Abstract
JAK-STAT signaling is involved in the regulation of cell survival, proliferation, and differentiation. JAK tyrosine kinases can be transiently activated by cytokines or growth factors in normal cells, whereas they become constitutively activated as a result of mutations that affect their function in tumors. Specifically, the JAK2V617F mutation is present in the majority of patients with myeloproliferative disorders (MPDs) and is implicated in the pathogenesis of these diseases. In the present study, we report that the kinase CK2 is a novel interaction partner of JAKs and is essential for JAK-STAT activation. We demonstrate that cytokine-induced activation of JAKs and STATs and the expression of suppressor of cytokine signaling 3 (SOCS-3), a downstream target, are inhibited by CK2 small interfering RNAs or pharmacologic inhibitors. Endogenous CK2 is associated with JAK2 and JAK1 and phosphorylates JAK2 in vitro. To extend these findings, we demonstrate that CK2 interacts with JAK2V617F and that CK2 inhibitors suppress JAK2V617F autophosphorylation and downstream signaling in HEL92.1.7 cells (HEL) and primary cells from polycythemia vera (PV) patients. Furthermore, CK2 inhibitors potently induce apoptosis of HEL cells and PV cells. Our data provide evidence for novel cross-talk between CK2 and JAK-STAT signaling, with implications for therapeutic intervention in JAK2V617F-positive MPDs.
Introduction
The JAK-STAT pathway is crucial in transmitting signals from many cytokines and growth factors into the nucleus, regulating gene expression. Cytokines of the IL-6 family, type I and II IFNs, and growth factors such as growth hormone (GH) activate the JAK-STAT signaling pathway. Oncostatin M (OSM), a cytokine belonging to the IL-6 family, is a potent activator of the JAK-STAT signaling pathway.1 Binding of OSM induces heterodimerization of its receptors, gp130 and OSMRβ, and the receptor-associated JAKs, JAK1 and JAK2, become activated, leading to phosphorylation of gp130 tyrosine residues. The phosphorylated residues direct the recruitment of STAT proteins, including STAT-3, STAT-1, and STAT-5, which in turn become JAK substrates. Activated tyrosine–phosphorylated STATs form homodimers or heterodimers, translocate to the nucleus, and bind to consensus sequences in the promoters of OSM-responsive genes, inducing transcription.2
The JAK tyrosine kinase family comprises 4 mammalian members: JAK1, JAK2, JAK3, and TYK2. JAK2 is essential in erythropoiesis,3 and its dysfunction has been implicated in myeloproliferative disorders (MPDs) and leukemias.4 MPDs are a group of clonal hematopoietic disorders including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF).4 Recent studies have revealed that JAK2V617F, a somatic, activating point mutation, occurs in most PV patients and in > 50% of ET and PMF patients, and is involved in the pathogenesis of MPDs.5–8 Overexpression of JAK2V617F in murine Ba/F3 cells leads to cytokine-independent growth,5 and expression of JAK2V617F in mice recapitulates many pathologic characteristics observed in PV, ET, and PMF patients.9–11 Among the signaling pathways activated by JAK2V617F are the STATs, predominantly STAT-5.12 Therefore, JAK2V617F represents an ideal target for therapeutic intervention, especially in JAK2V617F-positive MPDs.
Protein kinase CK2 (formerly known as casein kinase 2 or II) is a ubiquitous, highly conserved serine/threonine kinase, and recent studies have shown that it can also phosphorylate tyrosine residues.13 CK2 phosphorylates > 300 substrates involved in DNA replication, gene transcription, signal transduction, and cell growth and apoptosis.14,15 CK2 presents as a tetramer composed of 2 catalytic subunits (α or α′) and 2 β regulatory subunits. CK2 is essential for cell viability, because disruption of either CK2α or CK2β is embryonically lethal.16,17
CK2 expression and activity are up-regulated in blood tumors, including multiple myeloma18 and leukemia,19 and in solid tumors, including kidney, mammary gland, lung, prostate, and head and neck cancers.20 In mouse models, CK2 cooperatively promotes oncogenesis and tumor progression with overexpression of other oncogenes such as c-myc,21 or with loss of tumor suppressors such as p53.22 Prosurvival genes such as β-catenin; oncogenes such as c-Myc, c-Myb, and c-Jun; and tumor suppressors such as promyelocytic leukemia (PML) protein, phosphatase and tensin homolog (PTEN), and p53 have been shown to be targets and/or interactors of CK2.20 Several signaling pathways, such as the NF-κB, Wnt, and PI3K pathways, are regulated by CK2 in a manner that promotes cell survival signaling.20
Both CK2 and JAK-STAT signaling play pivotal roles in cell survival, proliferation, and antiapoptotic mechanisms, and their dysregulation is associated with human malignancies. However, little is known of potential cross-talk between CK2 and the JAK-STAT pathway. To test this, we used small interfering RNA (siRNA) against CK2α and/or CK2β, as well as the pharmacologic inhibitors 4,5,6,7-tetrabromobenzotriazole (TBB) and emodin to knock down CK2 expression or inhibit its activity, respectively, and then examined the effects on activation of the JAK-STAT pathway. Our results indicate that OSM-, IFN-γ-, and GH-induced JAK-STAT activation are dependent on the presence or activity of CK2. In addition, CK2 is constitutively associated with JAK2 and JAK1, but not STAT-3. In vitro kinase assays suggest that JAK2 is phosphorylated by CK2, which is inhibited in the presence of TBB. Identifying CK2 as a novel interactor of JAKs and a positive regulator of JAK and STAT activation, we extended these studies to examine JAK2V617F activation in MPD cells. Using JAK2V617F-expressing erythroid leukemia cells HEL as a model, we demonstrate that the CK2 inhibitor TBB suppresses autonomous JAK2V617F activation and downstream signaling and also potently induces apoptosis of HEL cells. Furthermore, TBB suppresses constitutive activation of STAT-3, STAT-5, and ERK in primary cells from PV patients and induces apoptosis. These results suggest that CK2 inhibitors are of potential value for the treatment of JAK2V617F-positive MPDs.
Methods
Recombinant proteins and reagents
Recombinant human and murine OSM and human and murine IFN-γ were obtained from R&D Systems. Recombinant human full-length JAK2 was purchased from OriGene Technologies, and recombinant human CK2 (α and β) was from New England Biolabs. Antibodies to p-Y-STAT-3, total STAT-3, p-Y-STAT-1, p-Y-STAT-5, total STAT-5, p-JAK1, p-ERK, ERK, and caspase 8 were from Cell Signaling Technology. Antibodies to gp130, OSMRβ, p-Y1007/1008-JAK2, CK2α, JAK1, and IFN-γ receptor-1 (IFNGR1) were from Santa Cruz Biotechnology. STAT-1, JAK2, and anti-phosphotyrosine antibodies were from Millipore. Antibodies to caspase 3 were from Abcam. TBB (218697), P6, and antibody to CK2β were from EMD Biosciences. Emodin and antibodies to actin and hemagglutinin (HA) were from Sigma-Aldrich. Monoclonal antibodies specific for Bcl-xL were the generous gift of Dr Tong Zhou (University of Alabama at Birmingham). Murine CK2α and CK2β siRNA and transfection reagents were from Dharmacon.
