Abstract
The purpose of our study was to determine whether self-reported physical activity (PA), including recreational, household, and exercise activities, is associated with intra-abdominal fat (IAF) in community-dwelling white and black midlife women. We performed a cross-sectional study of 369 women from the Chicago site of the Study of Women’s Health Across the Nation (SWAN) ancillary study, the SWAN Fat Patterning Study. PA level was the independent variable, and IAF, assessed by computerized tomography (CT) scan, was the dependent variable. Measures were obtained at SWAN Fat Patterning Baseline visit between August 2002 and December 2005. Linear regression models explored the association between PA and IAF. The first model included IAF as the outcome and total score PA as the main predictor, adjusting for total percent fat mass, age, and ethnicity. The second model included education, parity, sex hormone–binding globulin (SHBG) level, and depressive symptoms, measured by Center for Epidemiological Studies-Depression (CES-D) scale. Each 1-point higher total PA score was associated with a 4.0 cm2 lower amount of IAF (P = 0.004), independent of total percent fat mass, age, ethnicity, SHBG level, educational level, CES-D, and parity. Associations did not differ between white and black women. This study demonstrates a significant negative association between PA and IAF independent of multiple covariates in midlife women. Our findings suggest that motivating white and black women to increase PA during midlife may lessen IAF, which may have a positive impact on subsequent development of diabetes and cardiovascular disease.
INTRODUCTION
Intra-abdominal fat (IAF) is an independent risk factor for cardiovascular disease and diabetes mellitus (1–4). Despite decades of public health messaging regarding the health dangers of obesity, the number of individuals with abdominal obesity continues to rise. More than half of adults in the United States in 2004 had abdominal obesity, measured as exceeding waist circumference cut-points from the American Heart Association (5). In women, between 1960 and 2000, mean waist circumference increased from 77.1 to 94.3 cm and the prevalence of abdominal obesity, defined at >88 cm (>35 inches), tripled from 19 to 60% (6). In women, particularly obese women, the accumulation of IAF was associated with elements of the metabolic syndrome, including hyperinsulinemia, dyslipidemia, and hypertension (7–11). In a recent meta-analysis, BMI was found to be the poorest predictor of hypertension, diabetes mellitus, and dyslipidemia when compared to measures of abdominal obesity including waist circumference, waist-to-hip ratio, and waist-to-height ratio (12). Several age-adjusted studies of midlife women have found that central adiposity is related to the menopausal transition (13–16). Although BMI, subcutaneous abdominal fat (SAF), and total fat increase linearly through the menopausal transition, only IAF has been shown to be significantly greater in post- vs. premenopausal women, adjusting for age (17).
Because of the menopause-related increase in IAF, it is clinically useful to identify behaviors that may help midlife women to reduce IAF, and thereby reduce risk for diabetes mellitus and cardiovascular disease. Exercise interventions to date have suggested short-term benefits on reducing IAF mass (18–20). It has been shown that exercise and weight loss, via caloric restriction or pharmacological therapy, cause a preferential loss of IAF when adjusted for loss of body fat, especially in individuals with greater IAF mass (21). A recent meta-analysis reported the value of solo aerobic exercise in promoting a preferential reduction in IAF (22). Energy expenditure was expressed as metabolic equivalents (METs) per hour per week. The meta-analysis included clinical exercise interventions with 18- to 65-year-old male and female subjects enrolled in aerobic exercise programs only and instructed to maintain energy intake. The authors concluded that 10 METs/h/week, grossly equivalent to 2.5 h/week of moderate (4 METs) physical activity (PA), was sufficient to reduce IAF. None of the interventions included in the meta-analysis focused on menopause-related IAF or on African-American women.
African-American women have higher rates of coronary heart disease morbidity and mortality, and more total body and subcutaneous fat than white women (23). Although the associations between visceral fat and disease risk appear to be equally strong for women of both racial/ethnic groups, of interest, most studies have found that for a given level of total body fat, African-American women have lower levels of IAF than their white counterparts (24).
In this study, we examined the association between PA and IAF in midlife women at different stages of the menopausal transition. We hypothesized a menopause-related increase in IAF, with higher levels of IAF in postmenopausal compared to pre- and perimenopausal women. In addition, we hypothesized that women reporting higher levels of PA would have lower IAF. Further, given that African-American women suffer a higher burden of coronary heart disease than white women, we explored whether the hypothesized association between PA and IAF differed by race/ethnicity. Finally, we hypothesized that reported exercise of moderate intensity and duration would be associated with lower levels of menopause-related increased IAF.
