Abstract
Particulate pollution has been linked to risk of cardiac death; possible mechanisms include pollution-related increases in cardiac electrical instability. T-wave alternans (TWA) is a marker of cardiac electrical instability measured as differences in the magnitude between adjacent T waves. In a repeated-measures study of 48 patients aged 43-75 years, we investigated associations of ambient and home indoor particulate pollution including black carbon (BC) and report of traffic exposure, with changes in half-hourly maximum TWA (TWA-MAX), measured by 24 hour Holter electrocardiogram monitoring. Each patient was observed up to 4 times within one year after percutaneous intervention for myocardial infarction, acute coronary syndrome without infarction, or stable coronary artery disease for a total of 5,830 half-hour observations. Diary data for each half-hour period defined whether the patient was home or not home, or in traffic. Increases in TWA-MAX were independently associated both with the previous 2-h mean ambient BC (2.1%; 95% C.I.: 0.9-3.3) and with being in traffic in the previous 2 hours (6.1%; 95% C.I.: 3.4-8.8). When subjects were home, indoor home BC effects were largest and most precise; when subjects were away from home, ambient central site BC effects were strongest. Increases in pollution increased the odds of TWA-MAX ≥ 75th percentile (OR 1.4; 95% CI: 1.2-1.6 for 1 μg/m3 increase in 6-h mean BC). In conclusion, following hospitalization for coronary artery disease, being in traffic, and short-term ambient or indoor BC exposures increase TWA, a marker of cardiac electrical instability.
Keywords: Air pollution, coronary disease, myocardial infarction, T-wave alternans, circadian rhythm
Traffic has been proposed as a specific source of pollution that may trigger myocardial infarction 1, ventricular arrhythmias, and cardiac death. In the recent European study by Peters and colleagues,1 the adverse cardiac effects of having been in traffic in the previous hour on risk of myocardial infarction were similar regardless of type of transportation. The risk of ICD shock for ventricular tachycardia/ventricular fibrillation was transiently elevated in the 30-minute period after driving in the Triggers of Ventricular Arrhythmias study2. The emotional stress of being in traffic, as well as the toxic effects of traffic pollution may have been responsible for the increased traffic-associated cardiac risk. We investigated the associations of ambient and indoor black carbon (BC) (a marker for traffic particulate pollution) and a report of being in traffic, with risk of increase in TWA in a population of patients with coronary artery disease, after hospital admission with percutaneous intervention for myocardial infarction, acute coronary syndrome without infarction, or stable coronary artery disease. Using our indoor measures of BC, we assessed whether pollution from traffic sources might increase TWA, not only when people with coronary artery disease were outside the home or in traffic, but also when they were indoors at home.
Methods
The study design has been previously described3. Briefly, prior to discharge we recruited a panel of patients with documented coronary artery disease from the greater Boston area (within route 495, a 40 Km radius of our central monitoring site) who had undergone percutaneous coronary intervention for an acute coronary syndrome (acute myocardial infarction or unstable angina pectoris) or for worsening stable coronary artery disease. We excluded those with atrial fibrillation and left bundle branch block (LBBB); coronary artery bypass graft surgery within the last 3 months; patients with psychiatric illness or drug abuse problems. Active cigarette smoking was an exclusion criterion at entry to the study, and no participant reported current smoking at the time of recruitment. While we did have 4 subjects who reported recidivist smoking, each reported smoking only at one visit, and only 1 reported more than 0 cigarettes per day at that visit (Table 1). Analyses excluding these subjects did not significantly change the associations of pollution with TWA. Given these findings, we chose to keep the data of all subjects in the analyses, because it provided us with more observations and more power to evaluate interactions and to do the subanalyses of interest. The protocol included a home visit within 2 to 4 weeks after hospital discharge, followed by three additional follow-up visits at approximately 3-month intervals. At the first visit a baseline screening questionnaire was administered regarding medications, pulmonary and cardiac symptoms, and smoking history.
Table 1.