Cells
Primary or immortalized mouse embryonic fibroblasts (MEFs) were kindly provided by Dr P-P. Pandolfi (Harvard University, Boston, MA). The CH235-MG human astroglioma cell line was cultured as described previously.23 γ2A-JAK2 is a JAK2-deficient human fibrosarcoma cell line (γ2A) that was stably transfected with JAK2, and γ2A-GHR-JAK2 (clone 14) is a γ2A cell line that was stably transfected with JAK2 and GH receptor (GHR) and maintained as described previously.24 γ2A-GHR-JAK2KD cells were maintained as described previously.25 MDA-MB-231 and 293T cells were maintained in DMEM and HEL 92.1.7 cells were maintained in RPMI 1640 medium. Primary murine astrocytes were prepared as described previously.26 Peripheral blood samples were obtained from the Mayo Clinic Cell Bank facility. All cells were collected under Mayo Clinic institutional review board–approved protocols for both collection to store and permission to use. Mononuclear cells from blood were obtained from JAK2V617F-positive PV patients as described previously,27 and maintained in StemSpan H3000 medium supplemented with CC100 cytokine cocktail (StemCell Technologies).
Plasmid constructs
HA-CK2α and the HA-CK2α-inhibitor–resistant mutant, which contains V66A and I174A double mutations, were constructed as described previously.28 HA-tagged full-length and deletion constructs of JAK2 were prepared as described previously.29
RNA interference
MEFs were seeded at 0.5 × 105/well in 6-well plates overnight and transfected with 100nM CK2α or CK2β siRNA or 50nM CK2α plus β siRNA for 48 hours using Dharmacon transfection reagent 4 according to the protocol provided by the manufacturer.
Immunoprecipitation and immunoblotting
For co-immunoprecipitation (co-IP) assays, confluent cells in 150-mm dishes were harvested in cell lysis buffer containing 50mM Tris (pH 7.5), 150mM NaCl, 1% Ipegal, 2mM EDTA, 1mM PMSF, 25 μg/mL of aprotinin, 25 μg/mL of leupeptin, and 1 × phosphatase inhibitor (ThermoScientific). Cell lysate (1-2 mg) was incubated with 1-2 μg of antibody overnight at 4°C and then with 35 μL of protein A/G beads for 1 hour. The beads were washed, boiled in 2 × loading buffer, separated by SDS-PAGE gel, and immunoblotted with antibody. For immunoblotting, ∼ 20-30 μg of total protein was used to detect STATs or CK2 on 8% or 10% gels, respectively, and ∼ 50-80 μg of total protein was used to detect JAK2, JAK1, gp130, or OSMRβ on 6% gels.
ELISA
Cell lysates were prepared from MEFs, HEL, and primary PV cells, and 65 μg of total protein was assayed using the JAK2 pYpY1007/ 1008 ELISA kit (Invitrogen) according to the protocol provided.
RNA isolation, riboprobes, RPA, and RT-PCR
Total RNA was extracted using TRIzol (Invitrogen). Riboprobes for murine suppressor of cytokine signaling 3 (SOCS-3) and GAPDH were prepared and the RNase protection assay (RPA) was performed as described previously.30 For RT-PCR, 1 μg of total RNA was used and the primer pairs 5′-aggagagcggattctactgga-3′ and 5′-tggccgttgacagtcttccgaca-3′ were used to amplify murine SOCS-3.
Apoptosis assay
HEL and primary PV cells were treated with TBB for 24 hours, stained with Annexin V and propidium iodide using the ApoAlert Annexin V-FITC Apoptosis Kit (Clontech), and examined by flow cytometry. The percentage of annexin V–positive cells was determined using FlowJo 7.5.5 software.
Cell-cycle analysis
HEL cells were treated with TBB for 24 hours, fixed with 70% ethanol overnight, stained with propidium iodide, digested with RNase for 45 minutes, and examined by flow cytometry. The percentage of cells in different stages was determined with FlowJo 7.5.5 software.
Cell-survival assay
Primary PV cells were seeded in triplicate at 1 × 105/mL in a 100-μL volume in the absence or presence of TBB; then, 10 μL of WST-1 (Roche) was added to the cells and absorbance was measured at 450 nm against 655 nm after 2 hours.
CK2 kinase activity assay
Cell lysates were prepared, immunoprecipitated, and assayed according to the provided protocol using a CK2 assay kit (17-132; Upstate Biotechnology).
In vitro phosphorylation assay
Purified recombinant human JAK2 was incubated with CK2 reaction buffer, 200μM ATP, 5 μCi γ–labeled ATP, and recombinant CK2 (α and β) at 30°C for 2 hours, boiled in 2 × loading buffer, separated by SDS-PAGE gel, and subjected to autoradiography.
Densitometric and statistical analysis
Densitometric quantitation of immunoblotting, RPA, or RT-PCR images in the linear range was performed using an image analysis program (ImageJ 1.41o; National Institutes of Health). Levels of significance for comparison between samples were determined by Student t test distribution. P ≤ .05 was considered statistically significant. All experiments were performed a minimum of 3 times.
Results
CK2 is required for cytokine-induced STAT activation and downstream gene expression
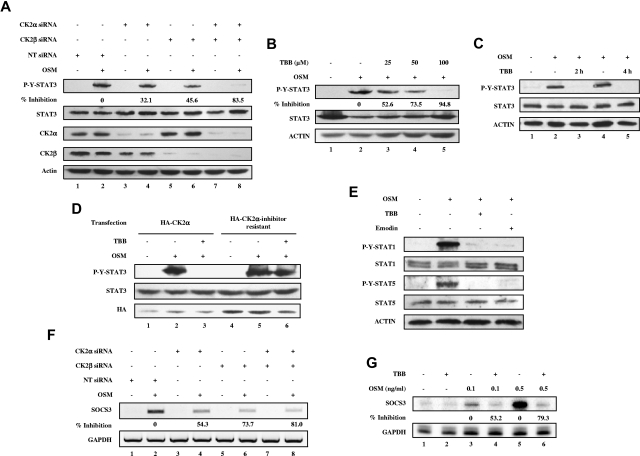
Previous studies in our laboratory identified the tumor suppressor PML as a regulator of the JAK-STAT pathway.31 Furthermore, PML was shown to be a substrate of CK2 and targeted for degradation by the proteosome.32 Therefore, we were interested in determining whether the JAK-STAT pathway is regulated by CK2 in a PML-dependent or a PML-independent manner. To investigate whether there is cross-talk between CK2 and the JAK-STAT pathway, we initially examined whether CK2 is involved in the regulation of STAT-3 activation. We used siRNA to specifically inhibit expression of CK2α and/or CK2β, and then examined the influence on OSM-induced signaling cascades. MEFs were transfected with 100nM CK2α or CK2β siRNAs, or 50nM CK2α and CK2β siRNAs for 48 hours, stimulated with OSM, and assayed for phospho-tyrosine STAT-3 and total STAT-3 levels. Silencing of CK2α or CK2β expression led to reduced OSM-induced STAT-3 activation (Figure 1A). The inhibitory effect was most pronounced when both CK2α and CK2β were diminished in expression (Figure 1A lane 8). We observed that CK2α siRNA also inhibited CK2β levels (Figure 1A lanes 3-4). This has been previously observed in vivo with CK2α knockout mice16 and in vitro in MCF7 cells.33 The CK2 pharmacologic inhibitor TBB is one of most selective inhibitors of CK2 available. We confirmed that TBB suppresses CK2 catalytic activity in a dose-dependent manner (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). MEFs pretreated with TBB for 30 minutes exhibited reduced OSM-induced STAT-3 tyrosine phosphorylation in a dose-dependent manner (Figure 1B). Longer preincubation times with TBB (2 and 4 hours) completely inhibited OSM-induced STAT-3 activation (Figure 1C lanes 3 and 5) without any observable toxic effect on cell survival of MEFs. Comparable results were obtained using PML−/− MEFs (data not shown), indicating that CK2-mediated effects on STAT activation occurred in a PML-independent manner. To confirm that the inhibitory effect of TBB on STAT-3 activation is dependent on the inhibition of CK2, we overexpressed either wild-type CK2α or CK2α-inhibitor resistant, which contains V66A and I174A double mutations, thus rendering cells resistant to 3 specific CK2 inhibitors including TBB.28 As shown in Figure 1D, TBB inhibited OSM-induced STAT-3 activation in HA-CK2α–transfected cells (Figure 1D compare lanes 3 and 2), whereas the inhibitory effect of TBB on STAT-3 activation was abolished by introducing HA-CK2α-inhibitor resistant (Figure 1D compare lanes 6 and 5). We conclude that CK2 expression and activity is required for OSM-induced STAT-3 tyrosine phosphorylation.
