Abstract
Human immunodeficiency virus type 1 (HIV-1) primarily infects CD4+ T cells and cells of the monocyte-macrophage lineage, resulting in immunodeficiency in an infected patient. Along with this immune deficiency, HIV-1 has been linked to a number of neurological symptoms in the absence of opportunistic infections or other co-morbidities, suggesting that HIV-1 is able to cross the blood-brain barrier (BBB), enter the central nervous system (CNS), and cause neurocognitive impairment. HIV-1-infected monocyte-macrophages traverse the BBB and enter the CNS throughout the course of HIV-1 disease. Once in the brain, both free virus and virus-infected cells are able to infect neighboring resident microglia and astrocytes and possibly other cell types. HIV-1-infected cells in both the periphery and the CNS give rise to elevated levels of viral proteins, including gp120, Tat, and Nef, and of host inflammatory mediators such as cytokines and chemokines. It has been shown that the viral proteins may act alone or in concert with host cytokines and chemokines, affecting the integrity of the BBB. The pathological end point of these interactions may facilitate a positive feedback loop resulting in increased penetration of HIV into the CNS. It is proposed in this review that the dysregulation of the BBB during and after neuroinvasion is a critical component of the neuropathogenic process and that dysregulation of this protective barrier is caused by a combination of viral and host factors including secreted viral proteins, components of the inflammatory process, the aging process, therapeutics, and drug or alcohol abuse.
Keywords: HIV, blood-brain barrier, Tat, Nef, gp120, drugs of abuse
1. Introduction
Human immunodeficiency virus type-1 (HIV-1) infection is a global health concern, with approximately 33 million individuals currently infected (Joint United Nations Programme on HIV/AIDS (UNAIDS/World Health Organization), 2009). HIV-1 has demonstrated the ability to cause adverse neurological complications within a subpopulation of those infected with the virus. Two classifications of HIV-associated neurocognitive disease (HAND) existed in the early stages of the HIV epidemic: HIV-associated dementia (HAD) and minor cognitive motor disorder. Approximately 15% of individuals infected with HIV-1 developed HAD, and approximately 30% to 60% of individuals developed less severe forms of HAND (Gendelman, 2005; McArthur, 2004). With the introduction of highly active antiretroviral therapy (HAART), survival rates associated with HIV-1 infection have improved (Joint United Nations Programme on HIV/AIDS (UNAIDS/World Health Organization), 2009). Although HAART therapy has increased survival (Graham et al., 1992; Hammer et al., 1997), reduced viral loads (Li et al., 1998), increased CD4 cell counts (Hunt et al., 2003), and reduced opportunistic infections (Chene et al., 1998) (Miller et al., 1999), one outcome has not changed: HAND is still a serious concern (Sacktor et al., 2002). Although the incidence of the most severe form of HAND, HAD, has decreased (McArthur, 2004), the overall prevalence of HAND has not declined, potentially because patients with the more mild forms of HAND, including asymptomatic neurocognitive impairment and mild neurocognitive disorder are living longer with persistent impairment (Cysique et al., 2009; McArthur, 2004; Nath et al., 2008).
Because of the more chronic nature of HIV/AIDS in the HAART era, the disease state is becoming more the result of a chronic inflammatory state induced by the production of both HIV-1 virions and viral proteins in the periphery and in the central nervous system (CNS). The integrity of the BBB is compromised by the actions of the HIV-1 virions, viral proteins, and host cytokines and chemokines from both sides of this barrier. This compromise results in disruption and dysregulation of the normal function of the BBB, allowing more rapid and greater entry of cell-free HIV-1 and HIV-1-infected cells into the CNS through a combination of passage mechanisms. This review discusses the regulation of BBB permeability within the context of HIV-1 disease progression with the goal of providing more clarity with respect to the molecular mechanisms associated with HIV-1 neuroinvasion and neuropathogenesis.
2. Structure and function of the normal BBB
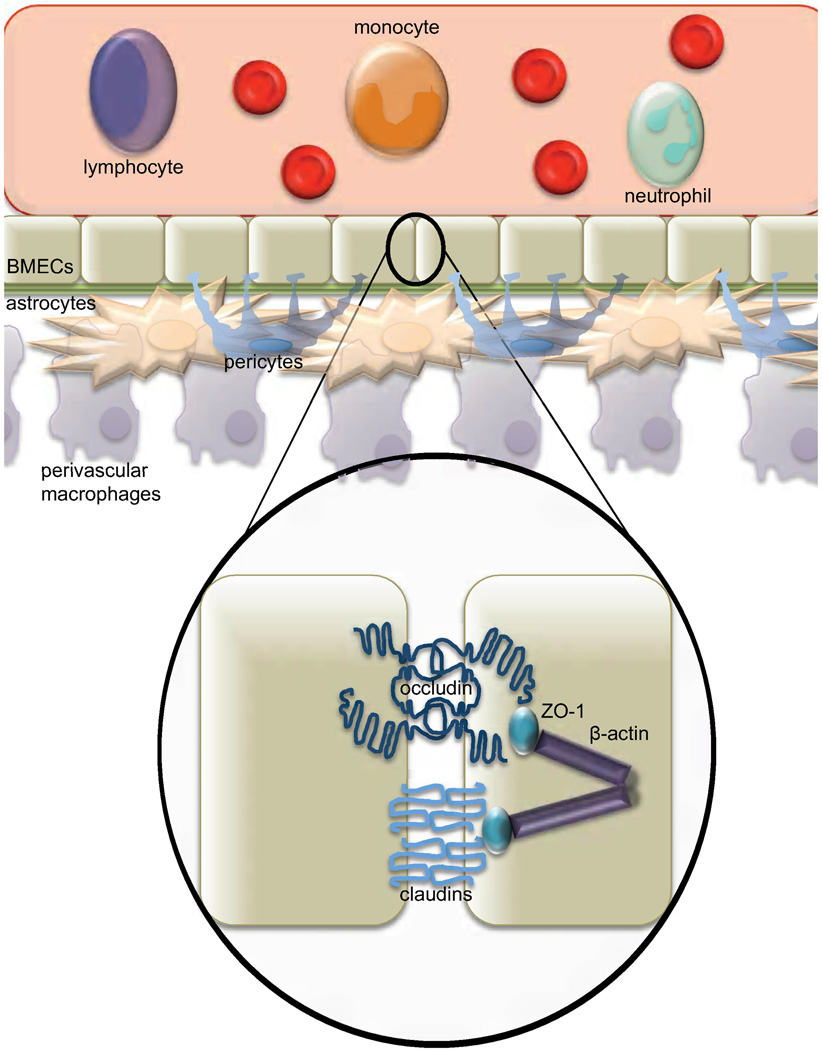
The BBB has been defined as a critically important protective barrier that is involved in providing essential biologic, physiologic, and immunologic separation between the CNS and the periphery. To understand how HIV-1 affects the BBB, one needs first to understand the molecular structure and regulation of its permeability. The barrier endothelium is composed of brain microvascular endothelial cells (BMECs) (Fig. 1), a distinct form of endothelial cell (Ivey et al., 2009). Underlying the BMECs is the basal lamina, followed by other cell types, including astrocytes, pericytes, perivascular macrophages, parenchymal microglia, and neurons, which are all involved in maintaining the microenvironment that preserves the functionality of BMECs (Ivey et al., 2009). Altogether, this neurovascular unit is responsible for the precise homeostasis between peripheral circulation and the CNS (Dore-Duffy et al., 1990). Astrocytes play an essential role in supporting the endothelial cells biochemically, involving physical interactions of astrocytic foot processes that cross the basal lamina and come in contact with BMECs (Ivey et al., 2009). It has been accepted that astrocytes are necessary for endothelial cell differentiation into BMECs. More recently, astrocytes have also been shown to be required for maintaining the BMEC differentiation state (Hamm et al., 2004). In fact, when BMECs were cocultured with astrocytes in vitro, removal of the astrocytes alone was sufficient to cause opening of tight junctions (TJs) and increased permeability across the BMEC monolayer (Hamm et al., 2004). Though astrocytes are sufficient to induce differentiation, pericytes also play a role in BMEC proliferation and survival (Lai and Kuo, 2005). Pericytes are connective tissue cells that interact physically with BMECs and neurons through cellular projections that cross the basal lamina and form gap junctions (Lai and Kuo, 2005). Extracellular communication between pericytes and astrocytes is thought to occur but is not yet well understood. Interestingly, recent studies have shown pericytes to be susceptible to HIV-1 infection in vitro (Nakagawa and Toborek, 2011), though the full implications of this finding are not fully understood. Given that pericytes play an important role in BBB function (for review see (Bonkowski et al., 2011) and that loss of pericytes increases focal permeability (Bonkowski et al., 2011), the impact of HIV-1 viral proteins and direct viral infection of these cells is an important area for future research.