Participant characteristics (n=48)
| Characteristic | ||
|---|---|---|
| Age, years (median, range) | 56.8 | (43.2-75.5) |
| Heart rate (bpm) (median, range) | 63.7 | (40.9-129.9) |
| Maximum TWA (median, range) | 21.1 | (1.7-61.2) |
| Male | 39 | (81%) |
| Female | 9 | (19%) |
| Hospital Discharge diagnosis | ||
| Myocardial Infarction (MI) | 19 | (40%) |
| Acute coronary syndrome, no MI | 19 | (40%) |
| Stable coronary artery disease, no MI | 10 | (20%) |
| Initial number of coronary arteries >=50% occluded at catheterization | ||
| 0 | 0 | (0%) |
| 1 | 26 | (54%) |
| 2 | 17 | (36%) |
| 3 | 5 | (10%) |
| Final number of coronary arteries >=50% narrowed | ||
| 0 | 37 | (77%) |
| 1 | 6 | (13%) |
| 2 | 5 | (10%) |
| 3 | 0 | (0%) |
| Ever used Medication | ||
| β-blocker | 44 | (92%) |
| Calcium channel blocker | 5 | (10%) |
| Angiotensin-converting enzyme inhibitors | 25 | (52%) |
| Theophylline or β-antagonist | 2 | (4%) |
| Digoxin | 2 | (4%) |
| Statin | 44 | (92%) |
| Diabetes | ||
| No | 36 | (75%) |
| yes | 12 | (25%) |
| Cigarette smoking | ||
| Never | 17 | (36%) |
| Former | 29 | (60%) |
| Current | 2 | (4%) |
| White | 45 | (94%) |
| Black | 1 | (2%) |
| Asian+other | 2 | (4%) |
| Ever had MI | 30 | (63%) |
Twenty-four-hour 3-lead Holter ECG monitoring (Marquette Seer Digital Recorder, Marquette Inc., Milwaukee, WI) was also performed with electrodes in modified V5 and aVF positions. For subsequent visits, participants were administered a brief questionnaire regarding cardiac and respiratory symptoms and medication use, and then received 24-hour Holter monitoring. The study design was reviewed and approved by the human subjects committees of the Brigham and Women’s Hospital and the Harvard School of Public Health. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Modified moving average analysis is a time-domain nonspectral technique that was developed to allow TWA measurement in ambulatory individuals. Briefly, a stream of beats is divided into odd and even bins and the morphology of the beats in each bin is averaged over a few beats successively to create a moving average complex.4 TWA is computed as the maximum difference in magnitude between the odd-beat and the even-beat average complexes from the J point to the end of the T wave. This analysis can be performed from standard Holter monitoring records.5 Because of the greater variability in measured ECG outcomes, we present results from analyses using the Holter data from the modified V5 precordial lead position. Each 24 hour Holter monitoring period was divided into 30 minutes intervals, and the maximum TWA magnitude in the 30 minutes interval was computed (TWA-MAX).
From diary data, the location of each participant was determined for each half hour period, with location noted as home or not home, and in traffic defined as in a car or riding a bus, subway or train. In relation to each half-hour TWA-MAX measurement, we classified each subject as either home or not home in the previous 2 hours, or at home only part of the previous 2 hours.
Ambient concentrations of particulate air matter with aerodynamic diameter < 2.5 μm (PM2.5), and black carbon (BC) were measured at a central monitoring site located on the roof of Countway Library, Harvard Medical School, in downtown Boston, MA. PM2.5 concentrations were measured using a Tapered Element Oscillation Microbalance (TEOM, Model 1400A, Rupprecht and Pastashnick, East Greenbush, NY). Ambient BC was measured using an aethalometer (Model 8021, McGee Scientific, and Berkeley, CA). The median distance of participant homes from the central site monitoring station was 17.6 km.
PM2.5 and BC concentrations were summarized in half-hour intervals with analyses based on both the half-hour up to and including 12 hour lagged and cumulative averaged pollution exposures as predictors of TWA-MAX.
Indoor PM2.5 and indoor and outdoor BC concentrations were also measured continuously at the homes of participants using the TEOM (Model 1400A, Rupprecht & Patashnick, Albany, NY) and model AE-14 Aethalometer (Magee Scientific Inc., Berkeley CA), respectively. For BC, identical measurement methods were used to measure corresponding concentrations outside the home. Technicians installed the equipment in the house, placing the equipment in the family room or in the room with the greatest activity. Hourly temperature was obtained from the National Weather Service First Order Station at Logan Airport.