METHODS
Subjects
Subjects were 369 women (55% whites; 45% African Americans) enrolled in the Study of Women Across the Nation (SWAN) Chicago site who were participating in the SWAN Fat Patterning Study, an ancillary study of the accumulation of IAF during the menopausal transition. SWAN is a multisite, multiethnic, longitudinal, prospective study of women transitioning through menopause. Inclusion criteria at the SWAN baseline were as follows: age of 42–52 years, not pregnant or breastfeeding, an intact uterus and at least 1 ovary, reported menstrual bleeding within the past 3 months, not currently using medications known to affect pituitary or ovarian function, and not using exogenous hormones within the 3 months preceding the baseline interview. Subjects completed annual interviews and assessments. Details of SWAN recruitment and protocol have been reported previously (25).
Enrollment for the ancillary Chicago SWAN Fat Patterning Study was coincidental with annual SWAN follow-up visits 4–8 from August 2002 through December 2005. Chicago SWAN women were ineligible for the ancillary study if they: (i) had a history of diabetes, chronic liver disease, and/or renal disease; (ii) self-reported a history of anorexia nervosa or alcohol or drug abuse; (iii) were currently pregnant or planning to become pregnant; (iv) had undergone surgical menopause (hysterectomy and/or bilateral oophorectomy); (v) had breast implants, hip replacements, or weighed ≥299 pounds which would preclude accurate imaging. Seventy-seven percent of eligible Chicago SWAN participants enrolled in the Fat Patterning Study. Because interest was in studying a full range of menopausal status and because many SWAN participants were already postmenopausal by the time the Fat Patterning Study began, we recruited an additional 138 pre- and perimenopausal women who were screened as part of the original SWAN recruitment effort in 1996 but were too young to participate. These newly recruited women did not differ from the SWAN women in level of PA, BMI, education, or age-adjusted total fat or IAF. The final cohort consisted of 435 women (200 African Americans and 235 whites). Due to missing data on IAF (due to equipment malfunction) (n = 3) and PA score (n = 1), new surgical menopause (n = 23), new hormone use (n = 24), or missing covariates (n = 15), 369 women (166 African Americans, 203 whites) were included in the current analyses.
Procedures
Full details of the SWAN protocol are provided elsewhere (25). At study entry and annually thereafter, women in SWAN completed a standard assessment including self-administered and interviewer-administered questionnaires assessing social, economic, behavioral, psychological, health and lifestyle characteristics, anthropometric measures, blood pressure readings, and laboratory measures based on fasting blood specimens. Laboratory measures assessed reproductive hormones, glucose and insulin levels, clotting factors, lipid and lipoprotein profiles. Covariates for the present analyses were measured as part of the annual SWAN assessment coincident with recruitment to the SWAN Fat Patterning Study. The 138 women recruited specifically to the Fat Patterning Study completed the same protocol as the SWAN participants, including the measurements obtained for the annual SWAN visit and the specific assessments of body composition for the Fat Patterning Study. Data presented here are from the baseline visit for the Fat Patterning Study. The study was approved by the Rush University Medical Center Institutional Review Board and all women provided written, informed consent for their participation.
Assessment of physical activity
PA was assessed using an adapted version of the Kaiser Physical Activity Survey, a standard questionnaire assessing the frequency of sport/exercise, recreational activities and household/childcare activities (26). The Kaiser Physical Activity Survey was administered at SWAN follow-up years 4, 6, 7, and 8 for women drawn from the SWAN cohort (corresponding to the SWAN visit that was closest to their fat patterning assessment), and at the fat patterning baseline for the 138 additional participants. A total activity score was created by summing across Kaiser Physical Activity Survey domains, with a higher score indicative of greater activity (27). Item responses in each domain are Likert-scale, with domain-specific activity indices ranging from 1 to 5, with 5 indicating the highest level of activity in that domain. We used the normally distributed continuous total PA score for the independent variable. Analyses using total PA score tertiles resulted in similar results.
