Abstract
Purpose
Antivascular endothelial growth factor (anti-VEGF) therapy is a promising treatment approach for patients with recurrent glioblastoma. This single-arm phase II study evaluated the efficacy of aflibercept (VEGF Trap), a recombinantly produced fusion protein that scavenges both VEGF and placental growth factor in patients with recurrent malignant glioma.
Patients and Methods
Forty-two patients with glioblastoma and 16 patients with anaplastic glioma who had received concurrent radiation and temozolomide and adjuvant temozolomide were enrolled at first relapse. Aflibercept 4 mg/kg was administered intravenously on day 1 of every 2-week cycle.
Results
The 6-month progression-free survival rate was 7.7% for the glioblastoma cohort and 25% for patients with anaplastic glioma. Overall radiographic response rate was 24% (18% for glioblastoma and 44% for anaplastic glioma). The median progression-free survival was 24 weeks for patients with anaplastic glioma (95% CI, 5 to 31 weeks) and 12 weeks for patients with glioblastoma (95% CI, 8 to 16 weeks). A total of 14 patients (25%) were removed from the study for toxicity, on average less than 2 months from treatment initiation. The main treatment-related National Cancer Institute Common Terminology Criteria grades 3 and 4 adverse events (38 total) included fatigue, hypertension, and lymphopenia. Two grade 4 CNS ischemias and one grade 4 systemic hemorrhage were reported. Aflibercept rapidly decreases permeability on dynamic contrast enhanced magnetic resonance imaging, and molecular analysis of baseline tumor tissue identified tumor-associated markers of response and resistance.
Conclusion
Aflibercept monotherapy has moderate toxicity and minimal evidence of single-agent activity in unselected patients with recurrent malignant glioma.
INTRODUCTION
Glioblastoma is the most common malignant primary brain tumor with an expected median progression-free survival (PFS) of 6.9 months and median overall survival (OS) of 10 to 14 months.1 Although the prognosis is better for patients with anaplastic glioma, these tumors ultimately transform into glioblastoma with increased vascular endothelial growth factor (VEGF) production secondary to an angiogenic switch. Radiotherapy plus temozolomide followed by adjuvant temozolomide has significantly improved the outcome for patients with glioblastoma1; however, tumor recurrence is inevitable and no curative treatment options exist for patients with recurrent malignant glioma. Patients with recurrent malignant glioma respond to therapy less than 15% of the time and have median PFS of 9 weeks for glioblastoma and 13 weeks for anaplastic astrocytoma.2
Vascular proliferation is one of the pathologic hallmarks of glioblastoma. Recruitment of tumor vessels from surrounding tissues requires angiogenic growth factors, including VEGF and the related placental growth factor (PlGF), which preferentially act on VEGF receptor 1.3 VEGF expression by glioma cells and infiltrating bone marrow–derived cells stimulate endothelial cell proliferation, migration, and survival, and increase vascular permeability. Tumor angiogenesis is a complex process whereby multiple molecules in normal and tumor tissue activate a series of signaling events leading to cooption of new blood vessels4,5 which may underlie the angiogenic switch and progression of anaplastic astrocytoma to glioblastoma. Preclinical studies highlight the potential efficacy of targeting VEGF and VEGF receptor (VEGFR) in the treatment of glioblastoma.6 Recent clinical trials7,8 in glioma with small-molecule inhibitors of VEGFR and VEGF antibody (bevacizumab [Avastin]) alone and in combination with cytotoxic chemotherapy9–12 have shown promising results.
PlGF stimulates angiogenesis, in part, by enhancing the activity of VEGF signaling by activation of VEGF receptor 1, and it is known to contribute to the angiogenic switch in cancer.3,13 Recent studies demonstrate that PlGF may mediate angiogenic escape and resistance to treatment,14 and blocking PlGF alone has evidence of preclinical efficacy.15 PlGF levels were recently shown to be markedly increased in patients with recurrent glioblastoma following treatment with a pan-VEGF receptor tyrosine kinase inhibitor,16 which supports the rationale for inhibiting both VEGF and PlGF in patients with glioma.
Aflibercept (VEGF Trap) is a recombinant fusion protein of the extracellular domains of VEGF fused to the Fc portion of immunoglobulin G1; it binds with high affinity to both VEGF (Kd: VEGF [165] 0.5 pmol/L) and PlGF (Kd: 39 pmol/L). Aflibercept was safe in a phase I clinical trial in solid tumors17 and is currently in phase II/III trials in Several cancer types, including lung, prostate, and ovarian cancer. Preclinical studies in glioma animal models demonstrate efficacy of aflibercept.18 In this trial, we sought to determine whether aflibercept-mediated inhibition of VEGF and PlGF leads to an improvement in 6-month PFS in patients with recurrent glioblastoma and anaplastic glioma.
