Abstract
Background
More than 90% of children with congenital heart defects now survive into adulthood; just a few decades ago, survival was rare, particularly among patients with complex defects. The new population of adults with congenital heart disease presents a special challenge to physicians from all of the involved specialties.
Methods
Selective literature review.
Results and conclusion
A complete cure of the congenital heart defect in childhood is exceptional, and most adult patients continue to suffer from residual problems and sequelae. Further surgery or catheter interventions may be needed. Potential late complications include arrhythmias, heart failure, pulmonary hypertension, endocarditis, and thromboembolic events. The management of these patients during pregnancy or non-cardiac surgery remains a challenge. If this evolving patient population is to receive the best possible care, the adequate provision of specialized medical services is a necessary, but not sufficient, condition: patients and their referring physicians will also need to be aware that these services are available, and then actually make use of them. Moreover, optimal communication among all of the involved physicians is essential.
Thanks to advances in pediatric cardiology and pediatric heart surgery in the past couple of decades, a new group of patients has emerged—adults with congenital heart defects. This continually growing population presents a particular challenge for cardiologists (e1). The estimated total number of adults with congenital heart defects in Germany is in excess of 250 000 (20% to 50% of these patients are estimated to have complex anomalies) (1, e2). Recommendations for the organization of healthcare services (2) and the qualification of doctors whose remit it is to care for adults with congenital heart defects were published by an interdisciplinary task force (3). Information is available, among others, from the academy for continuing medical education at the German Association for Pediatric Cardiologists (Deutsche Gesellschaft für Pädiatrische Kardiologie, Akademie für Fort-und Weiterbildung, Düsseldorf [theisen@dgpk.org]). Further information on certified cardiologists/pediatric cardiologists is available on the internet (4). Most adult patients with congenital heart defects are primarily being treated by their general practitioners and specialists in general internal medicine/cardiology but they should be referred at regular intervals—depending on the complexity of the underlying condition—to a center for adults with congenital heart disease or a cardiologist specializing in this subject. Accordingly, all doctors from all specialties involved in looking after such patients need to have basic knowledge of congenital heart defects.
This article provides an overview of the most important congenital heart defects, especially of common complications and therapeutic options in patients with congenital heart disease.
The aim is to enable readers to recognize predictable problems in adult congenital heart disease patients and prevent these, if possible. Since hardly any randomized controlled studies exist in this clinical area, the evidence level is comparatively low. The recommendations in this article are based mostly on the results of non-randomized prospective and retrospective studies and on expert opinion.
Method
The article is based on national and international guidelines (5, 6, e3). The authors also searched PubMed (www.ncbi.nlm.nih.gov) and selected articles on the basis of their own subjective assessment of their clinical relevance. The authors also used relevant textbooks and their own personal literature archives.
Overview of common heart defects in adult patients with congenital heart disease
Figures 1– 4 provide an overview of the anatomy of common congenital heart defects. Further information is summarized in a supplementary table (available at: http://www.aerzteblatt.de/download/files/2011/07/down155856.pdf). This table also contains information about the clinical presentation and common problems in the long term as well as therapeutic considerations for the most important congenital heart defects.
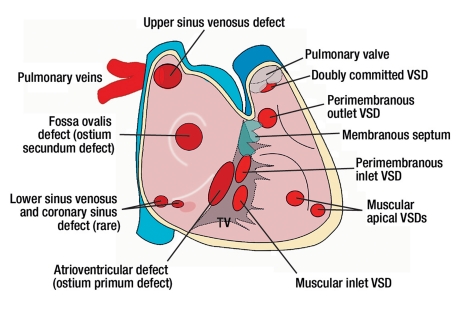
Figure 1.
Overview of the localizations (view from right atrium and ventricle to interatrial and interventricular septum) of atrial septal defects (ASD), atrioventricular defects (AVSD), and ventricular septal defects (VSD). The figure shows that especially in upper sinus venosus defects, attention should be paid to associated anomalous pulmonary venous connection. TV, tricuspid valve
Figure 4.