To provide a clinically meaningful measure of aerobic exercise dosing to test our hypothesis that reported exercise of moderate intensity and duration would be associated with lower levels of menopause-related increased IAF, we calculated a new measure, which we called the Clinical PA Index. The Kaiser Physical Activity Survey sport/exercise index includes a write-in section of sport/exercise with related questions on duration (h/week) and intensity (none, small, moderate, or large increase in heart rate and breathing). Those who reported exercising for ≥2 h/week for at least 9 months of the year and who had a moderate increase in their heart rate during exercise were classified as the Clinically Significant PA Group with those not meeting this level classified as the No Clinically Significant PA Group. Thirteen women made no response to the sport/ exercise leaving a total of 356 women for this analysis.
Assessment of fat patterning
IAF and SAF were assessed by computerized tomography (CT) of the abdomen using a General Electric Lightspeed VCT scanner (General Electric Medical Systems, Milwaukee, WI). Each participant was scanned in the supine position with her arms folded across her chest. In women still cycling, the test was done during the first 12 days of her menstrual cycle. After a scout view, a single 10-mm thick image at the L4–L5 vertebral space was obtained, stored on optical disk, and transferred to the reading center at the University of Colorado Health Sciences Center for analysis using their software (RSI, Boulder, CO). The radiologist reading the scans was blinded to the participants’ information. Total abdominal fat area was defined by using a cursor to delineate the area within the muscle wall surrounding the abdominal cavity (28,29). IAF was defined as all adipose tissue within this area with an attenuation range between −190 and −30 Hounsfeld Units (28). IAF area was subtracted from total abdominal fat area to quantify SAF (29).
Assessment of covariates/descriptives
Total body fat was assessed by dual-energy X-ray absorptiometry, using a General Electric Lunar Prodigy scanner (GE-Lunar, Madison, WI), which was calibrated daily to insure accuracy. The dual-energy X-ray absorptiometry scans were performed on the same day as the CT scan, with the participant supine with arms at her side. Scans were analyzed using enCORE software (GE-Lunar). Total body fat was quantified as the percent of fat in the total body, in order to represent the amount of fat for a given body size. Total percent fat mass was calculated as total fat mass/(total fat mass + total lean mass).
Race/ethnicity was self-reported as either non-Hispanic African-American or non-Hispanic white (referent). Age was self-reported in years and modeled continuously in all analyses. Respondents reported 1 of 5 educational levels (less than a high-school diploma, high-school diploma, some college, college degree, postgraduate education ( referent category)). Parity was calculated by report of number of live births at baseline. Depressive symptoms are assessed annually in SWAN with the 20-item Center for Epidemiological Studies-Depression (CES-D) scale. A score of ≥16 on the CES-D is considered indicative of clinically significant symptomatology (30). In analyses, we modeled the CES-D dichotomously with CES-D score ≥16 vs. <16 (referent).
Continuous values of sex hormone–binding globulin (SHBG) were used as a biological measure of menopausal status and were assessed from fasting blood specimens. Blood was drawn on the morning following an overnight fast, with study visits scheduled during days 2–5 of the menstrual cycle in subjects still cycling. All samples were maintained at 4 °C until separated and then were frozen at −80 °C and shipped on dry ice to central laboratory. SHBG was then measured by a competitive chemiluminescent assay (Bayer Diagnostics, Norwood, MA) with coefficients of variation of 3–12%. For 8% of participants without an SHBG value from the concurrent SWAN visit, the SHBG value from the most recent previous SWAN visit within ≤12 months was used.
Data analysis
Descriptive statistics were used to characterize the study sample on age, education, parity, CES-D score, PA, and amount of total body fat, SAF, and IAF. T-tests and χ2-tests were conducted to examine differences in these characteristics by race/ethnicity and to compare the mean values of SAF and IAF in the cohort by menopausal status and PA group. A series of linear regression models explored the association between PA and IAF. The first model included IAF as the outcome and PA score as the main predictor, adjusting for total percent fat mass, age, and ethnicity. The second model included additional covariates including education, parity, SHBG level, and depressive symptoms, measured by the CES-D Scale. Secondarily, we examined whether the association between PA and IAF differed by race, by testing a race × PA interaction.
