Abstract
Purpose
To examine the association between beta-blocker (BB) intake, pathologic complete response (pCR) rates, and survival outcomes in patients with breast cancer treated with neoadjuvant chemotherapy.
Patients and Methods
We retrospectively reviewed 1,413 patients with breast cancer who received neoadjuvant chemotherapy between 1995 and 2007. Patients taking BBs at the start of neoadjuvant therapy were compared with patients with no BB intake. Rates of pCR between the groups were compared using a χ2 test. Cox proportional hazards models were fitted to determine the association between BB intake, relapse-free survival (RFS), and overall survival (OS).
Results
Patients who used BBs (n = 102) were compared with patients (n = 1,311) who did not. Patients receiving BBs tended to be older and obese (P < .001). The proportion of pCR was not significantly different between the groups (P = .48). After adjustment for age, race, stage, grade, receptor status, lymphovascular invasion, body mass index, diabetes, hypertension, and angiotensin-converting enzyme inhibitor use, BB intake was associated with a significantly better RFS (hazard ratio [HR], 0.52; 95% CI, 0.31 to 0.88) but not OS (P = .09). Among patients with triple-negative breast cancer (TNBC; n = 377), BB intake was associated with improved RFS (HR, 0.30; 95% CI, 0.10 to 0.87;P = .027) but not OS (HR, 0.35; 95% CI, 0.12 to 1.00;P = .05).
Conclusion
In this study, BB intake was associated with improved RFS in all patients with breast cancer and in patients with TNBC. Additional studies evaluating the potential benefits of beta-adrenergic blockade on breast cancer recurrence with a focus on TNBC are warranted.
INTRODUCTION
The stress response is executed by the sympathetic nervous system (SNS) and the hypothalamic pituitary adrenal (HPA) axes.1 The SNS and the HPA pathways mediate their downstream effects through modulation of adrenergic and glucocorticoid signaling, respectively.2 A dynamic interaction exists at the molecular level between the mediators of the HPA and SNS. Adrenergic signaling enhances glucocorticoid receptor (GR) stability and binding to DNA.3,4 Conversely, glucocorticoids increase the expression and affinity of beta-2 adrenergic receptors and prevent their downregulation.5,6 In preclinical models, both pathways are thought to promote tumor growth.7,8 The activation of the GR in estrogen receptor (ER) –negative breast cancer cells has been shown to promote cell survival and growth.9 Similarly, the β-adrenergic system can affect cancer biology by promoting tumor invasion, angiogenesis, and ultimately increasing metastatic potential.10–14 However, epidemiologic studies examining the effect of beta-blocker intake on breast cancer incidence have consistently found no significant link.15–18 Recently, a single study suggested significantly lower rates of breast cancer recurrence in patients taking beta blockers.19 Overall, the preclinical and epidemiologic data point to a potential role for the beta-adrenergic system in breast cancer metastasis/recurrence rather than development.
Patients with triple-negative breast cancer (TNBC) have a higher prevalence of abdominal obesity and metabolic syndrome.20–23 Both have been linked to activation/disregulation of the SNS and HPA axis.2,24,25 This putative link coupled with the high expression of β-adrenergic receptors (ADRBs) in TNBC cell lines, and the effects of GR activation on ER-negative mammary tumor growth, point to a potential role for beta blockers in TNBC treatment.12,26–28 Patients with TNBC have limited therapeutic options after completion of conventional chemotherapy. They suffer higher rates of relapse compared with patients with ER-positive breast cancer, with most recurrences occurring within the first 3 years of breast cancer diagnosis.29,30 Consequently, there is a need to improve TNBC recurrence rates with therapies tailored to high relapse risk TNBC.
Neoadjuvant chemotherapy permits assessment of tumor sensitivity to a specific therapy thereby providing insight into tumor biology.31 In this single-institution study, we set out to determine whether beta-blocker use associates with breast cancer primary tumor response and survival outcome. We hypothesized that beta blockers might increase the effectiveness of neoadjuvant chemotherapy and improve relapse-free survival (RFS) in patients with breast cancer, more so in the TNBC subtype.
PATIENTS AND METHODS
Patients
The Breast Cancer Management System Database at The University of Texas MD Anderson Cancer Center was searched, and 1,449 patients with invasive breast cancer who were treated with anthracylines and taxane-based neoadjuvant chemotherapy from January 1995 to May 2007 were identified. The following exclusion criteria were applied: beta blockers after neoadjuvant chemotherapy, male sex, unknown ER, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) status, incomplete records (including medication records), longer than 9 months between neoadjuvant chemotherapy initiation and definitive surgery, and bilateral breast cancer. Stage was calculated according to the criteria of the American Joint Committee on Cancer (sixth edition). Information on medication use was retrieved from review of the patient medical and pharmacy records. Patients were asked about their medications during their first clinic visit and follow-up, this information is then updated in their medical record. The type of beta blockers, indication for intake, and use of other medications that may affect pathologic complete response (pCR) and relapse (metformin, bisphosphonates, insulin, angiotensin-converting enzyme inhibitors [ACEIs]/angiotensin receptor blockers [ARBs]) were tabulated. From the 1,449 patients, we excluded 33 patients who took beta blockers after completion of all neoadjuvant chemotherapy and three patients with incomplete records, the final study population consisted of 102 patients taking beta blockers during neoadjuvant chemotherapy and 1,311 patients on no beta blockers. Patients were followed according to practice guidelines at the time. As this is a retrospective study, there were no specified time points for follow-up. The status of the patients is updated yearly in the database and information on recurrence is obtained from their medical record. The institutional review board approved the retrospective review of the medical records for the purposes of this study.
Pathology
Breast pathologists reviewed all pathologic specimens. The histology, grade, pathologic stage, and analysis of ER, progesterone receptor, and HER2 status were determined as previously described.32 pCR was defined as no evidence of invasive carcinoma in the breast and axillary lymph nodes at time of surgery.
