Abstract
Purpose
To evaluate the clinical outcomes and relationship between tumor size, lymph node status, and prognosis in a large cohort of patients with confirmed triple receptor–negative breast cancer (TNBC).
Patients and Methods
We reviewed 1,711 patients with TNBC diagnosed between 1980 and 2009. Patients were categorized by tumor size and nodal status. Kaplan-Meier product limit method was used to calculate overall survival (OS) and relapse-free survival (RFS). A Sidak adjustment was used for multiple group comparisons. Cox proportional hazards models were fit to determine the association of tumor size and nodal status with survival outcomes after adjustment for other patient and disease characteristics.
Results
Median age was 48 years (range, 21 to 87 years). At a median follow-up of 53 months (range, 0.7 to 317 months), there were 614 deaths and 747 recurrences. The 5-year OS was 80% for node-negative patients (N0), 65% for one to three positive lymph nodes (N1), 48% for four to nine positive lymph nodes (N2), and 44% for ≥ 10 positive lymph nodes (N3; P < .0001). The 5-year RFS rates were 67% for N0, 52% for N1, 36% for N2, and 33% for N3 (P < .0001). Pairwise comparison by nodal status showed that when comparing N0 with node-positive disease, there was a significant difference in OS and RFS (P < .001 all comparisons). However, when comparing N1 with N2 and N3 disease regardless of tumor size, there were no significant differences in OS or RFS.
Conclusion
In patients with TNBC, once there is evidence of lymph node metastasis, the prognosis may not be affected by the number of positive lymph nodes.
INTRODUCTION
Breast cancer prognosis and survival have improved substantially over the last four decades. Such advances are mainly due to the systematic use of adjuvant chemotherapy and the development of directed therapies for hormone receptor–positive and C-erbB-2 (HER2) –positive tumors. However, human breast carcinomas are heterogeneous in nature and present with a wide spectrum of clinical, pathologic, and molecular profiles and with a variety of clinical outcomes. DNA microarray analysis has classified tumors based on gene expression patterns and their correlation with prognostic markers of overall survival (OS) and relapse-free survival (RFS).1 Expression patterns have defined five different subtypes: luminal A and B, HER2-enriched, basal-like, and normal breast-like tumors.1 Prospective studies have shown the shortest time to relapse and OS for the basal-like and HER2-enriched/estrogen receptor (ER) –negative subtype of tumors.1,2 Despite the poor prognosis seen in HER2/neu-positive tumors, the effectiveness of trastuzumab in this setting has had a significant impact in the outcome of these patients.3
Correlation studies between immunohistochemistry markers and genetic expression profiles have shown that the basal-like category of tumors overlap in approximately 70% to 80% with tumors that are negative for ER, progesterone receptor (PR), and HER2. This “triple-negative” category comprises 10% to 15% of all breast cancers.4,5 In retrospective studies, patients with TNBC have worse clinical outcomes when compared with those with non-TNBC.4–7 These tumors tend to present at a younger age,4 with higher histologic grade, larger size, high rate of p53 mutations, and Ki-67 staining and have a tendency toward local and visceral metastases rather than bone metastases.4–9
The factors responsible for the poor clinical outcomes seen in TNBC have been studied using gene expression profiles and more advanced genomic techniques. However, these approaches are still experimental and not widely available. Currently, assessment of prognosis and appropriate treatment is based on patient and standard tumor-related characteristics. Among these factors, tumor size and nodal status are known to be independent prognostic factors and play an important role in treatment decisions.10
It is well known that in breast cancer, the size of the primary tumor and the number of positive lymph nodes has an inverse linear relationship with prognosis and survival.10 The sixth edition of staging in breast cancer11 has included the size of the tumor and the nodal status as the most important prognostic factors. Previous analyses within patients with TNBC have shown that tumor size and lymph node status may not be linearly correlated with survival outcomes,4,12 raising the doubt for the accuracy and independence of tumor size and number of positive lymph nodes in the prediction of clinical outcomes. These studies were based on small numbers of patients. In this retrospective study, we sought to analyze the clinical outcomes and relationship between tumor size, lymph node status, and prognosis in a large cohort of patients with confirmed TNBC.
