Abstract
The stage of development between birth and weaning in mammals is a period of very rapid growth that is crucial for the long-term well-being of the animal. The rate of protein deposition in neonatal animals is very high because dietary protein is efficiently utilized to increase body protein mass. Our studies in neonatal pigs have shown that this high efficiency of protein deposition is largely due to the marked increase in protein synthesis after feeding, and this response is particularly profound in the skeletal muscle. The enhanced stimulation of muscle protein synthesis in neonates after feeding is independently mediated by the rise in insulin and amino acids and this response declines with age. Intracellular signaling components that respond to the postprandial rise in amino acids and insulin have been identified and their activation has been shown to be elevated in skeletal muscle of neonatal pigs after a meal and to decrease with development. The enhanced activation of these components in the amino acid and insulin signaling pathways in neonatal muscle contributes to the high rate of muscle protein synthesis and rapid gain in skeletal muscle mass in newborn pigs, which are essential determinants of efficient growth during development.
Keywords: growth, newborn, protein metabolism, translation initiation, mammalian target of rapamycin
Implications
The skeletal muscles of healthy newborn mammals grow at rapid rates. Using baby pigs as an animal model, we have shown that this is because the rate at which their muscles synthesize protein increases profoundly when they eat. The rise in amino acids, which are the building blocks of proteins, and the hormone, insulin, after eating stimulates the synthesis of proteins in muscle. Intracellular signaling proteins have been identified that stimulate the synthesis of muscle proteins by becoming activated in response to the rise in amino acids and insulin. We have shown that the activity of these signaling proteins is elevated in muscle of the newborn pig and decreases with age. Thus, the high capacity of muscle in the newborn mammal to activate these signaling components contributes to the rapid growth of muscle in neonates. These studies are providing physiologically relevant information on the molecular mechanisms that regulate protein accretion and growth in a model of high agricultural relevance.
Developmental regulation of protein deposition
The rate of growth is higher during the neonatal period than at any other stage of postnatal life in mammals (Young, 1970). In the newborn, the rate of protein deposition is very rapid and is greater in skeletal muscle than in other tissues of the body (Reeds et al., 1992; Reeds et al., 1993). As a result, the amount of muscle protein relative to body protein increases substantially during the neonatal period (Davis et al., 1989; Fiorotto et al., 2000). However, the fractional rate of growth of skeletal muscle, that is, the amount of muscle weight gained in relation to the existing muscle mass, decreases markedly during the neonatal period.
Changes in the rate of protein deposition can be driven by changes in the rate of protein synthesis and (or) protein degradation. Protein deposition occurs when the rate of protein synthesis is higher than the rate of protein degradation. The high rate of muscle protein deposition in the newborn and the decline in the fractional rate of muscle protein deposition during early postnatal development are due to an elevated fractional rate of protein synthesis at birth, which declines with age (Kelly et al., 1984; Davis et al., 1989; Davis et al., 1996; Fiorotto et al., 2000). Indeed, fractional rates of muscle protein synthesis in the pig and rat are about threefold higher at birth than at weaning. In contrast, fractional rates of protein degradation in skeletal muscle are modestly elevated in early life and decline slightly with age.
Rates of protein synthesis are determined by the abundance of ribosomes in a tissue and the efficiency with which they translate mRNA into protein. The high rate of protein synthesis in newborn muscle and its developmental decline are in part driven by an elevated number of ribosomes at birth and a reduction in ribosome concentration as the skeletal muscle matures (Davis et al., 1989; Fiorotto et al., 2000). The elevated capacity for protein synthesis in skeletal muscle of the neonatal pig is also driven by an increased efficiency of the translation process, which is markedly enhanced in response to ingestion of a meal (Davis et al., 1996).
Feeding stimulates muscle protein synthesis
Dietary protein is utilized very efficiently for the deposition of whole-body protein in the neonate, with little lost through catabolic processes (Davis et al., 1993a). Our study in rats and pigs suggests that this high efficiency is due to a marked increase in the synthesis of body protein after eating (Davis et al., 1993b; Burrin et al., 1995; Davis et al., 1996). Although feeding stimulates protein synthesis in all tissues, the magnitude of the increase is greater in skeletal muscle than in other tissues of the body (Davis et al., 1996). Feeding stimulates protein synthesis in growing animals and humans (Garlick et al., 1983; Oddy et al., 1987; Denne et al., 1991) and the response decreases with age (Melville et al., 1989; Davis et al., 1996). The high rate of muscle protein synthesis in neonates in response to food ingestion should be anticipated because the rate of protein deposition during the postprandial period must be higher than the rate of protein loss during the postabsorptive period to allow growth of skeletal muscle.