Fig. 1. The physiologically normal blood-brain barrier (BBB).
The BBB is made up of specialized endothelial cells, brain microvascular endothelial cells (BMECs), surrounded by astrocytes and pericytes, which are involved in the creation of the biochemical environment. The BBB functions to separate peripheral circulation from the central nervous system (CNS). The limited permeability of the barrier allows highly regulated transport of cells and other substances into the CNS. It also separates the CNS from peripheral immune surveillance. Circulating monocytes that gain entry into the CNS develop into resident macrophages. The function of the BBB is established by the tight junctions (TJs) between BMECs. TJ proteins include the transmembrane proteins claudin and occludin along with zonula occludens 1 (ZO-1) which connect the transmembrane proteins to the cytoskeleton.
BMECs form an unfenestrated endothelium, which, in combination with intervening TJs and the low number of intracellular vesicles under normal conditions, causes the BBB to have selective permeability to peripheral proteins and other molecules (Lossinsky and Shivers, 2004; Lv et al., 2010; Stamatovic et al., 2008). Unlike other TJs, the TJs of the BBB provide high transendothelial electrical resistance (TEER), further limiting pass-through of many types of macromolecules (Butt et al., 1990). Therefore, to regulate the molecules able to pass this barrier, multiple transporters are expressed on BMECs including the blood-to-brain influx transport system, the brain-to-blood efflux transport system, and efflux pumps located on the luminal membrane of BMECs, thereby creating a precisely regulated system of permeability (Ueno et al., 2010). Through facilitated transport, secondary active transport, saturable transport systems (Elali and Hermann, 2011), or adsorptive transcytosis (Herve et al., 2008), molecules from peripheral circulation can gain entry into BMECs, including the HIV-1 proteins Tat and gp120 (Banks et al., 1997; Banks et al., 2005) as discussed in more detail below. Despite this fact, small lipophilic molecules that are able to diffuse across the plasma membrane of BMECs may be substrates or partial substrates for primary active or secondary active efflux transporters and, therefore, may not enter into or remain in the CNS, making the BBB not completely impermeable, but highly selective in permeability (Ohtsuki and Terasaki, 2007). Therefore, size and lipophilicity of a molecule alone are not enough to guarantee passage into, or even exclusion from, the CNS. Disruption of these precisely regulated processes controlling BBB permeability by factors including HIV-1 and its proteins and drugs of abuse has an important impact on HIV-1 neuropathogenesis. The majority of studies addressing this process have implicated TJ disruption in HIV-1-mediated increased BBB permeability; for this reason, this review centers around this topic. Investigation into the dysregulation of influx and efflux mechanisms throughout HIV-1 pathogenesis is an important area of future research because current HAART regimens do not quench circulating levels of viral proteins and proinflammatory cytokine surges are common throughout the course of HIV-1 disease pathogenesis in the HAART era.
TJs are made up of multiple proteins including the membrane proteins claudin, occludin, and junctional adhesion molecules (Fig. 1). Claudins are tetraspan membrane proteins that are important for the structure and function of TJs (Lal-Nag and Morin, 2009). The extracellular loops of claudins on adjacent BMECs interact, acting as a sealant for the endothelium (Lal-Nag and Morin, 2009). Although the importance of claudins has been shown through numerous mutational analyses, the exact mechanism of claudin regulation is not known. It has been shown, however, that under certain conditions claudins can be localized to the cytoplasm, which is thought to involve phosphorylation (Lal-Nag and Morin, 2009). Claudins have also been shown to be downregulated at a transcriptional and post-transcriptional level by growth factors and cytokines in cells other than BMECs (Lal-Nag and Morin, 2009). Studies concerning occludin regulation have focused mainly on tissue-specific analyses of dysregulation. However, phosphorylation of occludin can occur at multiple sites, and the level of phosphorylation has been shown to affect the localization of the protein (Dorfel et al., 2009). The complete functional role of this phosphorylation is not yet understood, but phosphorylation at the C-terminus of either protein has been shown to disrupt interactions with accessory proteins. Interestingly, Rho kinase has been shown to directly phosphorylate the C-termini of claudin-5 and occludin preferentially in autopsied brain tissue samples from patients diagnosed with HIV encephalitis (HIVE) (Yamamoto et al., 2008). The mechanistic relationship between Rho kinase and HIVE is not yet fully understood, but it is thought that the link is in the generation of reactive oxygen species (ROS) (Yamamoto et al., 2008), which are discussed below. Therefore, it is important to identify any additional kinases that may play a role during the course of HIVE.
Accessory proteins, such as zonula occludens (ZO)-1 and ZO-2, are other key components of TJs that link other TJ proteins to the actin cytoskeleton (Fig. 1). The link to the actin cytoskeleton, particularly through ZO-1, is essential for TJ formation. This fact was demonstrated through studies that knocked down ZO-1 with siRNA, thereby demonstrating defects in barrier permeability to large compounds; however, these defects were not observed with knockdown of ZO-2 (Van Itallie et al., 2009). This observation demonstrates that ZO-1 is necessary for both establishment and maintenance of TJs. The dysregulation of TJ proteins is a direct mediator of increased BBB permeability and is important in the context of HIV-1 infection because levels and localization of these proteins have been disrupted in multiple studies of autopsied brain tissue samples derived from patients who died of HIVE. The regulatory pathways linking HIV-1 pathogenesis and the functional properties of TJ proteins have not been fully characterized and are an important area of ongoing research. Proposed mechanisms associated with the dysregulation of the pathways involved in this important pathogenic process are also explored in this review.
3. HIV-1 infection and monocytic chemotaxis across the BBB
The current model of CNS infection centers around infected circulating monocytes crossing the BBB, carrying virus into the CNS early in infection, and continuing this process throughout infection, particularly during late-stage disease. These changes can lead to an enhancement of an activated monocyte subset that more readily invades tissues as evidenced from increased numbers of monocytes and brain macrophages, without increased proliferation of these populations, in the CNS of patients with HIVE (Fischer-Smith et al., 2004; Glass et al., 1995). In addition, infected monocytes cross the BBB more readily than uninfected monocytes (Persidsky et al., 1999). Infected monocytes that have crossed into the CNS can then repopulate the resident macrophages. HIV-1 infection of macrophages is thought to be a more productive infection than that in monocytes. Normally, the repopulation of resident macrophages by monocytes from the periphery is a precisely regulated process; however, the rate of this repopulation process can be enhanced by inflammatory conditions (Persidsky et al., 1999). Additionally, normal repopulation does not disturb the BBB; however, once the BBB is disrupted, the precise regulation of the process is lost.
