, an estimated 7,470 women will die due to their disease in 2008 (1, 2). Thus, a better understanding of the underlying molecular mechanisms is needed to plan effective therapeutic strategies, especially for women with advanced and recurrent endometrial cancer.
Receptor tyrosine kinases play an important role in diverse molecular functions such as cellular proliferation, differentiation and migration (3, 4). The Eph receptors are the largest family of tyrosine kinases and are divided into two subclasses based on interaction with their ligands, ephrin-A and ephrin-B (5). There is growing evidence that several Eph receptors play critical roles in cancer development and progression (6). EphA2 is a transmembrane protein that is primarily found on adult human epithelial cells, unlike other Eph kinases which are mainly expressed during embryogenesis (5, 6). While the specific role for EphA2 in normal epithelium is not fully known, EphA2 may potentially regulate cell growth, invasion, angiogenesis and survival of cancer cells (6, 7). EphA2 overexpression has been demonstrated in several solid tumors including melanoma, breast, prostate, lung and ovarian cancer (7–10). However, the clinical relevance of EphA2 expression in endometrial cancer is unknown.
In the present study, we sought to examine the role of EphA2 expression in patients with endometrial carcinoma. In addition, we investigated the relationship of EphA2 with known prognostic factors in endometrial cancer including the expression of steroid hormone receptors and markers of cellular proliferation.
Materials and Methods
Samples for Immunostaining
Following IRB approval, archived formalin-fixed, paraffin embedded samples were obtained from 139 patients with endometrioid endometrial carcinoma who were surgically treated at the University of Texas M. D. Anderson Cancer Center and the Methodist Hospital between 2000 and 2004, and who had adequate tissue available for immunohistochemical evaluation. All patients were surgically staged based on the International Federation of Gynecology and Obstetrics (FIGO) staging system. None of the patients received pre-operative chemotherapy or radiation. Endometrial samples from ten women who underwent hysterectomy for benign indications were included as controls for the expression of the study proteins.
Immunohistochemical Staining
The methods for immunohistochemical staining of paraffin-embedded samples have been previously described (10, 11). Briefly, formalin-fixed, paraffin-embedded samples were sectioned at 4 μm and stained with hematoxylin and eosin (H&E) for tumor confirmation. Sections adjacent to the H&E staining were used for immunohistochemical staining. Sections were deparaffinized and then probed with either a monoclonal EphA2 antibody (MedImmune, Gaithersburg, MD), mouse antihuman estrogen receptor-α (ER; dilution 1:10) mouse antihuman progesterone receptor (PR; dilution 1:75); and mouse antihuman Ki-67 (dilution 1:100; DakoCytomation, Carinteria, CA). After this, slides were rinsed with PBS, incubated with a secondary antibody for 30 minutes, and then with the Ready-to-Use avidin-biotin complex method reagent for 5-15 minutes and then counterstrained with 1:10 dilution of Mayer's hematoxylin for 35-60 seconds.
Semiquantitative analysis of immunostaining
All samples were reviewed by two investigators (A.A.K. and D.C.), who were blinded to the clinical outcome of patients. Semi-quantitative assessment of immunohistochemical expression was performed as previously described (11) by assessing the percentage of stained tumor cells and staining intensity. Briefly, the percentage of positively stained cells was rated as follows: 0 points, 0-5%; 1 point, 6-50%; 2 points, 51-75%; 3 points, >75%. The staining intensity was rated in the following manner: 2 points, weak intensity; 3 points, moderate intensity; 4 points, strong intensity. The product of the scores for intensity and percentage of positive cells were used to get an overall score index (SI) ranging from 0-3. Tumors were categorized into 3 groups based on the SI: negative or weak expression (SI of 0-4), moderate expression (SI of 5-8) and strong expression (SI of 9-12). For Ki-67 expression, the number of positively stained cells in 5-high powered fields in the areas of greatest proliferation were calculated and the percentage of positive cells per filed were calculated.