RESULTS
Participant characteristics
Table 1 presents characteristics of the sample by ethnicity. The participants were 50 years of age on average, well educated, and 72% exceeded normal weight (<25 kg/m2). African-American women had higher SAF, total body fat mass, total percent fat mass, waist circumference, and BMI but comparable IAF. They had lower PA scores and higher levels of depressive symptoms. Table 2 shows IAF and SAF by the Clinical PA Index with 171 women in the Clinically Significant PA Group and 185 women in the No Clinically Significant PA Group.
Table 1.
Demographic characteristics by race/ethnicity in SWAN Fat Patterning Study
| Total cohort | African American | White | |
|---|---|---|---|
| N | 369 | 166 | 203 |
| Age, mean (s.d.) | 50.7 (3.9) | 50.6 (3.7) | 50.9 (4.1) |
| Intra-abdominal fat (cm2), mean (s.d.) | 95.3 (53.1) | 96.8 (58.5) | 93.6 (45.8) |
| SAF (cm2), mean (s.d.) | 392.4 (162.7) | 440.3 (168.5) | 353.2 (147.1)*** |
| Total body fat mass, mean (s.d.) | 33,381.6 (13,008.2) | 36,950.4 (13,314.6) | 30,533.7 (12,052.3)*** |
| Total percent fat, mean (s.d.) | 43.3 (8.5) | 45.1 (8) | 41.8 (8.7)*** |
| Physical activity scorea, mean (s.d.) | 7.8 (1.6) | 7.3 (1.6) | 8.1 (1.6)*** |
| SHBG (nmol/l)b, mean (s.d.) | 54.2 (31.3) | 54.6 (31.2) | 53.9 (31.5) |
| CES-D score, mean (s.d.) | 6.9 (7.3) | 7.7 (7.8) | 6.2 (6.8)* |
| CES-D ≥16, N (%) | 47 (13.4) | 28 (18.1) | 19 (9.6)* |
| Current smoker, N (%) | 77 (20.9) | 40 (24.1) | 37 (18.2) |
| Education, N (%) | |||
| Less than an HS diploma | 7 (1.9) | 4 (2.4) | 3 (1.5)** |
| HS diploma | 36 (9.8) | 17 (10.2) | 19 (9.3) |
| Some college | 100 (27.1) | 60 (36.2) | 40 (19.7) |
| College degree | 90 (24.4) | 34 (20.5) | 56 (27.6) |
| Graduate school | 136 (36.8) | 51 (30.7) | 85 (41.9) |
| Parity, N (%) | |||
| 0 | 52 (14.1) | 21 (12.7) | 31 (15.3)* |
| 1–2 Children | 168 (45.5) | 89 (53.6) | 79 (38.9) |
| >2 Children | 149 (40.4) | 56 (33.7) | 93 (45.8) |
| Menopausal statusc, N (%) | |||
| Premenopausal | 47 (12.7) | 16 (9.6) | 31 (15.3) |
| Early perimenopausal | 135 (36.6) | 66 (39.7) | 69 (34.0) |
| Late perimenopausal | 35 (9.5) | 14 (8.5) | 21(10.3) |
| Postmenopausal | 152 (41.2) | 70 (42.2) | 82 (40.4) |
| Waist circumference (cm), mean (s.d.) | 89.9 (13.6) | 92.5 (13.4) | 87.8 (13.4)*** |
| BMI (kg/cm2), mean (s.d.) | 29.1 (6.2) | 31 (6.5) | 27.6 (5.6)*** |
| Weight categories, N (%) | |||
| Normal weight (BMI< 25 kg/cm2) | 104 (28.3) | 35 (21.3) | 69 (33.9)*** |
| Overweight (BMI 25 to <30 kg/cm2) | 122 (33.3) | 48 (29.2) | 74 (36.5) |
| Obese (BMI ≥30 kg/ cm2) | 141 (38.4) | 81 (49.5) | 60 (29.6) |
CES-D, Center for Epidemiological Study-Depression scale; HS, high school; PA, physical activity; SHBG, sex hormone–binding globulin; SWAN, Study of Women’s Health Across the Nation.
Range of PA score 3.5–12.5.
Range for SHBG 6.8–229.9 nmol/l.
SWAN used bleeding patterns to categorize menopausal status: premenopausal (no bleeding irregularity in past 3 months), early perimenopausal (less predictable menses in last 3 months), late perimenopausal (no menstrual bleeding for at least 3 months but not >12 months), and postmenopausal (no menstrual bleeding for at least 12 months).
