Abstract
Purpose
Vaccination with hybridoma-derived autologous tumor immunoglobulin (Ig) idiotype (Id) conjugated to keyhole limpet hemocyanin (KLH) and administered with granulocyte-monocyte colony-stimulating factor (GM-CSF) induces follicular lymphoma (FL) –specific immune responses. To determine the clinical benefit of this vaccine, we conducted a double-blind multicenter controlled phase III trial.
Patients and Methods
Treatment-naive patients with advanced stage FL achieving complete response (CR) or CR unconfirmed (CRu) after chemotherapy were randomly assigned two to one to receive either Id vaccine (Id-KLH + GM-CSF) or control (KLH + GM-CSF). Primary efficacy end points were disease-free survival (DFS) for all randomly assigned patients and DFS for randomly assigned patients receiving at least one dose of Id vaccine or control.
Results
Of 234 patients enrolled, 177 (81%) achieved CR/CRu after chemotherapy and were randomly assigned. For 177 randomly assigned patients, including 60 patients not vaccinated because of relapse (n = 55) or other reasons (n = 5), median DFS between Id-vaccine and control arms was 23.0 versus 20.6 months, respectively (hazard ratio [HR], 0.81; 95% CI, 0.56 to 1.16; P = .256). For 117 patients who received Id vaccine (n = 76) or control (n = 41), median DFS after randomization was 44.2 months for Id-vaccine arm versus 30.6 months for control arm (HR, 0.62; 95% CI, 0.39 to 0.99; P = .047) at median follow-up of 56.6 months (range, 12.6 to 89.3 months). In an unplanned subgroup analysis, median DFS was significantly prolonged for patients receiving IgM-Id (52.9 v 28.7 months; P = .001) but not IgG-Id vaccine (35.1 v 32.4 months; P = .807) compared with isotype-matched control-treated patients.
Conclusion
Vaccination with patient-specific hybridoma-derived Id vaccine after chemotherapy-induced CR/CRu may prolong DFS in patients with FL. Vaccine isotype may affect clinical outcome and explain differing results between this and other controlled Id-vaccine trials.
INTRODUCTION
Follicular lymphoma (FL), an indolent B-cell lymphoma, accounts for 22% of non-Hodgkin's lymphomas diagnosed worldwide.1 Although survival of patients with FL has improved with the addition of rituximab to chemotherapy, advanced stage FL is still considered incurable.2,3 Therefore, novel therapeutic strategies are needed to eliminate minimal residual disease after chemotherapy.
The variable regions of surface immunoglobulin (Ig) on a B-cell form a specific antigen-binding site unique to each Ig and contain molecular determinants, termed idiotype (Id), which can themselves be recognized as antigens. Because B-cell malignancies are clonal proliferations, Ig variable regions on the tumor cells are distinct from those of other normal B-cells. The idiotypic determinants of the surface Ig of B-cell lymphoma can therefore serve as a tumor-specific antigen for therapeutic vaccine development.4
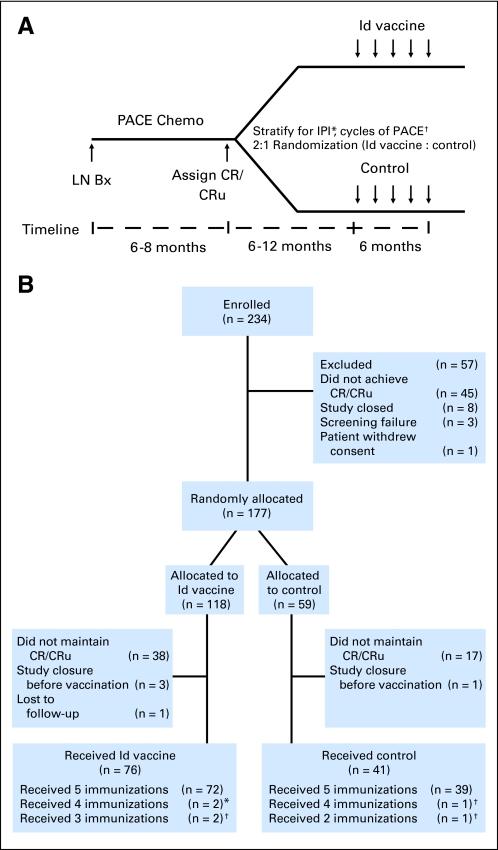
In experimental animal models, immunization with myeloma-Id protein induced the immune system of the host to reject tumor cells bearing idiotypic antigens.5,6 Induction of tumor-specific immune responses was optimized by conjugation of Id protein to keyhole limpet hemocyanin (KLH)7 and administration with granulocyte-monocyte colony-stimulating factor (GM-CSF) as an adjuvant.8 Kwak et al9 first demonstrated the immunogenicity of Id vaccines in patients with lymphoma using hybridoma-produced tumor Ig isotype–matched Id proteins. Subsequent pilot studies of this vaccine formulation demonstrated feasibility but primarily induced humoral immune responses.10,11 A landmark National Cancer Institute (NCI) phase II study of patients with FL vaccinated with autologous hybridoma-derived Id-KLH + GM-CSF in first complete remission after prednisone, doxorubicin, cyclophosphamide, and etoposide (PACE) chemotherapy demonstrated lymphoma-specific CD8+ T-cell responses in 95% of patients. Cellular immune responses correlated with molecular remissions, demonstrating potential for elimination of minimal residual disease by vaccination.12 These results provided the rationale for this randomized controlled double-blind trial, with the primary objective of confirming the effect of autologous hybridoma-derived Id vaccine on disease-free survival (DFS) in patients with FL using chemotherapy, vaccine formulation, and clinical setting identical to those in the NCI phase II study (Fig 1 A).
Fig 1.
(A) Clinical trial schema. Previously untreated patients with advanced stage follicular lymphoma underwent lymph node biopsy (LN Bx) after enrollment and were treated with prednisone (60 mg/m2 orally daily on days 1 to 14), doxorubicin (25 mg/m2 intravenously [IV] on days 1 and 8), cyclophosphamide (650 mg/m2 IV on days 1 and 8), and etoposide (120 mg/m2 IV on days 1 and 8; PACE) chemotherapy (chemo) every 28 days. Patients achieving complete response (CR)/CR unconfirmed (CRu) were stratified according to International Prognostic Index (IPI) and number of chemotherapy cycles and randomly assigned two to one to receive five injections of hybridoma-derived autologous tumor immunoglobulin idiotype (Id) conjugated to keyhole limpet hemocyanin (KLH) and administered with granulocyte-monocyte colony-stimulating factor (GM-CSF; Id-KLH + GM-CSF) or control vaccine (KLH + GM-CSF), respectively. (*) Low, low-intermediate or high-intermediate, high groups. (†) < eight or ≥ eight cycles. (B) CONSORT diagram of enrollment, randomization, and treatment. Two hundred thirty-four patients were enrolled and 117 patients were randomly assigned to receive at least one dose of the blinded vaccine; 76 received Id vaccine and 41 received control vaccine. Patients receiving fewer than five immunizations either (*) withdrew from the study or (†) relapsed before completion.