For statistical analyses, tumors were dichotomized into 2 groups based on the level of immunostaining as follows, for EphA2, low expression (negative, weak or moderate staining; SI= 0-8) and high expression (strong staining; SI = 9-12); for ER and PR, low expression (negative or weak staining; SI = 0-4) and high expression (moderate and strong staining; SI = 5-12); for Ki-67, low expression (Ki-67 positive cells ≤ 30%) and high expression (Ki-67 positive cells > 30%). For all immunohistochemical analyses, the independent scores from both investigators were consolidated into a final score, which is reported in this study. Any differences in the scores were adjudicated following discussion between the two investigators.
Clinicopathologic analysis
All patients underwent surgical exploration and primary surgical staging as the initial treatment. The extent of the surgical staging was based on the pre-operative endometrial biopsy, frozen section pathology and the surgeon's clinical judgment. The treating gynecologic oncologist determined the adjuvant therapy. The pathologic diagnosis was verified by the pathology reports. A gynecologic pathologist (D.C.) reviewed all the H&E slides to confirm the histopathologic diagnosis and tumor grading. Based on FIGO stage, patients were divided into two groups, low stage (FIGO stage I and II, n=108) and high-stage (FIGO stage III and IV, n=31). A clinical remission was defined as no evidence of disease based on physical examination and/or imaging studies. Disease specific survival (DSS) was defined as the time from diagnosis until the date of death or the date of last contact.
Statistical Analysis
Chi-square or Fisher's exact tests were used, as appropriate, to test for the association in the proportions across levels of a single covariate factor and expression of EphA2, ER, PR and Ki-67. Patients who were alive at last follow-up or died from causes other than uterine cancer were censored at the date of last follow-up. Disease specific survival (DSS) estimated using the Kaplan-Meier product limit method. A two-sided log-rank test was used to test for differences between survival curves. DSS was assessed using both univariate and multivariate Cox proportional hazards regression. A p value of <0.05 on two-tailed testing was considered significant.
Results
Demographic Factors
To determine the clinical significance of EphA2 expression in human endometrial cancers, we examined 139 samples stained for EphA2, ER, PR and Ki-67 expression. The demographic features of the patients are listed in Table 1. The mean age of patients was 63 years (range, 27 - 91). Seventy-eight percent of patients had stage I or II disease, all tumors were of endometrioid histology and only 18% were poorly-differentiated. The median follow-up for patients in this study was 24.9 months.
Table 1. Demographic features of patients with endometrioid endometrial cancer.
| Variable | N=139 | |
|---|---|---|
| Age* (y) | 63 (27-91) | |
| Menopausal | Premenopausal | 24 (17) |
| Postmenopausal | 112 (81) | |
| Unknown | 3 (2) | |
| Stage | Low (I / II) | 108 (78) |
| High (III / IV) | 31 (22) | |
| Grade | 1 | 35 (25) |
| 2 | 79 (57) | |
| 3 | 25 (18) | |
| Depth of invasion | ≤ ½ myometrium | 100 (72) |
| > ½ myometrium | 39 (28) | |
| Node status | Negative | 74 (53) |
| Positive | 49 (35) | |
| Not done | 16 (12) | |
Mean (range)
EphA2 expression in Human Endometrioid Endometrial Cancer Samples
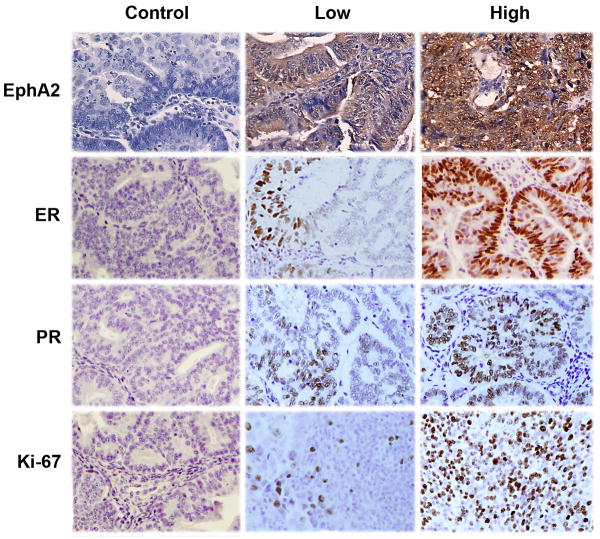
Representative photomicrographs illustrating negative, low and high expression of EphA2 are presented in Figure 1. In addition, 10 samples of benign endometrium were also stained for EphA2 expression. Majority of the benign samples (90%) demonstrated negative or weak expression of EphA2. Among the invasive endometrioid endometrial cancers, 72 (52%) had low or weak expression and 67 (48%) tumors overexpressed EphA2. In 2 samples, EphA2 expression could not be assessed due to poor sample quality.