P <0.05;
P < 0.01;
P < 0.001.
Table 2.
Intra-abdominal and subcutaneous abdominal fat area by menopausal status and Clinical Physical Activity Index
| Pre/peri
|
Post
|
No Clinically Significant PA group (<2 h/week exercise with moderate increase in heart rate)
|
Clinically Significant PA group (≥2 h/week exercise with moderate increase in heart rate)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| N = 211 | N = 145 | P value | Pre/peri (N = 88) | Post (N = 97) | P value | Pre/peri (N = 123) | Post (N = 48) | P value | |
| IAF area | 85.1 (46.9) | 110.03 (58.1) | <0.0001 | 92.3 (46.2) | 115.3 (55.9) | 0.003 | 79.9 (46.9) | 99.3 (61.6) | 0.03 |
| SAF area | 378.7 (162.6) | 409.4 (162.2) | 0.081 | 396.7 (163.6) | 426.3 (156.8) | 0.210 | 365.8 (161.3) | 375.1 (169.2) | 0.738 |
IAF, intra-abdominal fat; PA, physical activity; SAF, subcutaneous abdominal fat.
Associations between PA, menopause, and IAF
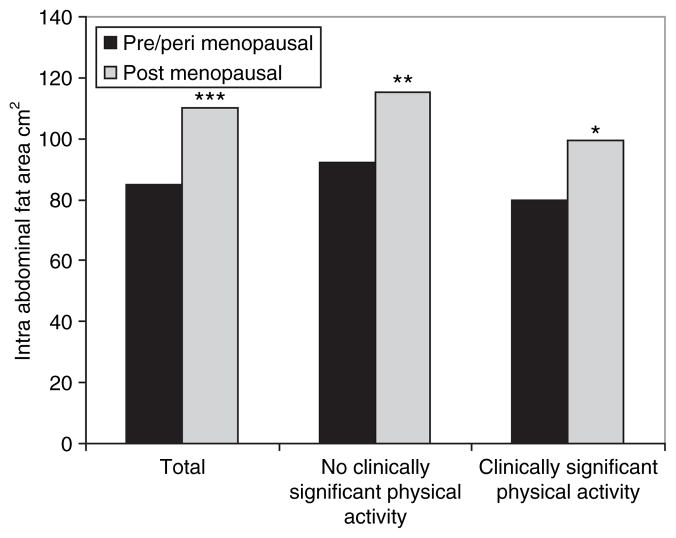
Figure 1 presents IAF by menopausal status and Clinical PA Index. In the overall cohort, there was a statistically significant difference in the postmenopausal subjects compared to pre- and perimenopausal subjects in mean IAF (110.03 (58.1) vs. 85.1 (46.9) cm2, P < 0.0001) (Table 2). In those in the Clinically Significant PA Group (exercising for ≥2 h/week and reported a moderate increase in their heart rate during exercise) there was a moderate increase in IAF with menopausal status. However, this increase in mean IAF was more pronounced in those in the No Clinically Significant PA Group. We did not find a statistically significant difference in SAF by menopausal status or Clinical PA Index.
Figure 1.
Intra-abdominal fat area by menopausal status and Clinical Physical Activity Index.
Associations between PA and IAF
Table 3 presents multivariate modeling PA on IAF. In model 1, each additional point on the PA scale related to a 5.0 cm2 decrease in IAF (estimate = −5.0; s.e. = 1.4; P = 0.0005), independent of percent total fat mass, age, and ethnicity. Adding SHBG, education, CES-D, and parity to the model (model 2) slightly reduced the strength of the impact of PA on IAF (estimate = −4.0; s.e. = 1.4; P = 0.004). The associations between PA and IAF did not differ between black and white women (test of interaction, PA × ethnicity = P = 0.7488; data not shown).
Table 3.