Overview of different modifications of Fontan palliation in patients with a univentricular heart. In patients with Fontan-type repair, the systemic venous return is re-directed to the pulmonary circulation (without a subpulmonary ventricle). Over time, several modifications have been developed. The “classic” Fontan repair: direct connection between right atrium and pulmonary artery. In total cavopulmonary anastomosis (TCPC), a connection is made between the superior and inferior vena cava and the right pulmonary artery. The superior vena cava is anastomosed directly end-to-side with the pulmonary artery, the connection to the inferior vena cava can be established either via a lateral tunnel within the right atrium or via an extracardiac conduit. TCPC is the preferred procedure because of better hemodynamics, a lower incidence of arrhythmias, and better long-term survival. Typical problems in the long term are ventricular dysfunction, intraatrial thrombi, and a proneness to arrhythmias. These problems are encountered in particular after the “classic” Fontan repair
In addition to a merely anatomical description, congenital heart defects should be categorized according to their complexity and the presence of cyanosis (Box 1). This is of fundamental importance for planning follow-up care and in gauging what problems and complications might be expected (6). Except in rare conditions (early correction of uncomplicated patent ductus arteriosus and uncomplicated secundum atrial septal defects, which may be considered “cured,” patients with congenital heart defects usually require lifelong care in order to secure an optimal outcome. According to current guidelines, patients with congenital heart defects of low complexity should be seen at a center for adults with congenital heart disease at least once, in order to decide on their further management. Patients with congenital heart defects of moderate and high complexity should be referred to a center and be assessed regularly (6).
Box 1. Complexity of common heart defects*1.
-
Heart defects of low complexity
-
Uncorrected heart defects
Isolated small atrial septal defects (ASD II)
Isolated restrictive ventricular septal defects (VSD)
Mild pulmonary stenosis (gradient <30 mm Hg)
Restrictive persistent ductus arteriosus
Isolated congenital aortic stenosis
Isolated congenital mitral valve disorder
-
Heart defects after correction
After closure of ASD (except for atriventricular defect)
After uncomplicated closure of VSD
After closure of persistent ductus arteriosus
Heart defects of moderate complexity
Atrioventricular septal defects (AVSD) / ASD I
Sinus venosus defects
Aortic isthmus stenosis
Ebstein’s anomaly
Tetralogy of Fallot
Ventricular septal defects with associated anomalies
Moderate and higher grade pulmonary stenosis or regurgitation
Anomalous pulmonary venous connection
Persistent ductus arteriosus (uncorrected)
-
-
Heart defects of high complexity
Cyanotic heart defects (independent of anatomy)
Complex heart defects with functionally univentricular anatomy
Patients after Fontan repair
Transposition of the great arteries (TGA)
Any form of discordant connection between atria, ventricles, and great arteries
Patients with implanted conduits
ASD, atrial septal defect; AVSD, atrioventricular septal defect; TGA, transposition of the great arteries; VSD, ventricular septal defect. *1 Adapted from Warnes et al (6)
Cardiac problems during follow-up
Interventions and repeat interventions
Although most patients undergo surgery in childhood, further surgical or interventional procedures are often required in the long term. The decision about the optimal timing of the intervention is a particular challenge in adults with congenital heart disease. In some cases, interventions are necessary before patients develop any symptoms, so as to prevent irreversible damage to the cardiac muscle and pulmonary vascular disease. On the other hand, procedures where reinterventions are anticipated (for example, implantation of a homograft or a biological valve replacement) should not be performed at an unnecessarily early stage.
Occasionally, congenital heart defects are detected only in adulthood. Apart from valvular disease (especially bicuspid valve), the most common abnormalities are atrial septal defects, occasionally Ebstein’s anomaly, and, more rarely, complex defects such as congenitally corrected transposition of the great arteries without associated abnormalities. If cardiac anatomy is unclear or ventricular dilatation and dysfunction occur (e.g., right ventricular dysfunction in the setting of atrial septal defects or partial anomalous pulmonary venous connection [PAPVC]); left ventricular dysfunction, e.g., in ductus arteriosus), physicians should consider an undetected congenital heart defect, even in adults, and seek further diagnostic evaluation.
A particular challenge is posed by patients with complex congenital heart defects in whom surgery was contraindicated for historical reasons or in whom only alleviating procedures have been performed, and in whom it needs to be considered whether surgical correction is still possible in adult life.
Arrhythmias
Arrhythmias are a common late complication in adults with congenital heart defects, owing to the underlying cardiac disorder, hemodynamic problems, and surgical corrections resulting in scarring (7, e4). They range from bradycardiac arrhythmias to atrial tachycardias to life threatening ventricular tachycardias or atrial fibrillation.