Insulin is an important regulator of muscle protein synthesis in neonatal pigs
Insulin increases rapidly in response to feeding and plays a key role in regulating the assimilation of nutrients. Early studies performed in incubated muscles obtained from rodents showed that insulin stimulates the synthesis of protein (Harmon et al., 1984). Because of these findings, studies to identify the mechanism responsible for the increase in protein synthesis in skeletal muscle after eating have focused on insulin. In weaned rats, it was shown that the stimulation of muscle protein synthesis by feeding can be blocked by anti-insulin serum (Preedy and Garlick, 1986). The infusion of insulin stimulates protein synthesis in skeletal muscle of overnight-fasted weaned rats (Garlick et al., 1983), whole-body protein synthesis in fetal sheep (Liechty et al., 1992) and the synthesis of protein in the hindlimb of young lambs (Wester et al., 2000). In marked contrast to studies conducted in growing animals, most studies performed in adult animals and humans show little, if any, response of muscle protein synthesis to variations in insulin concentration within the physiological range (Gelfand and Barrett, 1987; Baillie and Garlick, 1992). Altogether, these results suggest that the response of muscle protein synthesis to insulin is characteristic of the immature muscle and may be developmentally regulated.
Circulating concentrations of amino acids decrease during experimentally induced hyperinsulinemia. Because a decrease in amino acids during the infusion of insulin could limit the ability of insulin to stimulate protein synthesis, we developed a hyperinsulinemic–euglycemic–euaminoacidemic clamp technique to examine the role of insulin in the regulation of protein synthesis, independent of changes in circulating amino acids and glucose (Wray-Cahen et al., 1997). This technique allows the maintenance of amino acids and glucose at fasting or any other desired level during the infusion of insulin. More recently, we modified this technique by the addition of somatostatin, which blocks insulin and glucagon secretion by the pancreas, so that these hormones also can be maintained at any desired level (including zero). This technique is termed the pancreatic–substrate clamp (Vann et al., 2000). Using the hyper-insulinemic–euglycemic–euaminoacidemic clamp technique in overnight-fasted 7- and 26-day-old pigs, we showed that when amino acids and glucose are maintained at fasting levels, the infusion of insulin increases the uptake and utilization of amino acids by the body (Wray-Cahen et al., 1997). Furthermore, the younger the pig, the greater are both the sensitivity and responsiveness to insulin. This suggests that the age-related decline in the insulin sensitivity of whole-body amino acid disposal may underlie the age-related decline in the efficiency with which dietary amino acids are utilized for protein deposition.
In further studies using the hyperinsulinemic–euglycemic–euaminoacidemic clamp technique, we showed that raising circulating insulin concentrations from fasting to fed levels can increase the rate of protein synthesis in skeletal muscle of neonatal pigs, even when amino acids and glucose are maintained at fasting levels (Wray-Cahen et al., 1998). The rate of muscle protein synthesis achieved is similar to that normally present in the fed state. This response to insulin, like the response to feeding, is greater the younger the animal is. Using the more refined pancreatic–substrate clamp technique, we showed that insulin stimulates protein synthesis in skeletal muscle in a dose-dependent manner within the physiological range (O’Connor et al., 2003a) until maximal rates are achieved at the level of insulin normally present in the fully-fed state. Moreover, insulin stimulates protein synthesis in muscle of the neonatal pig even when amino acids are allowed to fall to half of the level normally present in the fasting state. This suggests that insulin mediates the stimulation of protein synthesis by feeding in skeletal muscle of the neonate and this effect of insulin is independent of amino acids. The response to insulin is specific for skeletal muscle as protein synthesis in the visceral tissues does not respond to insulin infusion (Davis et al., 2001; Suryawan et al., 2009), suggesting that the feeding-induced stimulation of protein synthesis in visceral tissues is not mediated by insulin and that other anabolic factors are involved.
Amino acids are critical regulators of muscle protein synthesis in neonatal pigs
The rise in circulating amino acids that occurs after a meal is critical to the upregulation of whole-body rates of protein synthesis. In growing, adult and older mammals, amino acid infusion stimulates protein synthesis in skeletal muscle (Preedy and Garlick, 1986; Bennet et al., 1990; Volpi et al., 1998). Thus, amino acids appear to have the ability to stimulate protein synthesis in skeletal muscle throughout life, in contrast to the loss with age in the ability of insulin to stimulate muscle protein synthesis.