We analyzed the association of TWA-MAX with air pollution using generalized additive regression models. The models included indicator variables for each subject, which removes the potential for confounding of subject-specific (time-invariant) factors. The model also controlled for time-varying factors, including day of the week and being in traffic as categorical variables. The factors average heart rate for the relevant half-hour period of TWA measurement, hour of the day, date, and mean temperature were included as smooth penalized spline terms, to account for potential non-linear associations between these continuous factors and the outcome.6 TWA-MAX was log-transformed to achieve normality, and was specified as a continuous outcome in primary analyses. After assessing the main effects of pollution, we evaluated whether the TWA effects of pollution (centrally measured or measured in the home) were modified by whether subjects were at home, not at home, or at home during only part of the exposure period of interest.
In sensitivity analyses, with a focus on potential clinical implications of our findings,7 TWA-MAX was treated as a binary outcome, evaluating pollution effects on the probability of TWA-MAX at or above the 75th percentile vs below the 75th percentile of all subject observations. The odds of TWA-MAX ≥ 75th percentile were estimated by fitting logistic regression models, controlling for the same covariates as specified above.
The results are presented as percent change scaling the PM2.5 effects to 10 μg/m3 and the BC effects to 1 μg/m3. The analysis was performed using the statistical package R (The Comprehensive R Archive Network: http://cran.r-project.org/).
Results
Table 1 shows the median and range for TWA-MAX and half-hour averaged heart rate for the 48 subjects who had a total of 5830 half-hour observations. The range of values for TWA-MAX at or above the 75th percentile was 26 to 61 μV. At the first home visit, 13 of 48 subjects described at least one episode of pain or discomfort in their chest since hospital discharge; 11 of the 13 with any chest pain described chest pain at rest. Over the subsequent visits 2-4, as activity levels increased, when asked “since we last saw you, have you had any pain or discomfort in your chest?”, 29 (36%) described any chest pain or discomfort, with 7 of the 29 describing chest pain/discomfort walking uphill or hurried; 13 describing chest pain/discomfort walking at a regular pace on the level, and 19 describing chest pain/discomfort at rest over the interim period since the previous visit. However discrete episodes of ischemia were infrequent during the Holter ECG recordings 3. Diabetes was most strongly linked to chest pain/discomfort. At the first visit, 5 of 12 subjects with diabetes had experienced chest pain (42%), whereas 8 of 36 non-diabetic subjects (22%) had experienced chest pain.
In Table 2 we summarize the distributions of temperature, of PM2.5 and BC measurements from the ambient central site, and of PM2.5 and BC measurements from inside and outside each participant home. The mean level for the 24-hour averages of the criteria pollutant PM2.5 at 24- hour averaging time were below the current or proposed National Air Quality Standards8. Compared to the ambient central site data, fewer indoor home measurements were available for analysis. PM2.5 was only measured at the ambient central site and inside the home; BC was measured at the ambient central site and both inside and outside of the home. Ambient PM2.5 levels measured at the central site were similar to levels inside the home and were correlated, reflecting the regional distribution of particle mass. In contrast, BC levels varied markedly between the ambient and the home sites, reflecting the variation in local sources for this traffic-derived pollutant. BC concentrations measured at the home of the participants (indoors or outdoors) were half that measured at the central site, which was located near a busy road in downtown Boston. BC concentrations inside homes were, on average, slightly lower than outside homes, though indoor maximal half-hour BC values exceeded maximal values outside the home, likely because of transient indoor sources such as cooking.
Table 2.