PATIENTS AND METHODS
Patient Eligibility
Patients were required to have histologically confirmed glioblastoma, gliosarcoma, or anaplastic glioma (anaplastic astrocytoma, anaplastic oligodendroglioma, anaplastic mixed oligoastrocytoma) with evidence of unequivocal progression after chemoradiotherapy and no more than one adjuvant temozolomide-containing regimen. Additional eligibility requirements were as follows: tissue available from primary surgery at initial diagnosis or at relapse; Karnofsky performance status of ≥ 60; baseline magnetic resonance imaging (MRI) ≤ 14 days of registration on a stable dosage of steroids; life expectancy more than 8 weeks; recovered from the toxic effects of prior therapy; adequate bone marrow (absolute neutrophil count ≥ 1,500/mL; platelet count ≥ 100,000/mL), liver (ALT/alkaline phosphatase < 2 × normal; bilirubin < 1.5 mg/dL), and renal (blood urea nitrogen and creatinine < 1.5 × normal) functions; urine protein creatinine ratio (UPCR) less than 1; informed consent; more than 28 days from cytotoxic chemotherapy or investigational agent; and more than 4 weeks from surgery. Patients were excluded if they were pregnant or nursing, had a history of intracerebral or intratumoral hemorrhage, had treatment with prior bevacizumab or other antiangiogenic agent, had a history of significant cardiovascular disease, had severe concurrent illness or prior malignancy, and for any reason for full dose anticoagulation (such as deep venous thrombosis or pulmonary embolism). Patients who developed a requirement for anticoagulation were removed from the study. The study was approved by the Cancer Treatment Experimental Program (CTEP) and the institutional review board of each participating North American Brain Tumor Coalition NABTC site.
Treatment Plan
Aflibercept was provided by CTEP and administered to patients at the recommended phase II dose of 4 mg/kg intravenously on day 1 of every 14-day cycle. A 2-day window on either side of the 14-day treatment was allowed. Patients were allowed up to two dose reductions for grade 3 or 4 toxicities and were allowed to recover from treatment-related toxicities for up to 2 weeks or longer with the permission of the principal investigator and CTEP medical monitor. Patients received treatment until they experienced tumor progression or toxicity or withdrew consent.
Evaluations During Study
CBC with differential and serum chemistries were performed weekly for the first 12 weeks and then every 2 weeks thereafter. Urinalysis and UPCR were evaluated every week for the first 4 weeks and then every 4 weeks thereafter. Prothrombin time and partial thromboplastin time were performed every 2 weeks. Detailed physical examination, including complete neurologic examination and determination of Karnofsky performance status, was performed every 4 weeks. Blood pressure was carefully monitored every 2 weeks before each cycle. UPCR was required to be less than 1 or a 24-hour urinary protein concentration was required to be less than 2 g before treatment. Standard MRI evaluations were performed at baseline, at 4 and 8 weeks after initiating therapy, and every 8 weeks thereafter. The National Cancer Institute Common Terminology Criteria, version 3.0, was used to evaluate and grade treatment-related toxicities.
End Point and Assessment of Response
The primary end point for patients with glioblastoma was a 6-month (26-week) PFS rate for patients treated with aflibercept defined as the time of study registration to tumor progression. The anaplastic glioma cohort was considered exploratory, with emphasis on estimating the potential success rate in this patient group. Secondary end points included radiographic response rate, time to progression (TTP), OS, and toxicity. Response to treatment was determined by using modified MacDonald criteria,19 including a requirement for a confirmatory scan. Clinical decisions regarding progression were performed by the treating investigator on the basis of radiographic findings and evidence of clinical deterioration with response and progression at 6 months verified by central radiologic assessment. Final determination of response was provided by NABTC central radiologic review. A special response review sponsored by CTEP confirmed the central reviewer's assessment of response for the first 48 patients.
Correlative Studies
Tumor tissue obtained at the time of tumor diagnosis was evaluated for markers predictive of response or resistance to aflibercept. Slides from each site were used to isolate RNA from paraffin-embedded tissue as previously described.20 A whole-genome cDNA-mediated annealing, selection, extension and ligation assay (Illumina, San Diego, CA) was used to interrogate gene expression profiles.21,22 After normalization and batch effect removal by using ComBat,23 we used Cox regression analysis with a random forest approach to identify candidate genes associated with TTP. We then performed unsupervised clustering on expression from the top TTP candidate genes.
Advanced Neuroimaging
Vascular MRI scans were performed on a cohort of patients with glioblastoma at baseline and following 24 hours of treatment with aflibercept. Dynamic contrast enhanced MRI measures of vascular to extravascular extracellular space (EES) transfer constant (Ktrans), EES to vascular space rate constant (kep), and EES volume fraction (ve) were assessed at the indicated time points by using a two-compartment pharmacokinetic model.24,25 MRI sequence parameters were standardized between the two centers participating in the advanced imaging portion of this study (Dana-Farber Cancer Institute and MD Anderson Cancer Center).