Figure 1.
CK2 is required for OSM-induced STAT activation and gene expression. (A-E) Cell lysates were immunoblotted with the indicated antibodies. (A) MEFs were transfected with nontarget (NT) siRNA (100nM), CK2α siRNA (100nM), CK2β siRNA (100nM), or CK2α (50nM) plus CK2β siRNA (50nM) for 48 hours, then stimulated with 1 ng/mL of OSM for 10 minutes. The densitometric ratios of p-Y-STAT-3 versus STAT-3 were calculated. The values shown in lanes 4, 6, and 8 were compared with that of lane 2 (control, no inhibition) and the percentage of inhibition was determined. (B) MEFs were pretreated with 25-100μM TBB for 2 hours and then stimulated with 1 ng/mL of OSM for 10 minutes. The densitometric ratios of p-Y-STAT-3 versus STAT-3 were calculated. The values shown in lanes 3, 4, and 5 were compared with that of lane 2 (control, no inhibition) and the percentage of inhibition was determined. (C) MEFs were pretreated with TBB (50μM) for 2 and 4 hours and then stimulated with 0.1 ng/mL of OSM for 30 minutes. (D) MEFs were transfected with HA-CK2α or CK2α-inhibitor–resistant constructs for 24 hours, pretreated with TBB (50μM) for 2 hours and then stimulated with 1 ng/mL of OSM for 30 minutes. (E) MEFs were pretreated with CK2 inhibitors for 2 hours and then treated with OSM (10 ng/mL) for 30 minutes. (F) MEFs were transfected with nontarget (NT) siRNA (100nM), CK2α siRNA (100nM), CK2β siRNA (100nM), or CK2α (50nM) plus CK2β siRNA (50nM) for 48 hours, then stimulated with 0.1 ng/mL of OSM for 30 minutes. RNA was prepared and analyzed by RT-PCR for expression of SOCS-3 and GAPDH. The densitometric ratios of SOCS-3 versus GAPDH were calculated. The values of lanes 4, 6, and 8 were compared with that of lane 2 (control, no inhibition) and the percentage of inhibition was determined. (G) MEFs were pretreated with TBB (50μM) for 2 hours and then stimulated with different concentrations of OSM (0.1-0.5 ng/mL). Total mRNA was extracted and analyzed by RPA with probes specific to SOCS-3 and GAPDH (loading control). The densitometric ratios of SOCS-3 versus GAPDH were calculated. The value of lane 4 was compared with that of lane 3 (control, no inhibition), the value of lane 6 was compared with that of lane 5, and the percentage of inhibition was determined.
We next investigated whether the activation of other STATs, including STAT-1 and STAT-5, is also dependent on CK2. As shown in Figure 1E, OSM-induced activation of STAT-1 and STAT-5 tyrosine phosphorylation was inhibited by TBB or emodin, another CK2 inhibitor that inhibits CK2 catalytic activity in a dose-dependent manner (supplemental Figure 1B). We examined the effect of inhibition of CK2 on SOCS-3, a downstream STAT target gene. OSM induced expression of SOCS-3 mRNA (Figure 1F lane 2 and Figure 1G lanes 3 and 5), which was inhibited by CK2 siRNAs (Figure 1F lanes 4, 6, and 8) or TBB (Figure 1G lanes 4 and 6). These observations indicate that OSM-induced SOCS-3 gene expression is dependent on CK2.
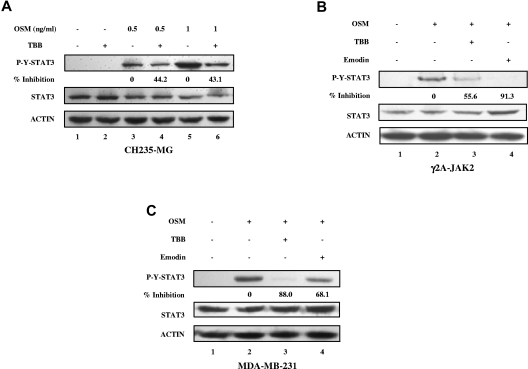
We also observed the inhibitory effects of CK2 inhibitors on OSM-induced STAT-3 tyrosine phosphorylation in human cancer cell lines, including CH235-MG human astroglioma cells, γ2A-JAK2 human fibrosarcoma cells, and MDA-MB-231 breast tumor cells (Figure 2), indicating that this effect is not restricted to MEFs.
Figure 2.
Inhibition of OSM-induced STAT-3 activation in human solid tumor cell lines by CK2 inhibitors. (A-C) Cell lysates were immunoblotted with the indicated antibodies. (A) CH235 human astroglioma cells were pretreated with TBB (50μM) for 2 hours and then stimulated with different concentrations of human OSM (0.5 and 1 ng/mL) for 30 minutes. The densitometric ratios of p-Y-STAT-3 versus STAT-3 were calculated. The value of lane 4 was compared with that of lane 3 (control, no inhibition), the value of lane 6 was compared with that of lane 5, and the percentage of inhibition was determined. γ2A-JAK2 human fibrosarcoma cells (B) and MDA-MB-231 human breast cancer cells (C) were pretreated with TBB (50μM) or emodin (50μM) for 2 hours and then stimulated with 0.5 ng/mL of human OSM for 30 minutes. The densitometric ratios of P-Y-STAT-3 versus STAT-3 were calculated. The values of lanes 3 and 4 were compared with that of lane 2 (control, no inhibition) and the percentage of inhibition was determined.
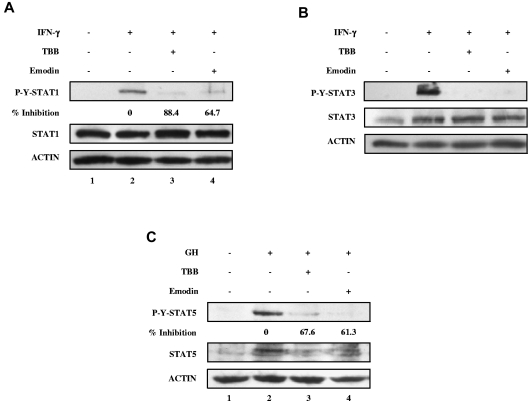
We next examined whether signaling by other cytokines was affected by CK2. We used IFN-γ, an activator of STAT-1 and STAT-3, and GH, an activator of STAT-5. As shown in Figure 3, the CK2 inhibitors TBB and emodin suppressed IFN-γ–induced tyrosine phosphorylation of both STAT-1 and STAT-3, and GH-induced tyrosine phosphorylation of STAT-5. These results indicate that CK2 is required for cytokine (OSM and IFN-γ)- and GH-induced activation of the JAK-STAT signaling pathway.