PATIENTS AND METHODS
Patients
Eligible patients had a diagnosis of FL, grade 1, 2, or 3a, confirmed by central pathology review (E.S.J.); had monoclonal surface IgM or IgG on tumor; were chemotherapy naive; and had bulky (> 5 cm) stage II, III, or IV disease with a lymph node larger than 2 cm accessible for biopsy.
Study Design
This prospective randomized double-blind controlled trial was initiated in January 2000 at the NCI and subsequently expanded to 17 centers in the United States and Europe under sponsorship of Biovest International after institutional review board approval at each center. After informed consent, patients underwent an excisional lymph node biopsy to confirm pathology and provide material for Id protein production (Fig 1A). Patients who achieved complete response (CR)/CR unconfirmed (CRu)13 after PACE chemotherapy12,14,15 were stratified by International Prognostic Index risk group (0 to 2 v 3 to 4)16 and number of chemotherapy cycles (≤ eight v > eight) and randomly assigned two to one to receive either Id vaccine (Id-KLH + GM-CSF) or control (KLH + GM-CSF). Randomization was performed centrally through a concealed Web-based random allocation system (EMMES Corporation, Rockville, MD). Patients with less than CR/CRu were excluded from randomization. Because of changes in standard of care for FL during the course of the trial, the protocol was amended in 2007 to allow cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab (R-CHOP) as induction therapy.17
Vaccine Therapy
Tumor isotype-matched Id protein was manufactured by heterohybridoma technology (Data Supplement).12,18,19 At study initiation, estimated time for Id-vaccine production was 6 to 12 months. To ensure that physicians and patients remained blinded to treatment, release dates for Id vaccine and control were matched using an algorithm (Data Supplement). Depending on release date, randomly assigned patients who remained in CR/CRu received five blinded Id-vaccine or control injections at 1, 2, 3, 4, and 6 months, starting between 6 and 12 months after completion of chemotherapy. Patients received isotype-matched (IgM/IgG) Id-KLH or KLH 0.5 mg each subcutaneously on day 1, with GM-CSF 100 μg/m2/d subcutaneously on days 1 to 4. Patients randomly assigned to receive Id vaccine for whom Id protein could not be made received KLH + GM-CSF but were analyzed as randomized.
Study Evaluations
Physical examination; computed tomography scans of chest, abdomen, and pelvis; and bone marrow examination were performed before chemotherapy, after cycle four, and every two cycles thereafter before first vaccination and 4 weeks after fifth vaccination. Thereafter, physical examination and computed tomography scans were performed every 6 months until relapse. Tumor response was assessed by study investigators blinded to treatment assignment according to the International Workshop response criteria for non-Hodgkin's lymphoma.13 Common Toxicity Criteria version 2.0 were used for adverse event (AE) reporting.
Statistical Analysis
The primary objective of the study was to determine whether Id vaccination prolonged DFS compared with control in patients with FL in durable CR/CRu after chemotherapy. Two prospective efficacy analyses were performed to compare DFS between treatment arms: first, all randomly assigned patients, and second, randomly assigned patients remaining in CR/CRu at time of vaccination and receiving at least one blinded vaccination. Secondary objectives were to evaluate safety, overall survival (OS), and immunologic and molecular responses.
Complete statistical methods are described in the Data Supplement. The study intended to enroll 563 patients, and 375 were expected to attain CR/CRu. Of these 375 patients, 250 would be randomly assigned to receive Id vaccine and 125 to receive control. This number is sufficient to allow approximately 80% power to detect 50% reduction in hazard in the experimental arm with minimum follow-up of 8 months. DFS was calculated from date of randomization until date of relapse or last follow-up. OS was calculated from date of randomization until death or last follow-up. Kaplan-Meier survival curves were constructed and the log-rank statistic used to test statistical differences using SAS (SAS Institute, Cary, NC). The trial was monitored annually by an independent data monitoring committee (DMC). All patients were observed for as long as possible to obtain survival information.
In an unplanned exploratory analysis, we compared DFS of Id-vaccinated patients with control patients separately depending on tumor Ig isotype. To address whether there was a differential treatment effect on DFS depending on Ig isotype, we used Cox proportional hazards modeling; in addition to both as main effects, we included an interaction term between treatment and Ig isotype and International Prognostic Index and number of chemotherapy cycles as covariates.
RESULTS
Study Population
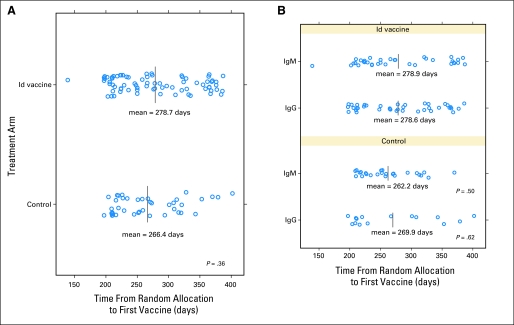
Starting in January 2000, a total of 234 patients were enrolled onto the study (Fig 1B; Data Supplement). Because of protracted enrollment (Data Supplement), the trial was terminated before full accrual, and data were locked on June 30, 2008, following DMC recommendation. Efficacy results did not play a role in the DMC decision to stop the study. At study termination, 219 patients had completed PACE chemotherapy, and six had completed R-CHOP chemotherapy. Of patients who received PACE, 177 (81%) achieved CR/CRu and were stratified and randomly assigned to receive either Id vaccine (n = 118) or control (n = 59; Data Supplement). Fifty-seven patients (24%) were excluded from randomization because of failure to achieve CR/CRu (n = 45), study closure (n = 8), screening failure (n = 3), or withdrawal of consent (n = 1). Patients who received R-CHOP were among the 57 patients excluded because of either study closure (n = 3) or failure to achieve CR/CRu (n = 3). Before vaccination, 55 randomly assigned patients (31%) relapsed (Id-vaccine arm, 38; control arm, 17), and five randomly assigned patients were excluded because of study closure (Id-vaccine arm, three; control arm, one) or loss to follow-up (Id-vaccine arm, one). Of 117 patients who received at least one blinded vaccination, 76 received Id vaccine, and 41 received control. As expected from the vaccine release algorithm, median time between randomization and initiation of vaccination was not significantly different between the Id-vaccine (8.74 months) and control (8.31 months) arms (P = .279; Appendix Fig A1, online only). Id protein was successfully produced in 71 (93%) of 76 patients assigned to receive Id vaccine. Five patients assigned to the experimental arm received KLH + GM-CSF because Id protein could not be produced, but they were analyzed as randomized. Six patients did not complete the five intended vaccinations because of either withdrawal (n = 2) or relapsed disease (n = 4), but they were analyzed as randomized. All baseline characteristics were well balanced between the groups that received blinded vaccinations (n = 117; Table 1) as well as between the two groups of 60 randomly assigned patients who did not receive vaccinations (Table 2).