Statistical Considerations
Patients were stratified on the basis of tumor histology: glioblastoma or gliosarcoma (n = 42) and anaplastic glioma (anaplastic astrocytoma, anaplastic oligodendroglioma, anaplastic mixed oligoastrocytoma; n = 16). All efficacy analyses were based on the principle of intent to treat and included all eligible patients. Safety analyses included all patients who received at least one dose of study medication. The primary end point of 6-month PFS was assessed from the time of study registration. Patients not known to be progression free at the time of the 6-month scan (24 weeks) were considered to have experienced treatment failure. Efficacy was based on comparison with historical data for 225 patients with recurrent glioblastoma treated on protocol in which patients with glioblastoma treated with ineffective agents had a 6-month PFS of 15%.2 The trial was sized to be able to discriminate between a 15% and 30% rate of 6-month PFS for the glioblastoma group. A sample size of 42 patients provided approximately 85% power to detect a true 30% 6-month PFS rate while maintaining 0.91 probability of rejecting for a true 15% 6-month PFS rate. In supplementary analyses, PFS and OS were estimated by using the Kaplan-Meier method. For the purpose of this supplementary PFS analysis, if a patient stopped treatment for reasons other than progression, the following rules were applied. If the patient continued to have regular scans and did not receive alternative treatment, the date of progression or death was used for assessing PFS. Otherwise the date of last scan or start of subsequent treatment was used to determine follow-up time, and the patient was censored for PFS at that date. If additional information post-treatment was not available, the patient was censored at the last dose date. All patients alive as of the last contact were censored for survival on the basis of that contact date.
RESULTS
Patient Characteristics
A total of 58 patients, including 16 patients with anaplastic glioma and 42 patients with glioblastoma or gliosarcoma, were enrolled from seven different institutions from February 2007 to November 2008. Patient demographic and pretreatment characteristics are provided in Table 1 . All patients underwent central pathologic review by the NABTC neuropathologist (K.A.). Of the 16 patients with anaplastic glioma, 12 (75%) had anaplastic astrocytoma, three (19%) had anaplastic oligodendroglioma, and one (6%) had an anaplastic mixed oligoastrocytoma. Of the 42 patients enrolled on the glioblastoma arm, all had pathologically confirmed glioblastoma with the exception of three patients (7%) with gliosarcoma. All 42 patients with glioblastoma received radiation with concurrent and adjuvant temozolomide. Three patients (7%) with glioblastoma were treated at their second recurrence and were excluded from the final efficacy analyses. Two patients in each cohort received only one cycle of treatment with one patient in each cohort withdrawing consent.
Table 1.
Patient Characteristics
| Characteristic | Anaplastic Glioma Arm |
Glioblastoma Arm |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| No. of Patients | 16 | 42 | ||
| Age, years | ||||
| Median | 53 | 55 | ||
| Range | 26-70 | 33-72 | ||
| Sex | ||||
| Male | 11 | 25 | ||
| Female | 5 | 17 | ||
| Karnofsky performance status | ||||
| Median | 90 | 90 | ||
| Range | 60-100 | 60-100 | ||
| Pathology | ||||
| Glioblastoma | 39 | 93 | ||
| Gliosarcoma | 3 | 7 | ||
| Anaplastic astrocytoma | 12 | 75 | ||
| Anaplastic oligodendroglioma | 3 | 19 | ||
| Anaplastic mixed oligoastrocytoma | 1 | 6 | ||
PFS and OS
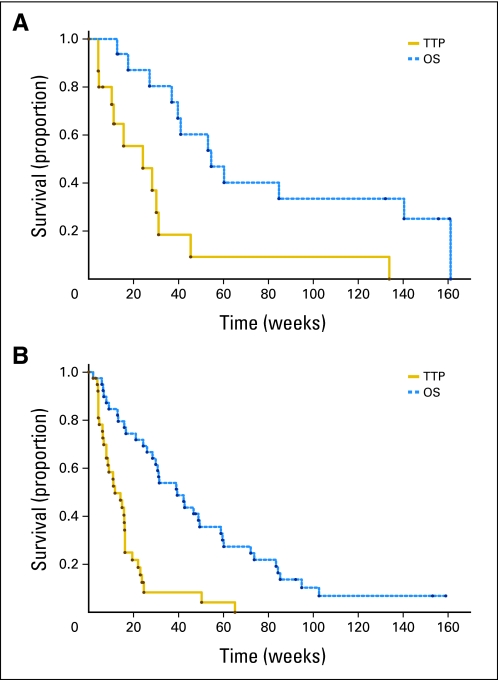
Three patients (7.7%) with glioblastoma had PFS of more than 6 months. Two patients experienced treatment failure at 50 and 65 weeks, and one was censored at 26 weeks. Thus, the primary efficacy end point for the glioblastoma cohort was not met. The 6-month PFS for patients with anaplastic glioma was 25%. The median PFS for the anaplastic cohort was 24 weeks (95% CI, 5 to 31 weeks), and the median PFS was 12 weeks (95% CI, 8 to 16 weeks) for patients with glioblastoma. Six grade 4 and four grade 3 patients were censored for progression before the 26-week time point. Median survival for patients with anaplastic glioma was 55 weeks with five patients living 2.5 years. For patients with glioblastoma, the median survival was 39 weeks with two patients alive beyond 150 weeks. Kaplan-Meier curves for PFS and OS for patients with glioblastoma and anaplastic glioma are shown in Figure 1.