These circulating monocytes can be divided into two subsets based on expression of CD14 and CD16, with the CD14hi/CD16− monocytes being the most prevalent and CD14low/CD16+ comprising 5% to 15% of circulating monocytes in a healthy individual (Thieblemont et al., 1995). This CD14low/CD16+ population is larger in patients infected with HIV-1, can expand to 40% of the monocyte population in the peripheral blood of patients with acquired immunodeficiency syndrome (AIDS) (Thieblemont et al., 1995), and correlates with elevated viremia and occurrence of HIVE (Fischer-Smith et al., 2001; Pulliam et al., 1997; Thieblemont et al., 1995; Williams et al., 2002; Williams et al., 2005; Williams et al., 2001; Williams and Hickey, 2002). Therefore, expansion of the CD14low/CD16+ subset is predictive of rapid disease progression and, likely, CNS involvement (Burdo et al., 2010). This finding remains relevant in the era of HAART because monocytic subset expansion may precede the development of early neurocognitive deficits. The expanded population of CD14low/CD16+ monocytes in this population of individuals infected with HIV-1 has an altered marker expression profile, having been shown to express MAX.1, p150,95 and HLA-DR, which are typically associated with resident tissue macrophages (Thieblemont et al., 1995). Additionally, cytokine production is altered in these cells by constitutive expression of interleukin (IL)-1 and tumor necrosis factor alpha (TNF-alpha) (Thieblemont et al., 1995). The altered marker profile and cytokine expression pattern are particularly interesting because the CD14low/CD16+ monocytes in a healthy individual do not share these traits. The expression of these cytokines and markers also would allow these cells to potentially dysregulate the transport mechanism of the BBB, including diapedesis, discussed below. In addition, CD14low/CD16+ monocytes are more susceptible to HIV-1 infection and to the establishment of productive replication (Ellery et al., 2007). The increased viral replication observed in CD14low/CD16+ monocytes could be a result of the increased cytokine secretion having an autocrine effect, upregulating transcription factors that bind and activate the HIV long terminal repeat. IL-1 is known to activate nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB), which is a known transcriptional regulator of the long terminal repeat. Given the increased level of infection, the monocytes homing to the BBB are likely harboring actively replicating virus. The fact that infection in these cells is productive leads to a double assault on BBB integrity in that these monocytes are likely secreting viral proteins in addition to proinflammatory cytokines.
These circulating monocytes are repopulated by differentiating, proliferating hematopoietic cell populations in the bone marrow. Hematopoietic stem cells residing in the bone marrow are resistant to HIV-1 infection (Weichold et al., 1998). However, hematopoietic progenitor cells, which are multipotent cells differentiated from HSCs, are susceptible (Folks et al., 1988). Monocytic progenitor cells, which are a specific lineage of HPCs, likely become infected as virus and/or virus-infected cells infiltrate the bone marrow (Carter et al., 2010). Virus can enter the bone marrow as cell-associated virus in HIV-1-infected CD4+ T cells and/or dendritic cells (Cavanagh et al., 2005). In addition, these infected monocytes may be involved in the periodic delivery of virus back to the bone marrow during the course of disease. Following infection, monocytic progenitor cells continue to differentiate and proliferate, leaving the bone marrow, entering into circulation, and traveling throughout the body carrying virus to many tissues including the brain (Carter et al., 2010). Acceleration of the myeloid differentiation process, leading to increased monocyte trafficking out of the bone marrow, is associated with increased trafficking into the brain and correlates with early onset of AIDS and severe simian immunodeficiency virus encephalitis in rhesus macaques (Burdo et al., 2010).
It is not known whether the increase in the CD14low/CD16+ monocyte subset is due to increased production of these monocytes in the bone marrow or if the increase in this subset is a result of enhanced differentiation of CD14hi/CD16− monocytes toward the CD14low/CD16+ phenotype, although an acceleration of peripheral monocyte turnover is associated with disease progression in simian immunodeficiency virus-infected macaques (Hasegawa et al., 2009). A recent study of the kinetics of monocyte proliferation suggests that CD14low/CD16+ monocytes differentiate from CD14hi/CD16− monocytes following migration out of the bone marrow or that CD14low/CD16+ monocytes traffick out of the bone marrow at a later time (Burdo et al., 2010). Increased generation of CD14low/CD16+ monocytes in the bone marrow could be caused by HIV-1-induced changes in cytokine production, such as the mentioned increase in IL-1 and TNF-alpha expression (Thieblemont et al., 1995) within the bone marrow that could influence hematopoiesis. This observation may suggest that the phenotypic changes in the population, including altered cytokine secretion, precede expansion.
The increase in circulating CD14low/CD16+ monocytes has been associated specifically with the development of HAND. It is not fully understood why this occurs, but this monocytic population is thought to migrate more effectively to the CNS than the normally predominant CD14hi/CD16− subset of monocytes, as evidenced by increased numbers of CD14low/CD16+ monocytes found in autopsied brain tissue samples from patients with HIVE (Fischer-Smith et al., 2001). Additionally, global gene expression analysis of CD14+ monocytes isolated from HIV-1-infected individuals with high viral loads indicated a significant increase in monocytes expressing CD16, CCR5, monocyte chemoattractant protein (MCP)-1, and sialoadhesin, monocyte markers associated with migration and chronic inflammation (Pulliam et al., 2004). This observation is important because the interaction between circulating immune cells and BMECs mediated through cytokines, chemokines, and adhesion molecules plays an important role in regulating diapedesis under both normal and inflammatory conditions. It is currently accepted that immune cells use both paracellular and transcellular diapedesis mechanisms to cross the BBB (Carman and Springer, 2004; Carman, 2009; Engelhardt and Wolburg, 2004), and it has been proposed that it is the carefully balanced regulation of these two processes that determines the mechanism of entry. Paracellular diapedesis is regulated through the interaction of cell surface molecules on leukocytes and BMECs and is enhanced through reciprocal activation of these cell populations that upregulate these surface molecules (Ley et al., 2007; Nourshargh et al., 2010). Immune surveillance of the CNS under normal, noninflammatory conditions is greatly reduced compared to that in other tissues, such as lung and spleen, owing to the limited expression of cell adhesion molecules (CAMs) on BMECs (Carrithers et al., 2000; Raine et al., 1990). Passage is mediated by the activation of intracellular pathways within BMECs that transiently upregulate surface expression of CAMs (Alvarez et al., 2011; Bo et al., 1996; Cayrol et al., 2008; Sobel et al., 1990; Steffen et al., 1994). The activation of BMECs can be a result of activated leukocyte binding or in response to leukocyte-secreted activating cytokines, including IL-17, TNF-alpha, interferon-gamma, IL-22, and IL-1beta (Alvarez et al., 2011; Bo et al., 1996; Cayrol et al., 2008; Sobel et al., 1990; Steffen et al., 1994). Therefore, the extent of diapedesis is greatly influenced by the activation state of both leukocytes and BMECs, and the increased recruitment of CD14+ monocytes expressing high levels of CD16, CCR5, MCP-1, and sialoadhesin (Pulliam et al., 2004) in individuals infected with HIV will lead to enhanced BMEC activation, a possible explanation for increased numbers of CD14low/CD16+ monocytes transmigrating into the CNS (Fischer-Smith et al., 2001). After initial interaction of activated leukocytes with BMECs, intracellular signaling cascades are triggered within the BMECs that relay back to the engaged leukocytes, causing changes in surface adhesion molecules that allow firm adhesion at high-avidity patches along the endothelium (Carman and Springer, 2003) and control the slow-crawling movement of the leukocytes that delivers the cells to these patches (Phillipson et al., 2006; Schenkel et al., 2004). The integrity of TJs is thought to influence the efficiency of this process because T cells crawl over shorter distances along in vitro cultured BMECs with disrupted TJs compared to BMEC monolayers with greater barrier function (Steiner et al., 2011).