Figure 3.
Inhibition of IFN-γ and GH signaling pathways by CK2 inhibitors. (A-C) Cell lysates were immunoblotted with the indicated antibodies. (A) MEFs were pretreated with TBB (50μM) or emodin (50μM) for 2 hours and then stimulated with 5 ng/mL of IFN-γ for 30 minutes. The densitometric ratios of P-Y-STAT-1 versus STAT-1 were calculated. The values of lanes 3 and 4 were compared with that of lane 2 (control, no inhibition) and the percentage of inhibition was determined. (B) MEFs were pretreated with TBB (50μM) or emodin (50μM) for 2 hours and then stimulated with 5 ng/mL of IFN-γ for 30 minutes. (C) γ2A-GHR-JAK2 cells were pretreated with TBB (50μM) or emodin (50μM) for 2 hours and then stimulated with 250 ng/mL of GH for 10 minutes. The densitometric ratios of P-Y-STAT-5 versus ACTIN were calculated. The values of lanes 3 and 4 were compared with that of lane 2 (control, no inhibition) and the percentage of inhibition was determined.
Given that OSM-induced activation of 3 STATs, STAT-3, STAT-1, and STAT-5, is suppressed by inhibiting CK2 expression or activity, the inhibitory effect may involve a common mediator(s) capable of activating the different STAT proteins, which could be JAKs or the cytokine receptors.
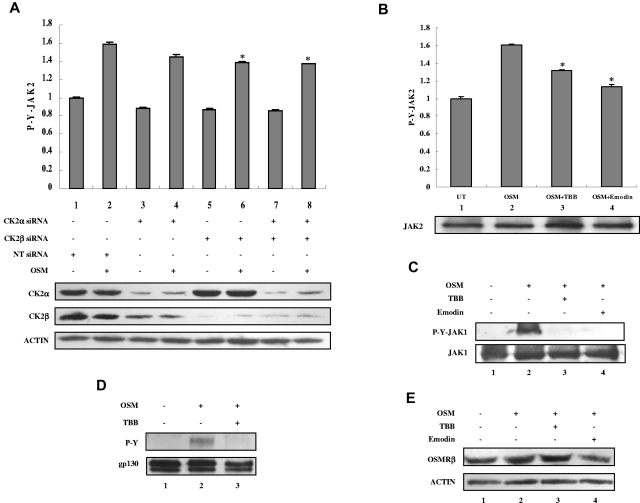
OSM-induced activation of JAKs is dependent on CK2
OSM binds to the gp130 receptor subunit, and recruits the OSMRβ subunit.1 The receptor-associated JAK tyrosine kinases JAK1 and JAK2 are tyrosine phosphorylated and activated and then phosphorylate gp130. To determine the effect of CK2 siRNAs or inhibitors on JAK2 activation, we assayed JAK2 tyrosine phosphorylation using ELISA (Figure 4A-B). Because of the low level of phosphorylation of JAKs and gp130, higher concentrations of OSM were used to examine JAK and gp130 activation. OSM induced JAK2 tyrosine phosphorylation (Figure 4A-B column 2), which was inhibited by CK2 siRNAs (Figure 4A columns 6 and 8) or by preincubation with TBB or emodin (Figure 4B columns 3-4). OSM also induced JAK1 tyrosine phosphorylation (Figure 4C lane 2), which was inhibited by preincubation with TBB or emodin (Figure 4C lanes 3-4). Total levels of JAK2 (Figure 4B lower panel) and JAK1 (Figure 4C) were not affected by treatment with TBB or emodin. OSM stimulation induced gp130 tyrosine phosphorylation (Figure 4D lane 2), which was inhibited by TBB (Figure 4D lane 3). Total levels of gp130 were also slightly inhibited (Figure 4D lane 3). OSM treatment did not affect the total levels of OSMRβ (Figure 4E lane 2), which were also largely unaffected by treatment with TBB or emodin (Figure 4E lanes 3-4). These findings suggest that CK2 may function to regulate JAK activation, thus controlling the activation of STATs and the expression of downstream genes.
Figure 4.
CK2 is required for OSM-induced JAK2 and JAK1 activation. (A) MEFs were transfected with nontarget (NT) siRNA (100nM), CK2α siRNA (100nM), CK2β siRNA (100nM), or CK2α (50nM) plus CK2β siRNA (50nM) for 48 hours and then stimulated with 5 ng/mL of OSM for 10 minutes. (B-C) MEFs were pretreated with TBB (50μM) or emodin (50μM) for 2 hours and then stimulated with 5 ng/mL OSM for 10 minutes. (A-B) The protein concentration of the cell lysates was measured in duplicate and 65 μg of total protein was analyzed for JAK2 pYpY 1007/1008 expression by ELISA and normalized to JAK2 expression, which is not affected by CK2 siRNAs. The value of the untreated sample was arbitrarily set as 1. Three independent experiments were performed and error bars show ± SD. *P < .05. Cell lysates were immunoblotted with the indicated antibodies (lower panels). (C) Cell lysates were blotted with the indicated antibodies. (D) MEFs were pretreated with TBB (50μM) for 2 hours and then stimulated with 10 ng/mL of OSM for 30 minutes. Lysates were immunoprecipitated with anti-gp130 antibody and analyzed by immunoblotting. The blot was detected with anti-phosphotyrosine antibody and then reprobed with gp130 antibody after stripping. (E) MEFs were pretreated with TBB (50μM) or emodin (50μM) for 2 hours and then stimulated with 0.1 ng/mL of OSM for 30 minutes. Cell lysates were immunoblotted with the indicated antibodies.