Table 1.
Characteristics of Randomly Assigned Patients Who Received Vaccination (n = 117)
| Characteristic | Id Vaccine (n = 76) |
Control (n = 41) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age at enrollment, years | .146 | ||||
| Mean | 49.7 | 51.7 | |||
| SD | 9.7 | 9.1 | |||
| Male sex | 39 | 51.3 | 28 | 68.3 | .083 |
| White race | 67 | 88.2 | 38 | 92.7 | .537 |
| ECOG performance status | .222 | ||||
| 0 | 64 | 84.2 | 30 | 73.2 | |
| 1 | 12 | 15.8 | 11 | 26.8 | |
| Histology | .845 | ||||
| FL, grade 1 | 34 | 44.7 | 17 | 41.5 | |
| FL, grade 2 | 42 | 55.3 | 24 | 58.5 | |
| IgM isotype | 35 | 46.1 | 25 | 61.0 | |
| IgG isotype | 40 | 52.6 | 15 | 36.6 | |
| IgM/IgG isotype | 1 | 1.3 | 1 | 2.4 | |
| Stage | .263 | ||||
| II | 2 | 2.6 | 1† | 2.4 | |
| III | 29 | 38.2 | 10‡ | 24.4 | |
| IV | 45 | 59.2 | 30§ | 73.2 | |
| International Prognostic Index | 1.000 | ||||
| Low or low intermediate (0-2) | 69 | 90.8 | 37 | 90.2 | |
| High intermediate or high (3-5) | 7 | 9.2 | 4 | 9.8 | |
| ≤ Eight induction chemotherapy cycles | 38 | 50.0 | 22 | 53.7 | .846 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; Id, hybridoma-derived autologous tumor Ig idiotype; Ig, immunoglobulin; SD, standard deviation.
Comparisons between age groups were performed with nonparametric t-tests using normal approximation (two-sided Wilcoxon test). Comparisons between groups for remaining variables were performed using two-sided Fisher exact test.
P = 1.000 for comparison for stage II representation between two arms.
P = .154 for comparison for stage III representation between two arms.
P = .160 for comparison for stage IV representation between two arms.
Table 2.
Characteristics of Randomly Assigned Patients Who Did Not Receive Vaccination (n = 60)
| Characteristic | Id Vaccine (n = 42) |
Control(n = 18) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age at enrollment, years | .276 | ||||
| Mean | 49.6 | 46.6 | |||
| SD | 10.3 | 10.8 | |||
| Male sex | 21 | 50.0 | 7 | 38.9 | .574 |
| White race | 37 | 88.1 | 14 | 77.8 | .431 |
| ECOG performance status | .163 | ||||
| 0 | 30 | 71.4 | 16 | 88.9 | |
| 1 | 11 | 26.2 | 1 | 5.5 | |
| 2 | 1 | 2.4 | 1 | 5.5 | |
| Histology | 1.000 | ||||
| FL, grade 1 | 20 | 47.6 | 8 | 44.4 | |
| FL, grade 2 | 22 | 52.4 | 10 | 55.6 | |
| IgM isotype† | 26 | 61.9 | 8 | 44.4 | |
| IgG isotype† | 15 | 35.7 | 8 | 44.4 | |
| IgM/IgG isotype† | 0 | 0.0 | 1 | 5.6 | |
| IgD isotype† | 1 | 2.4 | 1 | 5.6 | |
| Stage | .520 | ||||
| III | 11 | 26.2 | 3 | 16.7 | |
| IV | 31 | 73.8 | 15 | 83.3 | |
| International Prognostic Index | 1.000 | ||||
| Low or low intermediate (0-2) | 36 | 85.7 | 16 | 88.9 | |
| High intermediate or high (3-5) | 6 | 14.3 | 2 | 11.1 | |
| ≤ Eight induction chemotherapy cycles | 22 | 52.4 | 7 | 38.9 | .405 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; Id, hybridoma-derived autologous tumor Ig idiotype; Ig, immunoglobulin; SD, standard deviation.
Comparisons between age groups were performed with nonparametric t-tests using normal approximation (two-sided Wilcoxon test). Comparisons between groups for remaining variables were performed using two-sided Fisher exact test.
Isotypes reflect tumor biopsy isotype as determined by flow cytometry or immunohistochemistry.