The BBB can also be activated through stimulation of BMECs by cytokines or other factors not originating from leukocytes; in this situation, activated BMECs can in turn activate circulating leukocytes. Chemokines upregulated on the surface of BMECs will interact with receptors on circulating leukocytes, triggering integrin activation and strengthening leukocyte adhesion (Ley et al., 2007). Expression levels of CXCL12 on BMECs and its cognate receptor CXCR4 on circulating leukocytes mediate activation of infiltrating cells, as demonstrated in models of inflammatory disease (Holman et al., 2011; Krumbholz et al., 2006; McCandless et al., 2006). This evidence has important implications for HIV due to the role CXCR4 plays as a coreceptor in viral entry. Therefore, alterations in CXCR4 levels by virus or viral proteins can influence the activation state of the BBB and the overall regulation of diapedesis.
Cellular transmigration requires precisely regulated reversible disengagement of TJs; intercellular CAM-1 (ICAM-1) has been well characterized to mediate TJ changes during lymphocyte migration into the CNS (extensively reviewed in (Greenwood et al., 2011). It is thought that individual CAMs play different roles in mediating diapedesis of specific leukocyte populations, with vascular CAM-1 (VCAM-1) and platelet endothelial CAM-1 playing central roles in monocyte migration into the CNS (Floris et al., 2002; Graesser et al., 2002; Muller et al., 1993). These CAMs also play important roles in returning BMECs to an inactivated state following stimulation (Couty et al., 2007; Graesser et al., 2002; Muller et al., 1993; Newman et al., 2001; Newman and Newman, 2003). Therefore, interruption of the inactivation mechanisms is an alternative explanation for the increased migration observed in HIV-infected individuals. Understanding the intricacies of regulation of transmigration across the BBB, mechanisms of both activation and inactivation, sheds light on the numerous targets for viral proteins to alter the process and disrupt peripheral-CNS homeostasis.
The constitutive production of proinflammatory cytokines in the HIV disease state is likely to play an important role in dysregulation of these processes because the constitutive production of IL-1 and TNF-alpha at the BBB alters permeability (Farkas et al., 2006; Wong et al., 2004). An experimental knockout of TNF-alpha in mice showed decreased BBB leakage after treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, which is known to cause BBB leakage and increase TNF-alpha expression (Zhao et al., 2007). Cumulatively, these observations point to the possibility that CD14low/CD16+ monocytes are more likely to enter the CNS owing to their enhanced ability to increase passage through the BBB. Another possible explanation for increased migration of this monocytic subset is increased or constitutively high activation and expression levels of adhesion molecules on the monocyte cell surface, causing increased interaction with and activation of BMECs, altering the regulation of normal extravasation; investigation of this possibility is an important area of future research concerning this monocytic subset.
4. HIV-1 proteins in peripheral circulation alter the integrity of the BBB
Changes in BBB permeability during HIV-1 infection are likely the result of multiple intra- and intercellular events involving multiple cell types as well as both viral and host proteins. In addition to a change in cytokine expression profiles, an actively infected cell will also produce virions and secrete viral proteins. During acute HIV-1 infection, the BBB becomes activated; however, the cause of this activation remains an unanswered question (MacLean et al., 2004). More recently, it was shown using the simian immunodeficiency virus macaque model that the presence of virions at the BBB is not necessarily sufficient for activation; however, virus-infected mononuclear cells were consistently able to induce activation (MacLean et al., 2004), which suggested that an element secreted from infected cells is necessary for the effects on the BBB. Many studies have been performed to investigate the affects of viral proteins such as Tat, Nef, and gp120 on the BBB, and evidence collectively points to these proteins as necessary mediators in causing breakdown of the BBB. Although the roles of these viral proteins in mediating neural degeneration have been well characterized (for review see (Masliah et al., 1996), the focus of this review is on their effects on the BBB. Tat and gp120 play roles in altering BBB integrity from the peripheral side as well as the CNS, whereas the role of Nef has been established primarily through its presence in astrocytes after HIV-1 entry into the CNS.
4.1 Tat
The full-length HIV-1 Tat protein is a 101-amino acid protein with residues 1–72 encoded by a first exon and residues 73–101 encoded by a second exon (Jeang et al., 1999). It should be noted that, although an 86-amino acid form of Tat, which exists for a few laboratory-passaged virus strains (e.g., LAI, HXB2, pNL4–3), has been frequently used and is considered a full-length protein, this version actually represents a truncated protein. In fact, more than 90% of the more than 100 independently characterized HIV-1 Tat proteins maintain the 101- (and not the 86-) amino acid configuration (Myers et al., 1996). However, the functions of cellular uptake (Vives et al., 1997), transactivation (Churcher et al., 1993), neurotoxicity (Sabatier et al., 1991), and immune activation (Buonaguro et al., 1992; Li et al., 2005) are all present within the first 72 amino acids encoded by the first exon. For this reason, many studies use Tat1–72 protein encoded only from exon I or the truncated Tat1–86 protein rather than the 101-amino acid full-length protein. However, the functional properties contained within the second Tat exon are being explored in more detail (Jeang et al., 1999; Lopez-Huertas et al., 2010). Most relevant to the discussion posed in this review is the fact that the second exon may contribute to viral infectivity and binding to cell surface integrins (Barillari et al., 1999; Fiorelli et al., 1999; Smith et al., 2003). Two short motifs have been identified in the C-terminus of Tat: the RGD (arg-gly-asp) motif, which is a ligand for several integrins, and the highly conserved ESKKKVE motif, which may be related to optimal HIV-1 replication in vivo (Neuveut et al., 2003; Smith et al., 2003). Although the transactivation domain has been localized to Tat exon I, Tat exon II also plays a role in NF-κB-dependent control of HIV-1 transcription in T cells, enhancing HIV-1 replication in T cells (Mahlknecht et al., 2008). In addition, studies have also shown a requirement for the second coding exon of Tat in the optimal replication of macrophage-tropic HIV-1 (Neuveut et al., 2003). Overall this points to a scenario where exon 2 of Tat could contribute to BBB dysregulation through increased replication in cells in the periphery causing increased levels of inflammatory cytokines, viral proteins, and virus, all of which as described in this review impact the normal function of several mechanisms of the BBB.
The protein can also be secreted from infected cells. Because of its protein transfer domain, it has been shown to be able to cross membranes of uninfected cells, inducing a number of different effects depending on cell type (Ensoli et al., 1993; Magnuson et al., 1995). Tat protein crosses the BBB at a rate comparable to passage of IL-1 and TNF- alpha, possibly through a mechanism of adsorptive endocytosis (Banks et al., 2005). In addition to influx, brain efflux of Tat was also observed, suggesting that Tat produced on either side of the BBB can impact cellular function within the opposite compartment (Banks et al., 2005).