Figure 1.
Representative images of invasive endometrioid endometrial cancers following immunohistochemical staining for EphA2, ER, PR and Ki-67. All pictures were taken at original magnification, X 200.
Association of EphA2 expression with Clinical and Pathologic Variables
Based on the fact that EphA2 is overexpressed in a large proportion of endometrioid endometrial cancers, we next analyzed the association of EphA2 expression with known prognostic variables. The correlations of EphA2 overexpression with various clinical and pathologic variables are listed in Table 2. EphA2 was overexpressed in 65% of patients with high-stage disease compared to 43% with low-stage disease (p=0.04). Interestingly, high levels of EphA2 expression were also associated with higher grade tumors (grade 3; 76%, p=0.006) as well as increasing depth of myometrial invasion (p=0.047). There was no significant difference in EphA2 expression among patients with negative, positive or unknown lymph node status.
Table 2. Association of EphA2 overexpression with clinicopathologic variables in endometrioid endometrial cancer.
| N=139*** | ||||
|---|---|---|---|---|
| Low EphA2 | High EphA2 | p | ||
| Stage | Low (I/II) | 61 | 47 | |
| High (III/IV) | 10 | 19 | 0.04 | |
| Grade | Low (1 or 2) | 65 | 47 | |
| High (3) | 6 | 19 | 0.003 | |
| Depth of invasion | ≤ 1/2 | 56 | 42 | |
| > 1/2 | 15 | 24 | 0.047 | |
| Node status | Negative | 40 | 34 | |
| Positive | 25 | 24 | ||
| Not done | 6 | 8 | 0.7 | |
| ER* | Low | 28 | 41 | |
| High | 43 | 25 | 0.01 | |
| PR* | Low | 23 | 37 | |
| High | 48 | 29 | 0.006 | |
| Ki-67** | Low | 48 | 33 | |
| High | 23 | 33 | 0.04 | |
NOTE:
Low expression of ER and PR denotes negative, weak or moderate expression (SI = 0-8); high expression of ER and PR denotes strong expression (SI = 9-12).
Low expression of Ki-67 denotes ≤ 30% positive cells and high expression denotes > 30% positive cells.
Missing numbers denote samples that were damaged and could not be evaluated.
Since the presence of steroid hormone receptor expression is a good prognostic indicator in patients with endometrial cancer and may guide therapeutic strategies, we next evaluated the expression of ER and PR in our cohort. High expression of ER and PR (Figure 1) was demonstrated in 46% and 55% of the samples, respectively. When we analyzed the expression of EphA2 in relation to steroid hormone receptor expression, EphA2 overexpression was demonstrated in 59% of tumors with low ER expression compared to 32% of tumors with high ER expression (p=0.01). A similar finding was seen with PR, where tumors with low expression showed high EphA2 expression in 62% of cases compared to 38% in tumors with high PR expression, a highly significant difference (p=0.006). Finally, we studied the relationship of EphA2 expression with the degree of cellular proliferation as represented by the percentage of tumor cells that stained positive for Ki-67 (Figure 1D). Tumors with a higher fraction of proliferating cells demonstrated a higher level of EphA2 expression (59%) compared to tumors with lower levels of Ki-67 (41%, p=0.04).