Ambient (measured at the central site), indoor at home, and outdoor at home measurements of air pollution and temperature among all subjects (48 subjects)
| Variable | N (observations) | 25th percentile | 50th percentile | 75th percentile | Maximum |
|---|---|---|---|---|---|
| Ambient PM2.5 (μg/m3) | |||||
| 0-30 minutes | 5,830 | 5.44 | 9.05 | 13.87 | 49.96 |
| 2-hour mean | 5,830 | 5.71 | 9.06 | 13.71 | 46.50 |
| 6-hour mean | 5,830 | 5.97 | 9.03 | 13.54 | 43.47 |
| Indoor PM2.5 (μg/m3) | |||||
| 0-30 minutes | 3,825 | 4.50 | 7.88 | 12.37 | 170.50 |
| 2-hour mean | 3,825 | 4.74 | 8.16 | 12.49 | 124.40 |
| 6-hour mean | 3,825 | 5.07 | 8.47 | 12.84 | 113.60 |
| Ambient BC (μg/m3) | |||||
| 0-30 minutes | 5,830 | 0.41 | 0.70 | 1.07 | 6.66 |
| 2-hour mean | 5,830 | 0.43 | 0.71 | 1.07 | 5.77 |
| 6-hour mean | 5,830 | 0.47 | 0.72 | 1.07 | 4.59 |
| Indoor BC (μg/m3) | |||||
| 0-30 minutes | 2,993 | 0.22 | 0.39 | 0.63 | 12.04 |
| 2-hour mean | 2,993 | 0.22 | 0.40 | 0.63 | 8.90 |
| 6-hour mean | 2,993 | 0.24 | 0.41 | 0.65 | 5.80 |
| Outdoor BC (μg/m3) | |||||
| 0-30 minutes | 2,993 | 0.26 | 0.48 | 0.79 | 3.43 |
| 2-hour mean | 2,993 | 0.27 | 0.50 | 0.79 | 2.98 |
| 6-hour mean | 2,993 | 0.30 | 0.50 | 0.80 | 2.55 |
| Mean temperature | 5,830 | 2.8 | 10.0 | 18.3 | 34.4 |
Regarding the mode of transportation for our study participants, 90% of transportation time consisted of driving a car and the remaining 10% using public transportation.
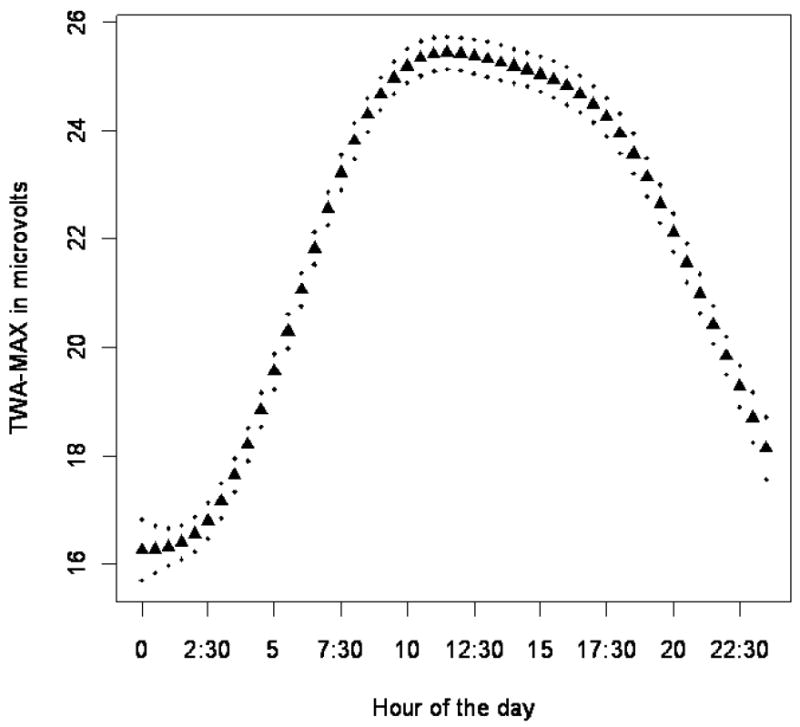
Figure 1 shows the circadian rhythm of TWA-MAX. T-wave alternans magnitude was lowest early in the morning, rising during the day, reaching a maximum at approximately 10 am.
Figure 1.
Twenty-four hour estimated circadian pattern of TWA-MAX, plotted as the mean of TWA-MAX values for each half-hour period of the day, controlling for subject, day of the week, being in traffic, average heart rate, hour of the day, date, mean temperature, and BC. The curve and pointwise 95% confidence interval (dotted line) are estimated using a penalized spline.