PATIENTS AND METHODS
Patient Population
Using the Breast Cancer Management System database of The University of Texas MD Anderson Cancer Center, we retrospectively identified all the patients with ER-negative, PR-negative, HER2-negative primary breast cancer diagnosed at our institution between December 1980 and June 2009 who underwent primary surgery. For the patients diagnosed before 1997, the immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) tests were performed as part of institutional review board–approved retrospective laboratory studies. Patients with ductal carcinoma in situ with microinvasion and patients with lack of information on tumor size, receptors, or lymph node status were excluded. We also excluded patients who received neoadjuvant chemotherapy. A total of 1,711 patients were identified and included in the analysis. Patients were categorized according to their tumor size and lymph node status. All patients were treated with a multidisciplinary approach. After definitive surgery, all patients received adjuvant chemotherapy with an anthracycline-based, a taxane-based, or an anthracycline/taxane-based and a nonanthracycline/taxane-based regimen. The institutional review board of The University of Texas MD Anderson Cancer Center approved the retrospective study.
Pathology Methods
Staging of primary tumors was based on the TNM system of the Sixth American Joint Committee on Cancer criteria.11 Grading of tumors and histologic classification were based on the modified Black's nuclear grading system13 and WHO criteria,14 respectively; evaluation was performed centrally by dedicated breast pathologists. Immunohistochemical analysis to determine ER and PR status was performed using standard procedures on 4-μm sections of paraffin-embedded tissues stained with monoclonal antibodies: 6F11 (Novacastra Laboratories, Burlingame, CA) for ER and 1A6 (Novacastra Laboratories) for PR. Nuclear staining ≥ 10% was considered a positive result. Before 1993, the dextran-coated charcoal ligand-binding method was used to determine ER and PR. Hormone receptor concentration ≥ 10 femtomole/mg protein was considered positive. Patients were considered hormone receptor–positive in case of ER and/or PR positivity. Nuclear staining ≤ 10% of either estrogen receptor or progesterone receptor was considered a negative result. HER2 status was evaluated by IHC or by FISH. HER2-negative was defined as negative receptor expression on IHC staining (membranous staining in < 10% of cells) and/or negative gene amplification found on FISH. A gene copy/CEP-17 ratio greater than 2.0 was considered amplified.
Statistical Analysis
Patients were categorized according to their tumor size into one of three groups: T1 (tumor size ≤ 2 cm), T2 (tumor size 2 to 5 cm), and T3/4 (tumor size ≥ 5 cm or with extension to chest wall or skin). On the basis of the pathology review, the number of positive lymph nodes was categorized into one of four groups: N0 (negative lymph nodes), N1 (one to three positive lymph nodes), N2 (four to nine positive lymph nodes), and N3 (≥ 10 positive lymph nodes). Patient and tumor characteristics were tabulated and compared between the three tumor size groups with the χ2 test. RFS was measured from the date of diagnosis to the date of first documented local or distant recurrence or last follow-up. Patients who died before experiencing a disease recurrence were considered censored at their dates of death.
OS was defined as the time from the date of diagnosis to the date of death or last follow-up. The Kaplan-Meier product limit method was used to estimate the survival outcomes of all patients by tumor size and nodal status; groups were compared using the log-rank statistic. Survival outcomes were also estimated by nodal status within each tumor size subgroup; a Sidak adjustment15 was used for multiple group comparisons. Cox proportional hazards models were fit to determine the association of tumor size and nodal status with survival outcomes after adjustment for other patient and disease characteristics (age > 50 v <50, lymphovascular invasion, histology, adjuvant radiation, and type of adjuvant chemotherapy). Results are expressed in hazard ratios (HRs) and 95% CIs. P values less than .05 were considered statistically significant; all tests were two-sided. Statistical analyses were carried out using SAS 9.2 (SAS Institute, Cary, NC) and S-Plus 7.0 (Insightful, Seattle, WA).
RESULTS
Patient Demographics, Tumor Characteristics, and Patterns of Distant Metastases
There were 19,474 patients with primary nonmetastatic breast cancer identified, of whom 14,447 did not receive neoadjuvant chemotherapy, and 1,711 had TNBC.