In patients with symptoms such as palpitations, tachycardia, dizziness, and syncope, but also signs of heart failure, arrhythmias should be considered. We will now present some examples of cardiac abnormalities that predispose to arrhythmias.
Tetralogy of Fallot (ToF)—Patients with corrected ToF are predisposed to atrial and ventricular arrhythmias and are at increased risk for sudden cardiac death (about 2.5% per 10 years) (6). The substrate for these arrhythmias is represented by myocardial fibrosis (e5) and re-entry circuits around ventriculotomy and atriotomy scars or the VSD (ventricular septal defect) patch (e6). Identified risk markers for sudden cardiac death include a wide QRS complex >180 ms (8), severe pulmonary insufficiency, right and left ventricular dysfunction, extensive myocardial fibrosis, late corrective surgery, and palliative shunts that have been in place for a long time. The importance of regular Holter-ECGs is the subject of controversial debate (e7). In selected symptomatic patients, electrophysiological studies can yield prognostic information, and, in ventricular re-entry circuits ablation treatment may be an option (9). Implantation of an implantable cardioverter defibrillator (ICD) is widely accepted in the context of secondary prophylaxis (“survived sudden cardiac death”), but criteria for primary preventive implantation are currently not well defined (e8).
Patients with transposition of the great arteries (TGA) after atrial switch surgery—Most of the patients with TGA develop relevant cardiac arrhythmias during their lifetime. These include bradycardiac and tachycardiac arrhythmias. As a result of the procedure and owing to scarring in the atrial region, more than 10% of patients with TGA develop early postoperative bradycardiac arrhythmias after atrial switch operation, which require pacemaker insertion (e9). It has been reported that 20 years postoperatively, only 40% of patients are in sinus rhythm (e10). Symptoms of arrhythmia, heart failure, and documented arrhythmias in the patient’s medical history have been identified as a predictor for sudden cardiac death, whereas findings from resting ECG and Holter-ECG monitoring do not seem to carry prognostic information (10). Little is currently known about the importance of electrophysiological examination for prognostic assessment.
Patients with functional single ventricle after Fontan repair—Recurrent supraventricular arrhythmias are common in patients after Fontan repair, especially patients who have undergone the classic Fontan procedure (connection between right atrium and pulmonary artery). In such patients, intra-atrial macro-re-entry circles with a long cycle-length are common, which are hemodynamically poorly tolerated. Patients with recurrent supraventricular tachycardias should be referred to an experienced center for electrophysiological evaluation (11). This applies in particular to patients after a “classic” Fontan repair, whose prognosis is limited (5 year survival 85%) (e11, e12).
Heart failure
In the long term, the development of heart failure is common in patients with congenital heart disease, especially those with complex abnormalities and systemic right ventricle. It has been described that heart failure develops in 22% of patients with congenitally corrected transposition of the large vessels, 32% of TGA patients after atrial switch surgery, and up to 40% of patients after Fontan repair (12). Potentially correctable causes (such as hemodynamically relevant shunts, valve insufficiency and stenosis, and other intracardial or vascular obstructions, arrhythmias, pulmonary arterial hypertension) should be identified and treated accordingly. Angiotensin converting enzyme (ACE) inhibitors and beta blockers are used empirically, but evidence supporting the prognostic benefit of these medications is lacking in adult patients with congenital heart defects (13). Transplantation is the treatment of last resort in patients with end-stage heart failure, but this may be technically demanding and has an increased risk compared with other groups of patients (e13).
Pulmonary hypertension and Eisenmenger’s syndrome
Pulmonary arterial hypertension (PAH) is a common complication in adults with congenital heart defects, especially with uncorrected shunt lesions and unprotected pulmonary circulation or after a late repair. Patients with PAH after shunt closure pathophysiologically resemble patients with idiopathic PAH, but severe PAH in patients who have not had surgery usually results in shunt reversal with Eisenmenger’s syndrome (a combination of PAH and cyanosis). Endothelin receptor antagonists, phosphodiesterase inhibitors, and prostacyclines are effective drugs for the treatment of PAH (14, e14); however, far fewer data exist for adults with congenital heart defects than for those with idiopathic PAH.