Treatment
In general, all patients received the following anthracycline/taxane-based chemotherapy regimens: docetaxel, doxorubicin, and cyclophosphamide, fluorouracil, doxorubicin or epirubicin, and cyclophosphamide; or doxorubicin and cyclophosphamide; with sequential taxane chemotherapy (paclitaxel or docetaxel). At the completion of chemotherapy, all patients underwent surgery and radiation therapy as indicated. Patients had axillary staging with lymph node dissection and/or sentinel node biopsy. Radiation therapy was delivered in the event of breast conservation surgery, locally advanced disease, primary tumor measurement before chemotherapy of larger than 5 cm, and ≥ 4 involved axillary nodes. Adjuvant hormone therapy and/or trastuzumab were administered according to standard practice at the time. None of the included patients received trastuzumab in the neoadjuvant setting.
Statistical Analysis
Patient characteristics were tabulated between the beta-blocker and non–beta-blocker groups. Groups were compared with the χ2 test or Fisher's exact test. Results are expressed in odds ratios and 95% CIs. Survival analyses were carried out to examine RFS and overall survival (OS). RFS was measured from the date of diagnosis to the date of first documented local or distant recurrence or last follow-up. Patients who died before experiencing a disease recurrence were considered censored at their date of death. OS was measured from the date of diagnosis to the date of death or last follow-up. Kaplan-Meier product limit method was used to estimate the survival outcomes and groups were compared with the log-rank statistic. Cox proportional hazards models were fitted to determine the association of beta-blocker intake with survival outcomes after adjustment for other patient and disease characteristics. Results are expressed in hazard ratios (HR) and 95% CIs. Missing data for the covariates were multiply imputed.33 A total of five imputations were used and the SAS procedure PROC MIANALYZE (SAS Institute, Cary, NC) was used to generate P values. A P value less than .05 was considered statistically significant; all tests were two sided. Subset analyses were explored by tumor subtype with specific interest in patients with TNBC. Statistical analyses were carried out using SAS version 9.1 (SAS Institute Inc) and S-Plus 7.0 (Insightful Corporation, Seattle, WA).
RESULTS
Patient Demographics and Clinical Characteristics
A total of 102 patients used beta blockers and 1,311 patients did not. All hormone receptor–positive patients were treated with endocrine therapy after completion of systemic chemotherapy except for 25 patients (1.9%) in the group on no beta blockers and two patients (2%) in the beta-blocker group who received it concurrently with neoadjuvant chemotherapy (P = .97). Of these, 14 (1.1%) received neoadjuvant tamoxifen in the group on no beta blockers and 1 (1%) in the beta-blocker group (P = .93). Patient characteristics are summarized in Table 1. Patients on beta blockers tended to be older (P < .001), median age was 57 versus 49 years, and consequently this group had a higher proportion of postmenopausal patients (P < .001). Fifty-five percent of the patients were obese in the group on beta blockers versus 32% in the nonusers (P < .001). More patients (98%) carried a diagnosis of hypertension in the beta-blocker group when compared to the group not on beta blockers (19.6%; P < .001). The patients on beta blockers were also more likely to be on ACEIs/ARBs (P < .001). Other prognostic factors were not significantly different between the groups. We also evaluated the use of other medications that may affect pCR and/or RFS, specifically metformin, bisphosphonate, and insulin, and there was no significant difference in use between the groups.34–36 The most commonly prescribed beta blockers were selective beta blockers (89%), mainly metoprolol (42%) followed by atenolol (37%). When evaluating patients with TNBC, we found a total of 377 patients (27%) with triple-negative tumors, 29 patients (7.6%) had TNBC and were on beta blockers versus 348 (92.4%) not on beta blockers. Characteristics of the TNBC patients are presented in Table 2. The significant differences between the two groups were age, BMI, hypertension, ACEIs/ARBs, and metformin use. All patients in the beta-blocker group completed planned anthracycline taxane–based therapies despite older age and concomitant comorbidities. In the TNBC group, 27 of 29 patients on beta blockers carried a diagnosis of hypertension. Patients with hormone receptor–negative breast cancer were more likely to be obese (39%) compared with hormone receptor–positive (30%) breast cancer regardless of beta-blocker use (P = .002). The rates of hypertension were not significantly different between the hormone receptor–positive and TNBC patients, at 25% and 26%, respectively (P = .18).
Table 1.
Baseline Patient Characteristics by Beta-Blocker Intake
| Characteristic | No Beta Blockers(n = 1,311) |
Beta Blockers (n = 102) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | |||||
| Median | 49.0 | 57.0 | |||
| Mean | 49.1 | 56.6 | < .001* | ||
| < 50 | 694 | 52.9 | 28 | 27.5 | |
| ≥ 50 | 617 | 47.1 | 74 | 72.5 | < .001 |
| Menopausal status | |||||
| Pre | 655 | 50.0 | 20 | 19.8 | |
| Post | 654 | 50.0 | 81 | 80.2 | < .001 |
| Body mass index, kg/m2 | |||||
| Median | 27 | 31.4 | |||
| < 25 | 452 | 35.6 | 16 | 16.3 | |
| 25-29 | 413 | 32.5 | 28 | 28.6 | |
| 30+ | 406 | 31.9 | 54 | 55.1 | < .001 |
| Race | |||||
| White/other | 1,128 | 86.0 | 83 | 81.4 | |
| Black | 183 | 14.0 | 19 | 18.6 | .20 |
| Clinical stage | |||||
| I | 54 | 4.1 | 3 | 2.9 | |
| II | 706 | 54.1 | 60 | 58.8 | |
| III | 546 | 41.8 | 39 | 38.2 | .60 |
| Nuclear grade | |||||
| I | 49 | 3.8 | 1 | 1.0 | |
| II | 413 | 32.4 | 37 | 37.4 | |
| III | 812 | 63.7 | 61 | 61.6 | .25 |
| LVI | |||||
| Negative | 861 | 68.0 | 74 | 76.3 | |
| Positive | 405 | 32.0 | 23 | 23.7 | .09 |
| Hormone receptor status | |||||
| Negative | 470 | 35.9 | 35 | 34.3 | |
| Positive | 841 | 64.1 | 67 | 65.7 | .76 |
| HER2 status | |||||
| Negative | 1,062 | 82.1 | 83 | 81.4 | |
| Positive | 232 | 17.9 | 19 | 18.6 | .86 |
| Triple-negative tumor | |||||
| No | 946 | 73.1 | 73 | 71.6 | |
| Yes | 348 | 26.9 | 29 | 28.4 | .74 |
| Diabetes | |||||
| No | 1,240 | 94.6 | 96 | 94.1 | |
| Yes | 71 | 5.4 | 6 | 5.9 | .87 |
| Insulin use among diabetics | |||||
| No | 46 | 64.8 | 6 | 100.0 | |
| Yes | 25 | 35.2 | 0 | 0.0 | .17† |
| Hypertension | |||||
| No | 1,054 | 80.4 | 4 | 3.9 | |
| Yes | 257 | 19.6 | 98 | 96.1 | < .001 |
| ACEIs/ARBs | |||||
| No | 1,211 | 92.4 | 62 | 60.8 | |
| Yes | 100 | 7.6 | 40 | 39.2 | < .001 |
| Bisphosphonates | |||||
| No | 1,276 | 97.3 | 99 | 97.1 | |
| Yes | 35 | 2.7 | 3 | 2.9 | .87 |
| Metformin use | |||||
| No | 1,281 | 97.7 | 98 | 96.1 | |
| Yes | 30 | 2.3 | 4 | 3.9 | .30 |
Abbreviations: LVI, lymphovascular invasion; HER2, human epidermal growth factor receptor 2; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Two samplet-test.