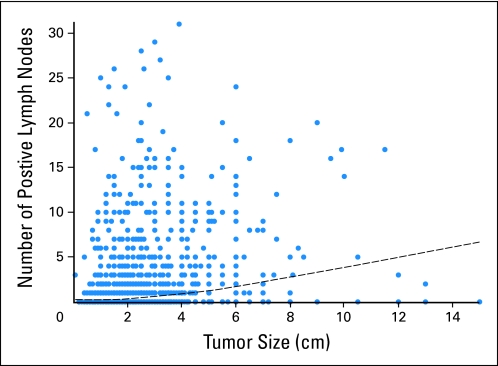
The total of 1,711 patients with TNBC were included. Patient characteristics are listed in Table 1. The median age was 48 years (range, 21 to 87 years). Eight hundred thirty-four patients (48.7%) had tumor size that was less than 2 cm (T1), 768 patients (44.9%) had tumor size that was between 2 and 5 cm (T2), and 109 patients (6.4%) had tumor size that was greater than 5 cm (T3/4). Figure 1 shows the distribution of positive lymph nodes against tumor size. The superimposed locally weighted scatter plot smoothing fit shows that as tumor size increases, the number of positive lymph nodes also increases. Compared with patients with smaller tumors, patients who had larger tumors tended to be younger (median age was 46 years for T3/4; P = .047), presented with greater numbers of positive lymph nodes (P < .0001), and were more likely to have lymphovascular invasion (P < .0001). Patients with T3/T4 tumors received anthracycline- and taxane-based chemotherapy more frequently than T1 and T2 patients (55%, 49%, and 39% for T3/T4, T2, and T1 patients, respectively; P < .0001).
Table 1.
Patient Characteristics by Tumor Size
| Characteristic | T1 (n = 834) |
T2 (n = 768) |
T3/4 (n = 109) |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age, years | |||||||
| Median | 49 | 48 | 46 | ||||
| ≤ 50 | 467 | 56.0 | 461 | 60.0 | 73 | 67.0 | |
| > 50 | 367 | 44.0 | 307 | 40.0 | 36 | 33.0 | .047 |
| Lymph nodes | |||||||
| N0 | 553 | 67.4 | 341 | 44.8 | 26 | 25.0 | |
| N1 | 201 | 24.5 | 280 | 36.8 | 29 | 27.9 | |
| N2 | 39 | 4.8 | 89 | 11.7 | 22 | 21.2 | |
| N3 | 27 | 3.3 | 51 | 6.7 | 27 | 26.0 | < .0001 |
| Lymphovascular space invasion | |||||||
| Negative | 631 | 76.2 | 484 | 63.5 | 45 | 42.1 | |
| Positive | 197 | 23.8 | 278 | 36.5 | 62 | 57.9 | < .0001 |
| Grade | |||||||
| 1 or 2 | 93 | 11.6 | 65 | 8.9 | 12 | 11.5 | |
| 3 | 708 | 88.4 | 664 | 91.1 | 92 | 88.5 | .21 |
| Histology | |||||||
| Ductal | 780 | 93.5 | 685 | 89.2 | 90 | 82.6 | |
| Other | 54 | 6.5 | 83 | 10.8 | 19 | 17.4 | < .0001 |
| Adjuvant radiation | |||||||
| No | 290 | 34.8 | 304 | 39.6 | 29 | 26.6 | |
| Yes | 544 | 65.2 | 464 | 60.4 | 80 | 73.4 | .012 |
| Adjuvant chemotherapy | |||||||
| Anthracycline based | 371 | 44.5 | 281 | 36.6 | 30 | 27.5 | |
| Taxane based | 31 | 3.7 | 37 | 4.8 | 3 | 2.8 | |
| Anthracycline/taxane based | 325 | 39.0 | 378 | 49.2 | 60 | 55.0 | |
| Nonanthracycline/taxane based | 107 | 12.8 | 72 | 9.4 | 16 | 14.7 | < .0001 |
| Metastatic sites | |||||||
| Visceral | 147 | 64.2 | 200 | 57.1 | 40 | 62.5 | |
| Bone only | 36 | 15.7 | 58 | 16.6 | 8 | 12.5 | |
| Other | 46 | 20.1 | 92 | 26.3 | 16 | 25.0 | .40 |
| No. of metastasis | |||||||
| 1 | 125 | 54.6 | 190 | 54.3 | 38 | 59.4 | |
| 2 | 55 | 24.0 | 84 | 24.0 | 13 | 20.3 | |
| 3+ | 49 | 21.4 | 76 | 21.7 | 13 | 20.3 | .96 |
Fig 1.