Infectious endocarditis
In principle, all congenital heart defects are associated with an increased lifetime risk for infectious endocarditis (IE). However, since it has to be assumed that most cases of IE are not caused by medical or dental interventions, and since the assumption that administration of an antibiotic can prevent IE has never been conclusively proved in humans, the current guidelines of the international societies recommend IE prophylaxis only in high-risk patients and patients who have previously had IE (15). This high-risk group comprises patients with complex, usually cyanotic conditions, patients with implanted prosthetic valves, and patients with residual defects in immediate proximity to implanted patches or conduits. Furthermore, prophylaxis against endocarditis for 6 months is recommended after cardiac surgery with implantation of patch or conduit material and after catheter intervention using implants.
By type of intervention, prophylaxis is restricted to dental procedures that may be accompanied by injury to the mucosa, gingiva, or apical dental region, as well as bronchoscopy with incision of the mucosa or biopsy, as well as tonsillectomies and adenectomies. We cannot emphasize enough that patients need to be reminded to observe good oral hygiene and visit their dentist regularly.
It is of particular importance in adults with congenital heart disease to consider endocarditis early in case of suspicious symptoms (raised temperature, night sweats, embolism, etc) and initiate the necessary diagnostic steps early on—especially echocardiography and blood cultures, before an antibiotic is given (e15, e16).
Common non-cardiac problems in the context of aftercare for adults with congenital heart defects
Contraception and pregnancy
Cardiovascular disorders are currently the most common cause of maternal mortality during pregnancy (16). An individual risk assessment in any adult woman with a congenital heart defect should be done early on, in order to prevent unwanted high-risk pregnancies. The physiological changes and their possible implications for different congenital heart defects have just been discussed in detail in a review article in Deutsches Ärzteblatt International (16). Because of the increase in cardiac volume and circulating blood volume, stenotic abnormalities and increased pulmonary vascular resistance are particularly problematic. Cyanotic abnormalities also present a particular problem, because of the drop in peripheral vascular resistance and the increase in right-to-left shunts. The combination of severe pulmonary arterial hypertension and cyanosis (as in Eisenmenger’s syndrome) is associated with particularly high maternal mortality of up to 50% (17); the deaths often occur during the puerperium. Generally, symptoms of heart failure (NYHA >2), reduced left ventricular function, previous cardiovascular problems during pregnancy, and severe pulmonary valve insufficiency—especially combined with reduced right ventricular function—are all associated with increased risk for pregnant women (18– 20). Patients with Marfan syndrome and a dilated aortic root (>4 cm) have an increased risk for dissection (5). Treating patients with mechanical prosthetic valves is problematic because of the need for adequate anticoagulation and the increased risk for fetal abnormalities owing to treatment with vitamin K antagonists (for example, Marcumar [phenprocoumon]). Alternative strategies for anticoagulation are not easily controlled with standard coagulation tests; they are associated with an increased risk of thrombosis, barely evidence based, and thus not without problems. The management of patients with a moderate to high pregnancy risk (Table 1) requires particular experience, an interdisciplinary team, and should be undertaken by specialized centers only.
Table 1. Pregnancy risk depending on the underlying heart defect.
| Low risk (risk of cardiac complications or death <1%) | Moderate risk (risk of cardiac complications or death ≈ 1–5%) | High risk (risk of cardiac complications or death >5%) |
|
|
|
Cyanosis and erythrocytosis
Patients with uncorrected complex cardiac defects and patients after palliative procedures (for example, Glenn surgery) may present with cyanosis. Chronic cyanosis is characterized by notably reduced exercise capacity, frequent infections (for example, endocarditis and cerebral abscesses), and multiple end organ damage in the sense of a multiorgan disorder (e17). Typical complications in addition to the infections include hemorrhage, thromboembolism, arrhythmias, impaired renal function, gallstones, and joint problems. A comprehensive explanation of the problems would exceed the scope of this article.
Erythrocytosis develops secondary to chronic hypoxia, is associated with an increased oxygen carrying capacity of the blood, and improves tissue oxygenation; however, it requires sufficient availability of iron. Preventing iron deficiency is of particular importance (e18). Phlebotomy should be limited to patients with confirmed symptoms of hyperviscosity (headache, dizziness, impaired vision, paresthesias, muscle aches, etc.) after iron deficiency has been excluded; the hematocrit is usually in excess of 65% in such cases (5). However, even hematocrit values above 70% are commonly tolerated without any symptoms. Repeated unnecessary phlebotomy will then result in iron deficiency, reduced exercise capacity, and increased risk of stroke (e17, e19). Dehydration needs to be avoided in such patients; if it does develop it requires immediate treatment.