Fisher's exact test.
Table 2.
Characteristics by Beta-Blocker Intake Within Triple-Negative Breast Cancer Subgroups
| Characteristic | No Beta Blockers(n = 348) |
Beta Blockers (n = 29) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | |||||
| Median | 47.5 | 55.0 | |||
| < 50 | 198 | 56.9 | 9 | 31.0 | |
| ≥ 50 | 150 | 3.1 | 20 | 69.0 | .007 |
| Menopausal status | |||||
| Pre | 171 | 49.1 | 7 | 24.1 | |
| Post | 177 | 50.9 | 22 | 75.9 | .01 |
| Body mass index, kg/m2 | |||||
| Median | 27.8 | 33.2 | |||
| < 25 | 105 | 31.1 | 3 | 11.1 | |
| 25-29 | 104 | 30.8 | 7 | 25.9 | |
| 30+ | 129 | 38.2 | 17 | 63.0 | .02 |
| Race | |||||
| White/other | 283 | 81.3 | 21 | 72.4 | |
| Black | 65 | 18.7 | 8 | 27.6 | .24 |
| Clinical stage | |||||
| I | 13 | 3.8 | 0 | 0.0 | |
| II | 186 | 53.8 | 16 | 55.2 | |
| III | 147 | 42.5 | 13 | 44.8 | .57 |
| Nuclear grade | |||||
| I | 2 | 0.6 | 0 | 0.0 | |
| II | 34 | 10.0 | 3 | 10.7 | |
| III | 304 | 89.4 | 25 | 89.3 | .79* |
| LVI | |||||
| Negative | 245 | 71.4 | 24 | 88.9 | |
| Positive | 98 | 28.6 | 3 | 11.1 | .05 |
| Diabetes | |||||
| No | 328 | 94.3 | 25 | 86.2 | |
| Yes | 20 | 5.7 | 4 | 13.8 | .10* |
| Insulin among diabetics | |||||
| No | 14 | 70.0 | 4 | 100.0 | |
| Yes | 6 | 30.0 | 0 | 0.0 | .21 |
| Hypertension | |||||
| No | 275 | 79.0 | 2 | 6.9 | |
| Yes | 73 | 21.0 | 27 | 93.1 | < .001 |
| ACEIs/ARBs | |||||
| No | 319 | 91.7 | 17 | 58.6 | |
| Yes | 29 | 8.3 | 12 | 41.4 | < .001 |
| Bisphosphonates | |||||
| No | 342 | 98.3 | 29 | 100.0 | |
| Yes | 6 | 1.7 | 0 | 0.0 | 1.00* |
| Hyperlipidemia | |||||
| No | 339 | 97.4 | 28 | 96.6 | |
| Yes | 9 | 2.6 | 1 | 3.4 | .55* |
| Metformin use | |||||
| No | 340 | 97.7 | 25 | 86.2 | |
| Yes | 8 | 2.3 | 4 | 13.8 | .009* |
Abbreviations: LVI, lymphovascular invasion; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Fisher's exact test.
Beta Blockers and pCR Rates
There was no difference in the estimates of pCR rates between the groups. The proportion of pCR was 16.4% (95% CI, 14% to 18%) in the patients not on beta blockers and 13.7% (95% CI, 7% to 20%) in the patients on beta blockers (P = .48; Table 3). For the patients with TNBC, in the group not on beta blockers, the pCR rate was 27.3% (95% CI, 22.6 to 32.0%). In the group on beta blockers, the pCR rate was 24.1% (95% CI, 8.6 to 39.7%). There was no statistically significant difference in pCR rate between the groups (P = .71). In the multivariate analysis, the use of beta blockers was not associated with pCR after adjustment for age, stage, grade, hormone receptor status, HER2 status, lymphovascular invasion (LVI), metformin use, and BMI (Appendix Table A1, online only).
Table 3.
Pathologic Complete Remission by Beta-Blocker Use
| Patients by Beta-Blocker Use | Residual Disease(n = 1,184) |
pCR(n = 229) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| All | |||||
| No | 1,096 | 83.6 | 215 | 16.4 | |
| Yes | 88 | 86.3 | 14 | 13.7 | .48 |
| Patients with TNBC | |||||
| No | 253 | 72.7 | 95 | 27.3 | |
| Yes | 22 | 75.9 | 7 | 24.1 | .71 |
Abbreviations: pCR, pathologic complete response; TNBC, triple-negative breast cancer.