Fig 1.
Kaplan-Meier estimates of time to progression (TTP) and overall survival (OS) for patients with anaplastic glioma (A) and glioblastoma (B).
Response
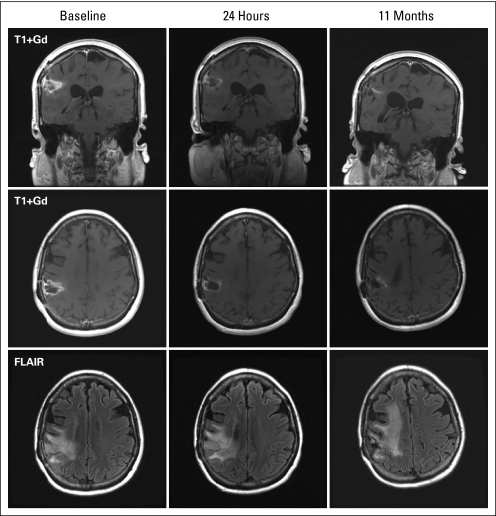
Of the 39 patients with glioblastoma, seven (18%) achieved a partial response (PR). One patient stopped treatment after 2 weeks because of toxicity but had a 4-week scan showing a PR. The PFS for the remaining six patients ranged from 15 to 65 weeks with a median of 23 weeks. Figure 2 shows an example of a patient with recurrent glioblastoma with a PR to aflibercept. Of 16 patients in the anaplastic glioma cohort, one patient (6%) achieved a complete response, and six patients (38%) showed a PR. Two patients with anaplastic glioma stopped treatment early and were censored at 6 and 10 weeks, respectively. PFS for the remaining five patients ranged from 24 to 134 weeks with a median of 45 weeks.
Fig 2.
Coronal (top) and axial (middle) T1-weighted post gadolinium (Gd) and fluid attenuated inversion recovery (FLAIR; bottom) magnetic resonance imaging of a patient with recurrent glioblastoma achieving a partial response to aflibercept.
Toxicity
Aflibercept was only moderately well tolerated in this single-agent phase II study. Table 2 lists the grade 3 (35 events) and grade 4 (three events) toxicities. No grade 5 toxicities were observed. Dose reductions for toxicity occurred in 19 patients with 11 grade 3 and 4 adverse events observed at −1 (nine events) and −2 (two events) dose levels. A total of 14 patients (eight anaplastic glioma, 50%; six glioblastoma, 14%) developed toxicity that required their discontinuing treatment. The median number of cycles received before removal from study for toxicity was five (range, one to 39 cycles) for the anaplastic glioma cohort and 3.5 cycles (range, zero to eight cycles) for the glioblastoma cohort. The most common adverse events leading to study drug discontinuation were fatigue, thromboembolic complications, wound healing complications, and CNS ischemia.
Table 2.
Grade 3 and 4 Toxicities per Patient (n = 58)
| Toxicity | Grade 3 |
Grade 4 |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Ataxia | 1 | 2 | 0 | |
| CNS ischemia | 1 | 2 | 2 | 3 |
| Confusion | 1 | 2 | 0 | |
| Dysphagia | 1 | 2 | 0 | |
| Fatigue | 3 | 5 | 0 | |
| GI hemorrhage | 0 | 1 | 2 | |
| Hand-foot syndrome | 1 | 2 | 0 | |
| Headache | 1 | 2 | 0 | |
| Hypertension | 6 | 10 | 0 | |
| Hyponatremia | 2 | 3 | 0 | |
| Hypophosphatemia | 2 | 3 | 0 | |
| Hypoxia | 1 | 2 | 0 | |
| Increased LFTs | 1 | 2 | 0 | |
| Lymphopenia | 4 | 7 | 0 | |
| Mucositis | 2 | 3 | 0 | |
| Neutropenia | 1 | 2 | 0 | |
| Pain | 2 | 3 | 0 | |
| Pericarditis | 1 | 2 | 0 | |
| Proteinuria | 2 | 3 | 0 | |
| Rash | 1 | 2 | 0 | |
| Thrombosis/embolism | 2 | 3 | 0 | |
| Wound complication | 1 | 2 | 0 | |
Abbreviation: LFT, liver function test.