Clinical Outcome Based on EphA2 Expression
Prior to testing the prognostic significance of EphA2 expression, we first performed univariate analyses of traditional clinical variables for DSS (Table 3). Age was analyzed as a continuous variable and advancing age was significantly associated with an increased risk of death due to EEC (p=0.002). As expected, high-stage (p=0.01), high tumor grade (p=0.001) and depth of myometrial invasion (p=0.003) were all associated with a shorter DSS. Interestingly, lymph node status was not a significant determinant of DSS. Of note, there were 5 deaths out of 12 patients in whom nodal status was not known; all of whom demonstrated high EphA2 expression in their tumors. Among other variables tested, low ER (p=0.001), low PR (p=0.002) and high Ki-67 (p<0.001) were all significantly associated with a significantly shorter DSS among patients with EEC. The median DSS for patients with tumors overexpressing EphA2 compared to those with low EphA2 expression was highly significant (p<0.001, Figure 2). In analysis restricted to tumors with low ER, those with EphA2 overexpression had a median DSS of 43 months compared to those with low ER and low EphA2 expression (median DSS of 80 months; p =0.01).
Table 3. Univariate survival analysis of prognostic variables for DSS in endometrioid endometrial cancer patients.
| N=139 | ||||
|---|---|---|---|---|
| Variable | Median | Survival | HR (95% CI) | p |
| Age (per yr) | NR | NR | 1.05 (1.02-1.09) | 0.002 |
| Stage* | NR | NR | 2.67 (1.21-5.87) | 0.01 |
| FIGO grade* | NR | 25 | 4.44 (2.11-9.35) | 0.001 |
| Depth of invasion > ½* | NR | NR | 3.18 (1.49-6.78) | 0.003 |
| Positive nodes | NR | NR | 0.7 (0.27-1.81) | 0.4 |
| High ER | 93 | NR | 0.22 (0.09-0.56) | 0.001 |
| High PR | 93 | NR | 0.27 (0.12-0.63) | 0.002 |
| High Ki-67 | NR | 93 | 4.19 (1.78-9.86) | 0.001 |
| High EphA2 | NR | NR | 4.66 (1.87-11.59) | <0.001 |
For the clinical variables, the groups are sub-classified as low and high as follows: Stage: low (I/II) and high (III/IV); FIGO grade: low (1 or 2) and high (3); Histology: low (endometrioid) and high (non-endometrioid); Depth of invasion: low (≤ ½ myometrial thickness) and high (> ½ myometrial thickness). The same criteria for low and high expression of immunohistochemical variables were used as in Table 2. NR = not reached
Figure 2.
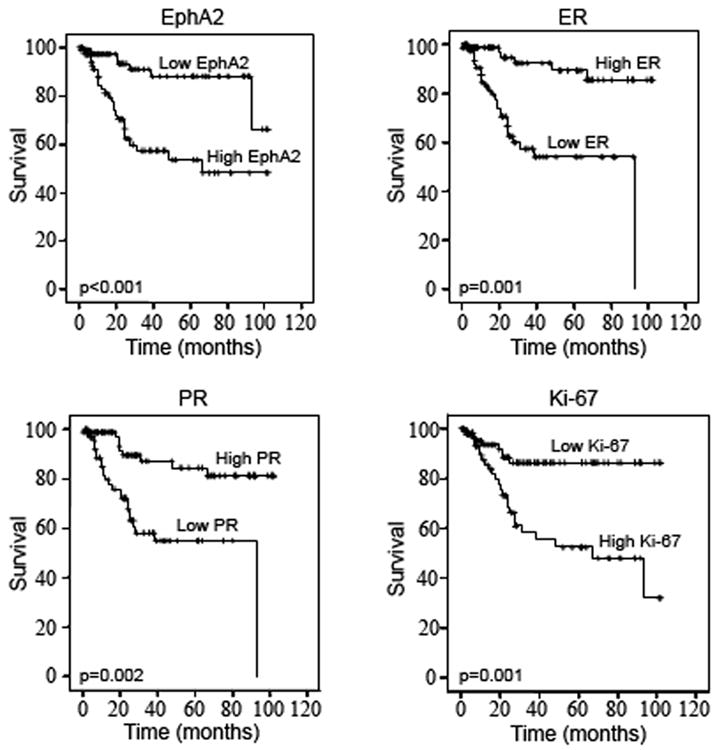
Kaplan Meier Curves showing the effect of study markers on disease-specific survival in patients with endometrioid endometrial cancer.
