Abstract
Background
Laboratory and cross-sectional human studies suggest a potential role for prolactin in the pathogenesis of hypertension; however, the prospective association between prolactin and hypertension has not been previously reported.
Methods
We prospectively examined the association between day-time plasma prolactin levels and the risk of incident hypertension among 874 postmenopausal women who were participants of the Nurses' Health Study. Cox proportional hazards regression models were used to adjust for potential confounders.
Results
We identified 183 incident cases of hypertension during 8-years of follow-up. After adjusting for potential confounders, the relative risk of hypertension for a 1-standard deviation higher plasma prolactin was 1.31 (95% confidence interval 1.05-1.62). Compared to women with plasma prolactin levels ≤ 8.0 ng/mL, the multivariable relative risk of incident hypertension for those with prolactin levels > 8.0 ng/mL was 1.34 (95% CI 0.90-1.99).
Conclusions
A higher day-time plasma prolactin level is independently associated with an increased risk of incident hypertension among post-menopausal women.
Introduction
Hypertension is the most important cardiovascular risk factor among both men and women, contributing to one half of the coronary heart disease and approximately two thirds of the cerebrovascular disease burdens1. As a whole, men have higher blood pressure (BP) than women through middle age2. However, the prevalence of hypertension in women increases after the average age of menopause2. By 60 to 69 years of age, the prevalence of hypertension is higher in women than in men2.
Prolactin, a polypeptide hormone released by the anterior pituitary gland, is best-known for its involvement with lactation and reproduction. Recent in vitro and animal experiments indicate that prolactin has other physiological effects, including vasoconstriction3, reduction of nitric oxide production3, stimulation of leukocyte adhesion via induction of intracellular adhesion molecule-14, and proliferation of vascular smooth muscle cells5. These effects are all potentially related to the pathogenesis of hypertension. Over 30 years ago, it was reported that prolactin levels were up to four times higher in patients with essential hypertension than in normotensive controls6. A recent cross-sectional study of 76 postmenopausal women demonstrated that prolactin levels were positively correlated with BP and aortic stiffness independent of traditional risk factors7, and a prolactin level >8.0 ng/mL was 100% sensitive in predicting hypertension7. Therefore, it is possible that prolactin contributes to the development of hypertension in postmenopausal women. However, there are no published prospective studies of prolactin and hypertension risk in postmenopausal women.
Therefore, we prospectively investigated the association between plasma prolactin levels and the risk of incident hypertension among non-hypertensive post-menopausal women from the Nurses' Health Study (NHS).
Methods
Source Population
The NHS cohort was assembled in 1976, when 121 700 female nurses aged 30 to 55 years returned a mailed questionnaire. Participants are followed via biennial questionnaires that gather updated information on health-related behaviors and medical events. From 1989 to 1990, 32 826 consenting women provided blood samples returned with a cold pack by overnight mail; 97% of samples were received within 24 hours of collection. All blood samples were stored in liquid nitrogen (-130 °C or less) until laboratory analysis. This study was approved by the institutional review board at Brigham and Women's Hospital. Receipt of each questionnaire implied participant's consent.
Study Population
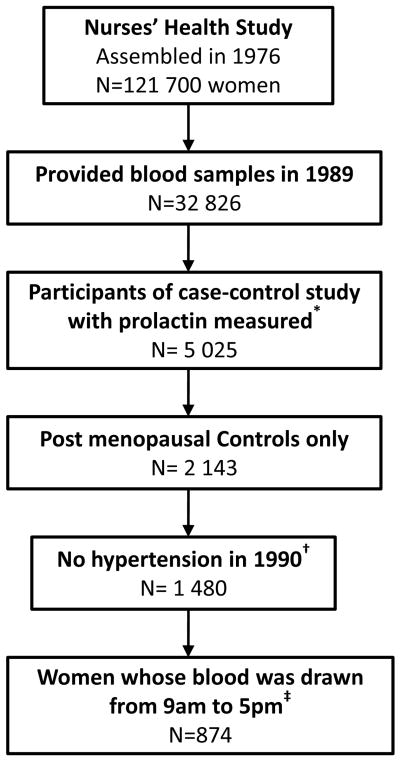
Prolactin was measured in a subset of NHS participants who were selected for a nested case-control study designed to examine breast cancer. This study has been described in detail elsewhere8. To minimize potential bias, cases (women who developed breast cancer between 1990 to June 1998) were excluded from the present study (Figure 1). We limited our analysis to post-menopausal women. We considered a woman to be postmenopausal if (1) her natural menstrual periods had ceased permanently, (2) she had a bilateral oophorectomy, or (3) she had a hysterectomy with at least one ovary remaining and was 56 years or older (nonsmokers) or 54 years or older (smokers). From the remaining participants, we excluded individuals with prevalent hypertension at the time of blood collection so that our study would be prospective. Participants were also excluded if they reported use of antihypertensive medication, or had a history of cancer at baseline (except for non-melanoma skin cancer).