To examine the role of amino acids as regulators of muscle protein synthesis during the neonatal period, neonatal pigs were infused with an amino acid mixture that is similar in composition to the amino acid composition of body protein (Davis et al., 2002). We showed that infusion of amino acids to raise the circulating amino acid concentrations to the fed level increases protein synthesis in skeletal muscle. The response to amino acids decreases with age in parallel with the age-related decline in the response of muscle protein synthesis to eating. Using the pancreatic clamp technique, we showed that the stimulation of muscle protein synthesis by amino acids occurs in the presence of fasting levels of insulin, at levels of insulin that are intermediate between the fasting and the fully fed levels, and even when circulating insulin levels are reduced to undetectable levels (O’Connor et al., 2003a). The magnitude of the increase in muscle protein synthesis in response to fed levels of amino acids is similar to the magnitude of the increase in protein synthesis in response to fed levels of insulin and the magnitude of the increase in protein synthesis that occurs after a meal. The acute increase in muscle protein synthesis in response to fed levels of amino acids can be largely reproduced by the provision of fed levels of the branched-chain amino acid, leucine (Escobar et al., 2005; Escobar et al., 2006).
Thus, amino acids and insulin appear to act independently to stimulate protein synthesis in skeletal muscle of the neonate after a meal, although the responses are not additive. The ability of skeletal muscle to respond to both insulin and amino acids likely contributes to the more rapid gain in protein mass in skeletal muscle, compared to other tissues of the body, in the neonate. Because amino acids also stimulate protein synthesis in most visceral tissues of the neonate (O’Connor et al., 2004; Suryawan et al., 2009), it appears that the rise in amino acids after a meal mediates the post-prandial increase in protein synthesis in visceral as well as peripheral tissues of the neonate.
Insulin signaling pathway leading to protein synthesis
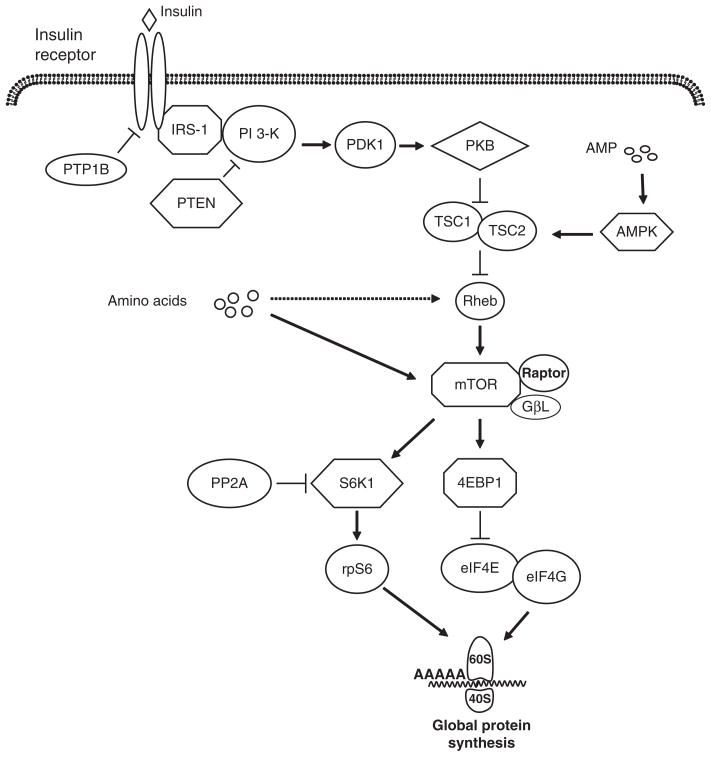
Genetic and biochemical studies have shown the importance of the insulin signaling pathway in the regulation of protein synthesis. The insulin signaling cascade is initiated by the binding of insulin to its receptor (Figure 1), inducing autophosphorylation of the receptor on its tyrosine residues, followed by the activation of its tyrosine kinase activity (Di Guglielmo et al., 1998; Bevan, 2001). Insulin binding to its receptor leads to the activation of the insulin receptor substrate (IRS)-1/2 proteins (White and Kahn, 1994). IRS 1/2 proteins acts as docking proteins that transmit the insulin signal to several signaling molecules including phosphoinositide 3-kinase (PI 3-kinase) and phosphoinositide-dependent kinase 1 (PDK-1). Their activation triggers downstream signaling pathways that lead to various insulin-stimulated biological responses including protein synthesis (Egawa et al., 1999). Protein kinase B (PKB) phosphorylates and inactivates an inhibitor of cell growth, called tuberous sclerosis complex 1 and 2 (TSC2), to inactivate the function of the TSC1/2 complex, resulting in the activation of Rheb, followed by the induction of a mammalian target of rapamycin (mTOR), a master protein kinase involved in cellular growth (Inoki et al., 2003; Kwiatkowski and Manning, 2005).
Figure 1.