Based on the findings that EphA2 overexpression is associated with aggressive features in patients with endometrioid endometrial cancer, we next assessed whether there was an independent association between EphA2 expression and DSS. Using a Cox proportional hazards model, we performed a multivariate survival analysis (Table 4). Using a model that included age, stage and grade of disease, depth of invasion, nodal status and markers including EphA2, ER, PR and Ki-67, we found that increasing age (p<0.001), high-stage (p=0.002) and EphA2 overexpression (p=0.04) were each independent predictors of shorter DSS. In this model, patients whose tumors overexpressed EphA2 had a 3-fold increased risk of death compared to those with low EphA2 expression. Since positive lymph node status is a known predictor of poor prognosis for endometrial cancer patients, we reanalyzed our cohort with the exclusion of the 16 patients in whom lymph node status was unknown. Among patients with known lymph node status, there remained a significant association of high EphA2 expression with shorter DSS (p=0.002). On multivariate analysis in this sub-group of patients, once again, high EphA2 expression was independently associated with a shorter DSS (HR 3.0, 95% CI 1.05 – 8.56; p=0.04).
Table 4. Multivariate survival analysis using Cox proportional hazards model.
| N=139 | ||
|---|---|---|
| Variable | HR (95% CI) | p |
| Age (per year) | 1.09 (1.04 - 1.14) | <0.001 |
| High-stage (III/IV) | 5.64 (1.89 – 16.8) | 0.002 |
| High-grade (grade 3) | 1.78 (0.62 – 5.13) | 0.3 |
| Depth of invasion > ½ | 1.96 (0.79 – 4.84) | 0.14 |
| Positive lymph nodes | 0.56 (0.19 – 1.63) | 0.3 |
| High ER expression | 0.4 (0.12 – 1.3) | 0.12 |
| High PR expression | 0.66 (0.2 – 2.12) | 0.48 |
| High Ki-67 expression | 2.07 (0.69 – 6.25) | 0.2 |
| High EphA2 expression | 3.05 (1.05 – 8.87) | 0.04 |
Discussion
The major findings of the present study are that a large proportion of endometrioid endometrial cancers overexpress EphA2, compared to weak or negative expression in majority of benign endometrial samples. EphA2 overexpression was significantly associated with several aggressive clinical variables and was inversely associated with the expression of steroid hormone receptors. Significantly, EphA2 overexpression was an independent predictor of shorter disease-specific survival in these patients. Together, these results indicate that EphA2 may be an attractive therapeutic target, especially in patients with steroid receptor-negative endometrial cancer.
Our work adds to the growing evidence that EphA2 may play an important role in progression and development of several malignancies (5, 6). EphA2 is a receptor tyrosine kinase that is found at low levels on non-transformed epithelial cells (12). Although the role of EphA2 in normal epithelia is not fully understood, the cellular consequences of ligand binding include negative regulation of cell growth, migration and invasion (7, 13, 14). EphA2 overexpression has been reported in many cancers, including breast, melanoma, prostate, lung and ovarian carcinoma (10). Kinch and colleagues showed that EphA2-transfected non-transformed cells demonstrate increased growth in vitro and form larger and more aggressive tumors in vivo (15). Moreover, EphA2 was found to be an independent prognostic factor for survival in patients with ovarian cancer, with more that 75% of tumors overexpressing this oncoprotein (10). In our cohort, tumors overexpressing EphA2 were associated with deep myometrial invasion. Recently, overexpression of EphA2 was found to be associated with elevated levels of several matrix metalloproteinases (MMPs) including MMP-2, MMP-9 and MT1-MMP, all of which facilitate migration and invasion of tumor cells (16). Interestingly, EphA2 expression was associated with poor clinical outcome independent of lymph node status. In our study, EphA2 overexpression was noted in tumors with higher levels of proliferation. These findings are supported by other studies regarding EphA2's role in tumor cell growth, angiogenesis and differentiation (17, 18).