Survival Estimates
The median follow-up time among patients in the beta-blocker group was 55 months (range, 3 to 145 months); the median follow-up time among patients not on beta blockers was 63 months (range, 5 to 121 months). The univariate log-rank test between survival outcomes and patients clinical characteristics are listed in Table 4. Patients taking beta blockers had a better 3-year RFS (87%) compared with patients not taking beta blockers (77%;P = .008). The 3-year OS was 91% in patients taking beta blockers compared to 85% in nonusers (P = .09). Missing data for LVI (3.5% missing), BMI (3.1% missing), grade (2.8% missing), and HER2 status (1.2% missing) were imputed. Younger age, African American ancestry, advanced stages, high-grade tumors, TNBC, LVI, and HER2-positive tumors were significantly associated with worse RFS. A diagnosis of diabetes was significantly associated with a worse OS (P = .002) but not a worse RFS (P = .42). Hypertension did not significantly associate with RFS or OS (P = .37 and 0.61, respectively). After adjustment for age, race, stage, grade, hormone status, HER2 status, LVI, BMI, diabetes, hypertension, and ACE/ARBs, the use of beta blockers remained associated with significantly better RFS (HR, 0.52; 95% CI, 0.31 to 0.88;P = .015). The association of beta blockers with OS did not achieve a statistical significance (HR, 0.64; 95% CI, 0.38 to 1.07;P = .09) after adjustment (Table 5). Only advanced stages, African American ancestry, and LVI remained associated with worse survival outcomes in the multivariate analysis.
Table 4.
Three-Year Survival Estimates: Patient and Clinical Characteristics
| Characteristic | No. of Patients | Relapse-Free Survival |
Overall Survival |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Events | 3-Year Estimate | 95% CI | P | No. of Events | 3-Year Estimate | 95% CI | P | ||
| Beta blocker | |||||||||
| No | 1,311 | 387 | 0.77 | 0.74 to 0.79 | 335 | 0.85 | 0.83 to 0.87 | ||
| Yes | 102 | 17 | 0.87 | 0.78 to 0.92 | .008 | 18 | 0.91 | 0.83 to 0.95 | .09 |
| Age, years | |||||||||
| < 50 | 722 | 229 | 0.75 | 0.71 to 0.78 | 179 | 0.85 | 0.82 to 0.88 | ||
| ≥ 50 | 691 | 175 | 0.81 | 0.77 to 0.83 | .003 | 174 | 0.86 | 0.83 to 0.88 | .75 |
| Race | |||||||||
| Non-black | 1,211 | 320 | 0.79 | 0.77 to 0.82 | 271 | 0.87 | 0.85 to 0.89 | ||
| Black | 202 | 84 | 0.67 | 0.6 to 0.73 | < .001 | 82 | 0.77 | 0.71 to 0.83 | < .001 |
| Body mass index | |||||||||
| Normal/underweight | 468 | 128 | 0.8 | 0.76 to 0.84 | 102 | 0.88 | 0.85 to 0.91 | ||
| Overweight | 441 | 117 | 0.8 | 0.75 to 0.83 | 100 | 0.87 | 0.83 to 0.89 | ||
| Obese | 460 | 145 | 0.73 | 0.69 to 0.77 | .07 | 138 | 0.82 | 0.78 to 0.85 | .001 |
| Clinical stage | |||||||||
| I/II | 823 | 182 | 0.85 | 0.82 to 0.87 | 151 | 0.91 | 0.89 to 0.93 | ||
| III | 585 | 218 | 0.68 | 0.64 to 0.71 | < .001 | 197 | 0.78 | 0.74 to 0.81 | < .001 |
| Nuclear grade | |||||||||
| I/II | 500 | 104 | 0.86 | 0.83 to 0.89 | 82 | 0.95 | 0.92 to 0.96 | ||
| III | 873 | 287 | 0.73 | 0.7 to 0.76 | < .001 | 257 | 0.8 | 0.77 to 0.83 | < .001 |
| LVI | |||||||||
| Negative | 935 | 212 | 0.83 | 0.8 to 0.85 | 182 | 0.88 | 0.86 to 0.9 | ||
| Positive | 428 | 172 | 0.67 | 0.63 to 0.72 | < .001 | 149 | 0.81 | 0.77 to 0.84 | < .001 |
| Hormone receptor status | |||||||||
| Negative | 505 | 187 | 0.66 | 0.62 to 0.71 | 172 | 0.74 | 0.7 to 0.78 | ||
| Positive | 908 | 217 | 0.84 | 0.81 to 0.86 | < .001 | 181 | 0.92 | 0.9 to 0.94 | < .001 |
| HER2 status | |||||||||
| Negative | 1,145 | 308 | 0.78 | 0.76 to 0.81 | 279 | 0.86 | 0.83 to 0.88 | ||
| Positive | 251 | 91 | 0.74 | 0.68 to 0.79 | .02 | 70 | 0.84 | 0.78 to 0.88 | .99 |
| Triple-negative tumor | |||||||||
| No | 1,019 | 263 | 0.82 | 0.79 to 0.84 | 216 | 0.9 | 0.88 to 0.92 | ||
| Yes | 377 | 136 | 0.66 | 0.61 to 0.71 | < .001 | 133 | 0.72 | 0.67 to 0.76 | < .001 |
| Diabetes | |||||||||
| No | 1,336 | 380 | 0.78 | 0.76 to 0.8 | 324 | 0.86 | 0.84 to 0.88 | ||
| Yes | 77 | 24 | 0.72 | 0.6 to 0.81 | .42 | 29 | 0.76 | 0.64 to 0.84 | .002 |
| Hypertension | |||||||||
| No | 1,058 | 307 | 0.77 | 0.75 to 0.8 | 258 | 0.85 | 0.83 to 0.87 | ||
| Yes | 355 | 97 | 0.79 | 0.74 to 0.83 | .37 | 95 | 0.86 | 0.82 to 0.89 | .61 |
| ACEIs/ARBs | |||||||||
| No | 1,273 | 371 | 0.77 | 0.75 to 0.79 | 316 | 0.86 | 0.84 to 0.88 | ||
| Yes | 140 | 33 | 0.81 | 0.74 to 0.87 | .21 | 37 | 0.83 | 0.76 to 0.88 | .58 |
Abbreviations: LVI, lymphovascular invasion; HER2, human epidermal growth factor receptor 2; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Table 5.