Cyanotic patients, especially those with Eisenmenger’s syndrome, are sometimes placed in grave danger by simple surgical procedures, but also by other medical interventions, if their particular characteristics are not considered accordingly. Any medication or surgical treatment, including semi-invasive and invasive examinations should be coordinated with a center for adults with congenital heart disease.
Non-cardiac surgical interventions
Many adult patients with congenital heart defects require general surgery during their lifetime. Because of the range of such procedures, and also depending on their urgency, medical centers in primary as well as secondary care are often confronted with this problem. The risk of a procedure may vary depending on the underlying defect and associated problems. The treating surgeon and anesthetist should consider the influence of anesthesia, mechanical ventilation, possible required vasoactive substances, and blood volume shifts on the underlying cardiac physiology and consult a cardiologist with experience in adult patients with congenital heart disease. High-risk patients (Fontan repair, severe PAH, cyanosis, complex heart defects especially in the presence of heart failure, and others) should be treated in specialized centers.
Psychosocial aspects, choice of job, and sports
The quality of life of adult patients with congenital heart disease is impaired by:
Reduced exercise capacity (21)
Cosmetic impairments (e20)
Repeatedly required catheter interventions or surgical procedures.
Furthermore, patients often have fears and anxieties regarding possible complications, increasing disability, and reduced life expectancy. These aspects have thus far not received much attention, but they are increasingly gaining importance. Appropriate psychosocial care is of great importance and should be offered at supraregional centers for adults with congenital heart disease. In addition, concrete questions regarding participation in sports and choice of job position should be answered. This requires experience and the objective assessment of maximal exercise capacity in the context of spiroergonomic examinations.
The organization of healthcare service structures
The treatment of patients with heart defects of moderate to high complexity should be coordinated by supraregional centers for adult patients with congenital heart disease. In a position paper published in 2001 (e21) it was stipulated that a supraregional center should provide care for a population of 5 to 10 million.
For Germany, this would mean that 8 to 16 supraregional centers are needed. Cardiologists at such centers should have particular experience in the treatment of adult patients with congenital heart disease (according to the American model, a minimum of 2 years’ experience with adult patients with congenital heart disease, ideally 5 or more years). Furthermore, an experienced cardiosurgical team should be available, as should be the option of electrophysiological investigations and treatments as well as all other diagnostic methods (cardiac MRI, CT, and invasive diagnostic approaches).
The interdisciplinary task force for adult patients with congenital heart disease that was mentioned earlier has published recommendations for improving the quality of interdisciplinary care for adults with congenital heart defects (2), which mainly follow the north American suggestions. The cooperation of basic care provided by general practitioners, regional services providing maximal care, and supraregional centers are explained and the requirements of the different structures are laid out. Good communication between the general practitioner, the cardiologist in private practice, and the center for adult patients with congenital heart disease is of crucial importance. Close collaboration between pediatric and adult cardiologists is also vital in order to provide a seamless transition for patients from pediatric cardiology services to adult cardiology services.
Figure 2.
Overview of the anatomy in uncorrected (left) and corrected tetralogy of Fallot. Common problems in adult patients after correction of a tetralogy of Fallot include relevant pulmonary valve regurgitation, right ventricular dilatation and functional impairment, and a tendency to arrhythmias
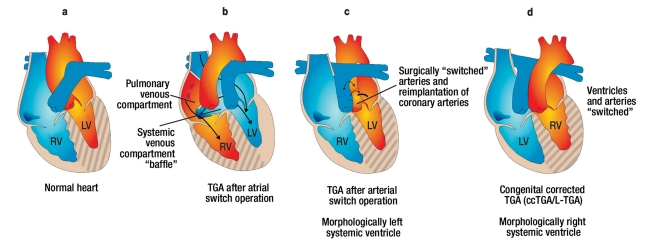
Figure 3.