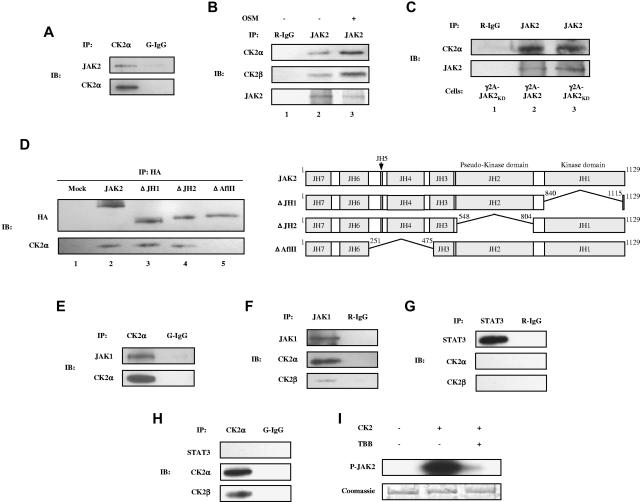
CK2 is associated with JAK2 and JAK1
Our results thus far suggested that JAK activation may be directly or indirectly dependent on the presence/activity of CK2. Therefore, we tested the possible association between endogenous JAKs and CK2 using co-IP experiments. JAK2 forms a complex with endogenous CK2α and CK2β (Figure 5A-B) in γ2A-JAK2 cells, and this association was enhanced in response to OSM (Figure 5B lane 3). We also observed an association between endogenous JAK2 and CK2α in MEFs, MDA-MB-231 cells, and primary astrocytes (supplemental Figure 2). In addition, to determine whether the association between JAK2 and CK2 is dependent on JAK2 kinase activity, we used a γ2A-JAK2KD stable cell line expressing kinase-dead JAK2.25 As shown in Figure 5C, CK2α was capable of associating with JAK2KD (Figure 5C lane 3), which suggests that JAK2 kinase activity is not required for association with CK2α. To identify the domain(s) of JAK2 that interacts with CK2, 293T cells were transfected with HA-tagged wild-type JAK2 and JAK2 deletion constructs (Figure 5D) and the co-IP experiment was performed. ΔJH1 and ΔJH2, which lack the JH1 and JH2 domains, respectively, co-precipitate with endogenous CK2α (Figure 5D lanes 3-4), whereas ΔAflII, which lacks the JH4 and JH5 domains and partial sequences of JH3 and JH6, does not (Figure 5D lane 5). This suggests that the JAK2 regions deleted in ΔAflII (JH3-JH6) are required for interaction with CK2. In addition to JAK2, endogenous CK2α and CK2β associate with JAK1 (Figure 5E-F). Co-IP experiments did not indicate an association between endogenous STAT-3 and CK2α or CK2β (Figure 5G-H). The association between JAKs and CK2 suggests that JAKs may be a substrate of CK2. Purified recombinant human JAK2, which was expressed in HEK293 cells, was examined in an in vitro phosphorylation assay. As shown in Figure 5I, JAK2 was phosphorylated by CK2 (Figure 5I lane 2), and inclusion of TBB inhibited JAK2 phosphorylation (Figure 5I lane 3). These findings suggest that JAK2 is a substrate for CK2.
Figure 5.
Co-IP of endogenous CK2 with JAK1 and JAK2 and phosphorylation of JAK2 by CK2 in vitro. Lysates of γ2A-JAK2 cells were immunoprecipitated (IP) with anti-CK2α and normal goat IgG (G-IgG, negative control) (A, E, H) or with anti-STAT-3, anti-JAK1, or normal rabbit IgG (R-IgG, negative control) (F-G). (B) γ2A-JAK2 cells were untreated or treated with 5 ng/mL of OSM for 15 minutes. Cell lysates were immunoprecipitated with the indicated antibodies. (C) Lysates of γ2A-GHR-JAK2 cells (lane 2) and γ2A-GHR-JAK2KD cells (lanes 1 and 3) were immunoprecipitated with the indicated antibodies. (D) Left panel: 293T cells were transfected with HA-tagged JAK2 full-length or deletion constructs, immunoprecipitated, and immunoblotted (IB) with the indicated antibodies. Right panel: schematic presentation of the proteins encoded by JAK2 constructs. The deleted amino acids are indicated. (I) 0.5 μg of JAK2 was incubated with 500 U of CK2 (α and β) in the absence or presence of 50μM of TBB and resolved by 6% SDS-PAGE, followed by autoradiography (top panel) and Coomassie blue staining (bottom panel).
Our results indicate that CK2 is required for IFN-γ–induced STAT-1 and STAT-3 activation (Figure 3A-B) and that it is associated with JAK1 (Figure 5E-F). IFN-γ–induced signaling requires ligand binding to the IFNGR1 subunit of the receptor. The IFNGR1 intracellular domain binds to JAK1 with different affinities in different cell types. Therefore, one issue that needed to be addressed was whether CK2 is associated with IFNGR1. A co-IP assay was performed in γ2A-JAK2 cells, and the results indicated that IFNGR1 does not co-precipitate with CK2α in these cells (supplemental Figure 3)
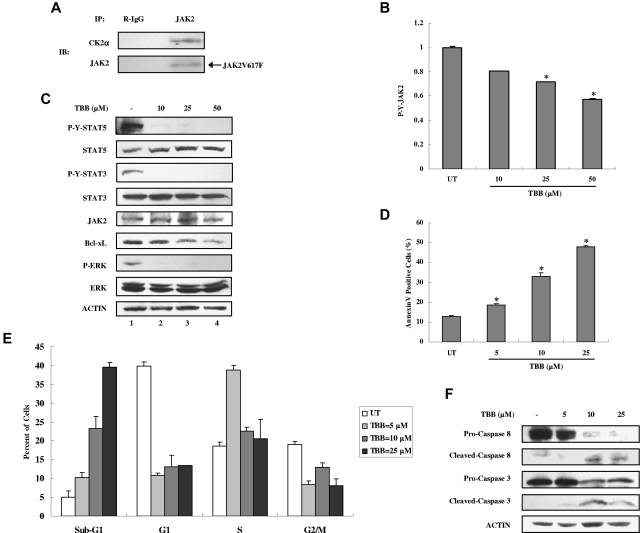
CK2 is required for JAK2V617F phosphorylation, JAK-STAT signaling, and survival of HEL cells
CK2 and JAKs are dysregulated in different types of blood tumors and in solid tumors. JAK2V617F, an acquired point mutation causing constitutive activation of JAK2 and STAT-5, is implicated in the pathogenesis of MPDs.5–8 Based on our findings that JAK2 activation is dependent on CK2 and that CK2 is constitutively associated with JAK2, we hypothesized that JAK2V617F activation may also depend on CK2. We first examined whether JAK2V617F could associate with CK2. We used the JAK2V617F mutant–expressing erythroid leukemia cell line HEL because it expresses homozygous JAK2V617F. As shown in Figure 6A, endogenous JAK2V617F is associated with CK2α. Furthermore, treatment with the CK2 inhibitor TBB for 4 hours suppressed autonomous JAK2V617F tyrosine phosphorylation in a dose-dependent manner in HEL cells (Figure 6B) without affecting the total levels of JAK2 (Figure 6C). We also detected constitutive activation of STAT-5, STAT-3, and ERK, which were inhibited by TBB at all concentrations (Figure 6C). Constitutive expression of the downstream antiapoptotic protein Bcl-xL was inhibited by TBB in a dose-dependent manner (Figure 6C lanes 2-4).
Figure 6.
Inhibition of autonomous activation of JAK-STAT signaling and induction of apoptosis in HEL cells by the CK2 inhibitor TBB. (A) Lysates of HEL cells were immunoprecipitated with anti-JAK2 and R-IgG and then immunoblotted with the indicated antibodies. (B) HEL cells were treated with different concentrations of TBB (10-50μM) for 4 hours. The protein concentration of cell lysates was measured in duplicate, and 65 μg of total protein was analyzed for JAK2 pYpY 1007/1008 expression with ELISA, and then normalized to JAK2 expression. The value of the untreated sample was arbitrarily set as 1. Three independent experiments were performed and error bars show ± SD. *P < .05. (C) HEL cells were treated with different concentrations of TBB (10-50μM) for 4 hours. Cell lysates were immunoblotted with the indicated antibodies. (D-F) HEL cells were treated with different concentrations of TBB (5-25μM) for 24 hours. (D) Cells were stained with annexin V and propidium iodide and examined by flow cytometry. Experiments were performed in triplicate and error bars show ± SD. *P < .05. (E) HEL cells were fixed overnight, stained with propidium iodide, and digested with RNase. The percentage of cells in the sub-G1, G1, S, and G2/M phases was examined by flow cytometry. (F) Cell lysates were immunoblotted with the indicated antibodies.