Correlative Studies
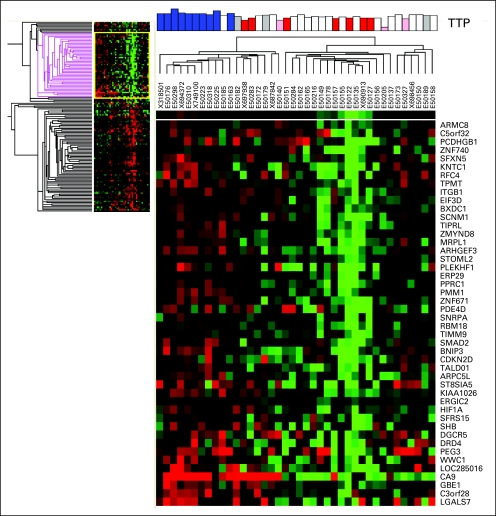
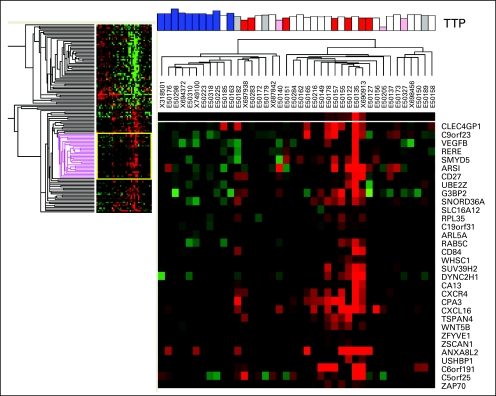
Unstained, fresh-frozen, paraffin-embedded tumor tissue from the patients' original surgery was obtained for 40 of the 58 patients (69%) enrolled on this trial (35 glioblastoma and five anaplastic glioma). RNA was isolated and analyzed by genome-wide gene expression patterns. Although the number of samples was small, we observed several genetic markers associated with TTP following treatment with aflibercept (see Appendix Figs A1 and A2, online only). Genes associated with a longer time to progression included carbonic anhydrase 9 (CA9), hypoxia inducible factor 1 alpha (HIF-1α), and SMAD2. Conversely, expression of CXCL16, CXCR4, ZAP70, CD27, CD84, and VEGF-B were associated with a shorter TTP (Table 3 and Appendix Table A1, online only).
Table 3.
Selected Gene Expression Changes Associated With TTP
| Longer TTP | P | Shorter TTP | P |
|---|---|---|---|
| ANAPC7 | .00213 | SLC16A13 | .00051 |
| GRM8 | .00329 | EPHB4 | .00491 |
| PCDHGB1 | .00551 | VEGFB | .00431 |
| KNTC1 | .00496 | CD27 | .00497 |
| ITGB1 | .00363 | SLC16A12 | .00455 |
| PLEKHF1 | .00506 | CD84 | .00406 |
| PPRC1 | .00673 | CA13 | .00480 |
| SMAD2 | .00271 | CXCR4 | .00565 |
| CDKN2D | .00351 | CXCL16 | .00383 |
| HIF-1α | .00492 | TSPAN4 | .00275 |
| DRD4 | .00265 | WNT5B | .00018 |
| CA9 | .00222 | ZAP70 | .00507 |
Abbreviation: TTP, time to progression.
Dynamic contrast enhanced MRI analysis was performed on 14 of 42 patients with glioblastoma at baseline and 24 hours following aflibercept infusion. Pixel-by-pixel Ktrans maps of all slices containing contrast-enhancing tumor were computed, and median values were determined for each patient at each time point. Compared with baseline, a 59% ± 39% reduction in median Ktrans was observed in 12 of 14 patients at 24 hours (P < .001, t test). Changes in Ktrans at this early time point were not predictive of durable response or TTP.
DISCUSSION
Targeting angiogenesis has recently been shown to improve PFS in recurrent glioblastoma; however, tumor progression occurs because of the development of resistance. Therefore, there is a strong rationale for dual inhibition of VEGF and PlGF, a proangiogenic growth factor which is thought to play an important role in stimulating pathologic angiogenesis via direct stimulation of VEGFR1 and VEGFR2 and via the attraction of proangiogenic myeloid cells. This NABTC phase II trial was, to our knowledge, the first clinical trial of affibercept in recurrent gliobastoma. Unfortunately, the primary end point of this trial was not met because only three patients with glioblastoma (7.7%) were alive and free of progression at 6 months.