It has been observed that Tat decreased expression of occludin and ZO-1 in the TJs of the BBB (two proteins essential for BBB functional integrity), following Tat injection into the tail veins of C57BL/6 mice to model Tat secreted from circulating infected cells (Pu et al., 2007). The mechanism of the decreased expression occurred through activation of cyclooxygenase (COX)-2, an enzyme induced during inflammation, specifically in response to TNF-alpha (Pu et al., 2007) and known to be elevated in patients infected with HIV-1. The specific role of COX-2 in the mechanism of BBB disruption in response to Tat was shown by treating mice with a protein product of COX-2 activity and observing a decrease in expression of occludin and ZO-1 comparable to that with Tat treatment alone (Pu et al., 2007). Following treatment of the mice with an inhibitor of COX-2, an attenuation in the effects on occludin expression was observed, but ZO-1 protein levels were still decreased (Pu et al., 2007), suggesting a limited role of COX-2 in the mechanism of BBB disruption in response to Tat. These results corresponded with previous results showing that intracerebral injection of Tat caused an inflammatory response in the CNS with an increase in expression of chemokines, proinflammatory cytokines, and adhesion molecules. The observed decreases in occludin and ZO-1 also correlated with observations made in the autopsied brain tissue samples from patients with HIVE (Dallasta et al., 1999), which highlighted the importance of elucidating the mechanism connecting the increase in COX-2 to TJ protein regulation.
Treatment with Tat also induces oxidative stress in a number of cell types, including BMECs (Price et al., 2005; Price et al., 2006; Shiu et al., 2007), which is well known to affect TJs (Plateel et al., 1995). Studies that exposed BMECs to alcohol found that metabolism to ROS increased permeability of the BBB (Buonaguro et al., 1992; Li et al., 2005) with activation of kinases and increased phosphorylation and degradation of multiple TJ proteins, including occludin, claudin-5, and ZO-1 (Haorah et al., 2005). This finding presents the possibility that Tat-induced changes in cellular oxidative processes mediate the disruption of the BBB through a similar mechanism. Evidence for this mediation comes from results demonstrating that Tat activates extracellular-signal-regulated kinase 1/2 and leads to a decrease in ZO-1 mRNA levels in BMECs and that this effect is blocked by addition of antioxidants (Pu et al., 2007). Additional studies are needed to identify the mechanisms that mediate the role of ROS in TJ protein regulation. Given that much is known about the cellular response to oxidative stress, studies will likely focus on narrowing the field to factors influencing TJ proteins. Additionally, the possibility of an additive impact of alcohol and Tat on the functional properties of the BBB in patients infected with HIV-1 who abuse alcohol is an area that merits further investigation.
As indicated earlier, circulating monocytes in patients infected with HIV-1 often constitutively express TNF-alpha, which has been shown to induce endothelial activation, oxidative stress, and the activation of multiple proinflammatory factors, such as p38 mitogen-activated protein kinase, NF-kappaB, COX-2, and prostaglandin E2 (Wang et al., 2008). Further investigation into the mechanisms associated with the impact of TNF-alpha on endothelial cells has revealed the necessity for caveolin-1 (Wang et al., 2008), a scaffolding protein that is a major component of caveolae and necessary for the formation of caveolae in endothelial cells. Caveolae are specialized lipid rafts where many signaling molecules localize, and they are especially abundant on endothelial cells. Proteins involved in proinflammatory signal transduction, such as activators of the extracellular-signal-regulated kinase pathway and inhibitor of kappaB, the upstream regulator of NF-kappaB, and elements of the Ras signaling cascade are localized to caveolae. Tat activates Ras signaling in BMECs and decreases the amount of occludin, ZO-1, and ZO-2 in caveolae (Zhong et al., 2008). The Tat-induced decrease in these TJ proteins was abolished with loss of caveolin-1 (Zhong et al., 2008). Importantly, these studies demonstrated a possible synergy between Tat and host proinflammatory factors while both molecules are circulating throughout HIV-1 infection, with respect to the downregulation of TJ proteins through similar mechanisms ultimately leading to disruption of the BBB.
Tat has been shown to disrupt TJs, increasing the permeability of the barrier; it has also been shown to be able to interact with astrocytes along the barrier. When astrocytes were treated with Tat, matrix metalloproteinase (MMP)-9 mRNA and protein levels increased (Ju et al., 2009). MMPs are zinc-dependent endopeptidases that function in cleavage of extracellular matrix proteins. MMP regulation is achieved through a balance between the level of MMPs and their inhibitors. Increased enzymatic activity of certain MMPs, including MMP-9, leads to disruption of the BBB by degrading components such as laminin and type IV collagen (Yong et al., 1998). The mechanism of upregulation of MMP-9 by Tat is mediated by NF-kappaB and mitogen-activated protein kinase (Ju et al., 2009). TNF-alpha neutralization was also inhibited in astrocytes treated with Tat, and the TNF-alpha accumulation was necessary for the upregulation of MMP-9 (Ju et al., 2009). This finding is particularly important given the previously discussed constitutive expression of TNF-alpha. Taken together, these results suggest that the effect of Tat on astrocytes is through a mechanism similar to that in BMECs. Based on the multiple lines of evidence pointing to Tat activation of these pathways, research studies have continued to define the critical steps involved in these pathways.
Collectively, these results provide an outline of a basic framework of the many ways through which Tat and host factors can lead to an increase in BBB permeability (Fig. 2A). However, the results from these studies have raised the question as to whether the concentration of Tat in circulation is high enough, even in microenvironments, to induce these effects during the course of HIV disease. As indicated above, the migration of HIV-1-infected monoctyes to the BBB increases during the course of HIV disease; additionally, Tat acts as a chemoattractant for monocytes. Based on these observations, it has been proposed that infected monocytes in circulation accumulate at the BBB and secrete Tat, thereby recruiting more monocytes to the site possibly leading to increased Tat secretion if these newly recruited monocytes are also productively infected. The question remaining centers on whether there is sufficient Tat present at the BBB to induce changes in permeability. Most of the previous studies have shown dose-dependent results with increasing concentrations of Tat, including 5, 10, and 25 µg of Tat in 100 µL phosphate-buffered saline (Pu et al., 2005), 2–100 nM (Zhong et al., 2008), or 500 ng/mL (Ju et al., 2009). These results demonstrate that the impact of Tat is concentration dependent, but it remains questionable whether the threshold concentration to achieve a biological affect in vitro is reached in circulation during the course of a natural HIV-1 infection. In this regard, experiments involving the injection of Tat into circulation through the mouse tail vein have not yielded information concerning the concentration of Tat reaching the BBB, although additional evidence has indicated that pathological concentrations of Tat in patients infected with HIV-1 can reach the range of nanograms per milliliter of serum (Xiao et al., 2000).