Figure 1.
Study population for analysis of plasma prolactin levels and risk of incident hypertension.
* Nested case-control study of breast cancer, which is described elsewhere8.
† Participants were also excluded if they reported use of antihypertensive medication, or had a history of cancer at baseline (except for non-melanoma skin cancer).
‡ Women with prolactin levels <0.6 ng/mL (n=1) or ≥ 50.0 ng/mL (n=10) were excluded from analyses.
Assessment of plasma prolactin level
Prolactin was measured by microparticle enzyme immunoassay. In the original case-control study, about 20% of the samples were assayed in three initial batches at the University of Massachusetts Medical Center (Boston, MA) using the IMx System (Abbott Laboratory, Abbott Park, IL). The remaining samples (80%) were assayed in five batches at the Reproductive Endocrinology Unit Laboratory at the Massachusetts General Hospital (Boston, MA), using the AxSYM Immunoassay system (Abbott Diagnostics, Chicago, IL). Although there was some batch-to-batch variation in prolactin values across data sets, the correlation between the two laboratories was 0.91, and across different batches within the same data set was 0.95. The intra-assay coefficient of variation ranged from 5.4% to 9.3%. The detection limit for both laboratories was 0.6 ng/mL; one sample had a value below this limit and was excluded from analysis.
Assessment of Other Covariates
BMI (body mass index, calculated as weight in kilograms divided by height in meters squared), physical activity (metabolic equivalent tasks), systolic BP, diastolic BP, smoking status, and use of postmenopausal hormones were ascertained by questionnaire at baseline. Intakes of alcohol, sodium and folate were ascertained from a food frequency questionnaire in 1990. Except for intake of alcohol, nutrient values were adjusted for total energy intake by the residual method 9. The reproducibility and validity of the questionnaire has been documented10. Details about the blood collection time and fasting status at the time of blood collection were reported on the questionnaire receieved with the blood sample.
Plasma sex steroid hormone levels were measured in a subset of these participants. Hormone assay methods have been described previously in detail11. In brief, analyses were performed at Quest Diagnostic's Nichols Institute (San Juan Capistranco, CA), by sensitive and specific radio-immunoassays following organic solvent extraction and Celite column partition chromatography. With-in batch laboratory coefficients of variation ranged from 6.8% (estrone) to 8.7% (testosterone).
Assessment of Hypertension
The baseline and biennial follow-up questionnaires asked participants to report whether a clinician had made a new diagnosis of hypertension during the preceding 2 years. Self-reported hypertension was previously validated in NHS12. In a subset of women who reported hypertension, medical record review confirmed a documented systolic and diastolic BP, respectively, higher than 140 mmHg and 90 mmHg in 100% and higher than 160 mmHg and 95 mmHg in 77% of participants12. Additionally, self-reported hypertension was predictive of subsequent cardiovascular events12. A participant was considered to have prevalent hypertension if she reported this diagnosis on any questionnaire up to and including the 1990 questionnaire, and therefore was excluded from the present study. Incident cases included individuals who first reported hypertension on questionnaires after 1990.