Insulin and amino acid signaling pathways that lead to the stimulation of translation initiation. The binding of insulin to its receptor activates the insulin receptor and insulin receptor substrate-1 (IRS-1), followed by the activation of phosphoinositide-3 kinase (PI 3-kinase). Activated PI3 kinase stimulates phosphoinositide-dependent kinase 1 (PDK-1) and protein kinase B (PKB) activation. Phosphorylation of PKB inactivates tuberous sclerosis complex 1 and 2 (TSC1/2), inducing the activation of Rheb and mammalian target of rapamycin (mTOR). Both amino acids and insulin activate mTOR, which exists in a complex with raptor and G protein β-subunit-like protein (GβL). Activated mTOR phosphorylates ribosomal protein S6 kinase 1 (S6K1) and eukaryotic initiation factor (eIF) 4E-binding protein-1 (4EBP1). Phosphorylation of S6K1 enhances the activation of ribosomal subunit S6 (rpS6), which increases the translation of specific mRNA. Phosphorylated 4EBP1 releases eIF4E from an inactive complex with 4EBP1, enabling the formation of the active eIF4E-eIF4G complex that mediates mRNA binding to the ribosome. Insulin signaling can be attenuated by a number of signaling proteins. These include protein tyrosine phosphatase-1B (PTP-1B), which dephosphorylates the insulin receptor and IRS-1; phosphatase and tensin homolog deleted on chromosome 10 (PTEN), which inactivates PI 3-kinase; and protein phosphatase 2A (PP2A), which decreases activation of PKB and S6K1. An increase in adenosine monophosphate (AMP) levels enhances AMP kinase activation, increasing TSC1/2 complex activation and decreasing mTOR activation.
mTOR binds to other cellular effectors to form the sub-cellular complexes, mTORC1 and mTORC2, that regulate cellular growth and differentiation. mTOR binds with raptor and G protein β-subunit-like protein (GβL) in a complex referred to as mTOR complex 1 (mTORC1; Kim et al., 2002; Sabitini, 2006). The role of raptor, although apparently essential for mTORC1 function, is not well understood, but it may serve as a scaffold for the association of mTORC1 with its downstream effectors, ribosomal protein S6 kinase 1 (S6K1) and eIF4E-binding protein-1 (4EBP1; Avruch et al., 2005; Martin and Hall, 2005). mTORC2 consists of mTOR, GβL and rictor and this complex has recently been shown to activate PKB (Avruch et al., 2005), although it is less implicated in the direct regulation of mRNA translation. In contrast, activation of mTORC1 upregulates mRNA translation by enhancing the phosphorylation of S6K1 and 4EBP1. Phosphorylated 4EBP1 releases eIF4E from an inactive eIF4E-4EBP1 complex, allowing the formation of the active eIF4E-eIF4G complex that mediates the binding of mRNA to the 43S ribosomal complex (Niedzwiecka et al., 2002).
Insulin signaling is downregulated by the action of a number of phosphatases. These include protein tyrosine phosphatase-1B (PTP-1B), a negative regulator of tyrosine phosphorylation of the insulin receptor and IRS-1 (Egawa et al., 2001). Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inactivates PI 3-kinase, thereby reducing downstream signaling (Goberdhan and Wilson, 2003). Protein phosphatase 2A (PP2A) negatively regulates the phosphorylation state of PKB, S6K1 and 4EBP1 (Peterson et al., 1999).
Amino acid signaling pathway leading to protein synthesis
The signaling pathway by which amino acids stimulate protein synthesis is less well understood than that of insulin. Studies performed in cell culture suggest that mTOR integrates amino acid and insulin signals via multiple mechanisms including phosphorylation of S6K1 and 4EBP1, resulting in the modulation of protein synthesis and cell growth (Proud, 2004). However, little is known about the mechanism by which amino acids modulate the activation of signaling components upstream of mTORC1. The adenosine monophosphate-activated protein kinase (AMPK) phosphorylates TSC2 on specific sites that enhance its activity during conditions of energy starvation (Hardie, 2005). This results in the inhibition of mTORC1 activation and a reduction in protein synthesis. It has been suggested that amino acids modulate mTOR through TSC2, but this has been controversial (Kimball and Jefferson, 2006; Smith et al., 2005). It has also been postulated that amino acids regulate the binding of raptor to mTOR, resulting in the activation of downstream effectors of the mTORC1 complex, S6K1 and 4EBP1 (Hara et al., 2002; Kim et al., 2002). Amino acid stimulation may also convert Rheb guanosine diphosphate to Rheb-guanosine triphosphate, thereby enhancing the association between Rheb and mTOR, and resulting in mTOR activation (Kimball, 2007; Long et al., 2005).