Overview of the anatomy in patients with transposition of the great arteries (TGA or D-TGA) and congenital corrected TGA (ccTGA or L-TGA). ccTGA and TGA (after atrial switch operation). Both have a morphologically right systemic ventricle in common. This is associated with the development of heart failure in the long term and with increased morbidity/mortality. By means of atrial switch surgery, the venous blood is redirected at the atrial level. This surgical method has been superseded in the past 20 years by arterial switch surgery, which has the advantage of a morphologically left systemic ventricle.
In ccTGA, a combination of atrioventricular and ventriculoarterial discordance is present. The systemic ventricle is hatched. LV, left ventricle; RV, right ventricle
Key Messages.
A minority of adult patients with congenital heart defects can be regarded as “cured”, and long term complications are common.
In spite of surgical interventions in childhood, further surgical or other interventional procedures are often required in the longer term.
Patients with heart defects of moderate to high complexity should be referred to specialized centers for adults with congenital heart disease and should be evaluated regularly in those centers.
Since cardiovascular disorders are currently the most common cause for maternal mortality during pregnancy, an individual risk assessment should be made in women with congenital heart defects early on, in order to prevent unwanted high-risk pregnancies or ensure optimal care during the pregnancy.
Good communications between patients’ general practitioners, cardiologists in private practice, and specialist centers, as well as close cooperation between pediatric and adult cardiologists is required in order to ensure optimal treatment for adult patients with congenital heart defects.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Kaemmerer H, Hess J. Adult patients with congenital heart abnormalities: present and future. Dtsch Med Wochenschr. 2005;130:97–101. doi: 10.1055/s-2005-837381. [DOI] [PubMed] [Google Scholar]
- 2.Kaemmerer H, Breithardt G. Empfehlungen zur Qualitätsverbesserung der interdisziplinären Versorgung von Erwachsenen mit angeborenen Herzfehlern (EMAH) Clinical Research in Cardiology. 2006;95:76–84. doi: 10.1007/s00392-006-2003-1. [DOI] [PubMed] [Google Scholar]
- 3.Hess J, Bauer U, de Haan F, et al. Empfehlungen für Erwachsenen- und Kinderkardiologen zum Erwerb der Zusatz-Qualifikation „Erwachsene mit angeborenen Herzfehlern“ (EMAH) Clinical Research in Cardiology Supplements. 2007;2:19–26. [Google Scholar]
- 4. http://www.kinderkardiologie.org/dgpkAkademieEMAH.shtml.
- 5.Schmaltz AA, Bauer U, Baumgartner H, et al. Medical guideline for the treatment of adults with congenital heart abnormalities of the German-Austrian-Swiss Cardiology Specialty Society. Clin Res Cardiol. 2008;97:194–214. doi: 10.1007/s00392-008-0639-8. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010) Eur Hart J. 2010;31:2915–2957. doi: 10.1093/eurheartj/ehq249. [DOI] [PubMed] [Google Scholar]
- 7.Walsh EP, Cecchin F. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115:534–545. doi: 10.1161/CIRCULATIONAHA.105.592410. [DOI] [PubMed] [Google Scholar]
- 8.Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 9.Khairy P, Stevenson WG. Catheter ablation in tetralogy of Fallot. Heart Rhythm. 2009;6:1069–1074. doi: 10.1016/j.hrthm.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 10.Kammeraad JA, van Deurzen CH, Sreeram N, et al. Predictors of sudden cardiac death after Mustard or Senning repair for transposition of the great arteries. J Am Coll Cardiol. 2004;44:1095–1102. doi: 10.1016/j.jacc.2004.05.073. [DOI] [PubMed] [Google Scholar]
- 11.Abrams DJ, Earley MJ, Sporton SC, et al. Comparison of noncontact and electroanatomic mapping to identify scar and arrhythmia late after the Fontan procedure. Circulation. 2007;115:1738–1746. doi: 10.1161/CIRCULATIONAHA.106.633982. [DOI] [PubMed] [Google Scholar]