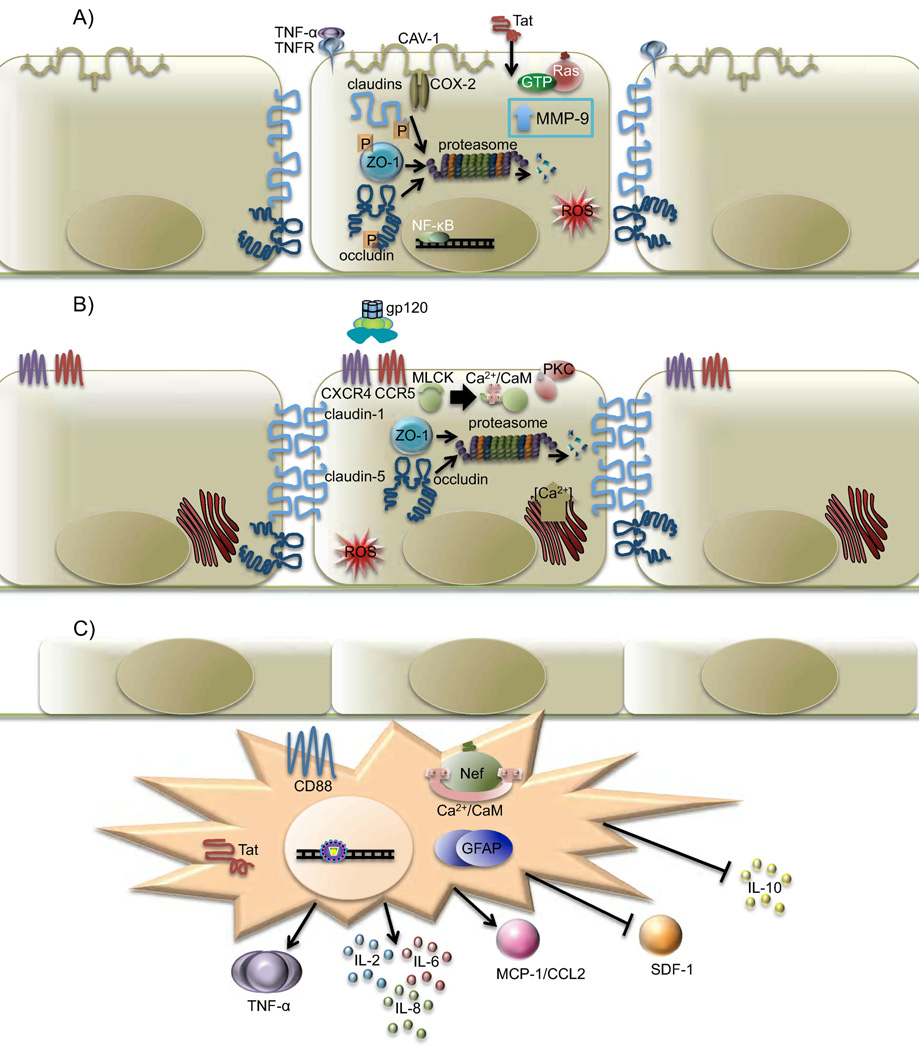
Fig. 2. The effects of HIV proteins on BMECs.
(A) Cellular responses to Tat resulting in increased blood-brain barrier (BBB) permeability. Tat activates NF-kappaB and mitogen-activated protein kinase through an unknown mechanism, thereby causing a decrease in TNF-alpha neutralization. The resulting accumulation of TNF-alpha causes an increase in MMP-9, which can function locally or may be secreted into the circulation. It is also possible that NF-kappaB and mitogen-activated protein kinase activity directly causes the increase in MMP-9 levels, but the mechanism is unknown. Active MMP-9 degrades laminin and type IV collagen, essential components of the BBB. Tat has been observed to increase ROS within the cell, an effect amplified in the presence of TNF-alpha and alcohol metabolism. ROS causes increased phosphorylation of occludin, claudin-5, and ZO-1 and causes a general decrease in tight junction (TJ) mRNA and protein levels. Tat and TNF-alpha lead to the activation of COX-2 at caveolae in the cell membrane, leading to a decrease in protein levels of occludin and ZO-1. Tat also activates Ras through an unknown mechanism requiring CAV-1, leading to a decrease in protein levels of occludin, ZO-1, and ZO-2. Overall, occludin, claudin-5, ZO-1, and ZO-2 are mislocalized in the cytoplasm, where they are likely degraded by the proteasome. (B) As gp120 binds to either CXCR4 or CCR5, changes within the cell lead to TJ dysfunction, a decrease in TEER, and an increase in BBB permeability. A resulting increase in intracellular concentrations of Ca2+ and activation of protein kinase C and myosin light chain kinase have been observed following gp120 binding. Surface levels of ICAM-1 and VCAM-1 increase significantly, leading to an increase in recruitment and transendothelial migration of lymphocytes and monocytes from the peripheral circulation. Although protein levels of claudin-1 and -5 are unaffected by gp120, the proteins ZO-1, ZO-2, and occludin are targeted to the proteasome for degradation. With loss of connection to the cytoskeleton, claudin-1 and -5 are unable to maintain TJ function. An increase in intracellular ROS is also observed as a result of decreased levels of glutathione peroxidase and glutathione reductase following exposure to gp120. (C) HIV-1 infection of astrocytes is characterized by expression of Tat, high levels of Nef, and increased secretion of proinflammatory cytokines TNF-alpha and IL-6. Nef expression is associated with astrocyte activation, as measured by increased CD88 surface expression and elevated glial fibrillary acidic protein. Myristoylated Nef anchored to the cell membrane can interact with calmodulin and has been associated with increased secretion of MCP-1/CCL2, a potent monocyte chemoattractant. MCP-1/CCL2 secretion decreases protein levels of occludin, claudin-5, ZO-1, and ZO-2 at the TJs. An increase in secretion of IL-2 and IL-8 has also been observed, along with a decrease in secretion of stromal cell-derived factor-1 and IL-10.
4.2 gp120
HIV-1 gp120 is localized on the surface of the viral envelope as trimers of heterodimers with gp41. It has been shown that gp120 plays a role in viral entry by binding both the CD4 receptor and CXCR4 or CCR5 coreceptor on the surface of a host cell. Damage to an infected cell or shedding events can lead to the release of gp120 protein into circulation, with subsequent detection in patient serum (Oh et al., 1992). Studies addressing the role of gp120 in BBB breakdown often use systems that mimic free gp120 in circulation. Circulating gp120 is able to cross the BBB through a lectin-like mechanism of inducing adsorptive endocytosis (Banks et al., 1997; Banks et al., 1998). This specific mechanism of passage results in direct access to neurons by gp120, a potent inducer of neurotoxicity, and allows for interaction with BMECs, astrocytes, and glial cells. The implications of these interactions are discussed below.
One model developed to examine the role of circulating gp120 on the BBB generated transgenic mice that expressed and secreted HIV-1 gp120 protein leading to serum levels of gp120 in a range comparable to levels measured in serum from patients infected with HIV-1 (Finco et al., 1997; Oh et al., 1992) to confirm gp120-induced functional impairment of the BBB in vivo (Toneatto et al., 1999). BBB permeability to albumin was significantly greater in brain samples from transgenic mice than from those of nontransgenic mice, evidenced by extravasation of albumin around large and small vessels in transgenic mice brains (Toneatto et al., 1999). ICAM-1 and VCAM-1 are adhesion proteins expressed on the surface of activated endothelial cells and are involved in the recruitment and transendothelial migration of lymphocytes and monocytes in peripheral circulation. Surface expression levels of ICAM-1 and VCAM-1 were significantly higher in transgenic mice brain samples than in those of nontransgenic mice (Toneatto et al., 1999). Although these results demonstrated that the BBB was activated and functionally impaired in gp120 transgenic mice, further studies were performed to confirm that these results were caused by serum levels of gp120 rather than by some intrinsic property of the brain vessel endothelium of transgenic mice. Brain endothelial cells cultured from either gp120 transgenic or nontransgenic brain microvessels were each treated with serum from gp120 transgenic or nontransgenic mice (Cioni and Annunziata, 2002). In brain endothelial cultures from both gp120 transgenic and nontransgenic mice, a significant increase in permeability to albumin was observed following exposure to serum from gp120 transgenic mice but not following exposure to serum from nontransgenic mice (Cioni and Annunziata, 2002). Increased permeabilization was completely blocked when serum was immunosorbed with gp120-specific monoclonal antibody, confirming that it is the gp120 protein in the serum that was required to induce the observed permeabilization (Cioni and Annunziata, 2002). The combination of in vitro and in vivo studies using these gp120 transgenic mice provides convincing evidence that gp120 protein present in serum of patients infected with HIV-1 would likely affect BBB integrity (Fig. 2B), and the possible mechanisms of this breakdown are an important point of future investigation.