Results of adjusted linear regression models examining physical activity in relation to intra-abdominal fat
| Model 1 (N = 369)
|
Model 2 (N = 369)
|
|||
|---|---|---|---|---|
| Estimate (s.e.) | P | Estimate (s.e.) | P | |
| Intercept | −109.9 (29.9) | 0.0003 | −84.6 (29.3) | 0.004 |
| Physical activity score | −5.0 (1.4) | 0.0005 | −4.0 (1.4) | 0.004 |
| African American | −18.9 (4.5) | <0.0001 | −18.4 (4.3) | <0.0001 |
| White | Referent | Referent | ||
| Total percent fat | 3.4 (0.3) | <0.0001 | 3.1 (0.3) | <0.0001 |
| Age | 2.1 (0.6) | 0.0004 | 2.1 (0.6) | 0.0003 |
| Education ≤HS diploma | 7.1 (6.5) | 0.2784 | ||
| At least some college | Referent | |||
| # Children | 0.1 (1.5) | 0.9301 | ||
| Depressive symptoms | 19.6 (6.3) | 0.0022 | ||
| SHBG | −0.4 (0.1) | <0.0001 | ||
HS, high school; SHBG, sex hormone–binding globulin.
DISCUSSION
This study demonstrates a significant negative association between PA and IAF independent of multiple covariates in a sample of African-American and white women at midlife. There is biologic plausibility for this association. Although SAF drains into the systemic circulation, IAF drains directly into the portal circulation, releasing free fatty acids and causing insulin resistance (10). Moreover, expanded IAF mass produces proinflammatory cytokines that could contribute to premature atherosclerosis and increase the risk of an acute coronary syndrome (31). Excess IAF has been associated with markedly reduced plasma levels of adiponectin, a cytokine synthesized by fat cells that has antiatherogenic and antidiabetic properties (32–34). In our study, subjects in the Clinically Significant PA group were able to blunt the menopause-related increase in IAF. This may be related to a hypothesized link between exercise and cytokine response.
Menopause is a time when PA decreases and IAF increases. A recent longitudinal study of African-American and white midlife women found reduced PA levels before and during menopause and decreased energy expenditure and fat oxidation (17). Both cross-sectional and longitudinal studies have shown an increase in IAF over the menopause, independent of age and total body fat (35–38). On average, women in our study had IAF of 95.3 (53.1) cm2. Each 1-point higher total PA score related to 4.0 cm2 lower IAF, roughly 4.2% of our subject’s average IAF.
Although we could not directly compare our findings to the meta-analysis of solo aerobic exercise interventions on IAF as our SWAN subjects were not constrained in energy intake, we wanted to explore how community-based, self-selected sports/exercise dosing might impact the menopause-related increase in IAF. The Clinical PA Index that we calculated did not have the same cut-points as that used in the recent meta-analysis with our Clinically Significant PA Group performing at ≥8 METs/h/week (≥2 h of moderate (4 METs)) not the 10 METs/h/week level they concluded was necessary for a preferential reduction in IAF (22). We found that in our Clinically Significant PA Group there was less of an increase in IAF in the pre-/pericompared to postmenopausal subjects relative to the No Clinically Significant PA Group. Eight METs/h/week may not completely blunt the menopause-related increase in IAF; however, it does weaken it.
Consistent with previous studies, African-American women in our cohort had higher total body fat mass and SAF than their white counterparts but lower IAF (24,39). Thus ethnic differences in such anthropometric measures as BMI are largely accounted for by differences in SAF. We found no differences between the white and African-American women in the relationship between PA and IAF, suggesting that for both ethnic groups PA could have benefits on IAF.
Strengths of this study include a large biracial sample of women from a well-characterized cohort study, CT assessments for quantifying IAF and SAF, and adjustment for important correlates of intra-abdominal adiposity, including total body fat mass by dual-energy X-ray absorptiometry scan. The study has limitations. PA was measured using a recall survey. There is no measure of physical or cardiovascular fitness, which may play a role in the association between PA and IAF. As the data presented here are cross-sectional, we cannot conclude that lower PA causes more IAF. Our findings suggest that motivating white and black women to increase their PA during their middle years can positively modify menopause-related increases in IAF, which may impact positively on cardiovascular disease risk profile with further determinations of the mode, frequency, duration, and timing of exercise warranted.
With repeat longitudinal assessments of IAF over 4 years in the SWAN Fat Patterning Study, we hope to further elucidate the temporal relationship between PA and IAF and determine a dose–response curve for the amount of PA needed to combat the accumulation of intra-abdominal adiposity over the menopause. Future research is needed to tease out the role of cytokines in this relationship, especially related to menopausal status, ethnic groups, body composition, and PA level.