Regulation of the insulin and amino acid signaling pathways leading to translation initiation in neonatal muscle
Most of the studies that have examined the regulation of the insulin and amino acid signaling pathways have been performed in cell culture, and there has been little study of intact animals under physiologically relevant conditions. Our studies in the neonatal pig showed that the abundance of the insulin-receptor protein in skeletal muscle of the newborn is twofold higher than at weaning (Suryawan et al., 2001). The abundance of some of the downstream signaling proteins, including PKB and mTOR, also decreases as the animal matures (Kimball et al., 2002). Moreover, the activation of the insulin receptor, IRS-1, PI 3-kinase, PDK-1 and PKB in skeletal muscle increases after a meal and these responses also decrease profoundly with age (Suryawan et al., 2001; Suryawan and Davis, 2005). Furthermore, we showed that the infusion of physiological levels of insulin increases the activation of these insulin signaling components in a dose-dependent manner and that the effect of insulin on the activation of these early steps in the insulin signaling pathway is not influenced by amino acids, regardless of age (Suryawan et al., 2004).
Recently, we showed that the higher rate of protein synthesis in neonatal compared to more mature muscle is in part due to a reduced activation of TSC1/2 and an enhanced activation of mTOR1 (Suryawan et al., 2006). We further showed that insulin, but not amino acids, increases PKB activation and decreases TSC2 activation in neonatal muscle. However, both insulin and amino acids increase the phosphorylation of mTOR (Suryawan et al., 2007).
Our studies have shown that the activation of translation initiation factors in skeletal muscle of the neonatal pig increases in response to feeding (Davis et al., 2000; Kimball et al., 2002; Suryawan et al., 2006). The phosphorylation and activation of mTOR increase after a meal, although the association of mTOR with raptor is not altered. Feeding also increases the phosphorylation of the downstream effectors of mTOR1, that is, S6K1 and 4EBP1, resulting in a decrease in association of the inactive eIF4E-4EBP1 complex, and an increase in formation of the active eIF4E-eIF4G complex that regulates the binding of mRNA to the ribosome. These responses to feeding decline with age in skeletal muscle.
The infusion of either insulin or amino acids, within the physiological range, increases the phosphorylation of mTOR, S6K1 and 4EBP1, decreases the binding of 4E-BP1 to eIF4E and increases eIF4E binding to eIF4G (O’Connor et al., 2003b). This suggests that the rise in both insulin and amino acids after a meal activates signaling components that are downstream of PKB, including mTOR, S6K1, 4EBP1 and eIF4E-eIF4G, and thus that both insulin and amino acids utilize a common signaling pathway downstream of PKB. In addition, the rise in the branched-chain amino acid, leucine, alone also increases mTOR signaling (Escobar et al., 2005; Escobar et al., 2006). However, only the rise in insulin after a meal, and not amino acids, activates PKB and the signaling components that are upstream of PKB, that is, the insulin receptor, IRS-1 and PI 3-kinase (Suryawan et al., 2004).
Besides some reports in cell culture, less is known about the negative regulators of the insulin and amino acid signaling pathways that lead to translation initiation in intact animals under physiologically relevant conditions. Our studies have shown that the activation of PTP1B, PTEN, PP2A and TSC2, which are negative regulators of insulin signaling, is low in muscle of neonatal animals and increases with development (Suryawan and Davis, 2003; Suryawan et al., 2006). These changes are consistent with the age-related decrease in muscle protein synthesis. The activation of TSC2 decreases in response to feeding, although feeding has no effect on PTP1B, PTEN and PP2A activation. Interestingly, the activation of AMPK, a negative regulator of the mTOR pathway and a sensor of cellular energy, does not change with feeding and is not affected by age.
Altogether, the results suggest that the age-related changes in the activation of both positive and negative regulators of the insulin and amino acid signaling pathways contribute to the high rate of protein synthesis in neonatal pigs and the decrease in response to insulin and amino acids that occurs as the skeletal muscle matures.
Conclusion
Protein deposition is very rapid during the early life of pigs and this is driven by the high fractional rate of protein synthesis in skeletal muscle. Neonates efficiently utilize their dietary amino acids for growth due to an elevated capacity for stimulation of skeletal muscle protein synthesis after food ingestion. This feeding-induced stimulation of muscle protein synthesis is mediated by an enhanced sensitivity to the postprandial rise in circulating insulin and amino acids. Components in the insulin and amino acid signaling pathways and translation initiation that are involved in the feeding-induced stimulation of protein synthesis in skeletal muscle have been identified. The enhanced activation of these signaling components in neonatal muscle contributes to the high rate of protein synthesis and rapid gain in skeletal muscle mass in neonates and determines their ability to grow rapidly during the developmental stage.
Acknowledgments
This study is based on an invited presentation at the 60th Annual Meeting of the European Association for Animal Production held in Barcelona, Spain during August 2009. This is a publication of the United States Department of Agriculture/Agriculture Research Service (USDA/ARS) Children’s Nutrition Research Center, Department of Pediatric, Baylor College of Medicine. This project was supported in part by the National Institute of Health (NIH) Grant AR-44474 and by the USDA/ARS under cooperative agreement no. 6250510000-040. The contents of this publication do not necessarily reflect the views or policies of the US Department of Agriculture, and nor does the mention of trade names, commercial products or organizations imply endorsement by the US Government.