Obesity and other hyperestrogenic states are known risk factors for the development of endometrial cancer (2). Endometrioid endometrial carcinomas, which account for greater than 80% of all endometrial cancers, are associated with a estrogen-driven model of carcinogenesis, where unopposed estrogen stimulation leads to malignant transformation of benign endometrium (19). In general, high levels of ER and PR are directly correlated with lower tumor grade, less myometrial invasion, lower incidence of nodal metastases and a good clinical outcome (20. 21). In our study, low expression of both steroid hormone receptors was significantly associated with a shorter DSS. There is some evidence that suggests a close interplay between EphA2 and ER (22). For example, EphA2 levels in breast cancer cells are inversely related to ER expression (22). Previous studies have also shown that EphA2 overexpression increases the malignant characteristics of ER-positive breast cancer cells (15). Moreover, these growth-promoting effects of EphA2 occur in the absence of estrogen, making these cells resistant to tamoxifen. Inhibition of EphA2 using an antibody can reverse these effects, making these cells once again sensitive to tamoxifen (15). Our results extend these findings and further indicate that the subset of patients whose tumors had low ER and high EphA2 expression had a worse prognosis than those with low EphA2 expression. These data suggest that EphA2 overexpression may contribute to the increased growth and invasiveness of ER-deficient cells, but additional mechanistic studies are required. In patients with recurrent or advanced endometrial cancers, hormonal therapies are commonly used, with best response rates seen in patients with well-differentiated, steroid hormone receptor positive tumors (23). Thus, these findings may have therapeutic implications for patients with poorly differentiated endometrial cancers that are steroid hormone deficient.
Efforts to target EphA2 are being actively pursued in our laboratory using a variety of different approaches. One approach using an agonistic antibody against EphA2 in conjunction with paclitaxel was shown to inhibit tumor growth and improve survival in mice with advanced ovarian cancer (24). Another novel approach for targeting EphA2 using siRNA incorporated in neutral liposomes has been recently described in an orthotopic ovarian cancer model (25). Intraperitoneal administration of liposomal EphA2-siRNA complexes along with paclitaxel resulted in significant reduction in tumor growth compared to non-silencing siRNA and paclitaxel treatment (25). Thus, EphA2-directed approaches in conjunction with chemotherapy appear promising and may provide alternative treatment options for patients with advanced endometrial cancer.
In summary, to the best of our knowledge, this is the first study to investigate the clinical relevance of EphA2 in endometrioid endometrial cancer. Tumors displaying EphA2 overexpression are associated with features of poor prognosis including high-stage, high-grade, deep myometrial invasion, high proliferative index and low expression of steroid hormone receptors. Notably, along with age and advanced stage, EphA2 overexpression is an important independent predictor of shorter DSS in patients with endometrioid endometrial cancer. These findings support the evaluation of EphA2-targeted therapies in patients with advanced or recurrent endometrial cancer.
Acknowledgments
Portions of this work were supported by the Betty Ann Asche-Murray Fellowship Award to A.A.K., National Cancer Institute - DHHS- NIH T32 Training Grant (T32 CA101642) to W.M.M, Y.G.L., and W.A.S.; and the U. T. M. D. Anderson Cancer Center SPORE in Ovarian Cancer grant (P50 CA083639), the Marcus Foundation grant, and the Betty Ann Asche Murray Distinguished Professorship to A.K.S.