Acknowledgments
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), Department of Health and Human Services, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The SWAN Fat Patterning Study is supported by the National Heart, Lung and Blood Institute (grant HL067128) and the Charles J. and Margaret Roberts Trust. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH. Clinical Center: University of Michigan, Ann Arbor—MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA—Robert Neer, PI 1994–1999; Joel Finkelstein, PI 1999–present; Rush University, Rush University Medical Center, Chicago, IL—Lynda Powell, PI 1994–2009; Howard Kravitz, PI 2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; Nanette Santoro, PI 2004–present; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI. NIH Program Office: National Institute on Aging, Bethesda, MD—Marcia Ory 1994–2001; Sherry Sherman 1994–present; National Institute of Nursing Research, Bethesda, MD–Program Officers. Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating Center: New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001; University of Pittsburgh, Pittsburgh, PA—Kim Sutton-Tyrrell, PI 2001–present. Steering Committee: Chris Gallagher, Chair; Susan Johnson, Chair. We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Disclosure
The authors declared no conflict of interest.
References
- 1.Després JP, Moorjani S, Lupien PJ, et al. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10:497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- 2.Després JP. Visceral obesity, insulin resistance, and dyslipidemia: contribution of endurance exercise training to the treatment of the plurimetabolic syndrome. Exerc Sport Sci Rev. 1997;25:271–300. [PubMed] [Google Scholar]
- 3.Matsuzawa Y, Shimomura I, Nakamura T, et al. Pathophysiology and pathogenesis of visceral fat obesity. Obes Res. 1995;3 (Suppl 2):187S–194S. doi: 10.1002/j.1550-8528.1995.tb00462.x. [DOI] [PubMed] [Google Scholar]
- 4.Peiris AN, Sothmann MS, Hoffmann RG, et al. Adiposity, fat distribution, and cardiovascular risk. Ann Intern Med. 1989;110:867–872. doi: 10.7326/0003-4819-110-11-867. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Ford ES, McGuire LC, Mokdad AH. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity (Silver Spring) 2007;15:216–224. doi: 10.1038/oby.2007.505. [DOI] [PubMed] [Google Scholar]
- 6.Okosun IS, Chandra KM, Boev A, et al. Abdominal adiposity in U.S. adults: prevalence and trends, 1960–2000. Prev Med. 2004;39:197–206. doi: 10.1016/j.ypmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Després JP, Nadeau A, Tremblay A, et al. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. 1989;38:304–309. doi: 10.2337/diab.38.3.304. [DOI] [PubMed] [Google Scholar]
- 8.Raison JM, Achimastos AM, Safar ME. Sex-dependence of body fat distribution in patients with obesity and hypertension. Clin Exp Hypertens A. 1992;14:505–525. doi: 10.3109/10641969209036203. [DOI] [PubMed] [Google Scholar]
- 9.Kanai H, Matsuzawa Y, Kotani K, et al. Close correlation of intra-abdominal fat accumulation to hypertension in obese women. Hypertension. 1990;16:484–490. doi: 10.1161/01.hyp.16.5.484. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita S, Nakamura T, Shimomura I, et al. Insulin resistance and body fat distribution. Diabetes Care. 1996;19:287–291. doi: 10.2337/diacare.19.3.287. [DOI] [PubMed] [Google Scholar]
- 11.Nicklas BJ, Penninx BW, Ryan AS, et al. Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diabetes Care. 2003;26:1413–1420. doi: 10.2337/diacare.26.5.1413. [DOI] [PubMed] [Google Scholar]
- 12.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61:646–653. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Svendsen OL, Hassager C, Christiansen C. Age- and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy X-ray absorptiometry. Metab Clin Exp. 1995;44:369–373. doi: 10.1016/0026-0495(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 14.Toth MJ, Tchernof A, Sites CK, Poehlman ET. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Metab Disord. 1996;20:213–219. doi: 10.1038/sj.ijo.0801118. [DOI] [PubMed] [Google Scholar]