References
- Avruch J, Lin Y, Long X, Murthy S, Ortiz-Vega S. Recent advances in the regulation of the TOR pathway by insulin and amino acids. Current Opinion in Clinical Nutrition and Metabolic Care. 2005;8:67–72. doi: 10.1097/00075197-200501000-00010. [DOI] [PubMed] [Google Scholar]
- Baillie AG, Garlick PJ. Attenuated responses of muscle protein synthesis to fasting and insulin in adult female rats. American Journal of Physiology. 1992;262:E1–E5. doi: 10.1152/ajpendo.1992.262.1.E1. [DOI] [PubMed] [Google Scholar]
- Bennet WM, Connacher AA, Scrimgeour CM, Rennie MJ. The effect of amino acid infusion on leg protein turnover assessed by L-[15N]phenylalanine and L-[1-13C]leucine exchange. European Journal of Clinical Investigation. 1990;20:41–50. doi: 10.1111/j.1365-2362.1990.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Bevan P. Insulin signalling. Journal of Cell Science. 2001;114:1429–1430. doi: 10.1242/jcs.114.8.1429. [DOI] [PubMed] [Google Scholar]
- Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S. Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatric Research. 1995;37:593–599. doi: 10.1203/00006450-199505000-00006. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Reeds PJ. Amino acid compositions of body and milk protein change during the suckling period in rats. Journal of Nutrition. 1993a;123:947–956. doi: 10.1093/jn/123.5.947. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Enhanced response of muscle protein synthesis and plasma insulin to food intake in suckled rats. American Journal of Physiology. 1993b;265:R334–R340. doi: 10.1152/ajpregu.1993.265.2.R334. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of suckling rats. American Journal of Physiology. 1989;257:R1141–R1146. doi: 10.1152/ajpregu.1989.257.5.R1141. [DOI] [PubMed] [Google Scholar]
- Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than 26-day-old pigs. American Journal of Physiology. 1996;270:E802–E809. doi: 10.1152/ajpendo.1996.270.5.E802. [DOI] [PubMed] [Google Scholar]
- Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. American Journal of Physiology –Endocrinology and Metabolism. 2000;279:E1226–E1234. doi: 10.1152/ajpendo.2000.279.6.E1226. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Beckett PR, Burrin DG, Reeds PJ, Wray-Cahen D, Nguyen HV. Differential effects of insulin on peripheral and visceral tissue protein synthesis in neonatal pigs. American Journal of Physiology –Endocrinology and Metabolism. 2001;280:E770–E779. doi: 10.1152/ajpendo.2001.280.5.E770. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O’Connor PM. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. American Journal of Physiology – Endocrinology and Metabolism. 2002;282:E880–E890. doi: 10.1152/ajpendo.00517.2001. [DOI] [PubMed] [Google Scholar]
- Denne SC, Rossi EM, Kalhan SC. Leucine kinetics during feeding in normal newborns. Pediatric Research. 1991;30:23–27. doi: 10.1203/00006450-199107000-00005. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo GM, Drake PG, Baass PC, Authier F, Posner BI, Bergeron JJ. Insulin receptor internalization and signaling. Molecular and Cellular Biochemistry. 1998;182:59–63. [PubMed] [Google Scholar]
- Egawa K, Sharma PM, Nakashima N, Huang Y, Huver E, Boss GR, Olefsky JM. Membrane-targeted phosphatidylinositol 3-kinase mimics insulin actions and induces a state of cellular insulin resistance. Journal of Biological Chemistry. 1999;274:14306–14314. doi: 10.1074/jbc.274.20.14306. [DOI] [PubMed] [Google Scholar]
- Egawa K, Maegawa H, Shimizu S, Morino K, Nishio Y, Bryer-Ash M, Cheung AT, Kolls JK, Kikkawa R, Kashiwagi A. Protein-tyrosine phosphatase-1B negatively regulates insulin signaling in L6 myocytes and Fao hepatoma cells. Journal of Biological Chemistry. 2001;276:10207–10211. doi: 10.1074/jbc.M009489200. [DOI] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. American Journal of Physiology – Endocrinology and Metabolism. 2005;288:E914–E921. doi: 10.1152/ajpendo.00510.2004. [DOI] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. American Journal of Physiology – Endocrinology and Metabolism. 2006;290:E612–E621. doi: 10.1152/ajpendo.00402.2005. [DOI] [PubMed] [Google Scholar]
- Fiorotto ML, Davis TA, Reeds PJ. Regulation of myofibrillar protein turnover during maturation in normal and undernourished rat pups. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2000;278:R845–R854. doi: 10.1152/ajpregu.2000.278.4.R845. [DOI] [PubMed] [Google Scholar]