Footnotes
None of the authors have any financial conflict of interest to disclose
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Rose P. Endometrial carcinoma. N Engl J Med. 1996;335:640–9. doi: 10.1056/NEJM199608293350907. [DOI] [PubMed] [Google Scholar]
- 3.Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene. 2000;19:5614–9. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- 4.Lamorte L, Park M. The receptor tyrosine kinases: role in cancer progression. Surg Oncol Clin A Am. 2001;10:271–88. [PubMed] [Google Scholar]
- 5.Walker-Daniels J, Hess AR, Hendrix MJC, Kinch MS. Differential regulation of EphA2 in normal and malignant cells. Am J Pathol. 2003;162:1037–42. doi: 10.1016/S0002-9440(10)63899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Moll Cell Biol. 2005;6:462–75. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 7.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–6. [PubMed] [Google Scholar]
- 8.Walker-Daniels J, Coffman K, Azimi M, et al. Overexpression of the EphA2 receptor tyrosine kinase in prostate cacner. Prostate. 1999;41:275–80. doi: 10.1002/(sici)1097-0045(19991201)41:4<275::aid-pros8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 9.Kinch MS, Moore MB, Harpole DH., Jr Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res. 2003;9:613–18. [PubMed] [Google Scholar]
- 10.Thaker PH, Deaver M, Celestino J, et al. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res. 2004;10:5145–50. doi: 10.1158/1078-0432.CCR-03-0589. [DOI] [PubMed] [Google Scholar]
- 11.Kamat AA, Fletcher M, Gruman LM, et al. The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin Cancer Res. 2006;12:1707–1714. doi: 10.1158/1078-0432.CCR-05-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindberg RA, Hunter T. cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Moll Cell Biol. 1990;10:6316–24. doi: 10.1128/mcb.10.12.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carles-Kinch K, Kilpatrick KE, Stewart JC, Kinch MS. Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res. 2002;62:2840–7. [PubMed] [Google Scholar]
- 14.Hess AR, Seftor EA, Gardner LM, et al. Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: role of epithelial cell kinase (Eck/EphA2) Cancer Res. 2001;61:3250–55. [PubMed] [Google Scholar]
- 15.Lu M, Miller KD, Gokmen-Polar Y, Jeng M, Kinch MS. EphA2 overexpression decreases estrogen dependence and tamoxifen sensitivity. Cancer Res. 2003;63:3425–29. [PubMed] [Google Scholar]
- 16.Lin YG, Han LY, Kamat AA, et al. EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer. 2007;109:332–9. doi: 10.1002/cncr.22415. [DOI] [PubMed] [Google Scholar]
- 17.Andres AC, Reid HH, Zurcher G, Blaschke RJ, Ablrecht D, Ziemiecki A. Expression of two novel eph-related receptor protein tyrosine kinases in mammary gland development and carcinogenesis. Oncogene. 1994;9:1461–7. [PubMed] [Google Scholar]
- 18.Pandey A, Shao H, Marks RM, Polverinin PJ, Dixit VM. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-α -induced angiogenesis. Science. 1995;268:567–9. doi: 10.1126/science.7536959. [DOI] [PubMed] [Google Scholar]
- 19.Sherman ME, Bur ME, Kurman RJ. p53 in endometrial cancer and its putative precursors: evidence for diverse pathways of tumorigenesis. Hum Pathol. 1995;26:1268–74. doi: 10.1016/0046-8177(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 20.Chambers JT, MacLusky N, Eisenfield A, Kohorn EI, Lawrence R, Schwartz PR. Estrogen and progesterone receptors in endometrial cancer and their prognostic relevance. Gynecol Oncol. 1990;38:59–65. doi: 10.1016/0090-8258(90)90012-a. [DOI] [PubMed] [Google Scholar]
- 21.Morris PC, Anderson JR, Anderson B, Butler RE. Steroid hormone receptor content and lymph node status in endometrial cancer. Gynecol Oncol. 1995;56:406–11. doi: 10.1006/gyno.1995.1072. [DOI] [PubMed] [Google Scholar]
- 22.Zelinski DP, Zantek ND, Walker-Daniels J, Peters MA, Taparowsky EJ, Kinch MS. Estrogen and Myc negatively regulate expression of the EphA2 tyrosine kinase. J Cell Biochem. 2002;85:714–20. doi: 10.1002/jcb.10186. [DOI] [PubMed] [Google Scholar]
- 23.Thigpen JT, Brady MF, Alvarez RD, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose response study by the Gynecologic Oncology Group. J Clin Oncol. 1999;17:1736–44. doi: 10.1200/JCO.1999.17.6.1736. [DOI] [PubMed] [Google Scholar]
- 24.Landen CN, Jr, Lu C, Han LY, et al. Efficacy and antivascular effects of EphA2 reduction with an agonist antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558–70. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- 25.Landen CN, Merritt WM, Mangala LS, et al. Intraperitoneal delivery of liposomal siRNA for therapy of advanced ovarian cancer. Cancer Biol Ther. 2006;5:1708–13. doi: 10.4161/cbt.5.12.3468. [DOI] [PubMed] [Google Scholar]