Efficacy
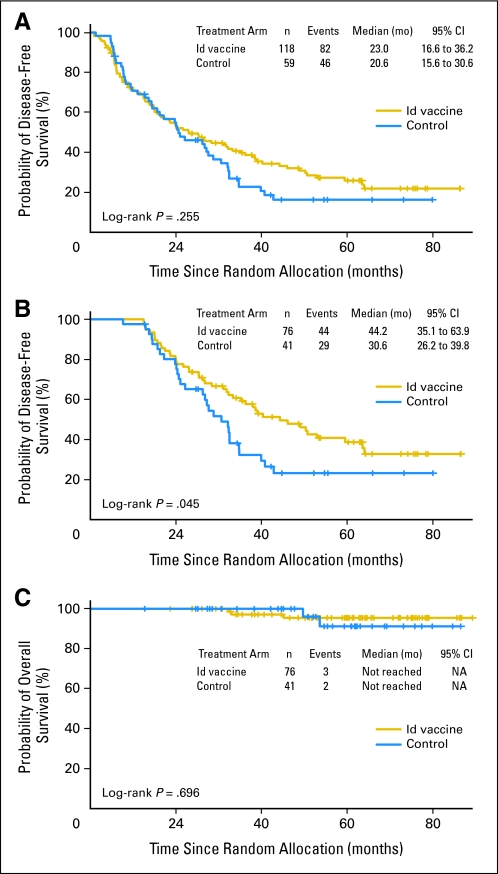
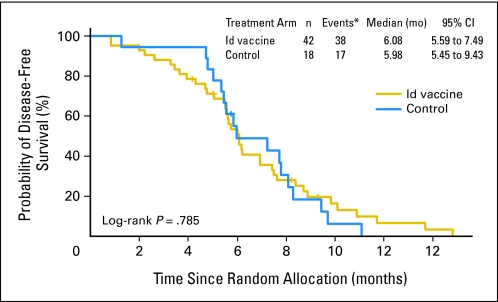
For all 177 randomly assigned patients, median DFS from randomization between Id-vaccine and control arms was 23.0 versus 20.6 months, respectively (hazard ratio [HR], 0.81; 95% CI, 0.56 to 1.16; P = .256; Fig 2A). There was no statistically significant difference in median DFS between arms for the 60 randomly assigned patients who did not receive vaccinations (6.08 months, Id-vaccine arm v 5.98 months, control arm; HR, 0.92; 95% CI, 0.51 to 1.65; P = .78; Appendix Fig A2, online only), suggesting that the arms were well balanced for baseline characteristics (Table 2). For 117 patients who received at least one blinded vaccination, median DFS was significantly prolonged in the Id-vaccine arm compared with that in the control arm (Fig 2B). At a median follow-up of 56.6 months (range, 12.6 to 89.3 months), median DFS after randomization to the Id-vaccine arm was 44.2 months versus 30.6 months for the control arm (P = .045). Using a Cox proportional hazard model, a statistically significant HR of 0.62 was achieved (95% CI, 0.39 to 0.99; P = .047). Median OS was not reached in either group; the number of deaths was too low to enable any conclusions about OS (Fig 2C).
Fig 2.
Disease-free survival (DFS) and overall survival (OS) according to treatment group for all randomly assigned patients (n = 177) and randomly assigned patients who received blinded vaccinations (n = 117). (A) Kaplan-Meier survival curves for DFS for all randomly assigned patients are shown according to treatment group: hybridoma-derived autologous tumor immunoglobulin idiotype (Id) vaccine (n = 118) and control vaccine (n = 59). Kaplan-Meier survival curves for (B) DFS and (C) OS for randomly assigned patients who received at least one dose of Id vaccine (n = 76) or control (n = 41).
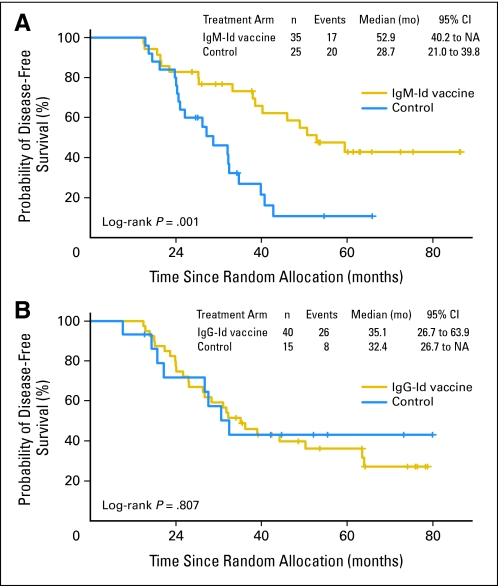
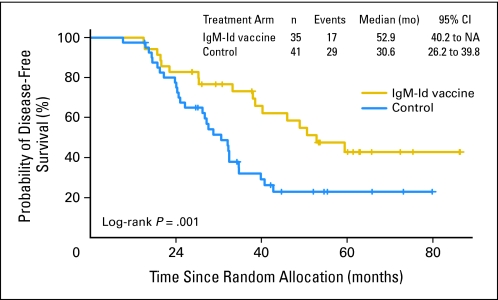
In an unplanned subgroup analyses, we also analyzed DFS of vaccinated patients by tumor Ig heavy and light chain isotypes (Data Supplement). For IgM and IgG heavy chain isotype groups, there were no statistically significant differences in baseline patient characteristics between experimental and control arms (IgM isotype, n = 35 v n = 25; IgG isotype, n = 40 v n = 15 for Id-vaccine and control arms, respectively). Two patients had mixed IgM/IgG biopsy isotypes and were excluded from this analysis (Data Supplement). Among patients receiving an IgM-Id vaccine, median time to relapse after randomization was 52.9 months versus 28.7 months in IgM tumor isotype control-treated patients (P = .001; HR, 0.34; 95% CI, 0.17 to 0.68; P = .002; Fig 3A) and 30.6 months in all controls (P = .010; Appendix Fig A3, online only). Among patients receiving IgG-Id vaccine, median time to relapse after randomization was 35.1 months versus 32.4 months in IgG tumor isotype control-treated patients (P = .807; HR, 1.1; 95% CI, 0.50 to 2.44; P = .807; Fig 3B). Cox proportional hazard modeling supports an interaction between treatment and Ig isotype (P = .039). When patients were grouped by light chain type, there was no difference in DFS (data not shown).
Fig 3.
Disease-free survival (DFS) according to tumor immunoglobulin (Ig) heavy chain isotype for randomly assigned patients who received blinded vaccination. Randomly assigned patients who received at least one dose of the hybridoma-derived autologous tumor immunoglobulin idiotype (Id) vaccine or control vaccine were grouped according to isotype of their tumor Ig heavy chain. Kaplan-Meier survival curves for DFS for Id vaccine and control groups according to (A) IgM and (B) IgG isotype.
Safety
Both Id vaccine and control were safe and well tolerated. There were no statistically significant differences in frequency or type of AE observed between groups. Grade 1 to 2 AEs, especially injection-site reactions (> 80% of patients) with erythema and induration lasting for a few days, were common in both groups (Data Supplement). However, grade 3 to 4 AEs were rare; there were no Id vaccine–related deaths (Table 3).
Table 3.