Statistical Analyses
Women with prolactin level ≥ 50.0 ng/mL (n=10) were excluded from analyses, since it is considered the threshold for “moderate prolactin excess”. Only women whose blood was drawn between 9am and 5pm were included in the primary analyses (Figure 1), because previous studies13, 14 and our own data show minimal fluctuation of plasma prolactin levels for post-menopausal women during these hours.
Person-time was truncated at the date of hypertension diagnosis, at the date of death, at the date of cancer diagnosis (except for non-melanoma skin cancer), or the end of follow-up, whichever came first.
Linear regression models were used to evaluate the relation between baseline characteristics and plasma prolactin level (after logarithmic transformation). The age-adjusted β coefficients were reported. Furthermore, the baseline variables were compared between those with plasma prolactin ≤ 8.0 ng/mL and >8.0 ng/mL7 using t test or Wilcoxon rank-sum test for continuous variables, and Fisher exact test for binary variables.
The association between prolactin and hypertension through 8 years of follow-up was analyzed using Cox proportional hazards regression models to estimate relative risks (RRs) and 95% confidence intervals (CIs). In our primary analysis, we evaluated the association between a 1- standard deviation (SD) increase in prolactin level (after logarithmic transformation) and the risk of incident hypertension. As a secondary analysis, we also examined prolactin as a dichotomous variable (≤ 8.0 ng/mL [as reference] vs. >8.0 ng/mL), as suggested by the recent cross-sectional paper7. Multivariable models were constructed to adjust for potential confounding variables that have either previously been associated with incident hypertension or else associated with prolactin level, including: age (continuous), BMI (6 categories), physical activity (quintiles), family history of hypertension (yes/no), current smoking (cigarettes/day), use of postmenopausal hormones (yes/no), fasting status (< 8 hours vs. ≥ 8 hours since last meal), laboratory batch number, and intakes of alcohol (continuous), folate (continuous) and sodium (continuous).
We also investigated whether the association between prolactin level and hypertension varied according to age (<60 yrs or ≥60 yrs, the median age at baseline) and postmenopausal hormone use. Stratified multivariable analyses were performed, and appropriate interaction terms were generated to test whether interactions were statistically significant.
We used partial Spearman correlations to investigate the association between plasma prolactin and other sex hormones.
We examined the prolactin level from women whose blood was drawn during the night (6pm to 8am) in a separate analysis because night time prolactin levels fluctuate to a great degree13. Cox proportional hazards regression models were constructed to investigate the association between prolactin and hypertension using the same strategy as the day time prolactin analysis.
All P values are 2-tailed. Statistical tests were performed using SAS version 9.1 for UNIX statistical software package (SAS Institute Inc, Gary, NC).
Results
During the 8 years (6 192 person-years) of follow-up in 874 postmenopausal women, 183 incident cases of physician-diagnosed hypertension were reported. The median prolactin level was 8.7 ng/mL (inter-quartile range 6.7-11.4 ng/mL). Participant characteristics are presented in Table 1. Age was significantly inversely associated with plasma prolactin levels. Compared to women with prolactin levels ≤ 8.0 ng/mL, participants whose prolactin was > 8.0 ng/mL were younger and had lower BMI.
Table 1.
Baseline characteristics of the study population*.