The response rate in this study was similar to that for other antiangiogenic agents in patients with recurrent glioblastoma, and the durability of response was sustained in the patients who responded (median, 5.8 months). However, the number of patients reaching the 6-month PFS end point was considerably lower than that reported in several trials of bevacizumab and VEGFR inhibitors.12,26 Patient attrition due to toxicity may have contributed to the lower PFS rate in this study. Fourteen percent of the patients with glioblastoma and 50% of the patients with anaplastic glioma came off study because of toxicity, thus contributing to patients' not reaching the 6-month PFS end point. Aflibercept targets both VEGF and PlGF and binds to VEGF with a greater affinity than does bevacizumab, yet this did not lead to greater efficacy compared with bevacizumab therapy alone. The reasons for this discrepancy need further study.
Glioma resistance to antiangiogenic therapy is inevitable for many reasons, including the release of alternate proangiogenic factors to promote VEGF-independent angiogenesis. PlGF has been reported to be one such potential growth factor responsible for stimulation of blood vessel growth in the setting of VEGF inhibition.3,13,15 Clinical support for this idea has been suggested in several studies16,26 showing that PlGF levels increase in the blood of patients treated with antiangiogenic therapies. However, it is not clear whether the observed increase in PlGF is a response to the drug itself or whether PlGF plays an active role in stimulating pathologic angiogenesis in patients receiving anti-VEGF therapy. If VEGF is the primary driver of angiogenesis in glioblastoma, inhibiting PlGF may not provide additional benefit.
We performed expression analysis of patient samples to determine whether there were any genes significantly associated with response or progression. Several markers were found to be significantly associated with longer TTP, including the hypoxia markers CA9, HIF-1α, and SMAD2. Hypoxic tumors are known to increase expression of CA9 and HIF-1α,27 the latter a known transcription factor that increases the expression of VEGF.28 Thus, we hypothesize that tumors with greater hypoxia have more VEGF-dependent angiogenesis and would respond better to anti-VEGF therapy. Conversely, there were several genes whose expression was significantly associated with shorter TTP, including CXCL16, CXCR4, ZAP70, CD27, and CD84, which are associated with T lymphocytes. T lymphocytes are thought to infiltrate gliomas at an early stage29 in which they mediate immunosuppression and resistance to treatment.29,30 CXCR4 is also expressed on macrophages, granulocytes, and myeloid suppressor cells.31 CXCL12 (SDF-1α), the ligand for CXCR4, has been shown to attract myeloid cells to gliomas in which they mediate resistance to antiangiogenic treatment in preclinical models.32 Although this analysis was performed on tissue from the initial surgery, these data provide valuable information about potential mediators of response and resistance. However, the absence of a control arm limits the interpretation of these markers, which could be prognostic.
In summary, single-agent aflibercept appears to have minimal activity in recurrent glioblastoma. Although the primary end point of this trial was not met, some patients had a durable response. Intensive plasma (reported elsewhere) and tissue biomarker analyses were performed to identify those patients most likely to benefit, and they identified potential mechanisms of resistance to anti-VEGF therapy. Preclinical data support a potential synergistic benefit of radiation combined with aflibercept.33 Future studies may include combinations with radiation or chemotherapy. If the biomarker analyses from the ongoing studies confirm those described in this report, then future trials could be enriched with patients most likely to obtain the greatest benefit.
Acknowledgment
We thank Nicholas Patronas, MD, for independently reviewing magnetic resonance imaging response data.
Appendix
Table A1.
Gene Expression Changes Associated With TTP
| Longer TTP | P | Shorter TTP | P |
|---|---|---|---|
| ZNF165 | .00194 | AKAP2 | .00316 |
| ANAPC7 | .00213 | FLII | .00163 |
| GRM8 | .00329 | SLC16A13 | .00051 |
| ARMC8 | .00337 | EPHB4 | .00491 |
| PCDHGB1 | .00551 | DLK1 | .00372 |
| ZNF740 | .00264 | ANKRD30A | .00004 |
| SFXN5 | .00513 | SGCZ | .00390 |
| KNTC1 | .00496 | RNF41 | .00267 |
| RFC4 | .00289 | STX18 | .00122 |
| TPMT | .00545 | SRD5A3 | .00221 |
| ITGB1 | .00363 | SLC14A2 | .00265 |