Summary of Grades 1 and 2 Adverse Events
| Adverse Event* | Id Vaccine(n = 76) |
Control(n = 41) |
P† | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Injection site reaction | 67 | 88.2 | 34 | 82.9 | .574 |
| Fatigue | 41 | 53.9 | 16 | 39.0 | .175 |
| Myalgia | 35 | 46.1 | 14 | 34.1 | .243 |
| Headache | 27 | 35.5 | 12 | 29.3 | .543 |
| Arthralgia | 25 | 32.9 | 14 | 34.1 | 1.000 |
| Infection | 16 | 21.1 | 2 | 4.9 | .029 |
| Nausea | 16 | 21.1 | 8 | 19.5 | 1.000 |
| Bone pain | 15 | 19.7 | 7 | 17.1 | .808 |
| Pruritus | 14 | 18.4 | 9 | 22.0 | .635 |
| Noncardiac chest pain | 13 | 17.1 | 6 | 14.6 | .799 |
| Pyrexia | 13 | 17.1 | 5 | 12.2 | .596 |
| Dyspepsia | 12 | 15.8 | 3 | 7.3 | .253 |
| Flushing | 11 | 14.5 | 4 | 9.8 | .571 |
| Influenza-like illness | 10 | 13.2 | 5 | 12.2 | 1.000 |
| Pain | 10 | 13.2 | 4 | 9.8 | .768 |
| Abdominal pain | 10 | 13.2 | 3 | 7.3 | .539 |
| Diarrhea | 10 | 13.2 | 2 | 4.9 | .211 |
| Sweating | 9 | 11.8 | 3 | 7.3 | .537 |
| Hyperglycemia | 8 | 10.5 | 1 | 2.4 | .158 |
Abbreviation: Id, hybridoma-derived autologous tumor immunoglobulin idiotype.
Most common ≥ 10% in either group.
Comparisons between groups were performed with two-sided Fisher exact test.
DISCUSSION
In this controlled clinical trial, treatment comparison on the basis of 177 patients, including 60 nonvaccinated patients, did not show a statistically significant difference in DFS between Id-vaccine and control arms (Fig 2A). However, a higher than expected relapse rate for randomly assigned patients, which precluded experimental therapy, may have obscured the treatment effect. Thus, the principal focus of our prospective efficacy analysis was on the group of 117 randomly assigned patients who received at least one blinded vaccination. For this group of patients with FL vaccinated during CR/CRu after PACE chemotherapy, our results demonstrate that the patient-specific hybridoma-derived Id protein vaccine significantly prolonged DFS compared with the control vaccine (Fig 2B). Although the ideal time for randomization is at initiation of experimental therapy, we made the decision to randomize well in advance, immediately after completion of chemotherapy, so that resources would not be expended manufacturing patient-specific vaccines for the control group. Nevertheless, our results should have the same validity as if randomization had occurred at initial vaccination, if patients allocated to each arm were equally likely to drop out of the study before vaccination. Indeed, DFS analysis of the 60 patients who were randomly assigned but not vaccinated showed no suggestion of treatment effect (Appendix Fig A2, online only), demonstrating that arms were well balanced in baseline characteristics (Table 2). Furthermore, the concealed randomization, double-blinded nature of the study, use of a vaccine release algorithm to achieve comparable times from randomization to vaccination for each treatment arm (Appendix Fig A1, online only), similar rate of injection site reaction in both groups (Table 3), and analysis of data by an independent statistician guarded against introduction of unintentional bias in the efficacy analysis of 117 vaccinated patients. The improvement in DFS observed for Id-vaccine treatment (Fig 2B) despite use of KLH + GM-CSF, a potentially active form of immunotherapy,20,21 in the control arm also suggests that the clinical benefit induced by Id vaccine may have been even greater had the control group received a placebo.
Although termination of the trial before completion of the planned accrual resulted in a smaller sample size than originally intended and decreased the power to detect a difference in DFS between treatment arms (Fig 2A), the study nevertheless showed a statistically significant improvement in DFS for Id-vaccinated patients (Fig 2B). As previously suggested, randomized trials may overcome limitations of small sample size and yield valid conclusions if baseline characteristics are well balanced and allocation is concealed, and if they are double-blinded.22,23 These features, built into our trial, together with the fact that the HR for DFS was 0.62 (Fig 2B), support the conclusion that the treatment effect observed by this vaccine was not exaggerated.
Although the epitopes after Id vaccination have been shown to be derived from the unique variable region of tumor Ig,24,25 the isotype of the constant region may influence the immunogenicity of variable region epitopes.26,27 Preclinical studies have shown that Ids switching to IgG became tolerogenic, whereas Ids of their IgM progenitors were highly immunogenic.26,27 Moreover, the fragment crystallizable (Fc) region of IgG has been shown to have highly promiscuous major histocompatibility complex class II T-cell epitopes that specifically activate regulatory T cells and skew immune responses toward tolerance rather than immunogenicity.28 Therefore, we analyzed DFS of vaccinated patients according to their tumor Ig isotype. We observed that vaccination with IgM-Id but not IgG-Id significantly prolonged DFS compared with isotype-matched controls (Fig 3). Although this was unplanned, and this trial was not powered to address such subset analysis, the observed treatment effects differed dramatically by isotype. Indeed, the 2-year improvement in DFS after IgM-Id vaccination (Fig 3A) was comparable to the magnitude of benefit observed with the addition of rituximab to induction chemotherapy3 or consolidation with yttrium-90 [90Y]ibritumomab tiuxetan after induction chemotherapy.29 However, formal comparison of these therapies would require randomized trials. It is also noteworthy that the DFS curves separated at approximately 20 months when the data were analyzed for all randomly assigned patients (Fig 2A), all vaccinated patients (Fig 2B), or all IgM vaccinated patients (Fig 3A), suggesting that the vaccine had a true clinical effect that was greater, more significant, and more readily apparent compared with the control when the correct patient population was analyzed.
The improvement in DFS observed for patients receiving Id vaccine in our trial stands in contrast to the results of the Genitope30 and Favrille31 phase III trials, which failed to show clinical benefit with recombinant Id vaccines in FL. The significant differences in trial design and vaccine formulation are likely responsible for the different clinical outcomes observed in these three phase III trials (Data Supplement). The present study used the phase II NCI treatment protocol and hybridoma Id protein manufacturing method.12,18 With regard to trial design, the Favrille and Genitope trials differed significantly from our trial by extending eligibility to patients with partial response and stable disease in addition to CR/CRu after chemotherapy, using less aggressive induction chemotherapy before vaccination, and not stratifying by clinical prognostic factors for treatment allocation. It is conceivable that the benefit of Id vaccination is discernable only in patients with minimal residual disease (ie, CR/CRu) after chemotherapy.
The hybridoma technique18 used in our trial yields Id proteins that more closely resemble the native Ig on the tumor cell surface, compared with the recombinant DNA–derived Id proteins used in the Genitope and Favrille studies.10 Production of recombinant protein may have altered post-translational modifications such as glycosylation, which can result in profound changes in final protein tertiary structure.32 In addition, the hybridoma technique yields Id proteins with IgM or IgG Fc regions identical to the tumor Ig isotype, as opposed to the universal IgG Fc used to produce Id vaccines for all patients in the Genitope and Favrille trials. It is possible that use of a universal IgG Fc may have altered the immunogenicity of Id vaccine (Fig 3).