| Variables | β coefficient† | P value | Plasma prolactin ≤ 8.0 ng/mL | Plasma prolactin > 8.0 ng/mL | P value |
|---|---|---|---|---|---|
| Number of participants | --- | --- | 373 | 501 | --- |
| Age (years) | -0.010 | <0.001 | 60 (56-64) | 58 (54-63) | <0.001 |
| Body mass index (kg/m2) | -0.007 | 0.06 | 24.2 (22.0-27.4) | 23.8 (21.6-26.6) | 0.007 |
| Alcohol consumption (g/d) | -0.0003 | 0.85 | 1.5 (0-6.7) | 1.8 (0-6.9) | 0.34 |
| Physical activity (METs/w) | 0.001 | 0.11 | 10.4 (4.0-22.2) | 10.2 (3.4-23.0) | 0.64 |
| Family history of hypertension | -0.006 | 0.84 | 40.2% | 41.3% | 0.74 |
| Current smoker | -0.109 | 0.01 | 14.5% | 11.6% | 0.20 |
| Female hormone user | 0.045 | 0.16 | 30.0% | 34.7% | 0.14 |
| Dietary factors (per day) | |||||
| Folate (mg) | 0.053 | 0.40 | 0.36 (0.28-0.60) | 0.38 (0.29-0.62) | 0.20 |
| Sodium (g) | -0.057 | 0.19 | 1.83 (1.62-2.04) | 1.78 (1.58-2.03) | 0.13 |
| Systolic blood pressure (mmHg) | -0.0008 | 0.56 | 120 (110-130) | 80 (70-80) | 0.09 |
| Diastolic blood pressure (mmHg) | -0.0005 | 0.79 | 120 (110-130) | 80 (70-80) | 0.52 |
| Plasma proclactin (ng/mL) | --- | --- | 6.3 (5.4-7.2) | 10.9 (9.3-13.7) | <0.001 |
Data are presented as median (inter-quartile range) or percentages; MET, metabolic equivalent.
Except for the β coefficient of age, all β coefficients were age-adjusted.
Plasma prolactin level was positively associated with the risk of incident hypertension in age-adjusted and multivariable-adjusted analyses (Table 2). After adjusting for age and BMI, a 1-SD higher prolactin level was associated with a 33% increased risk of developing hypertension (P=0.004). The multivariable RR of a 1-SD higher prolactin level was 1.31 (95% CI 1.05-1.62, P=0.01). Compared to women with plasma prolactin levels ≤ 8.0 ng/mL, the multivariable RR of incident hypertension for those whose prolactin levels were > 8.0 ng/mL was 1.34 (95% CI 0.90-1.99, P=0.15).
Table 2.
Plasma prolactin level and risk of incident hypertension.
| Hazards Ratio (95% CI) | P value | |
|---|---|---|
| Plasma prolactin level* (1-SD increase) | ||
| Age-adjusted | 1.32 (1.09-1.58) | 0.004 |
| Age- and BMI- adjusted | 1.33 (1.10-1.60) | 0.004 |
| Model a† | 1.31 (1.05-1.62) | 0.01 |
| Plasma prolactin level > 8.0 ng/mL vs. ≤ 8.0 ng/mL | ||
| Age-adjusted | 1.47 (1.03-2.08) | 0.03 |
| Age- and BMI- adjusted | 1.49 (1.04-2.13) | 0.03 |
| Model a | 1.34 (0.90-1.99) | 0.15 |
Plasma prolactin levels were logarithmic transformed.
Model a was adjusted for age, body mass index, physical activity, family history of hypertension, current smoking, female hormone use, fasting status, laboratory batch number, and intakes of alcohol, folate and sodium.
SD: standard deviation; CI, confidence interval; BMI, body mass index.
We did not observe effect modification by age (P value for interaction = 0.37) or by postmenopausal hormones use (P value for interaction = 0.34).
Among these 874 participants, the number who also had plasma estrone, estradiol, testosterone and androstenedione measured was 248, 529, 486 and 238, respectively. The age- and BMI- adjusted Spearman correlation coefficients between plasma estrone, estradiol, testosterone, androstenedione and prolactin levels were all less than 0.1.
Among the 1 480 postmenopausal women without hypertension at baseline (Figure 1), there were 606 women whose blood was drawn during the night (from 6pm to 8am). The median prolactin level was 10.9 ng/mL (inter-quartile range 8.1-15.1 ng/mL). During the 8 years (4 250 person-years) of follow-up, 135 incident cases of physician-diagnosed hypertension were reported. The multivariable RR for a 1-SD higher prolactin level was 1.01 (95% CI 0.76-1.34).
Discussion
We present the first prospective study to examine the relation between plasma prolactin level and the risk of incident hypertension. We found that a higher day-time plasma prolactin level was independently associated with the risk of incident hypertension among postmenopausal women.