Visual display of the positive lymph nodes against the tumor size. The superimposed locally weighted scatter plot smoothing fit shows that as tumor size increases, the number of positive lymph nodes also increases.
Complete information of distant metastatic sites was available in 643 patients. Irrespectively of tumor size, there was a higher rate of visceral metastases (64.2%, 57.1%, and 62.5% for T1, T2, and T3/T4 tumors, respectively) compared with bone metastasis (15.7%, 16.6%, and 12.5%, respectively; P = .40).
Survival Estimate and Pairwise Comparisons by Tumor Size and Lymph Node Status
Median follow-up of the entire cohort was 53 months (range, 0.7 month to 317 months). There were 614 deaths, and the 5-year OS rate was 70% (95% CI, 67% to 72%). The OS estimates with 95% CIs are listed in Table 2. Among all patients, the 5-year OS estimate was higher for N0 patients (80%; 95% CI, 77% to 83%) compared with N1 patients (65%; 95% CI, 60% to 69%), N2 patients (48%; 95% CI, 40% to 58%), and N3 patients (44%; 95% CI, 34% to 56%; P < .0001). We conducted six pairwise comparisons among the different lymph node status subgroups for OS and RFS; we accounted for the multiple comparisons using the Sidak adjustment. The adjusted P values from the pairwise comparisons and the statistical significance after adjusting for multiplicity are listed in Table 2.
Table 2.
Five-Year Survival Estimates and Pairwise Comparisons by Tumor Size and Positive Lymph Nodes
| Tumor Size/Nodal Status | No. of Patients | Overall Survival |
Relapse-Free Survival |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Events | 5-Year Estimates | 95% CI | Overall P | Pairwise | Adjusted P | No. of Events | 5-Year Estimates | 95% CI | Overall P | Pairwise | Adjusted P | ||
| Total | 1711 | 614 | 0.70 | 0.67 to 0.72 | N0 v N1* | < .001 | 747 | 0.57 | 0.55 to 0.60 | N0 v N1* | < .001 | ||
| N0 | 920 | 236 | 0.80 | 0.77 to 0.83 | N0 v N2* | < .001 | 307 | 0.67 | 0.64 to 0.70 | N0 v N2* | < .001 | ||
| N1 | 510 | 220 | 0.65 | 0.60 to 0.69 | N0 v N3* | < .001 | 265 | 0.52 | 0.47 to 0.56 | N0 v N3* | < .001 | ||
| N2 | 150 | 88 | 0.48 | 0.40 to 0.58 | N1 v N2 | 1 | 99 | 0.36 | 0.29 to 0.45 | N1 v N2 | 1 | ||
| N3 | 105 | 58 | 0.44 | 0.34 to 0.56 | < .0001 | N1 v N3 | 1 | 65 | 0.33 | 0.24 to 0.45 | < .0001 | N1 v N3 | .98 |
| N2 v N3 | .99 | N2 v N3 | .85 | ||||||||||
| T1 | |||||||||||||
| Total | 834 | 220 | 0.80 | 0.77 to 0.83 | N0 v N1 | .09 | 275 | 0.68 | 0.65 to 0.72 | N0 v N1* | .004 | ||
| N0 | 553 | 114 | 0.84 | 0.81 to 0.88 | N0 v N2* | < .001 | 147 | 0.74 | 0.70 to 0.78 | N0 v N2* | < .001 | ||
| N1 | 201 | 63 | 0.78 | 0.72 to 0.84 | N0 v N3* | < .001 | 81 | 0.66 | 0.59 to 0.73 | N0 v N3* | < .001 | ||
| N2 | 39 | 24 | 0.50 | 0.36 to 0.70 | N1 v N2 | .68 | 24 | 0.34 | 0.21 to 0.55 | N1 v N2 | 1 | ||
| N3 | 27 | 15 | 0.52 | 0.36 to 0.77 | < .0001 | N1 v N3 | .93 | 18 | 0.31 | 0.17 to 0.56 | < .0001 | N1 v N3 | 1 |