| EIF3D | .00303 | WBSCR17 | .00017 |
| BXDC1 | .00019 | VIT | .00046 |
| SCNM1 | .00323 | IGLL1 | .00421 |
| TIPRL | .00593 | TPSAB1 | .00210 |
| ZMYND8 | .00529 | CLEC4GP1 | .00177 |
| MRPL1 | .00569 | VEGFB | .00431 |
| ARHGEF3 | .00365 | RERE | .00424 |
| STOML2 | .00480 | SMYD5 | .00116 |
| PLEKHF1 | .00506 | ARSI | .00093 |
| ERP29 | .00195 | CD27 | .00497 |
| PPRC1 | .00673 | UBE2Z | .00286 |
| PMM1 | .00003 | G3BP2 | .00344 |
| ZNF671 | .00389 | SNORD36A | .00406 |
| PDE4D | .00332 | SLC16A12 | .00455 |
| SNRPA | .00320 | RPL35 | .00484 |
| RBM18 | .00392 | ARL5A | .00384 |
| TIMM9 | .00327 | RAB5C | .00559 |
| SMAD2 | .00271 | CD84 | .00406 |
| BNIP3 | .00140 | WHSC1 | .00152 |
| CDKN2D | .00351 | SUV39H2 | .00292 |
| TALDO1 | .00076 | DYNC2H1 | .00406 |
| ARPC5L | .00324 | CA13 | .00480 |
| ST8SIA5 | .00576 | CXCR4 | .00565 |
| KIAA1026 | .00139 | CPA3 | .00245 |
| ERGIC2 | .00452 | CXCL16 | .00383 |
| HIF-1α | .00492 | TSPAN4 | .00275 |
| SFRS15 | .00333 | WNT5B | .00018 |
| SHB | .00312 | ZFYVE1 | .00418 |
| DGCR5 | .00396 | ZSCAN1 | .00243 |
| DRD4 | .00265 | ANXA8L2 | .00359 |
| PEG3 | .00297 | USHBP1 | .00023 |
| WWC1 | .00208 | ZAP70 | .00507 |
| CA9 | .00222 | CLCC1 | .00465 |
| GBE1 | .00486 | SFRP4 | .00448 |
| LGALS7 | .00515 | CD3G | .00009 |
| SNORA32 | .00506 | ||
| TUBB4Q | .00343 | ||
| IQCE | .00276 | ||
| ZNF599 | .00303 | ||
| NCDN | .00509 | ||
| TCF7 | .00229 | ||
| TNFRSF10C | .00200 | ||
| ASB3 | .00513 | ||
| CD302 | .00150 | ||
| PAEP | .00056 | ||
| TRIM15 | .00003 | ||
| UNC119 | .00081 | ||
| XAGE3 | .00461 | ||
| TNFRSF11A | .00388 | ||
| ZNF544 | .00343 | ||
| CCDC142 | .00116 | ||
| MYADM | .00246 | ||
| TRIM5 | .00081 | ||
| ARRDC1 | .00003 |
Abbreviation: TTP, time to progression.
Fig A1.
Heat map showing unsupervised gene clustering for patients with longer time to progression (TTP) on the basis of whole genome cDNA-mediated annealing, selection, extension, and ligation analysis of original tumor tissue. Red, bottom quartile; blue, top quartile; white, middle 50%; pink and gray, censored samples.
Fig A2.
Heat map showing unsupervised gene clustering for patients with shorter time to progression (TTP) on the basis of whole genome cDNA-mediated annealing, selection, extension, and ligation analysis of original tumor tissue. Red, bottom quartile; blue, top quartile; white, middle 50%; pink and gray, censored samples.
Footnotes
Supported by Grants No. U01-CA62399 from the North American Brain Tumor Coalition, National Institutes of Health and No. 1R21A126127 from the National Cancer Institute (J.D.G.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00369590.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Minesh P. Mehta, Pharmacyclics (C) Consultant or Advisory Role: John F. de Groot, Genentech (C); Mark R. Gilbert, Genentech (C); Frank Lieberman, Merck (C), Genentech (C); Minesh P. Mehta, Adnexus Therapeutics (C), Bayer Pharmaceuticals (C), Genentech (C), Merck (C), Schering-Plough (C), TomoTherapy (C), Stemina Biomarker Discovery (C), Abbott Laboratories (U); Andrew B. Lassman, Bristol-Myers Squibb (C), Cephalon (C), Eisai (C), Genentech (C), ImClone Systems (C), Merck (C), Schering-Plough (C), Sigma Tau Pharmaceuticals (C), Campus Bio (C); W.K. Alfred Yung, Novartis (C), Eden Therapeutics, (C) Genentech (C) Stock Ownership: Minesh P. Mehta, Pharmacyclics, TomoTherapy Honoraria: Mark R. Gilbert, Genentech; Andrew B. Lassman, Schering-Plough, Merck; W.K. Alfred Yung, Merck, Schering-Plough Research Funding: John F. de Groot, AstraZeneca, Adnexus Therapeutics, Exelixis; Susan M. Chang, Novartis, Schering-Plough; Mark R. Gilbert, Genentech; Andrew B. Lassman, Keryx Biopharmaceuticals, Genentech, Sigma Tau Pharmaceuticals, Schering-Plough, Exelixis, NovoCure, AstraZeneca, Adnexus Therapeutics; W.K. Alfred Yung, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: John F. de Groot, Mark R. Gilbert, W.K. Alfred Yung, Alice Chen, Michael D. Prados, Patrick Y. Wen
Provision of study materials or patients: John F. de Groot, Susan M. Chang, Timothy F. Cloughesy, Kenneth Aldape, Frank Lieberman, H. Ian Robins, Minesh P. Mehta, Andrew B. Lassman, Lisa M. DeAngelis, W.K. Alferd Yung, Michael D. Prados, Patrick Y. Wen
Collection and assembly of data: John F. de Groot, Susan M. Chang, Timothy F. Cloughesy, Kenneth Aldape, Frank Lieberman, H. Ian Robins, Minesh P. Mehta, Andrew B. Lassman, Lisa M. DeAngelis, W.K. Alfred Yung, Michael D. Prados, Patrick Y. Wen
Data analysis and interpretation: John F. de Groot, Kathleen R. Lamborn, Jun Yao, Edward F. Jackson, Lisa M. DeAngelis, Michael D. Prados, Patrick Y. Wen