A possible role for prolactin in the pathogenesis of hypertension has been suggested by previous experimental studies. First, intravenous infusion of prolactin leads to widespread vasoconstriction3 and increased arterial pressure in decerebrate rabbits15. The mechanisms of this vascular response were shown to involve the inhibition of a tonic vasodialtory β2-adrenergic-receptor-mediated effect related to the intracellular nitric oxide pathway3. Enhanced pressor response to angiotensin-II was also observed in hyperprolactinemic humans, perhaps secondary to prolactin-induced up-regulation of adrenal and vascular angiotensin-II receptors16. Second, vascular wall inflammation plays a key role in the pathogenesis of hypertension17. Prolactin, which is also secreted by lymphocytes, has stimulatory effects on the immune system18. Prolactin induces the expression of intracellular adhesion molecule-1 and is associated with the local accumulation of monocytes and macrophages4. It has been demonstrated that treatment of human peripheral blood mononuclear cells with prolactin stimulates their adhesion to endothelial cells via chemokine receptors and tyrosine phosporylation signaling pathways19. Finally, experiments indicate that prolactin may induce proliferation of vascular smooth muscle cells through the protein kinase C pathway5, which in turn may be involved in the pathogenesis of hypertension and atherosclerosis.
It has long been proposed that prolactin might play an important role in the pathogenesis of preeclampsia20, and urinary prolactin level was closely associated with the severity of preeclampsia21. Over 30 years ago, it was reported that prolactin level was up to four times higher in patients with essential hypertension than in normotensive controls6. In a recent cross-sectional study of 76 postmenopausal women, plasma prolactin level was found to be correlated with systolic and diastolic BP (correlation coefficients of 0.33 and 0.27, respectively); these associations were independent of age and BMI7. Prolactin level > 8.0 ng/mL had a sensitivity of 100% and a specificity of 71% in predicting hypertension (systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90mmHg)7. However, the cross-sectional design of that study limits inferences about causality. Furthermore, use of antihypertensive medication was not considered in that study. It is known that several antihypertensive medications (such as verapamil, methyldopa and reserpine) may cause elevation of plasma prolactin level18, introducing the possibility of reverse causation. Results from the present study provide the first prospective evidence for the association between plasma prolactin and the risk of incident hypertension in a linear pattern. Our secondary analysis indicated that after controlling for potential confounders, plasma prolactin level > 8.0 ng/mL was not a statistically significant predicator of incident hypertension. The possible explanation may be that dichotomization results in loss of information and consequently reduced statistical power, or using 8.0 ng/mL as cut-off may not be appropriate.
Changes in sex hormone levels with onset of menopause, such as decreased estradiol and increased testosterone, have been implicated in the increased incidence of hypertension in postmenopausal women22. In our study, the highest correlation was between estradiol and prolactin and the correlation was weak (correlation coefficient=0.09). The lack of substantial association between prolactin and other sex hormones is consistent with observations from previous studies23, and suggests that other sex hormones were unlikely to be strong confounders of our observed association between prolactin and risk of hypertension.
Prolactin is secreted in a pulsatile fashion during the night13. The resulting fluctuation of prolactin levels at night may explain why we did not detect an association between prolactin and risk of hypertension among the 606 women with night time blood collections. In postmenopausal women, prolactin levels are relatively stable between 9am to 5pm13, and for this reason, previous studies of prolactin have analyzed daytime levels; therefore, we also chose this time period for our principal analysis.
Our study has limitations that deserve mention. First, our study population was derived from a pre-existing case-control study of breast cancer. Although we only included controls to avoid bias, the generalizability of the results from the present study might be limited. As our study was entirely female and mostly white, the results may not be generalizable to non-whites or men. Second, blood pressure was not directly measured and hypertension was self-reported. However, all of the participants were registered nurses, and hypertension reporting by these nurses is highly accurate12. Third, we only had a single measurement of plasma prolactin. Although prolactin level may vary over 8 years, this type of misclassification would tend to bias our study toward not finding an association; therefore, it is possible that we underestimated the true association. Fourth, we lacked information on renal function. Elevated prolactin level has been observed among patients with advanced renal dysfunction24. Yet, since all women in our study were free from hypertension at baseline, it is unlikely that many women had impaired renal function. Indeed, other studies have indicated a very low prevalence of renal dysfunction in this cohort 25-27. Finally, since our study was observational, the possibility of residual confounding by some unmeasured covariate exists.