- Garlick PJ, Fern M, Preedy VR. The effect of insulin infusion and food intake on muscle protein synthesis in postabsorptive rats. Biochemical Journal. 1983;210:669–676. doi: 10.1042/bj2100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand RA, Barrett EJ. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. Journal of Clinical Investigation. 1987;80:1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberdhan DC, Wilson C. PTEN: tumour suppressor, multi-functional growth regulator and more. Human Molecular Genetics. 2003;12:R239–R248. doi: 10.1093/hmg/ddg288. [DOI] [PubMed] [Google Scholar]
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- Hardie DG. New roles for the LKB1–AMPK pathway. Current Opinion in Cell Biology. 2005;17:167–173. doi: 10.1016/j.ceb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Harmon CS, Proud CB, Pain VM. Effects of starvation, diabetes, and acute insulin treatment on the regulation of polypeptide-chain initiation in rat skeletal muscle. Biochemical Journal. 1984;223:687–696. doi: 10.1042/bj2230687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Kelly F, Lewis SEM, Anderson P, Goldspink DF. Pre- and postnatal growth and protein turnover in four muscles of the rat. Muscle and Nerve. 1984;7:235–242. doi: 10.1002/mus.880070309. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a amino acid-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Kimball SR. The role of nutrition in stimulating muscle protein accretion at the molecular level. Biochemical Society Transactions. 2007;35:1298–1301. doi: 10.1042/BST0351298. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. New functions for amino acids: effects on gene transcription and translation. American Journal of Clinical Nutrition. 2006;83:500S–507S. doi: 10.1093/ajcn/83.2.500S. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Farrell PA, Nguyen HV, Jefferson LS, Davis TA. Developmental decline in components of signal transduction pathways regulating protein synthesis in pig muscle. American Journal of Physiology –Endocrinology and Metabolism. 2002;282:E585–E592. doi: 10.1152/ajpendo.00269.2001. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Human Molecular Genetics. 2005;14:R251–R258. doi: 10.1093/hmg/ddi260. [DOI] [PubMed] [Google Scholar]
- Liechty EA, Boyle DW, Moorehead H, Liu YM, Denne SC. Effect of hyperinsulinemia on ovine fetal leucine kinetics during prolonged maternal fasting. American Journal of Physiology. 1992;263:E696–E702. doi: 10.1152/ajpendo.1992.263.4.E696. [DOI] [PubMed] [Google Scholar]
- Long X, Ortiz-Vega S, Lin Y, Avruch J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. Journal of Biological Chemistry. 2005;280:23433–23436. doi: 10.1074/jbc.C500169200. [DOI] [PubMed] [Google Scholar]
- Martin DE, Hall MN. The expanding TOR signaling network. Current Opinions in Cell Biology. 2005;17:158–166. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Melville S, McNurlan MA, McHardy KC, Broom J, Milne E, Calder AG, Garlick PJ. The role of degradation in the acute control of protein balance in adult man: failure of feeding to stimulate protein synthesis as assessed by L-[1-13C]leucine infusion. Metabolism: Clinical and Experimental. 1989;38:248–255. doi: 10.1016/0026-0495(89)90083-8. [DOI] [PubMed] [Google Scholar]
- Niedzwiecka A, Stepinski J, Darzynkiewicz E, Conenberg N, Stolarski R. Positive heat capacity change upon specific binding of translation initiation factor eIF4E to mRNA 5′cap. Biochemistry. 2002;41:12140–12148. doi: 10.1021/bi0258142. [DOI] [PubMed] [Google Scholar]
- O’Connor PM, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. American Journal of Physiology – Endocrinology and Metabolism. 2003a;284:E110–E119. doi: 10.1152/ajpendo.00326.2002. [DOI] [PubMed] [Google Scholar]
- O’Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. American Journal of Physiology –Endocrinology and Metabolism. 2003b;285:E40–E53. doi: 10.1152/ajpendo.00563.2002. [DOI] [PubMed] [Google Scholar]
- O’Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of neonatal liver protein synthesis by insulin and amino acids in pigs. American Journal of Physiology – Endocrinology and Metabolism. 2004;286:E994–E1003. doi: 10.1152/ajpendo.00391.2003. [DOI] [PubMed] [Google Scholar]
- Oddy VH, Lindsay DB, Barker PJ, Northrop AJ. Effect of insulin on hindlimb and whole body leucine and protein metabolism in fed and fasted lambs. British Journal of Nutrition. 1987;58:437–452. doi: 10.1079/bjn19870112. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Desai BN, Hardwick JS, Schreiber SL. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycin associated protein. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4438–4442. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preedy VR, Garlick PJ. The response of muscle protein synthesis to nutrient intake in postabsorptive rats: the role of insulin and amino acids. Bioscience Reports. 1986;6:177–183. doi: 10.1007/BF01115004. [DOI] [PubMed] [Google Scholar]