In addition to these studies, experiments utilizing cultured human BMECs treated with gp120, from either CCR5- or CXCR4-utilizing viruses, allowed increased monocyte migration through the BMEC monolayer (Kanmogne et al., 2007). The exposure of the BMEC monolayer to CCR5 or CXCR4-utilizing gp120 resulted in a dose-dependent decrease in TEER (Kanmogne et al., 2007). Probing into the mechanism behind this effect revealed a role for myosin light chain kinase in increased permeability and for CCR5 and CXCR4 in decreased TEER, leading to increased cell migration (Kanmogne et al., 2007). Additionally, an increase in intracellular Ca2+ concentration and activation of protein kinase C (suggested that the increase in permeability may be linked to TJ assembly (Kanmogne et al., 2007) (Fig. 2B).
Finding that protein, but not mRNA, levels of ZO-1, ZO-2, and occludin were decreased following gp120 treatment of cultured human BMECs led to the discovery that ZO-1 and ZO-2 are targeted for proteasomal degradation as a result of treatment with gp120 (Kanmogne et al., 2005; Nakamuta et al., 2008). Protein levels of claudin-1 and -5 are unaffected by gp120, but the loss of connection to the cytoskeleton with loss of ZO-1 was enough to significantly decrease TJ function, thereby increasing BBB permeability and decreasing TEER (Kanmogne et al., 2005; Nakamuta et al., 2008). Under normal cellular conditions, ZO-1 has been shown to be linearly localized at the cell surface, along cell-cell borders (Nakamuta et al., 2008). Treatment with gp120, in the presence of a proteasome inhibitor, caused disruption of this characteristic distribution, with diffuse cytoplasmic localization of ZO-1 protein and discontinuous cell surface expression (Nakamuta et al., 2008). Inhibition of the proteasome prevented the decrease in protein levels following gp120 treatment (Nakamuta et al., 2008). Although this provided evidence that gp120 was inducing proteasomal degradation of ZO-1, it did not explain the mechanism of induction. It is possible that the cytoplasmic accumulation of ZO-1 was enough to cause proteasomal targeting; however, it was also possible that, in addition to disrupting localization, gp120 also specifically activated ubiquitination of ZO-1 leading to proteasomal targeting.
Importantly, withdrawal of gp120 from the BMEC monolayer restored near basal levels of TEER and permeability (Kanmogne et al., 2007). This observation was important because it suggested that the BBB was able to recover function if serum levels of gp120 could be controlled. As an extension of this observation, repair following exposure to and withdrawal from other factors found to be high in serum of patients infected with HIV-1, particularly those with neurocognitive symptoms, should be studied. Additionally, assessment of the protein levels of ZO-1 and ZO-2 in BMECs at different times after termination of gp120 exposure would provide insight into the recovery process.
As indicated previously, BMECs and TJs of the BBB are particularly sensitive to changes in redox balance. As a protective measure, BMECs express high levels of active antioxidant enzymes, such as glutathione peroxidase, glutathione reductase, and catalase, under normal physiological conditions (Tayarani et al., 1987). A study using an immortalized endothelial cell line from rat capillaries that were exposed to gp120 indicated the presence of decreased levels of glutathione peroxidase and glutathione reductase following exposure (Price et al., 2005). Significantly decreased levels of reduced glutathione were also observed with both time and dose dependency (Price et al., 2005). Although the relevance of this rat capillary cell line can be questioned, these results correlate with those from a more recent study using human BMECs in a BBB model system. Treatment of this in vitro BBB model with gp120 resulted in a 25% increase in permeability to [3H]-inulin and a 34% increase in permeability to propidium iodide, which has a smaller molecular mass (Shiu et al., 2007). Interestingly, pretreatment with a CCR5-specific antibody reduced this increased permeability significantly, indicating a role for CCR5 in this effect (Shiu et al., 2007). The role of ROS in mediating this increase in permeability was suggested by treating cells with diphenyleneiodonium to block superoxide production and observing a significantly reduced increase in BMEC monolayer permeability induced by gp120 treatment (Shiu et al., 2007). Cell viability assays were done, confirming that the increase in permeability was not the result of BMEC death (Shiu et al., 2007).
5. Functional alteration of the BBB by drugs of abuse
A correlation has been identified between selected drugs of abuse and increased incidence of HIVE and HAD. Introduction of these foreign substances into the host has been shown to play a role in altering the neuroinvasive properties of HIV-1, most likely owing to disruption of the BBB (Table 1). Cocaine abuse is associated with a higher incidence of HIVE and HAD and has been shown to bind BMECs through the sigma receptor, resulting in decreased TEER and increased transmigration of monocytes into the CNS (Fiala et al., 1998; Fiala et al., 2005; Gan et al., 1998). As cocaine interacts with BMECs, the sigma receptor is translocated to lipid rafts in the plasma membrane, and the platelet-derived growth factor-beta receptor is phosphorylated, leading to upregulation of activated leukocyte CAM (ALCAM) on the endothelial surface (Yao et al., 2011a; Yao et al., 2011b). This upregulation of ALCAM has been observed in autopsied brain microvessel tissue samples from cocaine abusers infected with HIV, and in vivo mouse studies correlated these increases with increased monocyte adhesion and transmigration (Yao et al., 2011b). Surface expression levels of ICAM-1 and VCAM-1 are also increased in vitro in response to cocaine (Gan et al., 1999). In addition, cocaine upregulates gene expression of MT1-MMP (Nair et al., 2004), a pro-MMP involved in MMP localization and activation (Kinoh et al., 1996), implying increased degradation of the extracellular matrix and increased BBB permeability. Translocation of the sigma receptor also induces upregulation of MCP-1 mRNA and protein in microglia, which in turn increases monocyte migration across the BBB as shown in vitro and in vivo (Yao et al., 2010; Yao et al., 2011b). Cocaine exposure also increases MCP-1 expression in infected monocytes and CCR2, the cognate receptor for MCP-1 (Dhillon et al., 2008). Upregulation of platelet-derived growth factor-beta in BMECs plays an essential role in mediating these cocaine-induced increases in BBB permeability (Yao et al., 2011a). Understanding the mechanism of this upregulation is an important area of ongoing research.
Table 1.