In conclusion, our prospective analysis suggests that higher day-time plasma prolactin level is independently associated with an increased risk of incident hypertension among post-menopausal women. The effects of a higher prolactin level may constitute a novel mechanism of hypertension in postmenopausal women, and may become a new target for treatment. However, whether prolactin > 8.0 ng/mL is a proper cut-off for predicting future risk of incident hypertension needs to be further investigated. Our findings should be tested in other cohorts to further evaluate this hypothesis.
Acknowledgments
Funding Sources: This study was funded by the American Heart Association grant 0535401T, NIH grants CA87969 and HL079929, and the Beijing NOVA program from the Beijing Municipal Science and Technology Commission. Additionally this work has been made possible through the International Society of Nephrology-funded Fellowship (LZh).
Footnotes
Disclosures: None
References
- 1.Whitworth JA. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 3.Molinari C, Grossini E, Mary DA, Uberti F, Ghigo E, Ribichini F, Surico N, Vacca G. Prolactin induces regional vasoconstriction through the beta2-adrenergic and nitric oxide mechanisms. Endocrinology. 2007;148:4080–4090. doi: 10.1210/en.2006-1577. [DOI] [PubMed] [Google Scholar]
- 4.Olson KK, Townson DH. Prolactin-induced expression of intercellular adhesion molecule-1 and the accumulation of monocytes/macrophages during regression of the rat corpus luteum. Biol Reprod. 2000;62:1571–1578. doi: 10.1095/biolreprod62.6.1571. [DOI] [PubMed] [Google Scholar]
- 5.Sauro MD, Zorn NE. Prolactin induces proliferation of vascular smooth muscle cells through a protein kinase C-dependent mechanism. J Cell Physiol. 1991;148:133–138. doi: 10.1002/jcp.1041480116. [DOI] [PubMed] [Google Scholar]
- 6.Stumpe KO, Kolloch R, Higuchi M, Kruck F, Vetter H. Hyperprolactinaemia and antihypertensive effect of bromocriptine in essential hypertension. Identification of abnormal central dopamine control. Lancet. 1977;2:211–214. doi: 10.1016/s0140-6736(77)92832-x. [DOI] [PubMed] [Google Scholar]
- 7.Georgiopoulos GA, Stamatelopoulos KS, Lambrinoudaki I, Lykka M, Kyrkou K, Rizos D, Creatsa M, Christodoulakos G, Alevizaki M, Sfikakis PP, Papamichael C. Prolactin and preclinical atherosclerosis in menopausal women with cardiovascular risk factors. Hypertension. 2009;54:98–105. doi: 10.1161/HYPERTENSIONAHA.109.132100. [DOI] [PubMed] [Google Scholar]
- 8.Tworoger SS, Eliassen AH, Sluss P, Hankinson SE. A prospective study of plasma prolactin concentrations and risk of premenopausal and postmenopausal breast cancer. J Clin Oncol. 2007;25:1482–1488. doi: 10.1200/JCO.2006.07.6356. [DOI] [PubMed] [Google Scholar]
- 9.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 10.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 11.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96:1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 12.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 13.Katznelson L, Riskind PN, Saxe VC, Klibanski A. Prolactin pulsatile characteristics in postmenopausal women. J Clin Endocrinol Metab. 1998;83:761–764. doi: 10.1210/jcem.83.3.4675. [DOI] [PubMed] [Google Scholar]