- Proud CG. Role of mTOR signalling in the control of translation initiation and elongation by nutrients. Current Topics in Microbiology and Immunology. 2004;279:215–244. doi: 10.1007/978-3-642-18930-2_13. [DOI] [PubMed] [Google Scholar]
- Reeds PJ, Fiorotto ML, Davis TA, Burrin DG. Homeorrhesis and homeostasis: problems of growth and maintenance. In: Takai K, editor. Frontiers and New Horizons in Amino Acid Research. Elsevier Science Publishers; Amsterdam, The Netherlands: 1992. pp. 133–144. [Google Scholar]
- Reeds PJ, Burrin DG, Davis TA, Fiorotto ML. Postnatal growth of gut and muscle: competitors or collaborators? Proceedings of the Nutrition Society. 1993;52:57–67. doi: 10.1079/pns19930037. [DOI] [PubMed] [Google Scholar]
- Sabitini DM. mTOR and cancer: insights into a complex relationship. Nature Reviews Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. Journal of Biological Chemistry. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Davis TA. Protein-tyrosine-phosphatase 1B activation is regulated developmentally in muscle of neonatal pigs. American Journal of Physiology – Endocrinology and Metabolism. 2003;284:E47–E54. doi: 10.1152/ajpendo.00210.2002. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Davis TA. Developmental regulation of protein kinase B activation is isoform specific in skeletal muscle of neonatal pigs. Pediatric Research. 2005;58:719–724. doi: 10.1203/01.PDR.0000180536.51032.AB. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Nguyen HV, Bush JA, Davis TA. Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. American Journal of Physiology – Endocrinology and Metabolism. 2001;281:E908–E915. doi: 10.1152/ajpendo.2001.281.5.E908. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Escobar J, Frank JW, Nguyen HV, Davis TA. Developmental regulation of the activation of signaling components leading to translation initiation in skeletal muscle of neonatal pigs. American Journal of Physiology – Endocrinology and Metabolism. 2006;291:E849–E859. doi: 10.1152/ajpendo.00069.2006. [DOI] [PubMed] [Google Scholar]
- Suryawan A, O’Connor PM, Bush JA, Nguyen HV, Davis TA. Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids. 2009;37:97–104. doi: 10.1007/s00726-008-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawan A, Orellana RA, Nguyen HV, Jeyapalan AS, Fleming JR, Davis TA. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. American Journal of Physiology – Endocrinology and Metabolism. 2007;293:E1597–E1605. doi: 10.1152/ajpendo.00307.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawan A, O’Connor PM, Kimball SR, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Amino acids do not alter the insulin-induced activation of the insulin signaling pathway in neonatal pigs. Journal of Nutrition. 2004;134:24–30. doi: 10.1093/jn/134.1.24. [DOI] [PubMed] [Google Scholar]
- Vann RC, Nguyen HV, Reeds PJ, Steele NC, Deaver DR, Davis TA. Somatotropin increases protein balance independent of insulin’s effects on protein metabolism in growing pigs. American Journal of Physiology –Endocrinology and Metabolism. 2000;279:E1–E10. doi: 10.1152/ajpendo.2000.279.1.E1. [DOI] [PubMed] [Google Scholar]
- Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. Journal of Clinical Investigation. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester TJ, Lobley GE, Birnie LM, Lomax MA. Insulin stimulates phenylalanine uptake across the hind limb in fed lambs. Journal of Nutrition. 2000;130:608–611. doi: 10.1093/jn/130.3.608. [DOI] [PubMed] [Google Scholar]
- White MF, Kahn CR. The insulin-signaling system. Journal of Biological Chemistry. 1994;269:1–4. [PubMed] [Google Scholar]
- Wray-Cahen D, Beckett PR, Nguyen HV, Davis TA. Insulin-stimulated amino acid utilization during glucose and amino acid clamps decreases with development. American Journal of Physiology. 1997;273:E305–E314. doi: 10.1152/ajpendo.1997.273.2.E305. [DOI] [PubMed] [Google Scholar]
- Wray-Cahen D, Nguyen HV, Burrin DG, Beckett PR, Fiorotto ML, Reeds PJ, Wester TJ, Davis TA. Response of skeletal muscle protein synthesis to insulin in suckling pigs decreases with development. American Journal of Physiology. 1998;275:E602–E609. doi: 10.1152/ajpendo.1998.275.4.E602. [DOI] [PubMed] [Google Scholar]
- Young VR. The role of skeletal and cardiac muscle in the regulation of protein metabolism. In: Munro HM, editor. Mammalian Protein Metabolism. Academic Press; New York, NY, USA: 1970. pp. 585–674. [Google Scholar]