| N2 v N3 | .89 | N2 v N3 | 1 | ||||||||||
| T2 | |||||||||||||
| Total | 768 | 333 | 0.62 | 0.59 to 0.66 | N0 v N1* | .004 | 399 | 0.48 | 0.45 to 0.52 | N0 v N1* | .008 | ||
| N0 | 341 | 115 | 0.73 | 0.68 to 0.79 | N0 v N2* | < .001 | 148 | 0.57 | 0.51 to 0.63 | N0 v N2* | < .001 | ||
| N1 | 280 | 137 | 0.57 | 0.51 to 0.64 | N0 v N3* | < .001 | 161 | 0.44 | 0.38 to 0.51 | N0 v N3* | < .001 | ||
| N2 | 89 | 51 | 0.48 | 0.38 to 0.61 | N1 v N2 | 1 | 57 | 0.39 | 0.29 to 0.51 | N1 v N2 | 1 | ||
| N3 | 51 | 26 | 0.46 | 0.32 to 0.65 | < .0001 | N1 v N3 | .99 | 30 | 0.30 | 0.18 to 0.51 | < .0001 | N1 v N3 | .99 |
| N2 v N3 | 1 | N2 v N3 | 1 | ||||||||||
| T3/4 | |||||||||||||
| Total | 109 | 61 | 0.47 | 0.38 to 0.59 | N0 v N1 | .36 | 73 | 0.35 | 0.27 to 0.47 | N0 v N1 | .44 | ||
| N0 | 26 | 7 | 0.83 | 0.68 to 1.00 | N0 v N2 | .21 | 12 | 0.49 | 0.30 to 0.78 | N0 v N2 | .47 | ||
| N1 | 29 | 20 | 0.44 | 0.28 to 0.68 | N0 v N3* | .029 | 23 | 0.25 | 0.13 to 0.49 | N0 v N3 | .82 | ||
| N2 | 22 | 13 | 0.45 | 0.27 to 0.76 | N1 v N2 | 1 | 18 | 0.29 | 0.15 to 0.56 | N1 v N2 | 1 | ||
| N3 | 27 | 17 | 0.29 | 0.14 to 0.59 | .04 | N1 v N3 | .97 | 17 | 0.41 | 0.26 to 0.66 | .31 | N1 v N3 | .99 |
| N2 v N3 | .96 | N2 v N3 | 1 | ||||||||||
NOTE. T1 (tumor size ≤ 2 cm), T2 (tumor size 2 to 5 cm), T3/4 (tumor size ≥ 5 cm or with extension to chest wall or skin); N0 (negative lymph nodes), N1 (one to three positive lymph nodes), N2 (four to nine positive lymph nodes), and N3 (≥ 10 positive lymph nodes). Bold font indicates the pairwise comparisons of groups with nonsignificant difference in overall survival and relapse-free survival.
Statistical significance after adjustment for multiple pairwise comparisons.
OS by Tumor Size Stratified by Lymph Node Involvement
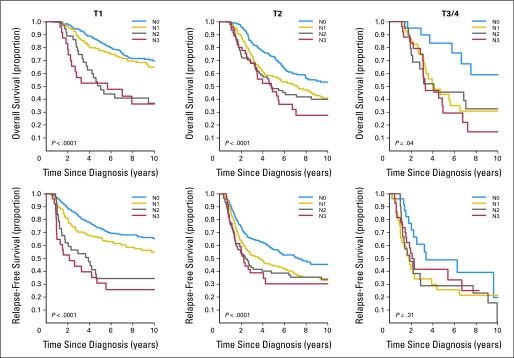
Among all patients, there was statistical significance in OS when comparing N0 with N1, N2, and N3 patients (P < .0001). We further evaluated the 5-year OS rates according to tumor size groups stratified by the number of lymph nodes involved. The Kaplan-Meier estimates of OS in each of the three tumor size subgroups stratified by nodal status are shown in Figure 2. For example, among T1 patients, the 5-year OS rate was 80% (95% CI, 77% to 83%). As the number of lymph nodes increased, the 5-year OS rate decreased from 84% for N0 patients to 52% for N3 patients (P < .0001). Pairwise comparisons after adjusting for multiplicity showed a trend to a difference in OS between patients with N0 and N1 disease (P = .09) and a significant difference when comparing N0 with N2 or N3 disease (P < .001 for both). However, when comparing N1 with N2 or N3 disease, there were no OS differences. Similar results were seen in T2 disease. When looking at T3/4 disease, the trends were similar in terms of OS rates by nodal status. However, the pairwise comparisons only showed significant differences between N0 and N3.