Given that JAK2V617F phosphorylation, STAT activation, and downstream protein expression of Bcl-xL can be inhibited by TBB, we hypothesized that inhibition of CK2 may promote apoptosis of HEL cells. As shown in Figure 6D, TBB induced an increase in apoptosis of HEL cells in a dose-dependent manner within 24 hours, as assayed by Annexin V staining. Cell-cycle analysis also showed that the number of HEL cells in the sub-G1 phase, which is used as an index for the degree of apoptosis, increased with TBB treatment in a dose-dependent manner (Figure 6E). The percentage of cells in each phase of the cell cycle was altered, and fewer cells were present in the G1 and G2/M phase after treatment with TBB (Figure 6E). To further confirm the incidence of apoptosis, we evaluated caspase activation by immunoblotting. After treatment with TBB for 24 hours, HEL cells displayed caspase 8 and caspase 3 activation (Figure 6F), indicating that TBB potently induces apoptosis in HEL cells.
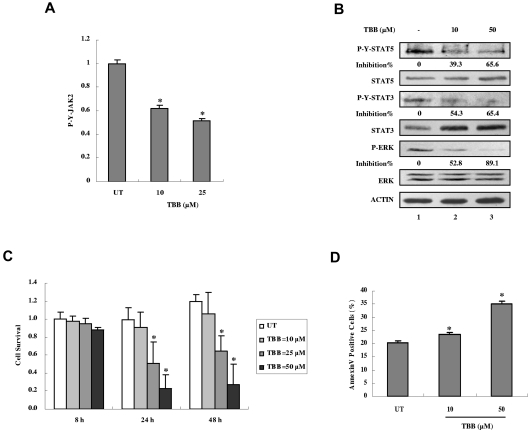
CK2 is required for autonomous activation of JAK-STAT signaling and survival of PV patient primary cells
We next examined the effects of inhibition of CK2 on JAK-STAT activation in mononuclear cells from JAK2V617F-positive PV patients. Treatment with TBB for 4 hours suppressed constitutive activation of JAK2 (Figure 7A), STAT-5, STAT-3, and ERK (Figure 7B). Furthermore, treatment with TBB for 24 hours suppressed cell survival (Figure 7C) and induced apoptosis (Figure 7D) in primary PV cells in a dose-dependent manner. These data indicate that CK2 is required for constitutive activation of JAK2V617F signaling and survival not only in HEL cells, but also in primary cells from JAK2V617F-positive PV patients.
Figure 7.
Inhibition of autonomous activation of JAK-STAT signaling and induction of apoptosis in primary PV cells by the CK2 inhibitor TBB. (A) Primary PV cells were treated with 10 or 50μM of TBB for 4 hours. The protein concentration of cell lysates was measured in duplicate and 100 μg of total protein was analyzed for JAK2 pYpY 1007/1008 expression with ELISA and then normalized to JAK2 expression. The value of the untreated sample was arbitrarily set as 1. Three independent experiments were performed and error bars show ± SD. *P < .05. (B) Primary PV cells were treated with 10 or 50μM TBB for 4 hours. Cell lysates were immunoblotted with the indicated antibodies. The densitometric ratios of P-Y-STAT-3 versus STAT-3, P-Y-STAT-5 versus STAT-5, and P-ERK versus ERK were calculated. The values of lanes 2 and 3 were compared with that of lane 1 (control, no inhibition) and the percentage of inhibition was determined. (C) Primary PV cells were treated with different concentrations of TBB (10-50μM) for the indicated times and cell survival was measured with the WST-1 assay. The value of the untreated sample (8 hours) was arbitrarily set as 1. Experiments were performed in triplicate and error bars show ± SD. *P < .05. (D) Primary PV cells were treated with 10 or 50μM TBB for 24 hours. Cells were stained with annexin V and propidium iodide and examined by flow cytometry. Experiments were performed in triplicate and error bars show ± SD. *P < .05.
Discussion
We provide evidence that activation of the JAK-STAT signaling pathway is dependent on the presence and/or activity of CK2 in normal and tumor cells. Silencing CK2 expression with CK2 siRNA or inhibiting CK2 activity with CK2 inhibitors abrogates OSM-induced activation of JAK2, STAT-3, STAT-1, and STAT-5, and expression of the SOCS-3 gene. In addition to OSM signaling, CK2 activity is also required for IFN-γ and GH activation of the JAK-STAT pathway. Furthermore, constitutive activation of the mutant JAK2 (JAK2V617F) and constitutive STAT-3, STAT-5, and ERK signaling were also abrogated by inhibition of CK2 in HEL cells and primary cells from PV patients. The suppressive effects of CK2 inhibition on activation of JAK-STAT signaling were observed in normal cells such as MEFs; in solid tumor cell lines such as CH235-MG, γ2A-JAK2, and MDA-MB-231; and in blood tumor cells such as HEL cells and primary cells from PV patients. This suggests that CK2-dependent activation of JAKs is a general regulatory mechanism of the JAK-STAT signaling pathway in different cell types. We also demonstrated that CK2α and CK2β are constitutively associated with JAK2 and JAK1, but not with STAT-3, suggesting that JAKs are the regulatory node linking CK2 and the JAK-STAT signaling pathway. To our knowledge, our studies are the first to demonstrate cross-talk between CK2 and the JAK-STAT pathway. Because JAK2 serves as a critical checkpoint for definitive erythropoiesis,34 it will be interesting to find out whether this physiologic process requires CK2 by performing in vivo studies in the future.
CK2 associates with a wide array of proteins. CK2α has an interaction network consisting of 154 proteins in yeast and 43 proteins in humans identified thus far, which are potential regulators or targets of CK2.35 We demonstrated that CK2 is constitutively associated with JAKs, which identifies JAKs as new members of the CK2 interactome. We have shown that JAK2 kinase activity is not required for the association of JAK2 with CK2, because kinase-dead JAK2 and JAK2 lacking the kinase domain (ΔJH1) are still associated with CK2. JAK2 kinase activity is also not required for CK2 activity, because pharmacologic inhibition of JAK2 by P6, a pan JAK inhibitor, does not affect CK2 catalytic activity (supplemental Figure 4). These results suggest that although CK2 and JAK2 interact, JAK2 may not be a regulator of CK2 activity. The JH6 and JH7 domains of JAKs mediate binding to cytokine and growth factor receptors.2 We determined that the JH3-JH6 domains of JAK2 mediate its interactions with CK2, suggesting that JAKs bind to CK2 and receptors through different N-terminal regions.