Multivariable Cox Proportional Hazards Model for All Patients
| Parameter | Relapse-Free Survival |
Overall Survival |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| Beta-blocker use, yesv no | 0.52 | 0.31 to 0.88 | .015 | 0.64 | 0.38 to 1.07 | .09 |
| Age, ≥ 50v < 50 years | 0.81 | 0.66 to 1.00 | .05 | 1.04 | 0.83 to 1.3 | .75 |
| Race, blackv non-black | 1.37 | 1.06 to 1.77 | .018 | 1.47 | 1.13 to 1.93 | .005 |
| Stage, IIIv I/II | 1.70 | 1.38 to 2.08 | < .001 | 1.77 | 1.42 to 2.21 | < .001 |
| Grade, IIIv I/II | 1.18 | 0.92 to 1.53 | .19 | 1.35 | 1.02 to 1.78 | .039 |
| Hormone receptor status, positivev negative | 0.74 | 0.48 to 1.13 | .16 | 0.76 | 0.47 to 1.23 | .26 |
| HER2 status, positivev negative | 1.31 | 0.92 to 1.87 | .14 | 1.03 | 0.69 to 1.53 | .90 |
| Triple-negative tumor, nov yes | 0.71 | 0.44 to 1.14 | .16 | 0.61 | 0.36 to 1.03 | .07 |
| LVI, positivev negative | 1.89 | 1.54 to 2.32 | < .001 | 1.75 | 1.4 to 2.18 | < .001 |
| BMI, kg/m2 | ||||||
| 25-29v < 25 | 0.99 | 0.77 to 1.27 | .92 | 1.03 | 0.78 to 1.36 | .83 |
| 30+v < 25 | 1.16 | 0.9 to 1.50 | .26 | 1.25 | 0.95 to 1.64 | .11 |
| Diabetes, yesv no | 1.20 | 0.77 to 1.88 | .41 | 1.63 | 1.07 to 2.48 | .022 |
| Hypertension, yesv no | 1.08 | 0.8 to 1.45 | .60 | 1.00 | 0.73 to 1.37 | .98 |
| ACEI/ARB use, yesv no | 0.82 | 0.54 to 1.26 | .37 | 0.99 | 0.65 to 1.51 | .96 |
Abbreviations: LVI, lymphovascular invasion; BMI, body mass index; HER2, human epidermal growth factor receptor 2; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
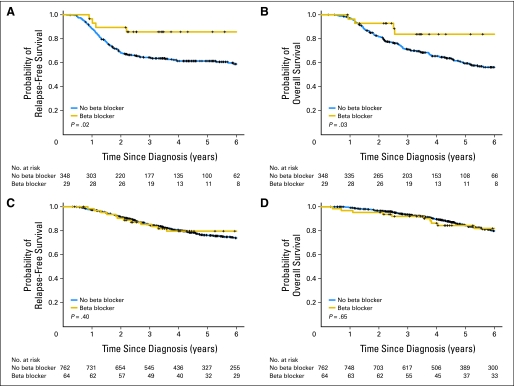
Among patients with TNBC, beta-blocker use remained associated with improved RFS (HR, 0.3; 95% CI, 0.1 to 0.87;P = .027) but not OS (HR, 0.35; 95% CI: 0.12 to 1.00;P = .05), after adjustment for age, stage, race, BMI, metformin use, diabetes, hypertension, and ACE/ARBs (Appendix Table A1, online only). The Kaplan-Meier estimates of RFS and OS between patients with TNBC on beta blockers and patients with TNBC not taking beta blockers are shown in Figures 1A and 1B. Beta-blocker intake was significantly associated with improved RFS and OS (P = .02 and P = .03, respectively). When evaluating the subset of patients with ER-positive breast cancer (n = 826), beta-blocker intake had no significant effects on RFS and OS (P = .4 and P = .65, respectively; Fig 1C, 1D).
Fig 1.
(A) Relapse-free survival (RFS) and (B) overall survival (OS) in patients with triple-negative breast cancer. (C) RFS and (D) OS in patients with estrogen receptor–positive breast cancer.
In view of the significant association between age and beta- blocker use, we also evaluated age as a continuous variable in addition to the categorical variable using age 50 as a cutoff value (Appendix Table A3, online only). This did not affect the survival analysis.
DISCUSSION
The objective of this retrospective study was to describe the effect of beta-blocker intake on pCR rates and subsequent survival outcomes in patients with breast cancer treated with anthracycline- and taxane-based neoadjuvant chemotherapy. We found that pCR rates were not associated with beta-blocker intake. Interestingly, despite a lack of effect on pCR, RFS was longer in patients who took beta blockers. The two groups were well balanced with regard to the amount of chemotherapy delivered. The improvement in OS approached significance in the TNBC subgroup.
Beta blockers have been shown previously to improve OS, likely related to their cardioprotective effects; however, the improvement in RFS suggests a cancer-specific effect.37 Our findings are concordant with a study by Powe et al19 where 43 patients with breast cancer taking beta blockers were found to have significant reduction in breast cancer recurrence compared to a similar cohort not on beta blockers. Interestingly, although beta-blocker intake in our study was associated with better RFS when all patients were analyzed, subset analysis also showed a highly significant association between beta-blocker use and improved RFS in the TNBC subgroup, while no significant RFS differences were noted for patients with ER-positive breast cancer (Figs 1C, 1D). This could be due to the relatively short follow-up time in this study; median follow-up was 55 months for patients on beta blockers. While this may be sufficient to detect an improvement in RFS for patients with TNBC due to shorter relapse times, this may not be sufficient for patients with ER-positive breast cancer. Another possible explanation may be related to tumor biology, whereby the presence of the ER may modulate the response to beta blockers. Furthermore, one can speculate that the lack of association between beta-blocker intake and pCR suggests an effect on the tumor metastases cascade rather than a primary effect on increasing cytotoxic sensitivity to systemic chemotherapy. It is important to point out that only pCR was analyzed in this retrospective study as a surrogate for response to therapy. Arguably, if beta blockers have cytostatic rather than cytotoxic properties, looking at pCR alone may not suffice to detect an effect on primary tumor.