This trial was initiated in the prerituximab era and used standard combination chemotherapy as the induction regimen. In current practice, chemotherapy is administered with rituximab because it improves overall response rate, progression-free survival, and OS in patients with FL.2,3,33,34 However, rituximab-containing immunochemotherapies do not seem to be curative, and complementary treatment strategies that are well tolerated are needed.33,34 Although rituximab induces prolonged B-cell deletion and delays induction of humoral response after Id vaccination, generation of tumor-specific cellular immunity is not affected.35 Phase I and II clinical trials have suggested that tumor-specific humoral and cellular immune responses after Id vaccination may each independently induce tumor regression and have been associated with improvement in clinical outcome in FL.10–12,36,37 Although the relative importance of humoral versus cellular immunity in efficacy of Id vaccination is unclear, cellular immunity induced by Id vaccination could conceptually complement rituximab-containing immunochemotherapies and minimize emergence of immune escape variants of the tumor.24 Furthermore, use of more effective induction therapies would minimize early relapse and facilitate vaccination in most patients (Fig 1B).
In conclusion, this trial shows that vaccination with patient-specific hybridoma-derived Id vaccine after chemotherapy-induced CR/CRu may prolong DFS in patients with FL. Furthermore, our results suggest that the isotype of the Fc region may influence immunogenicity of Id vaccines. If confirmed, our findings will have profound implications on Id vaccine production strategies and clinical development for FL and other B-cell malignancies.
Acknowledgment
We thank the investigators, pharmacy, and nursing staff at each of the participating institutions. We thank the patients for participating in this trial and their referring physicians. This trial was designed and initiated by the intramural research program at the National Cancer Institute in January 2000 and subsequently sponsored by Biovest International after transfer of technology through a cooperative research and development agreement in 2002.
Presented in part at the plenary session of the 45th Annual Meeting of the American Society of Clinical Oncology May 29-June 2, 2009, Orlando, FL.
Glossary Terms
- Epitope:
Region within an antigen that has the potential to give rise to an antibody response. With respect to protein antigens, epitopes may be defined on the basis of primary, secondary, or tertiary structure of the molecule and, consequently, maybe exposed or hidden within the molecule.
- Hybridoma:
A cell line generated by fusing a B cell expressing a unique surface antibody of interest and a myeloma cell that does not produce an antibody by itself. The fusion cell line secretes the antibody of interest.
- Idiotype:
The unique amino acid sequences in the variable regions of the heavy and light chains of an immunoglobulin molecule.
- Immunogenic:
Capable of inducing an immune response.
- Immunoglobulin isotype :
The isotype of the immunoglobulin refers to the different constant regions of the heavy and light chains of the immunoglobulin. Based on the constant region of the heavy chain, the isotype of the immunoglobulin may be IgM, IgG, IgA, IgD, or IgE. Based on the constant region of the light chain, the isotype of the immunoglobulin may be either kappa or lambda.
- Immunotherapy:
A therapeutic approach that uses cellular and/or humoral elements of the immune system to fight a disease.
- Keyhole limpet hemocyanin (KLH):
KLH is an extracellular respiratory protein isolated from hemolymph of the marine mollusk, the giant keyhole limpet Megathura crenulata, that is native to the Pacific coast of California and Mexico. KLH is known to be highly immunogenic in humans and animals.
- Regulatory T cells (known as suppressor T cells):
are a specialized subpopulation of T cells that act to suppress activation of the immune system and thereby maintain immune system homeostasis and tolerance to self-antigens. This is an important “self-check” built into the immune system so that responses do not go haywire. Regulatory T cells come in many forms, including those that express the CD8 transmembrane glycoprotein (CD8 T cells), those that express CD4, CD25 and Foxp3 (CD4CD25 regulatory T cells or “Tregs”) and other T cell types that have suppressive function. These cells are involved in closing down immune responses after they have successfully tackled invading organisms and also in keeping in check immune responses that may potentially attack one's own tissues (autoimmunity).
- Therapeutic vaccine:
A vaccine used for induction of humoral and/or cellular immune responses against an antigen or set of antigens to treat existing disease. In contrast, prophylactic vaccines are used to induce humoral and/or cellular immune responses against an antigen or set of antigens to prevent a future disease.
- Tolerogenic:
Not capable of inducing an immune response.
Appendix
BV301 Phase III Study Investigator Group
Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA: Stephen J. Schuster, MD, Elise A. Chong, BA, Lisa H. Downs, MSN, CRNP, Joanne T. Hinkle, BSN, RN; Center for Cancer Research, National Cancer Institute, Bethesda, MD: Barry L. Gause, MD, John E. Janik, MD, Elaine S. Jaffe, MD, Craig W. Reynolds, PhD, Maryalice Stetler-Stevenson, MD, PhD, Thelma M. Watson, RN, Sandra Snow, RN, Miriam Ferraro, Robin Pennington, Carol B. Kobrin, PhD, Sheldon Grove, Douglas J. Schwartzentruber, MD, David Danforth, MD, Richard Sherry, MD, Erik Kass, MD, Carter Van Waes, MD; The University of Texas MD Anderson Cancer Center, Houston, TX: Sattva S. Neelapu, MD, Donald A. Berry, PhD, Larry W. Kwak, MD, PhD, Ana Ayala, RN, Diana L. Rodriguez; Duke University Comprehensive Cancer Center, Durham, NC: Jon P. Gockerman, MD; Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL: Jane N. Winter, MD, Daina Variakojis, MD, Leo I. Gordon, MD, David Patton; New York University Cancer Institute, New York University School of Medicine, New York, NY: Franco M. Muggia, MD, Kenichi Takeshita, MD, Giorgio Inghirami, MD, Bruce Raphael, MD, Tatyana Feldman, MD, Andrea Downey, RN; Emory University Winship Cancer Institute, Atlanta, GA: Christopher R. Flowers, MD; SMDC Cancer Center and Duluth CCOP, Duluth, MN: Daniel A. Nikcevich, MD, PhD; H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL: Eduardo M. Sotomayor, MD, Thomas Loughran Jr, MD, Gregoire Bergier, MD; Virginia Oncology Associates, Norfolk, VA: Dean S. McGaughey, MD; Cancer and Blood Disease Center, Lecanto, FL: Gustavo Fonseca, MD; Southern Oncology Research, Greenville, NC: Jesse Lee, MD; North Mississippi Hematology and Oncology Associates, Tupelo, MS: Christopher Croot, MD; SI Russian Oncology Scientific Center, Moscow, Russia: Eugeny Osmanov, MD; Central Research Roentgen Radiological Institute, St Petersburg, Russia: Nikolay Vasilievich Ilyin, MD; Medical Radiological Research Center RAMS, Obninsk, Russia: Vyacheslav Vladimirovich Pavlov, MD; St Petersburg State Medical University, St Petersburg, Russia: Sergey I. Moiseev, MD.