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 3.Fischer C, Mazzone M, Jonckx B, et al. FLT1 and its ligands VEGFB and PlGF: Drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 5.Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 6.Cheng SY, Huang HJ, Nagane M, et al. Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 1996;93:8502–8507. doi: 10.1073/pnas.93.16.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yung WKA, Friedman C, Conrad D, et al. A phase I trial of single-agent PTK 787/ZK 222584 (PTK/ZK), an oral VEGFR tyrosine kinase inhibitor, in patients with recurrent glioblastoma multiforme. Proc Am Soc Clin Oncol. 2003;22:99. abstr 395. [Google Scholar]
- 8.Conrad C, Friedman H, Reardon D, et al. A phase I/II trial of single-agent PTK 787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in patients with recurrent glioblastoma multiforme (GBM) J Clin Oncol. 2004;22(suppl):110s. abstr 1512. [Google Scholar]
- 9.World Federation of Neuro-Oncology Meeting; May 2005. United Kingdom: Edinburgh; 2005. Stark-Vance V: Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma; p. 91. [Google Scholar]
- 10.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 11.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 12.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 14.Taylor AP, Rodriguez M, Adams K, et al. Altered tumor vessel maturation and proliferation in placenta growth factor-producing tumors: Potential relationship to post-therapy tumor angiogenesis and recurrence. Int J Cancer. 2003;105:158–164. doi: 10.1002/ijc.11059. [DOI] [PubMed] [Google Scholar]
- 15.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 16.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockhart AC, Rothenberg ML, Dupont J, et al. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol. 2010;28:207–214. doi: 10.1200/JCO.2009.22.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez-Manzano C, Holash J, Fueyo J, et al. VEGF Trap induces antiglioma effect at different stages of disease. Neuro Oncol. 2008;10:940–945. doi: 10.1215/15228517-2008-061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 20.Colman H, Zhang L, Sulman EP, et al. A multigene predictor of outcome in glioblastoma. Neuro Oncol. 2010;12:49–57. doi: 10.1093/neuonc/nop007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan JB, Yeakley JM, Bibikova M, et al. A versatile assay for high-throughput gene expression profiling on universal array matrices. Genome Res. 2004;14:878–885. doi: 10.1101/gr.2167504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.April C, Klotzle B, Royce T, et al. Whole-genome gene expression profiling of formalin-fixed, paraffin-embedded tissue samples. PLoS One 4. 2009:e8162. doi: 10.1371/journal.pone.0008162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker WL, Liao IH, Gilbert DL, et al. Empirical Bayes accommodation of batch-effects in microarray data using identical replicate reference samples: Application to RNA expression profiling of blood from Duchenne muscular dystrophy patients. BMC Genomics. 2008;9:494. doi: 10.1186/1471-2164-9-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997;7:91–101. doi: 10.1002/jmri.1880070113. [DOI] [PubMed] [Google Scholar]
- 25.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 26.Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28:2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plate KH, Breier G, Millauer B, et al. Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res. 1993;53:5822–5827. [PubMed] [Google Scholar]
- 28.Wang GL, Jiang BH, Rue EA, et al. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran Thang NN, Derouazi M, Philippin G, et al. Immune infiltration of spontaneous mouse astrocytomas is dominated by immunosuppressive cells from early stages of tumor development. Cancer Res. 2010;70:4829–4839. doi: 10.1158/0008-5472.CAN-09-3074. [DOI] [PubMed] [Google Scholar]
- 30.Humphries W, Wei J, Sampson JH, et al. The role of tregs in glioma-mediated immunosuppression: Potential target for intervention. Neurosurg Clin N Am. 2010;21:125–137. doi: 10.1016/j.nec.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murdoch C, Muthana M, Coffelt SB, et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 32.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wachsberger PR, Burd R, Cardi C, et al. VEGF trap in combination with radiotherapy improves tumor control in u87 glioblastoma. Int J Radiat Oncol Biol Phys. 2007;67:1526–1537. doi: 10.1016/j.ijrobp.2006.11.011. [DOI] [PubMed] [Google Scholar]