Fig 2.
Kaplan-Meier estimates of relapse-free survival and overall survival in each of the three tumor size subgroups stratified by nodal involvement.
RFS by Tumor Size Stratified by Lymph Node Involvement
Among all patients, there was statistical significance in RFS when comparing N0 with N1, N2, and N3 patients (P < .0001). We further evaluated the 5-year RFS rates according to tumor size groups stratified by the number of lymph nodes involved. The Kaplan-Meier estimates of RFS in each of the three tumor size subgroups stratified by nodal status are shown in Figure 2. For example, among T1 patients, the 5-year RFS rate was 68% (95% CI, 65% to 72%). As the number of lymph nodes increased, the 5-year OS rate decreased from 74% for N0 patients to 31% for N3 patients (P < .0001). Pairwise comparisons after adjusting for multiplicity showed a significant difference in RFS among patients with N0 compared with patients with N1, N2, or N3 disease (P = .004, P < .001, and P < .001, respectively). However, when comparing N1 with N2 or N3 disease, there were no RFS differences. Similar results were seen in T2 disease. When looking at T3 disease, there was no evidence of RFS differences by any nodal group.
Table 3 shows the multivariable models for OS and RFS. Using T1 as a reference, T2 patients had a higher risk of death (HR 1.64; 95% CI, 1.37 to 1.95) and a higher risk of recurrence (HR 1.64; 95% CI, 1.39 to 1.92) Patients with T3/4 tumors had a higher risk of death (HR 1.81; 95% CI, 1.32 to 2.48) and of disease recurrence (HR 2.01; 95% CI, 1.51 to 2.69). Compared with patients with negative lymph nodes, patients with positive lymph nodes had an increase in the risk of death (HR 1.40; 95% CI, 1.15 to 1.70, for N1 disease; HR 1.92; 95% CI, 1.47 to 2.50 for N2 disease; and HR 2.29; 95% CI, 1.66 to 3.16 for N3 disease). Compared with patients with negative lymph nodes, patients with positive lymph nodes had an increase in the risk of recurrence (HR 1.40; 95% CI, 1.17 to 1.66, for N1 disease; HR 1.83; 95% CI, 1.43 to 2.34 for N2 disease; and HR 1.95; 95% CI, 1.45 to 2.63 for N3 disease).
Table 3.
Cox Proportional Hazards Models
| Variable | Overall Survival |
Relapse-Free Survival |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Tumor size | ||||||
| T2 v T1 | 1.64 | 1.37 to 1.95 | < .001 | 1.64 | 1.39 to 1.92 | < .001 |
| T3/4 v T1 | 1.81 | 1.32 to 2.48 | < .001 | 2.01 | 1.51 to 2.69 | < .001 |
| Lymph nodes | ||||||
| N1 v N0 | 1.40 | 1.15 to 1.70 | < .001 | 1.40 | 1.17 to 1.66 | < .001 |
| N2 v N0 | 1.92 | 1.47 to 2.5 | < .001 | 1.83 | 1.43 to 2.34 | < .001 |
| N3 v N0 | 2.29 | 1.66 to 3.16 | < .001 | 1.95 | 1.45 to 2.63 | < .001 |
| Age, ≥ 50 years v < 50 years | 0.92 | 0.78 to 1.09 | .34 | 0.74 | 0.63 to 0.86 | < .001 |
| Lymphovascular invasion, yes v no | 1.55 | 1.29 to 1.85 | < .001 | 1.66 | 1.41 to 1.95 | < .001 |
| Histology, other v ductal | 1.14 | 0.86 to 1.52 | .37 | 1.12 | 0.87 to 1.44 | .39 |
| Adjuvant radiation, yes v no | 0.78 | 0.66 to 0.93 | .005 | 0.77 | 0.66 to 0.9 | .001 |
| Adjuvant chemotherapy | ||||||
| Taxane v anthracycline | 1.07 | 0.66 to 1.73 | .79 | 1.03 | 0.68 to 1.56 | .89 |
| Anthracycline/taxane v anthracycline | 1.14 | 0.95 to 1.38 | .16 | 0.98 | 0.83 to 1.16 | .79 |
| Nonanthracycline/taxane v anthracycline | 1.35 | 1.05 to 1.72 | .017 | 1.43 | 1.14 to 1.78 | .002 |
NOTE. T1 (tumor size ≤ 2 cm), T2 (tumor size 2 to 5 cm), T3/4 (tumor size ≥ 5 cm or with extension to chest wall or skin); N0 (negative lymph nodes), N1 (one to three positive lymph nodes), N2 (four to nine positive lymph nodes), N3 (≥ 10 positive lymph nodes).