Fig A1.
Distribution of randomized patients that received blinded vaccination according to days from randomization to first vaccination. (A) Days from randomization to first vaccination shown for randomly assigned patients that received at least one dose of hybridoma-derived autologous tumor immunoglobulin idiotype (Id) vaccine (N = 76) or control vaccine (N = 41). (B) Days from randomization to first vaccination shown for randomized patients according to heavy chain isotype: IgM-Id vaccine (N = 35), IgG-Id vaccine (N = 40), IgM control (N = 25), and IgG control (N = 15). The median for each group is indicated by a line. The p-values were calculated using two-sided Wilcoxon rank sum test of the days from randomization to first vaccine for the vaccine and the respective control group.
Fig A2.
Disease-free survival (DFS) according to treatment group for randomized patients who did not receive vaccination (N = 60). Kaplan-Meier survival curves for DFS for randomized patients who did not receive vaccination are shown according to treatment group: hybridoma-derived autologous tumor immunoglobulin idiotype (Id) vaccine (N = 42; yellow); control (N = 18; blue). The number of events, median DFS, and 95% confidence intervals for each group are also presented. (*) Five patients who remained in complete response/complete response unconfirmed did not receive vaccination because of study closure (n = 4) or noncompliance (n = 1).
Fig A3.
Disease-free survival (DFS) for the randomized patients that received IgM-Id vaccine vs all controls. Kaplan-Meier survival curves for DFS for the IgM-Id vaccinated patients (N = 35; yellow) and all patients in the control arm (N = 41; blue) are shown. The number of events, median DFS, and 95% confidence intervals for each group are also presented. Id, hybridoma-derived autologous tumor Ig idiotype; Ig, immunoglobulin; NA, not applicable.
Footnotes
See accompanying editorial on page 2748
Written on behalf of the BV301 phase III study investigators.
Supported by the National Cancer Institute and Biovest International.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00091676.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Carlos F. Santos, Accentia Biopharmaceuticals (C), Biovest International (C); Mihaela A. Popa, Biovest International (C); Amy M. McCord, Biovest International (C) Consultant or Advisory Role: Christopher R. Flowers, Biovest International (C), Genentech/Biogen Idec (U); Donald A. Berry, Biovest International (C); Larry W. Kwak, Biovest International (C), Antigenics (C) Stock Ownership: Barry L. Gause, Biovest International; Carlos F. Santos, Accentia Biopharmaceuticals, Biovest International; Mihaela A. Popa, Biovest International; Amy M. McCord, Biovest International; Larry W. Kwak, Antigenics, Xeme Biopharma Honoraria: None Research Funding: Stephen J. Schuster, Biovest International; Sattva S. Neelapu, Biovest International; Franco M. Muggia, Biovest International; Jane N. Winter, Biovest International; Christopher R. Flowers, Biovest International, Millennium Pharmaceuticals, Berlex/Bayer Pharmaceuticals; Larry W. Kwak, Celgene Expert Testimony: None Other Remuneration: Larry W. Kwak, Biovest International
AUTHOR CONTRIBUTIONS
Conception and design: Stephen J. Schuster, Barry L. Gause, Franco M. Muggia, Jane N. Winter, Craig W. Reynolds, Larry W. Kwak
Provision of study materials or patients: Stephen J. Schuster, Sattva S. Neelapu, Barry L. Gause, John E. Janik, Franco M. Muggia, Jon P. Gockerman, Jane N. Winter, Christopher R. Flowers, Daniel A. Nikcevich, Eduardo M. Sotomayor, Dean S. McGaughey
Collection and assembly of data: Stephen J. Schuster, Sattva S. Neelapu, Barry L. Gause, John E. Janik, Franco M. Muggia, Jon P. Gockerman, Jane N. Winter, Christopher R. Flowers, Daniel A. Nikcevich, Eduardo M. Sotomayor, Dean S. McGaughey, Elaine S. Jaffe, Elise A. Chong, Donald A. Berry, Carlos F. Santos, Mihaela A. Popa, Amy M. McCord, Larry W. Kwak
Data analysis and interpretation: Stephen J. Schuster, Sattva S. Neelapu, Barry L. Gause, John E. Janik, Franco M. Muggia, Jon P. Gockerman, Jane N. Winter, Christopher R. Flowers, Daniel A. Nikcevich, Eduardo M. Sotomayor, Dean S. McGaughey, Elaine S. Jaffe, Elise A. Chong, Donald A. Berry, Carlos F. Santos, Mihaela A. Popa, Amy M. McCord, Larry W. Kwak
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma: The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 2.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 3.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26:4579–4586. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson GT, Stevenson FK. Antibody to a molecularly-defined antigen confined to a tumour cell surface. Nature. 1975;254:714–716. doi: 10.1038/254714a0. [DOI] [PubMed] [Google Scholar]
- 5.Sirisinha S, Eisen HN. Autoimmune-like antibodies to the ligand-binding sites of myeloma proteins. Proc Natl Acad Sci U S A. 1971;68:3130–3135. doi: 10.1073/pnas.68.12.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch RG, Graff RJ, Sirisinha S, et al. Myeloma proteins as tumor-specific transplantation antigens. Proc Natl Acad Sci U S A. 1972;69:1540–1544. doi: 10.1073/pnas.69.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaminski MS, Kitamura K, Maloney DG, et al. Idiotype vaccination against murine B cell lymphoma: Inhibition of tumor immunity by free idiotype protein. J Immunol. 1987;138:1289–1296. [PubMed] [Google Scholar]
- 8.Kwak LW, Young HA, Pennington RW, et al. Vaccination with syngeneic, lymphoma-derived immunoglobulin idiotype combined with granulocyte/macrophage colony-stimulating factor primes mice for a protective T-cell response. Proc Natl Acad Sci U S A. 1996;93:10972–10977. doi: 10.1073/pnas.93.20.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwak LW, Campbell MJ, Czerwinski DK, et al. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992;327:1209–1215. doi: 10.1056/NEJM199210223271705. [DOI] [PubMed] [Google Scholar]