- 14.Kalleinen N, Polo-Kantola P, Irjala K, Porkka-Heiskanen T, Vahlberg T, Virkki A, Polo O. 24-hour serum levels of growth hormone, prolactin, and cortisol in pre- and postmenopausal women: the effect of combined estrogen and progestin treatment. J Clin Endocrinol Metab. 2008;93:1655–1661. doi: 10.1210/jc.2007-2677. [DOI] [PubMed] [Google Scholar]
- 15.Horrobin DF, Manku MS, Burstyn PG. Effect of intravenous prolactin infusion on arterial blood pressure in rabbits. Cardiovasc Res. 1973;7:585–587. doi: 10.1093/cvr/7.5.585. [DOI] [PubMed] [Google Scholar]
- 16.Arafah BM, Gordon NH, Salazar R, Douglas JG. Modulation of tissue responsiveness to angiotensin-II in hyperprolactinemic subjects. J Clin Endocrinol Metab. 1990;71:60–66. doi: 10.1210/jcem-71-1-60. [DOI] [PubMed] [Google Scholar]
- 17.Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15:152–158. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- 18.Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montes de Oca P, Macotela Y, Nava G, Lopez-Barrera F, de la Escalera GM, Clapp C. Prolactin stimulates integrin-mediated adhesion of circulating mononuclear cells to endothelial cells. Lab Invest. 2005;85:633–642. doi: 10.1038/labinvest.3700256. [DOI] [PubMed] [Google Scholar]
- 20.Oney T, Bellmann O, Kaulhausen H. Relationship between serum prolactin concentration, vascular angiotensin sensitivity and arterial blood pressure during third trimester pregnancy. Arch Gynecol Obstet. 1988;243:83–90. doi: 10.1007/BF00932973. [DOI] [PubMed] [Google Scholar]
- 21.Leanos-Miranda A, Marquez-Acosta J, Cardenas-Mondragon GM, Chinolla-Arellano ZL, Rivera-Leanos R, Bermejo-Huerta S, Romero-Arauz JF, Alvarez-Jimenez G, Ramos-Leon JC, Ulloa-Aguirre A. Urinary prolactin as a reliable marker for preeclampsia, its severity, and the occurrence of adverse pregnancy outcomes. J Clin Endocrinol Metab. 2008;93:2492–2499. doi: 10.1210/jc.2008-0305. [DOI] [PubMed] [Google Scholar]
- 22.Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension: an age-old debate. Hypertension. 2008;51:952–959. doi: 10.1161/HYPERTENSIONAHA.107.105742. [DOI] [PubMed] [Google Scholar]
- 23.Greendale GA, Huang MH, Ursin G, Ingles S, Stanczyk F, Crandall C, Laughlin GA, Barrett-Connor E, Karlamangla A. Serum prolactin levels are positively associated with mammographic density in postmenopausal women. Breast Cancer Res Treat. 2007;105:337–346. doi: 10.1007/s10549-006-9454-y. [DOI] [PubMed] [Google Scholar]
- 24.Hou SH, Grossman S, Molitch ME. Hyperprolactinemia in patients with renal insufficiency and chronic renal failure requiring hemodialysis or chronic ambulatory peritoneal dialysis. Am J Kidney Dis. 1985;6:245–249. doi: 10.1016/s0272-6386(85)80181-5. [DOI] [PubMed] [Google Scholar]
- 25.Knight EL, Rimm EB, Pai JK, Rexrode KM, Cannuscio CC, Manson JE, Stampfer MJ, Curhan GC. Kidney dysfunction, inflammation, and coronary events: a prospective study. J Am Soc Nephrol. 2004;15:1897–1903. doi: 10.1097/01.asn.0000128966.55133.69. [DOI] [PubMed] [Google Scholar]
- 26.Knight EL, Stampfer MJ, Rimm EB, Hankinson SE, Curhan GC. Moderate alcohol intake and renal function decline in women: a prospective study. Nephrol Dial Transplant. 2003;18:1549–1554. doi: 10.1093/ndt/gfg228. [DOI] [PubMed] [Google Scholar]
- 27.Curhan GC, Knight EL, Rosner B, Hankinson SE, Stampfer MJ. Lifetime nonnarcotic analgesic use and decline in renal function in women. Arch Intern Med. 2004;164:1519–1524. doi: 10.1001/archinte.164.14.1519. [DOI] [PubMed] [Google Scholar]