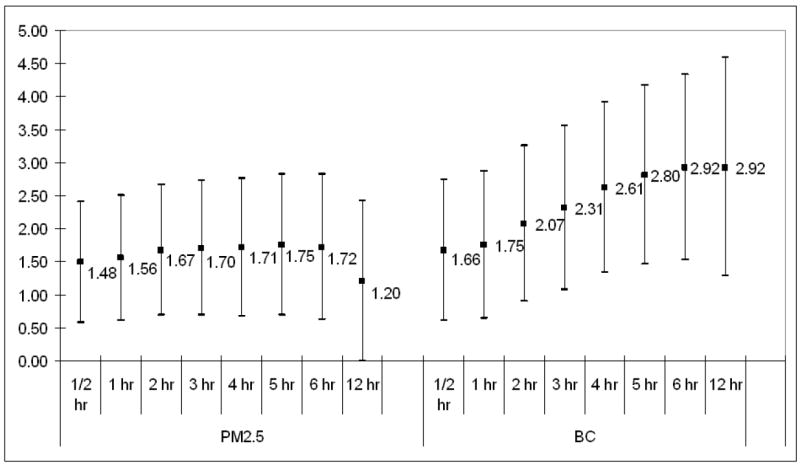
We found significant associations between TWA-MAX and both ambient pollutants and the pollutants measured indoors, adjusting for time of the day and other potential confounders (see methods section). Particularly for BC, up to 6 hours prior to TWA measurement, these pollution effects increased with the averaging time (Figure 2). TWA-MAX increased by 1.7% (95% C.I.: 0.6-2.8) for a 10 μg/m3 increase in PM2.5 and by 2.9% (95% C.I.: 1.5-4.3) for a 1 μg/m3 increase in central site BC for cumulative 6 hour exposure. We found significant associations between outdoor BC and TWA-MAX and these effects were similar to the associations with the central site BC, even if measurements were available in a subset of days.
Figure 2.
The percent change in maximum TWA for increasing averaging times for ambient PM2.5 and BC. PM2.5 effects are scaled to 10 μg/m3; BC effects are scaled to 1 μg/m3.
Traffic exposure in the 2 hours prior to and including the TWA measurement was associated with a 6% increase in half-hour averaged TWA-MAX (Table 3). The effects of being in traffic and of ambient central site-measured BC or PM2.5 were independent of each other. When subjects were away from home, 2-hour averaged ambient central site BC effects were stronger than when subjects were at home (P-value for interaction >0.001). When patients were home, indoor BC measurements were associated with larger and more precise estimates of change in TWA than ambient central site BC measurements. Compared to the effects of indoor PM2.5, indoor BC associations with TWA-MAX were greater.
Table 3.
Relation of maximum T-wave alternans (TWA) to ambient and indoor pollution and to being in traffic.
PM2.5 effects scaled to 10 μg/m3; BC effects scaled to 1 μg/m3 *
| Exposure | Change in TWA (μV) | 95% confidence interval | |
|---|---|---|---|
| Models including ambient PM2.5 | |||
| Model 1 | 30-minutes mean ambient PM2.5 | 1.48% | (0.58-2.40) |
| In traffic, past 2 hours | 6.00% | (3.33-8.74) | |
| Model 2 | 30-minutes mean ambient PM2.5 | 1.58% | (0.67-2.49) |
| Model 3 | 2-hour mean ambient PM2.5 | 1.67% | (0.68-2.66) |
| In traffic, past 2 hours | 5.97% | (3.30-8.71) | |
| Model 4 | 2-hour mean ambient PM2.5 | 1.78% | (0.79-2.77) |
| Model 5 | 2-hour mean ambient PM2.5 –Not home | 2.97% | (1.08-4.91) |
| 2-hour mean ambient PM2.5 – Home | 1.49% | (0.30-2.70) | |
| 2-hour mean ambient PM2.5 – Home, part of time | 0.11% | (-2.05-2.32) | |
| Models including indoor PM2.5 at home | |||
| Model 6 | 30 – minutes mean indoor PM2.5 | 1.83% | (1.01-2.66) |
| Model 7 | 2- hour mean indoor PM2.5 | 1.47% | (0.57-2.37) |
| Models including ambient BC | |||
| Model 1 | 30-minutes mean ambient BC | 1.66% | (0.60-2.74) |
| In traffic, past 2 hours | 6.09% | (3.41-8.83) | |
| Model 2 | 30-minutes mean ambient BC | 1.73% | (0.67-2.80) |
| Model 3 | 2-hours mean ambient BC | 2.07% | (0.89-3.26) |
| In traffic, past 2 hours | 6.12% | (3.45-8.86) | |
| Model 4 | 2-hour mean ambient BC | 2.12% | (0.94-3.31) |
| Model 5 | 2-hour mean ambient BC –Not home | 5.19% | (3.14-7.29) |
| 2-hour mean ambient BC – Home | 0.30% | (-1.24-1.85) | |
| 2-hour mean ambient BC – Home, part of time | 1.34% | (-1.39-4.13) | |
| Models including indoor BC at home | |||
| Model 6 | 30–minutes mean indoor BC | 3.23% | (1.59-4.89) |
| Model 7 | 2- hour mean indoor BC | 3.47% | (1.66-5.32) |
All models control for subject, day of the week, being in traffic, heart rate, hour of the day, date, and mean temperature (See Methods).