- 10.Park HJ, Neelapu SS. Developing idiotype vaccines for lymphoma: From preclinical studies to phase III clinical trials. Br J Haematol. 2008;142:179–191. doi: 10.1111/j.1365-2141.2008.07143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houot R, Levy R. Vaccines for lymphomas: Idiotype vaccines and beyond. Blood Rev. 2009;23:137–142. doi: 10.1016/j.blre.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Bendandi M, Gocke CD, Kobrin CB, et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999;5:1171–1177. doi: 10.1038/13928. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas: NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 14.Longo DL, DeVita VT, Jr, Duffey PL, et al. Superiority of ProMACE-CytaBOM over ProMACE-MOPP in the treatment of advanced diffuse aggressive lymphoma: Results of a prospective randomized trial. J Clin Oncol. 1991;9:25–38. doi: 10.1200/JCO.1991.9.1.25. [DOI] [PubMed] [Google Scholar]
- 15.Longo DL, Duffey PL, Gribben JG, et al. Combination chemotherapy followed by an immunotoxin (anti-B4-blocked ricin) in patients with indolent lymphoma: Results of a phase II study. Cancer J. 2000;6:146–150. [PubMed] [Google Scholar]
- 16.A predictive model for aggressive non-Hodgkin's lymphoma: The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 17.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 18.Carroll WL, Thielemans K, Dilley J, et al. Mouse x human heterohybridomas as fusion partners with human B cell tumors. J Immunol Methods. 1986;89:61–72. doi: 10.1016/0022-1759(86)90032-3. [DOI] [PubMed] [Google Scholar]
- 19.Lee ST, Jiang YF, Park KU, et al. BiovaxID: A personalized therapeutic cancer vaccine for non-Hodgkin's lymphoma. Expert Opin Biol Ther. 2007;7:113–122. doi: 10.1517/14712598.7.1.113. [DOI] [PubMed] [Google Scholar]
- 20.Jones SE, Schottstaedt MW, Duncan LA, et al. Randomized double-blind prospective trial to evaluate the effects of sargramostim versus placebo in a moderate-dose fluorouracil, doxorubicin, and cyclophosphamide adjuvant chemotherapy program for stage II and III breast cancer. J Clin Oncol. 1996;14:2976–2983. doi: 10.1200/JCO.1996.14.11.2976. [DOI] [PubMed] [Google Scholar]
- 21.Spitler LE, Grossbard ML, Ernstoff MS, et al. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2000;18:1614–1621. doi: 10.1200/JCO.2000.18.8.1614. [DOI] [PubMed] [Google Scholar]
- 22.Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias: Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 23.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135:982–989. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- 24.Baskar S, Kobrin CB, Kwak LW. Autologous lymphoma vaccines induce human T cell responses against multiple, unique epitopes. J Clin Invest. 2004;113:1498–1510. doi: 10.1172/JCI20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertinetti C, Zirlik K, Heining-Mikesch K, et al. Phase I trial of a novel intradermal idiotype vaccine in patients with advanced B-cell lymphoma: Specific immune responses despite profound immunosuppression. Cancer Res. 2006;66:4496–4502. doi: 10.1158/0008-5472.CAN-05-4233. [DOI] [PubMed] [Google Scholar]
- 26.Reitan SK, Hannestad K. Immunoglobulin heavy chain constant regions regulate immunity and tolerance to idiotypes of antibody variable regions. Proc Natl Acad Sci U S A. 2002;99:7588–7593. doi: 10.1073/pnas.052150899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reitan SK, Hannestad K. A syngeneic idiotype is immunogenic when borne by IgM but tolerogenic when joined to IgG. Eur J Immunol. 1995;25:1601–1608. doi: 10.1002/eji.1830250620. [DOI] [PubMed] [Google Scholar]
- 28.De Groot AS, Moise L, McMurry JA, et al. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes.”. Blood. 2008;112:3303–3311. doi: 10.1182/blood-2008-02-138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morschhauser F, Radford J, Van Hoof A, et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol. 2008;26:5156–5164. doi: 10.1200/JCO.2008.17.2015. [DOI] [PubMed] [Google Scholar]
- 30.Levy R, Robertson M, Leonard J, et al. Results of a phase 3 trial evaluating safety and efficacy of specific immunotherapy, recombinant idiotype (Id) conjugated to KLH (Id-KLH) with GM-CSF, compared with non-specific immunotherapy, KLH with GM-CSF, in patients with follicular non Hodgkin's lymphoma (FNHL) Ann Oncol. 2008;19(suppl 4):iv101–iv102. [Google Scholar]
- 31.Freedman A, Neelapu SS, Nichols C, et al. Placebo-controlled phase III trial of patient-specific immunotherapy with mitumprotimut-T and granulocyte-macrophage colony-stimulating factor after rituximab in patients with follicular lymphoma. J Clin Oncol. 2009;27:3036–3043. doi: 10.1200/JCO.2008.19.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redfern CH, Guthrie TH, Bessudo A, et al. Phase II trial of idiotype vaccination in previously treated patients with indolent non-Hodgkin's lymphoma resulting in durable clinical responses. J Clin Oncol. 2006;24:3107–3112. doi: 10.1200/JCO.2005.04.4289. [DOI] [PubMed] [Google Scholar]
- 33.Fisher RI, LeBlanc M, Press OW, et al. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23:8447–8452. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, Fayad L, Cabanillas F, et al. Improvement of overall and failure-free survival in stage IV follicular lymphoma: 25 years of treatment experience at The University of Texas M.D. Anderson Cancer Center. J Clin Oncol. 2006;24:1582–1589. doi: 10.1200/JCO.2005.03.3696. [DOI] [PubMed] [Google Scholar]
- 35.Neelapu SS, Kwak LW, Kobrin CB, et al. Vaccine-induced tumor-specific immunity despite severe B-cell depletion in mantle cell lymphoma. Nat Med. 2005;11:986–991. doi: 10.1038/nm1290. [DOI] [PubMed] [Google Scholar]
- 36.Weng WK, Czerwinski D, Timmerman J, et al. Clinical outcome of lymphoma patients after idiotype vaccination is correlated with humoral immune response and immunoglobulin G Fc receptor genotype. J Clin Oncol. 2004;22:4717–4724. doi: 10.1200/JCO.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Nelson EL, Li X, Hsu FJ, et al. Tumor-specific, cytotoxic T-lymphocyte response after idiotype vaccination for B-cell, non-Hodgkin's lymphoma. Blood. 1996;88:580–589. [PubMed] [Google Scholar]