These findings may also be explained in part by a recent study by Sloan et al38 linking breast cancer metastatic potential to activation of neuroendocrine pathways. Specifically, in an orthotopic mouse model of breast cancer, mice subjected to chronic stress had minimal growth of their primary breast tumor, but a significant increase in metastasis to distant tissues. These effects required β-adrenergic signaling, which increased the infiltration of macrophages into primary tumor and correlated with a pro-metastatic gene expression signature. Treatment with the β-antagonist propranolol reversed the macrophage infiltration and inhibited metastatic tumor spread. The effects of stress on distant metastasis were also inhibited by in vivo macrophage suppression using the CSF-1 receptor kinase inhibitor GW2580. CSF-1 receptor kinase is also known to be upregulated by glucocorticoids the other major effectors of the stress response.39
Interestingly, our study is consistent with previous observations that patients with TNBC have higher rates of obesity20–22; this in turn has been linked to increased activation of the stress response pathway and disruption of the SNS and HPA axis.40 A positive correlation between stress reduction and reduction of breast cancer recurrence has also been observed in a randomized biobehavoral intervention trial of 277 patients with early-stage breast cancer.41,42
This study may be limited by its retrospective nature and subset analyses for patients with TNBC. Information regarding beta-blocker use during neoadjuvant chemotherapy was obtained by medical record review and compliance could not be assessed. Furthermore, duration of beta-blocker intake after completion of neoadjuvant therapy could not be accurately determined for all patients, in view of the variability in follow-up care. It is also possible that not all the patients receiving beta blockers were correctly identified, likely diluting any possible association. However, it is important to note that the database used for this study is prospectively maintained and survival information is updated yearly. All the patients were treated at a single institution with fairly homogenous chemotherapeutic regimens and definitive surgical and radiation treatment. Other factors that could affect breast cancer relapse may also be confounding this study. These include aspirin use, alcohol intake, dietary factors, and lack of exercise. As this is a retrospective study not all factors could be controlled for.
To our knowledge, our study provides the first clinical evidence linking the use of beta blockers to TNBC relapse. In a population with limited targeted options and early relapse risk, this potentially beneficial intervention should be studied further.43 Future trials that prospectively examine the effects of low-dose beta blockade on breast cancer recurrence with a focus on patients with TNBC are needed. The future challenges in designing such trials will mainly involve appropriate patient selection. Specifically, should trials target patients with evidence of a hyperactive SNS, such as patients with the metabolic syndrome, or should all TNBC patients be included? Appropriate beta-blocker selection will also be important.44 In our study, most of the patients were on a selective β1-ADRB, the study by Sloane et al used a nonselective beta blocker. These have the potential to efficiently inhibit all ADRBs, such as the β2 and β3 ADRBs, which are involved in adipocyte lipolysis and thermogenic activity.45,46 However, it is important to note that although beta blockers are labeled as selective or nonselective, they still have affinity for both the β1 and β-2 ADRB. The β1 and β2 receptors are very similar and absolute selectivity has not been achieved.47 Conceivably, the benefits of more broad beta blockers may be even greater than the more selective ones. Optimal dose titration in the absence of a surrogate marker (eg, blood pressure) for activity must also be addressed. As both the adrenergic and the glucocorticoid-mediated HPA axis potentiate the stress response, and both are implicated in breast cancer progression, correlative studies examining the receptors for cortisol and epinephrine in primary tumors and stromal tissue should be performed. We predict that a subset of patients with TNBC may eventually be identified that are likely to benefit most from concomitant blockade of stress physiology and more traditional antitumor therapy.
Appendix
Table A1.
Multivariate Logistic Regression Model for pCR
| Parameter | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Beta-blocker use, yesv no | 1.38 | 0.83 to 2.29 | .21 |
| Age, ≥ 50v < 50 years | 0.66 | 0.47 to 0.93 | .016 |
| Stage, IIIv I/II | 0.67 | 0.48 to 0.94 | .019 |
| Grade, IIIv I/II | 3.46 | 2.16 to 5.52 | < .001 |
| Hormone receptor status, positivev negative | 0.36 | 0.26 to 0.51 | < .001 |
| HER2 status, positivev negative | 1.78 | 1.23 to 2.57 | .002 |
| LVI, positivev negative | 0.40 | 0.27 to 0.6 | < .001 |
| BMI, overweightv normal | 0.66 | 0.44 to 0.99 | .018 |
| BMI, obesev normal | 1.02 | 0.69 to 1.49 | .18 |
| Metformin use, yesv no | 0.87 | 0.32 to 2.35 | .79 |
Abbreviations: pCR, pathologic complete response; HER2, human epidermal growth factor receptor 2; LVI, lymphovascular invasion; BMI, body mass index.
Table A2.