- 12.Piran S, Veldtman G, Siu S, Webb GD, Liu PP. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation. 2002;105:1189–1194. doi: 10.1161/hc1002.105182. [DOI] [PubMed] [Google Scholar]
- 13.Dore A, Houde C, Chan KL, et al. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation. 2005;112:2411–2416. doi: 10.1161/CIRCULATIONAHA.105.543470. [DOI] [PubMed] [Google Scholar]
- 14.Gatzoulis MA, Beghetti M, Galie N, et al. Longer-term bosentan therapy improves functional capacity in Eisenmenger syndrome: results of the BREATHE-5 open-label extension study. Int J Cardiol. 2008;127:27–32. doi: 10.1016/j.ijcard.2007.04.078. [DOI] [PubMed] [Google Scholar]
- 15.Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the task force on the prevention, diagnosis, and treatment of infective endocarditis of the European Society of Cardiology (ESC) Eur Heart J. 2009;30:2369–2413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 16.Uebing A, Gatzoulis MA, von Kaisenberg C, Kramer HH, Strauss A. Congenital heart disease in pregnancy. Dtsch Arztebl Int. 2008;105:347–354. doi: 10.3238/arztebl.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J. 2009;30:256–265. doi: 10.1093/eurheartj/ehn597. [DOI] [PubMed] [Google Scholar]
- 18.Khairy P, Ouyang DW, Fernandes SM, Lee-Parritz A, Economy KE, Landzberg MJ. Pregnancy outcomes in women with congenital heart disease. Circulation. 2006;113:517–524. doi: 10.1161/CIRCULATIONAHA.105.589655. [DOI] [PubMed] [Google Scholar]
- 19.Siu SC, Colman JM, Sorensen S, et al. Adverse neonatal and cardiac outcomes are more common in pregnant women with cardiac disease. Circulation. 2002;105:2179–2184. doi: 10.1161/01.cir.0000015699.48605.08. [DOI] [PubMed] [Google Scholar]
- 20.Siu SC, Sermer M, Colman JM, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104:515–521. doi: 10.1161/hc3001.093437. [DOI] [PubMed] [Google Scholar]
- 21.Diller GP, Dimopoulos K, Okonko D, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112:828–835. doi: 10.1161/CIRCULATIONAHA.104.529800. [DOI] [PubMed] [Google Scholar]
- 22.Kaemmerer H, Bauer U, Pensl U, et al. Management of emergencies in adults with congenital cardiac disease. Am J Cardiol. 2008;101:521–525. doi: 10.1016/j.amjcard.2007.09.110. [DOI] [PubMed] [Google Scholar]
- 23.Kaemmerer H, Fratz S, Bauer U, et al. Emergency hospital admissions and three-year survival of adults with and without cardiovascular surgery for congenital cardiac disease. J Thorac Cardiovasc Surg. 2003;126:1048–1052. doi: 10.1016/s0022-5223(03)00737-2. [DOI] [PubMed] [Google Scholar]
- e1.Moons P, Bovijn L, Budts W, Gewillig M. Actual prospects to survive into adulthood in patients with congenital heart disease. Circulation. 2009;120 doi: 10.1161/CIRCULATIONAHA.110.946343. 18_MeetingAbstracts 561 Abstract 1866. [DOI] [PubMed] [Google Scholar]
- e2.Marelli A, Mackie A, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- e3.Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease) Circulation. 2008;118:e714–e833. doi: 10.1161/CIRCULATIONAHA.108.190690. [DOI] [PubMed] [Google Scholar]
- e4.Bouchardy J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Bottega N, et al. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. doi: 10.1161/CIRCULATIONAHA.109.866319. [DOI] [PubMed] [Google Scholar]
- e5.Babu-Narayan S, Kilner P, Li W, Moon J, Goktekin O, Davlouros P, et al. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006;113:405–413. doi: 10.1161/CIRCULATIONAHA.105.548727. [DOI] [PubMed] [Google Scholar]
- e6.Kalman J, VanHare G, Olgin J, Saxon L, Stark S, Lesh M. Ablation of ’incisional’ reentrant atrial tachycardia complicating surgery for congenital heart disease. Use of entrainment to define a critical isthmus of conduction. Circulation. 1996;93:502–512. doi: 10.1161/01.cir.93.3.502. [DOI] [PubMed] [Google Scholar]