Abbreviation: HR, hazard ratio.
DISCUSSION
In this study we evaluated the clinical outcomes and relationships between tumor size, lymph node status, and prognosis in a large cohort of patients with confirmed triple-negative breast cancer. Our study makes the important observation that in this cohort of patients, once there is any evidence of lymph node involvement, the OS and RFS estimates are not greatly affected by the number of additional positive lymph nodes. This finding suggests that TNBC has a distinct biologic behavior that differs from other subtypes of breast tumors in which the tumor size correlates with nodal involvement and the number of positive lymph nodes with prognosis. In our cohort, among all the patients with triple-negative breast cancer, the OS rate and RFS decreased as the size of tumor and number of positive lymph nodes increased. This has been reported in previous studies with lower number of patients.7 However, when we conducted pairwise comparison using the Sidak adjustment method, the only group that achieved significant difference in prognosis was the N0 group.
Our results support previous findings of biologic heterogeneity within the TNBC group, with different patterns of dissemination possibly causing early visceral involvement with major influence on mortality. Rakha et al,7 in a cohort of 282 patients with TNBC tested by IHC to detect the expression of basal cytokeratins (CK5/6, CK14), found that the basal phenotype comprised 55.7% of the triple-negative disease, and this subtype was associated with negative lymph node involvement and development of distant metastasis. In another study including 196 patients with the basal-like phenotype defined by IHC as ER-, PR-, HER2-negative and expressing cytokeratins 5/6 and 14, Foulkes et al12 reported a lack of relationship between tumor size and lymph node positivity (HR 1.30; 95% ,CI 0.70 to 2.41) in these patients.
In our patients, the size of the tumor and lymph node status did not correlate with the first site of distant metastasis. Even in tumors with small size or low lymph node involvement, the visceral involvement was substantially higher than bone-only or other types of metastases. The rate of visceral metastasis was not different in the patients classified by tumor size (P = .40) and nodal status (P = .37, full data not shown). This is in concordance with previous analysis in which patients with TNBC had a high risk for visceral metastasis on relapse and less risk for bone involvement compared with patients with other subtypes.8,16 The biologic explanation for this aggressive behavior is under investigation. Liu et al17 demonstrated higher levels of intratumoral and peritumoral lymphangiogenesis in early stages of triple-negative tumors. Rodriguez-Pinilla et al9 found an association between higher expression of the vimentin protein and development of visceral (lung) metastasis in hormone receptor–negative tumors. New hypotheses to explain the metastatic behavior in these aggressive types of malignancies have been proposed. Foulkes et al18 proposed that a higher fixed proportion of cancer cells with stem-cell properties of self-renewing and pluripotency may be present, conferring the capacity to small tumors to metastasize to distant sites. In triple-negative breast cancer, patients with small tumors and/or early nodal involvement may already have distant metastasis for which the OS and RFS is not affected by the additional number of positive lymph nodes.
To appreciate our findings, some strengths and limitations should be mentioned. To the best of our knowledge, this is the largest study evaluating the prognostic significance of tumor size and lymph node status in patients with TNBC. Despite the retrospective nature of our design, the database that we used was prospectively maintained. We need to account for the significant exclusion of patients treated with neoadjuvant chemotherapy. This leaves a highly selected and probably skewed patient population that might not reflect accurately the universe of triple-negative breast cancers. Another issue is the potential misclassification of TNBC if the recent American Society of Clinical Oncology/College of American Pathologists19 guidelines, which define ER and PR negativity as less than 1% nuclear expression, are applied to the cohort.