In the present study, we have shown that CK2 can phosphorylate JAK2 directly in vitro. JAK2 undergoes multisite phosphorylation after cytokine stimulation, including tyrosine and serine/threonine phosphorylation.36 Using the Scansite prediction program (http://scansite.mit.edu), T308, T310, and T817 of JAK2 were predicted to be potential CK2 sites, and are in agreement with consensus CK2 sites in that there are several negatively charged amino acids downstream from serine/threonine, especially at the n + 3 position, and there are no positively charged residues nearby.15 Our preliminary mass spectrometry (MS) results suggest that T308, T310, and T817 of human JAK2 are not phosphorylated by CK2. In addition, recent data have suggested that CK2 can phosphorylate tyrosine resides.13 Preliminary MS results suggest that CK2 may phosphorylate JAK2 on several tyrosine and serine residues. Experiments are ongoing to definitively identify the JAK2 site(s) phosphorylated by CK2 and to examine how this site(s) affects JAK2 function in cells. CK2 also regulates JAK-STAT signaling by targeting other regulators of this pathway. PIAS1, a negative regulator of activated STAT-1, is also a CK2 substrate, and its regulation of transcription factor function is affected by CK2 phosphorylation.37
Persistent STAT-3 and STAT-5 phosphorylation is prevalent in many types of human solid tumors and hematopoietic tumors, contributing to promoting the survival, growth, anti-apoptotic mechanisms, angiogenesis, and immune evasion of tumor cells.38 A recent study demonstrated that the JAKs, especially JAK2, are essential for constitutive STAT-3 signaling in solid tumor cells.39 Using CK2 siRNA and/or inhibitors, we found that cytokine-induced JAK2 phosphorylation and constitutive JAK2V617F phosphorylation are dependent on the presence or activity of CK2. OSM-induced JAK1 phosphorylation, which also contributes to STAT phosphorylation, is suppressed by inhibition of CK2. Therefore, inhibition of CK2 could be used to control persistent STAT phosphorylation in several types of tumors.
CK2 protects cells from apoptosis through the regulation of tumor suppressors and oncogenes; therefore, down-regulation of CK2 using various strategies potently induces apoptosis and inhibits tumor growth in several types of cancer.40 CK2 inhibitors such as TBB, TBCA, and DMAT induce apoptosis through activation of caspases in a variety of cancer cells.41–43 In a study using CK2 inhibitors to investigate the antitumorigenic function of PML, emodin was shown to inhibit tumor growth in a mouse model of lung cancer.32 Antisense CK2α oligodeoxynucleotides potently induced cell apoptosis and suppressed tumor growth in a xenograft mouse model of human prostate cancer.44 Considering the potential problems of drug resistance, human cancer therapy is moving away from drug monotherapy to treatment with more than one drug. Panobiostat, a pan-histone deacetylase inhibitor that can suppress the chaperone function of heat shock protein 90, has recently been proposed for combination therapy with JAK2V617F inhibitors because it can deplete mRNA levels and promote proteosomal degradation of JAK2V617F.45 CK2α has been shown to interact with the Bcr/Abl oncogene and promote proliferation of Bcr/Abl–expressing cells.46 Treatment of PLC1 Bcr/Abl lymphoblast leukemia cells with the CK2 inhibitor DMAT in combination with the Bcr/Abl inhibitor imatinib leads to a synergistic reduction of cell viability.47 We have shown that inhibition of CK2 is particularly potent in inducing apoptosis in JAK2V617F-expressing HEL cells and primary cells from PV patients, which is associated with suppression of constitutive activation of JAK2V617F, STAT-3, STAT-5, and ERK. Preclinical and clinical trials using several small-molecule JAK2 inhibitors has been reported for the treatment of MPDs.48 CK2 could be an additional therapeutic target for JAK2V617F-positive MPDs. In particular, CK2 inhibitors may be effective in tumors that display aberrant CK2, JAK, and STAT activation.
Supplementary Material
Acknowledgments
We acknowledge Dr Rosa Bernardi and Dr Pier Paolo Pandolfi for providing PML−/− and PML+/+ MEFs and Dr Olli Silvennoinen (University of Tampere, Finland) for JAK2 wild-type and deletion constructs. We thank Dr Xiaoyan Qiu (Peking University, China) and Dr Dewang Zhou (University of Alabama at Birmingham [UAB]) for assistance with culturing primary MPD cells. Dr Stephen Barnes and Landon Wilson provided invaluable assistance with preliminary MS experiments. We acknowledge the UAB AMC Flow Cytometry Core Facility for help with cell-cycle analysis.
The mass spectrometer used in this study was purchased with funds from a Shared Instrumentation grant from the National Center for Research Resources (S10 RR-27822 to S.B.). The operation of the UAB Targeted Metabolomics and Proteomics Laboratory is additionally supported by federal grants (U54 CA100949 to S.B., P30 AT050948 to C.E., and P30 DK079337 to A.A.) and the UAB Lung Health Center. This work was supported in part by National Institutes of Health grants NS-54158 (to E.N.B.), NS-45290 (to E.N.B.), NS-36765 (to E.N.B.), NS-66332 (to F-T. L.), GM-65959 (to B.D.S.), and DK-46395 (to S.J.F.); by a grant from the Army Research Office (51894LS to B.D.S.); and by the Canadian Cancer Society Research Institute (to D.W.L.). The Mayo Clinic Bank is supported by the MPD Foundation, Chicago, IL (to A.T. and A.P.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.Z. and E.N.B. designed the research plan, analyzed and interpreted the data, and wrote the manuscript; Y.Z. performed the experiments; and Y.Z., H.Q., S.J.F., L.D., D.W.L., A.T., A.P., F.-T.L., J.L., and B.S. contributed vital new reagents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Etty N. Benveniste, 1900 University Blvd, Tinsley Harrison Tower, Rm 926, Birmingham, AL 35294-0006; e-mail: tika@uab.edu.
References
- 1.Chen S-H, Benveniste EN. Oncostatin M: a pleiotropic cytokine in the central nervous system. Cytokine Growth Factor Rev. 2004;15(5):379–391. doi: 10.1016/j.cytogfr.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3(11):900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- 3.Parganas E, Wang D, Stravopodis D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93(3):385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112(6):2190–2198. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 7.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 8.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 9.Xing S, Wanting TH, Zhao W, et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008;111(10):5109–5117. doi: 10.1182/blood-2007-05-091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacout C, Pisani DF, Tulliez M, Gachelin FM, Vainchenker W, Villeval JL. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108(5):1652–1660. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- 11.Bumm TG, Elsea C, Corbin AS, et al. Characterization of murine JAK2V617F-positive myeloproliferative disease. Cancer Res. 2006;66(23):11156–11165. doi: 10.1158/0008-5472.CAN-06-2210. [DOI] [PubMed] [Google Scholar]