In sensitivity analyses, increases in PM2.5 and BC were associated with an increased risk of TWA-MAX ≥ 75th percentile for all averaging times (Table 4). While heart rate variability [the time domain variable RMSSD (the square root of the mean of the squared differences between adjacent normal RR intervals) and the frequency domain variable high frequency measure (HF)] and ST-segment level were correlated with TWA, the association of pollution with TWA was independent of these ECG measures (Supplementary Table 1).
Table 4.
Odds ratios (OR) and 95% confidence intervals (C.I.) for maximun T-wave alternans (TWA-MAX) >=26 microvolts for different averaging times for ambient PM2.5 and BC.
PM2.5 effects scaled to 10 μg/m3; BC effects scaled to 1 μg/m3
| Averaging Times | PM2.5 | BC | ||||
|---|---|---|---|---|---|---|
| OR | 95% C.I. | OR | 95% C.I. | |||
| 30 minutes | 1.11 | 0.99 | 1.25 | 1.17 | 1.03 | 1.34 |
| 2 hours mean | 1.14 | 1.00 | 1.29 | 1.26 | 1.09 | 1.46 |
| 4 hours mean | 1.16 | 1.02 | 1.33 | 1.40 | 1.19 | 1.64 |
| 6 hours mean | 1.14 | 0.99 | 1.31 | 1.42 | 1.19 | 1.69 |
| 12 hours mean | 0.97 | 0.83 | 1.14 | 1.24 | 1.01 | 1.53 |
Discussion
In the first 1-to-2 weeks following a myocardial infarction, the American College of Cardiology/American Heart Association Guidelines recommends avoiding driving, particularly under stressful circumstances such as heavy traffic9. These recommendations are based on studies suggesting that stress9, including the stress of driving in traffic, may increase the risk of post-myocardial infarction complications. Our study findings suggest that the particulate pollution from traffic sources itself, and not only the stress from traffic, may increase the risk of cardiac electrical instability both in the period post-myocardial infarction, and also in the period following successful percutaneous procedures for acute coronary syndrome without MI or worsening symptoms with stable coronary artery disease.
In our study we found that, when subjects were away from home, increases in the repolarization abnormality TWA were independently associated with two measures of traffic exposure: (1) a report of being in local traffic in the previous 2 hours and (2) ambient central-site measured BC, which is used to estimate a combination of regional and local traffic pollution. When subjects were at home, indoor BC was a better predictor of TWA than ambient BC.
In a German cohort of 56 men with stable ischemic heart disease, investigators10,11 showed pollution effects on another measure of T-wave repolarization abnormality, demonstrating a significant increase in QT duration and in variability of T-wave complexity in response to increases in organic carbon, as well as a significant decrease in T-wave amplitude and an increase in T-wave complexity in response to increases in PM2.5. TWA has been demonstrated to be the first step in a progression of T-wave complexity, cardiac electrical instability and ventricular fibrillation12. In the Triggers of Ventricular Arrhythmia study, Albert and colleagues found that in patients with implantable cardioverter-defibrillators (ICD), the risk of shocks for ventricular tachycardia or ventricular fibrillation (VT/VF) was greater in the 30 minutes after driving. Analyses suggested that exertion or anger could not explain the entire association between driving and ICG shocks for VT/VF; pollution exposure may have been a contributing factor2. Peters and co-authors1 also found consistent effects for patients traveling in cars, or using public transportation, motorcycles or bicycles, evidence supporting the hypothesis that the traffic effect was not all due to the stress of driving in heavy traffic13.