- e7.Cullen S, Celermajer D, Franklin R, Hallidie-Smith K, Deanfield J. Prognostic significance of ventricular arrhythmia after repair of tetralogy of Fallot: a 12-year prospective study. J Am Coll Cardiol. 1994;23:1151–1155. doi: 10.1016/0735-1097(94)90604-1. [DOI] [PubMed] [Google Scholar]
- e8.Khairy P, Harris L, Landzberg M, Viswanathan S, Barlow A, Gatzoulis M, et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008;117:363–370. doi: 10.1161/CIRCULATIONAHA.107.726372. [DOI] [PubMed] [Google Scholar]
- e9.Warnes C. Transposition of the great arteries. Circulation. 2006;114:2699–2709. doi: 10.1161/CIRCULATIONAHA.105.592352. [DOI] [PubMed] [Google Scholar]
- e10.Gelatt M, Hamilton R, McCrindle B, Connelly M, Davis A, Harris L, et al. Arrhythmia and mortality after the Mustard procedure: a 30-year single-center experience. J Am Coll Cardiol. 1997;29:194–201. doi: 10.1016/s0735-1097(96)00424-x. [DOI] [PubMed] [Google Scholar]
- e11.Diller G, Giardini A, Dimopoulos K, Gargiulo G, Muller J, Derrick G, et al. Predictors of morbidity and mortality in contemporary fontan patients: Results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J. 2010;31:3073–3083. doi: 10.1093/eurheartj/ehq356. [DOI] [PubMed] [Google Scholar]
- e12.Dimopoulos K, Giannakoulas G, Yuksel S, Inuzuka R, Pijuan-Domenech A, Hussain W, et al. Atrial tachyarrhythmias late after fontan operation are related to increase in mortality and hospitalization. Circulation. 2009;120 doi: 10.1016/j.ijcard.2010.12.049. 18_MeetingAbstracts S562-a-. Abstract 1871. [DOI] [PubMed] [Google Scholar]
- e13.Baumgartner H, Dabritz S. Congenital heart disease in adulthood. Med Klin (Munich) 2008;103:135–142. doi: 10.1007/s00063-008-1020-4. [DOI] [PubMed] [Google Scholar]
- e14.Galie N, Beghetti M, Gatzoulis M, Granton J, Berger R, Lauer A, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation. 2006;114:48–54. doi: 10.1161/CIRCULATIONAHA.106.630715. [DOI] [PubMed] [Google Scholar]
- e15.Durack D, Lukes A, Bright D. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96:200–209. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- e16.Naber C. S2 Guideline for diagnosis and therapy of infectious endocarditis. Z Kardiol. 2004;93:1005–1021. doi: 10.1007/s00392-004-0183-0. [DOI] [PubMed] [Google Scholar]
- e17.Diller G, Gatzoulis M. Pulmonary vascular disease in adults with congenital heart disease. Circulation. 2007;115:1039–1050. doi: 10.1161/CIRCULATIONAHA.105.592386. [DOI] [PubMed] [Google Scholar]
- e18.Spence M, Balaratnam M, Gatzoulis M. Clinical update: cyanotic adult congenital heart disease. Lancet. 2007;370(9598):1530–1532. doi: 10.1016/S0140-6736(07)61647-X. [DOI] [PubMed] [Google Scholar]
- e19.Broberg C, Bax B, Okonko D, Rampling M, Bayne S, Harries C, et al. Blood viscosity and its relationship to iron deficiency, symptoms, and exercise capacity in adults with cyanotic congenital heart disease. J Am Coll Cardiol. 2006;48:356–365. doi: 10.1016/j.jacc.2006.03.040. [DOI] [PubMed] [Google Scholar]
- e20.Bleiziffer S, Schreiber C, Burgkart R, Regenfelder F, Kostolny M, Libera P, et al. The influence of right anterolateral thoracotomy in prepubescent female patients on late breast development and on the incidence of scoliosis. J Thorac Cardiovasc Surg. 2004;127:1474–1480. doi: 10.1016/j.jtcvs.2003.11.033. [DOI] [PubMed] [Google Scholar]
- e21.Summary of recommendations—care of the adult with congenital heart disease. J Am Coll Cardiol. 2001;37:1167–1169. doi: 10.1016/s0735-1097(01)01281-5. [DOI] [PubMed] [Google Scholar]
- e22.Drenthen W, Pieper P, Roos-Hesselink J, van Lottum W, Voors A, Mulder B, et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J Am Coll Cardiol. 2007;49:2303–2311. doi: 10.1016/j.jacc.2007.03.027. [DOI] [PubMed] [Google Scholar]