In summary, we report that in this large cohort of patients with triple-negative breast cancer, independently of the size of the tumor, once there is evidence of lymph node involvement, the prognosis may not be affected by the number of positive lymph nodes. Taking into account the aggressive behavior of triple-negative breast cancer, clinicians struggle with therapeutic decisions, especially in early stages of the disease because of the scarcity of clinical data in this type of tumors. This large analysis indicates an aggressive and distinct biologic behavior of TNBC in which the classic tumor-node-prognosis relationships are not clear. More studies are needed to determine prognostic factors and develop new classifications able to accurately reflect the clinical behavior of this disease and guide treatment approaches.
Footnotes
Supported in part by National Cancer Institute Grant No. 1K23CA121994-01, National Cancer Institute Breast Specialized Program for Research Excellence Developmental Grant No. P50-CA116199 (A.M.G.), and National Cancer Institute through The University of Texas MD Anderson's Cancer Center Support Grant No. P30 CA016672. The MD Anderson Breast Cancer Management System and the Breast Tumor Bank are supported in part by the Nelly B. Connally Breast Cancer Research Fund.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Leonel F. Hernandez-Aya, Mariana Chavez-MacGregor, Thomas A. Buchholz, Gabriel N. Hortobagyi, Ana Maria Gonzalez-Angulo
Financial support: Ana Maria Gonzalez-Angulo
Administrative support: Gabriel N. Hortobagyi, Ana Maria Gonzalez-Angulo
Provision of study materials or patients: Gabriel N. Hortobagyi, Ana Maria Gonzalez-Angulo
Collection and assembly of data: Leonel F. Hernandez-Aya, Mariana Chavez-MacGregor, Xiudong Lei, Ana Maria Gonzalez-Angulo
Data analysis and interpretation: Leonel F. Hernandez-Aya, Mariana Chavez-MacGregor, Xiudong Lei, Gabriel N. Hortobagyi, Ana Maria Gonzalez-Angulo
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 4.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 5.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 6.Mersin H, Yildirim E, Berberoglu U, et al. The prognostic importance of triple negative breast carcinoma. Breast. 2008;17:341–346. doi: 10.1016/j.breast.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 7.Rakha EA, El-Sayed ME, Green AR, et al. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Pinilla SM, Sarrio D, Honrado E, et al. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res. 2006;12:1533–1539. doi: 10.1158/1078-0432.CCR-05-2281. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Pinilla SM, Sarrio D, Honrado E, et al. Vimentin and laminin expression is associated with basal-like phenotype in both sporadic and BRCA1-associated breast carcinomas. J Clin Pathol. 2007;60:1006–1112. doi: 10.1136/jcp.2006.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 11.Singletary SE, Allred C, Ashley P, et al. Staging system for breast cancer: Revisions for the 6th edition of the AJCC cancer staging manual. Surg Clin North Am. 2003;83:803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 12.Foulkes WD, Grainge MJ, Rakha EA, et al. Tumor size is an unreliable predictor of prognosis in basal-like breast cancers and does not correlate closely with lymph node status. Breast Cancer Res Treat. 2009;117:199–204. doi: 10.1007/s10549-008-0102-6. [DOI] [PubMed] [Google Scholar]
- 13.Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105:97–102. [PubMed] [Google Scholar]
- 14.The World Health Organization Histological Typing of Breast Tumors: Second Edition—The World Organization. Am J Clin Pathol. 1982;78:806–816. doi: 10.1093/ajcp/78.6.806. [DOI] [PubMed] [Google Scholar]
- 15.Sidak Z. Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc. 1967;62:626–633. [Google Scholar]
- 16.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 17.Liu HT, Ma R, Yang QF, et al. Lymphangiogenic characteristics of triple negativity in node-negative breast cancer. Int J Surg Pathol. 2009;17:426–431. doi: 10.1177/1066896909337505. [DOI] [PubMed] [Google Scholar]
- 18.Foulkes WD, Reis-Filho JS, Narod SA. Tumor size and survival in breast cancer: A reappraisal. Nat Rev Clin Oncol. 2010;7:348–353. doi: 10.1038/nrclinonc.2010.39. [DOI] [PubMed] [Google Scholar]
- 19.Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]