A limitation of this current study is the size of the cohort, the lack of longer term follow-up to evaluate clinical (e.g., sudden death) rather than subclinical outcomes, and the characterization of the cohort by amount of coronary artery atherosclerosis but not by ejection fraction. A second limitation is the lack of subject and visit specific cotinine levels, with reduced precision in estimation of personal smoking effects. Another potential limitation of our study may be the difficulty generalizing the results to a population of patients with more baseline cardiac electrical instability. However, it is worth noting that the circadian pattern of TWA (Fig. 1) parallels the early morning rise in frequency of SCD in the classic study by Muller and coworkers14.
The electrophysiologic basis for the link between TWA and arrhythmogenesis is that the alternating repolarization waveform is indicative of instabilities in cardiac membrane voltage and of disruptions in intracellular calcium cycling dynamics. TWA-associated electrophysiologic instabilities may be influenced by pollution-linked changes in autonomic tone15,16; pulmonary/systemic inflammation17,18 leading to myocardial or coronary artery inflammation; oxidative stress19,20; or thrombosis and myocardial ischemia21. Pollution has been shown to lead to changes in autonomic tone as reflected by reduced heart rate variability and increased heart rate22,23, and to increased risk of ST-segment depression consistent with ischemia3,24.
While our study participants did not have ventricular fibrillation or clinical adverse cardiac events during the periods of Holter monitoring, the range of values of maximal TWA that were at or above the 75th percentile (26 to 61 μV) was comparable to levels at or above the 75th percentile (42-53 μV) demonstrated to confer a 4 to 7-fold odds of life threatening arrhythmias in the post-myocardial infarction studies by Verrier and colleagues7. Thus the clinical relevance of our study is suggested by our findings that higher pollution levels predicted increased risk of having levels of TWA at or above the 75th percentile. Thus our data support the hypothesis that an important pathway by which pollution may increase the risk of ventricular fibrillation or sudden death may be through the repolarization abnormality TWA.
Supplementary Material
Acknowledgments
Funding Sources This work was supported in part by NIEHS (National Institute of Environment Health Sciences) P01 ES009825, NIEHS-00002, Environmental Protection Agency R832416-01-0, and National Science Council-095-SAF-I-564-602-TMS.
References
- 1.Peters A, von Klot S, Heier M, Trentinaglia I, Hormann A, Wichmann HE, Lowel H. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- 2.Albert CM, Rosenthal L, Calkins H, Steinberg JS, Ruskin JN, Wang P, Muller JE, Mittleman MA. Driving and implantable cardioverter-defibrillator shocks for ventricular arrhythmias: results from the TOVA study. J Am Coll Cardiol. 2007;50:2233–2240. doi: 10.1016/j.jacc.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 3.Chuang KJ, Coull BA, Zanobetti A, Suh H, Schwartz J, Stone PH, Litonjua A, Speizer FE, Gold DR. Particulate Air Pollution as a Risk Factor for ST-Segment Depression in Patients With Coronary Artery Disease. Circulation. 2008;118:1314–1320. doi: 10.1161/CIRCULATIONAHA.108.765669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nearing BD, Verrier RL. Modified moving average analysis of T-wave alternans to predict ventricular fibrillation with high accuracy. J Appl Physiol. 2002;92:541–549. doi: 10.1152/japplphysiol.00592.2001. [DOI] [PubMed] [Google Scholar]
- 5.Verrier RL, Nearing BD, Kwaku KF. Noninvasive sudden death risk stratification by ambulatory ECG-based T-wave alternans analysis: evidence and methodological guidelines. Ann Noninvasive Electrocardiol. 2005;10:110–120. doi: 10.1111/j.1542-474X.2005.10103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eilers PHC, Marx BD. Flexible smoothing with B-splines and penalties (with discussion) Statistical Science. 1996;11:89–121. [Google Scholar]