- 15.Simkin-Silverman LR, Wing RR. Weight gain during menopause. Is it inevitable or can it be prevented? Postgrad Med. 2000;108:47–50. 53–56. doi: 10.3810/pgm.2000.09.1.1204. [DOI] [PubMed] [Google Scholar]
- 16.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med. 2008;168:1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slentz CA, Aiken LB, Houmard JA, et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol. 2005;99:1613–1618. doi: 10.1152/japplphysiol.00124.2005. [DOI] [PubMed] [Google Scholar]
- 19.Giannopoulou I, Ploutz-Snyder LL, Carhart R, et al. Exercise is required for visceral fat loss in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:1511–1518. doi: 10.1210/jc.2004-1782. [DOI] [PubMed] [Google Scholar]
- 20.Ryan AS, Nicklas BJ, Berman DM. Aerobic exercise is necessary to improve glucose utilization with moderate weight loss in women. Obesity (Silver Spring) 2006;14:1064–1072. doi: 10.1038/oby.2006.122. [DOI] [PubMed] [Google Scholar]
- 21.Smith SR, Zachwieja JJ. Visceral adipose tissue: a critical review of intervention strategies. Int J Obes Relat Metab Disord. 1999;23:329–335. doi: 10.1038/sj.ijo.0800834. [DOI] [PubMed] [Google Scholar]
- 22.Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I. A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int J Obes (Lond) 2007;31:1786–1797. doi: 10.1038/sj.ijo.0803683. [DOI] [PubMed] [Google Scholar]
- 23.Rosamond W, Flegal K, Friday G, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 24.Stanforth PR, Jackson AS, Green JS, et al. Generalized abdominal visceral fat prediction models for black and white adults aged 17–65 y: the HERITAGE Family Study. Int J Obes Relat Metab Disord. 2004;28:925–932. doi: 10.1038/sj.ijo.0802563. [DOI] [PubMed] [Google Scholar]
- 25.Sowers MF, Crawford SL, Sternfeld B, et al. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. Academic Press; San Diego, CA: 2000. pp. 175–188. [Google Scholar]
- 26.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 27.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 28.Yoshizumi T, Nakamura T, Yamane M, et al. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–286. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 29.Seidell JC, Oosterlee A, Thijssen MA, et al. Assessment of intra-abdominal and subcutaneous abdominal fat: relation between anthropometry and computed tomography. Am J Clin Nutr. 1987;45:7–13. doi: 10.1093/ajcn/45.1.7. [DOI] [PubMed] [Google Scholar]
- 30.Myers JK, Weissman MM. Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry. 1980;137:1081–1084. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- 31.Després JP. Cardiovascular disease under the influence of excess visceral fat. Crit Pathw Cardiol. 2007;6:51–59. doi: 10.1097/HPC.0b013e318057d4c9. [DOI] [PubMed] [Google Scholar]
- 32.Côté M, Mauriège P, Bergeron J, et al. Adiponectinemia in visceral obesity: impact on glucose tolerance and plasma lipoprotein and lipid levels in men. J Clin Endocrinol Metab. 2005;90:1434–1439. doi: 10.1210/jc.2004-1711. [DOI] [PubMed] [Google Scholar]
- 33.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 34.Park KG, Park KS, Kim MJ, et al. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res Clin Pract. 2004;63:135–142. doi: 10.1016/j.diabres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Kanaley JA, Giannopoulou I, Tillapaugh-Fay G, Nappi JS, Ploutz-Snyder LL. Racial differences in subcutaneous and visceral fat distribution in postmenopausal black and white women. Metab Clin Exp. 2003;52:186–191. doi: 10.1053/meta.2003.50024. [DOI] [PubMed] [Google Scholar]
- 36.Tchernof A, Desmeules A, Richard C, et al. Ovarian hormone status and abdominal visceral adipose tissue metabolism. J Clin Endocrinol Metab. 2004;89:3425–3430. doi: 10.1210/jc.2003-031561. [DOI] [PubMed] [Google Scholar]
- 37.Guthrie JR, Dennerstein L, Dudley EC. Weight gain and the menopause: a 5-year prospective study. Climacteric. 1999;2:205–211. doi: 10.3109/13697139909038063. [DOI] [PubMed] [Google Scholar]
- 38.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 39.Lovejoy JC, Smith SR, Rood JC. Comparison of regional fat distribution and health risk factors in middle-aged white and African American women: the Healthy Transitions Study. Obes Res. 2001;9:10–16. doi: 10.1038/oby.2001.2. [DOI] [PubMed] [Google Scholar]