- 12.Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7(9):673–683. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- 13.Vilk G, Weber JE, Turowec JP, et al. Protein kinase CK2 catalyzes tyrosine phosphorylation in mammalian cells. Cell Signal. 2008;20(11):1942–1951. doi: 10.1016/j.cellsig.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369(pt 1):1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? Faseb J. 2003;17(3):349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 16.Lou DY, Dominguez I, Toselli P, Landesman-Bollag E, O'Brien C, Seldin DC. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol Cell Biol. 2008;28(1):131–139. doi: 10.1128/MCB.01119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchou T, Vernet M, Blond O, et al. Disruption of the regulatory β subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol Cell Biol. 2003;23(3):908–915. doi: 10.1128/MCB.23.3.908-915.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piazza FA, Ruzzene M, Gurrieri C, et al. Multiple myeloma cell survival relies on high activity of protein kinase CK2. Blood. 2006;108(5):1698–1707. doi: 10.1182/blood-2005-11-013672. [DOI] [PubMed] [Google Scholar]
- 19.Kim JS, Eom JI, Cheong JW, et al. Protein kinase CK2alpha as an unfavorable prognostic marker and novel therapeutic target in acute myeloid leukemia. Clin Cancer Res. 2007;13(3):1019–1028. doi: 10.1158/1078-0432.CCR-06-1602. [DOI] [PubMed] [Google Scholar]
- 20.Duncan JS, Litchfield DW. Too much of a good thing: The role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim Biophys Acta. 2008;1784(1):33–47. doi: 10.1016/j.bbapap.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Channavajhala P, Seldin DC. Functional interaction of protein kinase CK2 and c-Myc in lymphomagenesis. Oncogene. 2002;21(34):5280–5288. doi: 10.1038/sj.onc.1205640. [DOI] [PubMed] [Google Scholar]
- 22.Landesman-Bollag E, Channavajhala PL, Cardiff RD, Seldin DC. p53 deficiency and misexpression of protein kinase CK2α collaborate in the development of thymic lymphomas in mice. Oncogene. 1998;16(23):2965–2974. doi: 10.1038/sj.onc.1201854. [DOI] [PubMed] [Google Scholar]
- 23.Chen S-H, Gillespie GY, Benveniste EN. Divergent effects of Oncostatin M on astroglioma cells: Influence on cell proliferation, invasion and expression of matrix metalloproteinases. Glia. 2006;53(2):191–200. doi: 10.1002/glia.20264. [DOI] [PubMed] [Google Scholar]
- 24.Loesch K, Deng L, Cowan JW, et al. Janus kinase 2 influences growth hormone receptor metalloproteolysis. Endocrinology. 2006;147(6):2839–2849. doi: 10.1210/en.2005-1484. [DOI] [PubMed] [Google Scholar]
- 25.Deng L, He K, Wang X, et al. Determinants of growth hormone receptor down-regulation. Mol Endocrinol. 2007;21(7):1537–1551. doi: 10.1210/me.2007-0138. [DOI] [PubMed] [Google Scholar]
- 26.Dong Y, Tang L, Letterio JJ, Benveniste EN. The Smad3 protein is involved in TGF-beta inhibition of class II transactivator and class II MHC expression. J Immunol. 2001;167(1):311–319. doi: 10.4049/jimmunol.167.1.311. [DOI] [PubMed] [Google Scholar]
- 27.Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A. CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia. 2009;23(8):1441–1445. doi: 10.1038/leu.2009.50. [DOI] [PubMed] [Google Scholar]
- 28.Duncan JS, Gyenis L, Lenehan J, et al. An unbiased evaluation of CK2 inhibitors by chemoproteomics: characterization of inhibitor effects on CK2 and identification of novel inhibitor targets. Mol Cell Proteomics. 2008;7(6):1077–1088. doi: 10.1074/mcp.M700559-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Saharinen P, Takaluoma K, Silvennoinen O. Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol Cell Biol. 2000;20(10):3387–3395. doi: 10.1128/mcb.20.10.3387-3395.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin H, Wilson C, Roberts K, Baker B, Zhao X, Benveniste E. IL-10 inhibits lipopolysaccharide-induced cd40 gene expression through induction of suppressor of cytokine signaling-3. J Immunol. 2006;177(11):7761–7771. doi: 10.4049/jimmunol.177.11.7761. [DOI] [PubMed] [Google Scholar]
- 31.Choi YH, Bernardi R, Pandolfi PP, Benveniste EN. The promyelocytic leukemia protein functions as a negative regulator of IFN-gamma signaling. Proc Natl Acad Sci U S A. 2006;103(49):18715–18720. doi: 10.1073/pnas.0604800103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scaglioni PP, Yung TM, Cai LF, et al. A CK2-dependent mechanism for degradation of the PML tumor suppressor. Cell. 2006;126(2):269–283. doi: 10.1016/j.cell.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 33.Seldin DC, Landesman-Bollag E, Farago M, Currier N, Lou D, Dominguez I. CK2 as a positive regulator of Wnt signalling and tumourigenesis. Mol Cell Biochem. 2005;274(1–2):63–67. doi: 10.1007/s11010-005-3078-0. [DOI] [PubMed] [Google Scholar]
- 34.Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93(3):397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 35.Gyenis L, Litchfield DW. The emerging CK2 interactome: insights into the regulation and functions of CK2. Mol Cell Biochem. 2008;316(1–2):5–14. doi: 10.1007/s11010-008-9830-5. [DOI] [PubMed] [Google Scholar]
- 36.Robertson SA, Koleva RI, Argetsinger LS, et al. Regulation of Jak2 function by phosphorylation of Tyr317 and Tyr637 during cytokine signaling. Mol Cell Biol. 2009;29(12):3367–3378. doi: 10.1128/MCB.00278-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stehmeier P, Muller S. Phospho-regulated SUMO interaction modules connect the SUMO system to CK2 signaling. Mol Cell. 2009;33(3):400–409. doi: 10.1016/j.molcel.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hedvat M, Huszar D, Herrmann A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16(6):487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmad KA, Wang G, Slaton J, Unger G, Ahmed K. Targeting CK2 for cancer therapy. Anticancer Drugs. 2005;16(10):1037–1043. doi: 10.1097/00001813-200511000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Ruzzene M, Penzo D, Pinna LA. Protein kinase CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) induces apoptosis and caspase-dependent degradation of haematopoietic lineage cell-specific protein 1 (HS1) in Jurkat cells. Biochem J. 2002;364(pt 1):41–47. doi: 10.1042/bj3640041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pagano MA, Poletto G, Di Maira G, et al. Tetrabromocinnamic acid (TBCA) and related compounds represent a new class of specific protein kinase CK2 inhibitors. Chembiochem. 2007;8(1):129–139. doi: 10.1002/cbic.200600293. [DOI] [PubMed] [Google Scholar]
- 43.Pagano MA, Meggio F, Ruzzene M, Andrzejewska M, Kazimierczuk Z, Pinna LA. 2-Dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole: a novel powerful and selective inhibitor of protein kinase CK2. Biochem Biophys Res Commun. 2004;321(4):1040–1044. doi: 10.1016/j.bbrc.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 44.Slaton JW, Unger GM, Sloper DT, Davis AT, Ahmed K. Induction of apoptosis by antisense CK2 in human prostate cancer xenograft model. Mol Cancer Res. 2004;2(12):712–721. [PubMed] [Google Scholar]
- 45.Wang Y, Fiskus W, Chong DG, et al. Cotreatment with panobinostat and JAK2 inhibitor TG101209 attenuates JAK2V617F levels and signaling and exerts synergistic cytotoxic effects against human myeloproliferative neoplastic cells. Blood. 2009;114(24):5024–5033. doi: 10.1182/blood-2009-05-222133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mishra S, Reichert A, Cunnick J, et al. Protein kinase CKIIalpha interacts with the Bcr moiety of Bcr/Abl and mediates proliferation of Bcr/Abl-expressing cells. Oncogene. 2003;22(51):8255–8262. doi: 10.1038/sj.onc.1207156. [DOI] [PubMed] [Google Scholar]
- 47.Mishra S, Pertz V, Zhang B, et al. Treatment of P190 Bcr/Abl lymphoblastic leukemia cells with inhibitors of the serine/threonine kinase CK2. Leukemia. 2007;21(1):178–180. doi: 10.1038/sj.leu.2404460. [DOI] [PubMed] [Google Scholar]
- 48.Pardanani A. JAK2 inhibitor therapy in myeloproliferative disorders: rationale, preclinical studies and ongoing clinical trials. Leukemia. 2008;22(1):23–30. doi: 10.1038/sj.leu.2404948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.