Multivariable Cox Proportional Hazards Model Among Patients With Triple-Negative Tumors
| Parameter | Relapse-Free Survival |
Overall Survival |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| Beta-blocker use, yesv no | 0.30 | 0.1 to 0.87 | .027 | 0.35 | 0.12 to 1.00 | .05 |
| Age, ≥ 50v < 50 years | 1.00 | 0.69 to 1.45 | .98 | 1.04 | 0.72 to 1.51 | .84 |
| Race, Blackv non-black | 1.20 | 0.79 to 1.81 | .40 | 1.24 | 0.81 to 1.9 | .31 |
| Stage, IIIv I/II | 1.79 | 1.27 to 2.52 | < .001 | 1.81 | 1.28 to 2.57 | < .001 |
| LVI, positivev negative | 2.36 | 1.65 to 3.38 | < .001 | 1.90 | 1.33 to 2.73 | < .001 |
| BMI, kg/m2 | ||||||
| 25-29v < 25 | 1.04 | 0.67 to 1.62 | .86 | 1.08 | 0.68 to 1.7 | .75 |
| 30+v < 25 | 0.97 | 0.63 to 1.5 | .89 | 0.96 | 0.61 to 1.5 | .85 |
| Metformin, yes v no | 2.09 | 0.59 to 7.47 | .25 | 1.76 | 0.54 to 5.74 | .35 |
| Diabetes, yesv no | 1.20 | 0.47 to 3.09 | .7 | 1.71 | 0.75 to 3.93 | .20 |
| Hypertension, yesv no | 1.16 | 0.71 to 1.91 | .56 | 1.03 | 0.62 to 1.72 | .91 |
| ACEI/ARB use, yesv no | 0.72 | 0.35 to 1.52 | .39 | 0.89 | 0.43 to 1.82 | .74 |
Abbreviations: LVI, lymphovascular invasion; BMI, body mass index; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Table A3.
Multivariable Cox Proportional Hazards Model Among All Patients Using Age As a Continuous Variable
| Parameter | Relapse-Free Survival |
Overall Survival |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| Beta-blocker use, yesvno | 0.54 | 0.32 to 0.91 | .021 | 0.65 | 0.39 to 1.08 | .10 |
| Age (continuous) | 0.98 | 0.97 to 0.99 | .003 | 1.00 | 0.99 to 1.01 | .98 |
| Race, Blackv non-black | 1.38 | 1.06 to 1.78 | .015 | 1.48 | 1.13 to 1.93 | .004 |
| Stage, IIIv I/II | 1.69 | 1.38 to 2.08 | < .001 | 1.78 | 1.43 to 2.21 | < .001 |
| Grade, IIIv I/II | 1.18 | 0.92 to 1.51 | .19 | 1.35 | 1.03 to 1.78 | .03 |
| Hormone receptor status, positivev negative | 0.75 | 0.49 to 1.14 | .18 | 0.76 | 0.47 to 1.23 | .26 |
| HER2 status, positivev negative | 1.31 | 0.92 to 1.87 | .13 | 1.02 | 0.69 to 1.52 | .91 |
| Triple-negative tumor, yesv no | 1.40 | 0.87 to 2.26 | .17 | 1.62 | 0.95 to 2.77 | .08 |
| LVI, positivev negative | 1.85 | 1.5 to 2.28 | < .001 | 1.70 | 1.35 to 2.13 | < .001 |
| BMI, kg/m2 | ||||||
| 25-29v < 25 | 1.01 | 0.78 to 1.31 | .94 | 1.03 | 0.78 to 1.37 | .81 |
| 30+v < 25 | 1.14 | 0.89 to 1.48 | .3 | 1.23 | 0.93 to 1.61 | .14 |
| Diabetes, yesv no | 1.24 | 0.79 to 1.94 | .34 | 1.64 | 1.08 to 2.5 | .021 |
| Hypertension, yesv no | 1.12 | 0.83 to 1.5 | .46 | 1.01 | 0.74 to 1.39 | .94 |
| ACEI/ARB use, yesv no | 0.85 | 0.55 to 1.3 | .44 | 0.99 | 0.65 to 1.51 | .96 |
Abbreviations: HER2, human epidermal growth factor receptor 2; LVI, lymphovascular invasion; BMI, body mass index; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Footnotes
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Amal Melhem-Bertrandt, Mariana Chavez-MacGregor, Anil K. Sood, Suzanne D. Conzen, Ana-Maria Gonzalez-Angulo
Financial support: Ana-Maria Gonzalez-Angulo
Provision of study materials or patients: Ana-Maria Gonzalez-Angulo
Collection and assembly of data: Amal Melhem-Bertrandt, Mariana Chavez-MacGregor, Erika N. Brown, Ana-Maria Gonzalez-Angulo
Data analysis and interpretation: Amal Melhem-Bertrandt, Xiudong Lei, Richard T. Lee, Funda Meric-Bernstam, Anil K. Sood, Suzanne D. Conzen, Gabriel N. Hortobagyi, Ana-Maria Gonzalez-Angulo
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 2.Chrousos GP, Kino T. Glucocorticoid signaling in the cell: Expanding clinical implications to complex human behavioral and somatic disorders. Ann N Y Acad Sci. 2009;1179:153–166. doi: 10.1111/j.1749-6632.2009.04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rangarajan PN, Umesono K, Evans RM. Modulation of glucocorticoid receptor function by protein kinase A. Mol Endocrinol. 1992;6:1451–1457. doi: 10.1210/mend.6.9.1435789. [DOI] [PubMed] [Google Scholar]
- 4.Dong Y, Aronsson M, Gustafsson JA, et al. The mechanism of cAMP-induced glucocorticoid receptor expression: Correlation to cellular glucocorticoid response. J Biol Chem. 1989;264:13679–13683. [PubMed] [Google Scholar]
- 5.Mak JC, Nishikawa M, Shirasaki H, et al. Protective effects of a glucocorticoid on downregulation of pulmonary beta 2-adrenergic receptors in vivo. J Clin Invest. 1995;96:99–106. doi: 10.1172/JCI118084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies AO, Lefkowitz RJ. Agonist-promoted high affinity state of the beta-adrenergic receptor in human neutrophils: Modulation by corticosteroids. J Clin Endocrinol Metab. 1981;53:703–708. doi: 10.1210/jcem-53-4-703. [DOI] [PubMed] [Google Scholar]
- 7.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melhem A, Yamada SD, Fleming GF, et al. Administration of glucocorticoids to ovarian cancer patients is associated with expression of the anti-apoptotic genes SGK1 and MKP1/DUSP1 in ovarian tissues. Clin Cancer Res. 2009;15:3196–3204. doi: 10.1158/1078-0432.CCR-08-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu W, Pew T, Zou M, et al. Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J Biol Chem. 2005;280:4117–4124. doi: 10.1074/jbc.M411200200. [DOI] [PubMed] [Google Scholar]
- 10.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson MB, Armaiz-Pena G, Takahashi R, et al. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J Biol Chem. 2007;282:29919–29926. doi: 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- 12.Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: Biobehavioral signaling pathways and interventions. J Clin Oncol. 28:4094–4099. doi: 10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JW, Shahzad MM, Lin YG, et al. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009;15:2695–2702. doi: 10.1158/1078-0432.CCR-08-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang EV, Sood AK, Chen M, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 15.Largent JA, McEligot AJ, Ziogas A, et al. Hypertension, diuretics and breast cancer risk. J Hum Hypertens. 2006;20:727–732. doi: 10.1038/sj.jhh.1002075. [DOI] [PubMed] [Google Scholar]