- 7.Verrier RL, Nearing BD, La Rovere MT, Pinna GD, Mittleman MA, Bigger JT, Jr, Schwartz PJ. Ambulatory electrocardiogram-based tracking of T wave alternans in postmyocardial infarction patients to assess risk of cardiac arrest or arrhythmic death. J Cardiovasc Electrophysiol. 2003;14:705–711. doi: 10.1046/j.1540-8167.2003.03118.x. [DOI] [PubMed] [Google Scholar]
- 8.U.S. EPA. Agency U S EPA. Washington, DC: 2004. Air Quality Criteria for Particulate Matter. [Google Scholar]
- 9.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:148–304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 10.Henneberger A, Zareba W, Ibald-Mulli A, Ruckerl R, Cyrys J, Couderc JP, Mykins B, Woelke G, Wichmann HE, Peters A. Repolarization changes induced by air pollution in ischemic heart disease patients. Environ Health Perspect. 2005;113:440–446. doi: 10.1289/ehp.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yue W, Schneider A, Stolzel M, Ruckerl R, Cyrys J, Pan X, Zareba W, Koenig W, Wichmann HE, Peters A. Ambient source-specific particles are associated with prolonged repolarization and increased levels of inflammation in male coronary artery disease patients. Mutat Res. 2007;621:50–60. doi: 10.1016/j.mrfmmm.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Nearing BD, Verrier RL. Progressive increases in complexity of T-wave oscillations herald ischemia-induced ventricular fibrillation. Circ Res. 2002;91:727–732. doi: 10.1161/01.res.0000038887.17976.33. [DOI] [PubMed] [Google Scholar]
- 13.Rosenlund M, Picciotto S, Forastiere F, Stafoggia M, Perucci CA. Traffic-related air pollution in relation to incidence and prognosis of coronary heart disease. Epidemiology. 2008;19:121–128. doi: 10.1097/EDE.0b013e31815c1921. [DOI] [PubMed] [Google Scholar]
- 14.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–138. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- 15.Park SK, O’Neill MS, Vokonas PS, Sparrow D, Schwartz J. Effects of air pollution on heart rate variability: the VA normative aging study. Environ Health Perspect. 2005;113:304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 17.Adamkiewicz G, Ebelt S, Syring M, Slater J, Speizer FE, Schwartz J, Suh H, Gold DR. Association between air pollution exposure and exhaled nitric oxide in an elderly population. Thorax. 2004;59:204–209. doi: 10.1136/thorax.2003.006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adar SD, Adamkiewicz G, Gold DR, Schwartz J, Coull BA, Suh H. Ambient and microenvironmental particles and exhaled nitric oxide before and after a group bus trip. Environ Health Perspect. 2007;115:507–512. doi: 10.1289/ehp.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters A, Veronesi B, Calderon-Garciduenas L, Gehr P, Chen LC, Geiser M, Reed W, Rothen-Rutishauser B, Schurch S, Schulz H. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chahine T, Baccarelli A, Litonjua A, Wright RO, Suh H, Gold DR, Sparrow D, Vokonas P, Schwartz J. Particulate air pollution, oxidative stress genes, and heart rate variability in an elderly cohort. Environ Health Perspect. 2007;115:1617–1622. doi: 10.1289/ehp.10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verrier RL, Kumar K, Nearing BD. Basis for sudden cardiac death prediction by T-wave alternans from an integrative physiology perspective. Heart Rhythm. 2009;6:416–422. doi: 10.1016/j.hrthm.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, Allen G, Verrier M, Cherry R, Verrier R. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz J, Litonjua A, Suh H, Verrier M, Zanobetti A, Syring M, Nearing B, Verrier R, Stone P, MacCallum G, Speizer FE, Gold DR. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60:455–461. doi: 10.1136/thx.2004.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gold DR, Litonjua AA, Zanobetti A, Coull BA, Schwartz J, MacCallum G, Verrier RL, Nearing BD, Canner MJ, Suh H, Stone PH. Air pollution and ST-segment depression in elderly subjects. Environ Health Perspect. 2005;113:883–887. doi: 10.1289/ehp.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