- 16.Lindgren A, Pukkala E, Tuomilehto J, et al. Incidence of breast cancer among postmenopausal, hypertensive women. Int J Cancer. 2007;121:641–644. doi: 10.1002/ijc.22689. [DOI] [PubMed] [Google Scholar]
- 17.Davis S, Mirick DK. Medication use and the risk of breast cancer. Eur J Epidemiol. 2007;22:319–325. doi: 10.1007/s10654-007-9135-0. [DOI] [PubMed] [Google Scholar]
- 18.Fryzek JP, Poulsen AH, Johnsen SP, et al. A cohort study of antihypertensive treatments and risk of renal cell cancer. Br J Cancer. 2005;92:1302–1306. doi: 10.1038/sj.bjc.6602490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powe DG, Voss MJ, Zänker KS, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiti B, Kundranda MN, Spiro TP, et al. The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res Treat. 2010;121:479–483. doi: 10.1007/s10549-009-0591-y. [DOI] [PubMed] [Google Scholar]
- 21.Vona-Davis L, Rose DP, Hazard H, et al. Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiol Biomarkers Prev. 2008;17:3319–3324. doi: 10.1158/1055-9965.EPI-08-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litton JK, Gonzalez-Angulo AM, Warneke CL, et al. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol. 2008;26:4072–4077. doi: 10.1200/JCO.2007.14.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert GW, Straznicky NE, Lambert EA, et al. Sympathetic nervous activation in obesity and the metabolic syndrome–causes, consequences and therapeutic implications. Pharmacol Ther. 12:159–172. doi: 10.1016/j.pharmthera.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Kyrou I, Chrousos GP, Tsigos C. Stress, visceral obesity, and metabolic complications. Ann N Y Acad Sci. 2006;1083:77–110. doi: 10.1196/annals.1367.008. [DOI] [PubMed] [Google Scholar]
- 26.Slotkin TA, Zhang J, Dancel R, et al. Beta-adrenoceptor signaling and its control of cell replication in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2000;60:153–166. doi: 10.1023/a:1006338232150. [DOI] [PubMed] [Google Scholar]
- 27.Draoui A, Vandewalle B, Hornez L, et al. Beta-adrenergic receptors in human breast cancer: Identification, characterization and correlation with progesterone and estradiol receptors. Anticancer Res. 1991;11:677–680. [PubMed] [Google Scholar]
- 28.Williams JB, Pang D, Delgado B, et al. A model of gene-environment interaction reveals altered mammary gland gene expression and increased tumor growth following social isolation. Cancer Prev Res. 2009;2:850–861. doi: 10.1158/1940-6207.CAPR-08-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 30.Lin NU, Claus E, Sohl J, et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: High incidence of central nervous system metastases. Cancer. 2008;113:2638–2645. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 32.Guarneri V, Broglio K, Kau SW, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24:1037–1044. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 33.Rubin RD. New York, NY: John Willey & Sons; 1987. Multiple imputations for nonresponse in surveys. [Google Scholar]
- 34.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winter MC, Coleman RE. Bisphosphonates in breast cancer: Teaching an old dog new tricks. Curr Opin Oncol. 2009;21:499–506. doi: 10.1097/CCO.0b013e328331c794. [DOI] [PubMed] [Google Scholar]
- 36.Call R, Grimsley M, Cadwallader L, et al. Insulin–carcinogen or mitogen? Preclinical and clinical evidence from prostate, breast, pancreatic, and colorectal cancer research. Postgrad Med. 122:158–165. doi: 10.3810/pgm.2010.05.2153. [DOI] [PubMed] [Google Scholar]
- 37.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 38.Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tangir J, Bonafé N, Gilmore-Hebert M, et al. SGK1, a potential regulator of c-fms related breast cancer aggressiveness. Clin Exp Metastasis. 2004;21:477–483. doi: 10.1007/s10585-004-4226-8. [DOI] [PubMed] [Google Scholar]
- 40.Chrousos GP, Gold PW. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 41.Melhem-Bertrandt A, Conzen S. The relationship between psychosocial stressors and breast cancer biology. Curr Breast Cancer Rep. 2010;2:130–137. [Google Scholar]
- 42.Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: A randomized clinical trial. Cancer. 2008;113:3450–3458. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carey L, Winer E, Viale G, et al. Triple-negative breast cancer: Disease entity or title of convenience. Nat Rev Clin Oncol. 2010;7:683–692. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- 44.Basile JN. One size does not fit all: The role of vasodilating beta-blockers in controlling hypertension as a means of reducing cardiovascular and stroke risk. Am J Med. 2010;123(suppl 1):S9–S15. doi: 10.1016/j.amjmed.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Johnson M. Molecular mechanisms of beta(2)-adrenergic receptor function, response, and regulation. J Allergy Clin Immunol. 2006;117:18–24. doi: 10.1016/j.jaci.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Huang XE, Hamajima N, Saito T, et al. Possible association of beta2- and beta3-adrenergic receptor gene polymorphisms with susceptibility to breast cancer. Breast Cancer Res. 2001;3:264–269. doi: 10.1186/bcr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith C, Teitler M. Beta-blocker selectivity at cloned human beta 1- and beta 2-adrenergic receptors. Cardiovasc Drugs Ther. 1999;13:123–126. doi: 10.1023/a:1007784109255. [DOI] [PubMed] [Google Scholar]