The effects of drugs of abuse on the BBB.
| Cocaine | Morphine | Methamphetamine | |
|---|---|---|---|
| TEER value | Decrease (Fiala et al. 2005) | Decrease (Mahajan et al. 2008) | Decrease (Ramirez et al. 2005) |
|
Barrier permeability |
Increase monocyte transmigration and FITC-D passage (Fiala et al. 1998; Fiala et al. 2005; Gan et al. 1999) |
Increase PBMC transmigration (Mahajan et al. 2008) |
Increased Na-F passage (Ramirez et al. 2005; Banerjee et al. 2010) |
|
Cytokines and Chemokines |
Increase MCP-1 (Dhillon et al. 2008; Yao et al. 2010), CCR2 (Dhillon et al. 2008), TNF-α, IL-6 (Gan et al. 1999) |
Increase TNF-α and IL-8 secretion (Mahajan et al. 2008) |
ND |
|
Interendothelial gap formation |
Increase (Fiala et al. 2005) | ND | Increase (Ramirez et al. 2009) |
|
ZO-1 protein stability |
Decrease (Dhillon et al. 2008) | ND | ND |
|
TJ protein expression |
ND | Decrease ZO-1 and occludin (from RNA level) (Mahajan et al. 2008) |
Decrease ZO-1, claudin-5, and occludin (Ramirez et al. 2005; Banerjee et al. 2010) |
| ROS production | ND | ND | Increase (Banerjee et al. 2010; Kiyatkin and Sharma 2009) |
| Synergy | Tat (Ghandi et al. 2010) | Tat (Mahajan et al. 2008) | Tat, gpl20 (Banerjee et al. 2010) |
transendothelial electrical resistance (TEER), zonula occludens 1 (ZO-1), tight junctions (TJ), reactive oxygen species (ROS), tumor necrosis factor α (TNF-α), sodium-fluorescein (Na-F), FITC-dextran (FITC-D)
It has also been suggested that cocaine administration causes acute hyperthermia, which has been associated with disruption of the BBB; however, it is unknown if the hyperthermia is caused by a direct or indirect effect (Sharma et al., 2009). Studies have suggested that one of the indirect mechanisms may involve increases in levels of serotonin or proinflammatory cytokines (Sharma et al., 2009). Exposure of monocytes and BMECs in vitro to cocaine caused increased secretion of TNF-alpha and IL-6 (Gan et al., 1999). In addition to these specific modes of dysregulation, interendothelial gaps have been observed following treatment of BMECs with cocaine (Fiala et al., 2005). It is important to note that, although interendothelial gaps were observed following cocaine treatment of both BMECs and coronary artery endothelial cells, the gaps were not observed in human aortic endothelial cells or human umbilical vein endothelial cells (Fiala et al., 2005), indicating that the mechanism of action is at least partially specific for the endothelial cells of the BBB, BMECs. Pretreatment with a tyrosine kinase inhibitor blocked the formation of gaps, suggesting a role for tyrosine kinases in gap formation (Fiala et al., 2005); however, the details of this mechanism have not been established. Interestingly, a protein tyrosine phosphatase was observed to be downregulated following acute and chronic cocaine exposure (Fiala et al., 2005). Another study has linked cocaine treatment of BMECs with damage to the stability of ZO-1 and reorganization of the cytoskeleton causing stress fiber formation (Dhillon et al., 2008), another possible cause for gap formation and suggesting increased TJ opening.
Combined exposure of BMECs in vitro to cocaine and Tat protein caused a decrease in TEER values, an increase in FITC-dextran permeability, and an increase in monocyte transmigration, all with effects greater than those observed with treatment with cocaine or Tat alone (Gandhi et al., 2010). Interestingly, differences in interactions of cocaine with clade B Tat and clade C Tat were observed, which can be correlated with recorded differences in neuropathogenesis between the two clades (Gandhi et al., 2010).
In general, treatment of BMECs and macrophages with cocaine alters the transcriptional profile of the cells, resulting in changes in cell metabolism, function, and structure (Fiala et al., 2005). Specifically, following acute cocaine exposure, the downregulation of potassium voltage-gated channel expression has been observed, leading to the disruption of endothelial cell growth and integrity (Fiala et al., 2005). Additional genes are upregulated following chronic cocaine exposure, defined as treatment over 24 hours, including colony-stimulating factor 2 receptor and macrophage scavenger receptor 1, which can disrupt macrophage growth and endocytic function (Fiala et al., 2005). The impact on BBB integrity and HIV-1 neuroinvasion following acute and chronic exposure to and withdrawal from cocaine requires further investigation with the goal of elucidating the mechanistic cause of decreased TEER and altered gene expression profiles.
Studies of cocaine administration in rats have demonstrated a dependence on dose and route of administration. As mentioned previously, dose is an important factor to consider when ensuring relevance in a human drug-abusing population. Consideration of the route of administration is equally important. Breakdown of the BBB was observed most significantly when cocaine was administered to rats by intracerebroventricular injection (Sharma et al., 2009). Though this route of administration is likely not reflective of a natural system exposure, administering a certain dose directly into the cerebral ventricles may approximate the intravenous administration of a higher dose. When considering route and dose of administration, it is important to take into account the concentration administered as well as the approximate concentration expected to reach the proposed target area.
Treatment of BMECs with morphine also decreases TEER and enhances transendothelial migration across the BBB (Mahajan et al., 2008). As mentioned, Tat decreases ZO-1 and occludin expression in BMECs, and levels are further decreased by combined treatment with morphine and Tat (Mahajan et al., 2008). As a result, combined treatment with Tat and morphine additively decreased TEER. Interestingly, decreased levels of ZO-1 and occludin following treatment with morphine are statistically the same as levels following combined treatment with morphine and Tat (Mahajan et al., 2008). This result suggests that an individual abusing morphine will already have significantly increased BBB permeability at the time of infection, allowing more rapid entry of virus into the CNS. An individual who begins abusing morphine after being infected with HIV-1 will also potentially enhance the process of viral entry into the CNS by enhancing the disruption of the BBB.
Morphine and Tat act synergistically to increase production of the proinflammatory cytokines TNF-alpha and IL-8 (Mahajan et al., 2008). These studies show a dose-dependent response to Tat and morphine, but the kinetics of the dose response relevant to the pathogenic process during the course of HIV disease needs further consideration. The results suggest that morphine acted together with Tat to promote increased BBB permeability and provided a possible explanation for the increased risk of developing HIV-related neurological complications in an individual abusing opiates.
Treatment of BMECs with methamphetamine (METH) disrupted BBB structure in a time- and dose-dependent manner by decreasing claudin-5 and occludin protein levels, inducing gap formation, and enhancing production of ROS (Ramirez et al., 2009). Treatment of mice with gp120, Tat, or gp120 and Tat for 5 days followed by treatment with METH led to increased oxidative stress in brain tissue, with the highest levels observed with triple exposure (Banerjee et al., 2010). Increased oxidative stress was correlated with decreased TJ protein levels and increased permeability of the BBB (Banerjee et al., 2010). Overall, these alterations were prevented with treatment with an antioxidant (Banerjee et al., 2010; Ramirez et al., 2009). It is well accepted that METH-induced hypermetabolism in the brain leads to overproduction of ROS beyond levels that cells can naturally neutralize (Kiyatkin and Sharma, 2009). These results suggest that METH-potentiated effects of gp120 and Tat on BBB integrity may be mediated through ROS mechanisms. An important area for future research will center on the exploration of the effects of combined treatment with METH, Tat, and gp120 on the BBB to identify any additive or synergistic mechanisms of BBB damage.
Hyperthermia, as previously discussed, can cause breakdown of the BBB. This effect is not caused exclusively by cocaine administration; it has also been reported following administration of METH (Kiyatkin and Sharma, 2009). The effects of hyperthermia on BBB integrity were consistent with results reported for cocaine and were observed following experiments that involved the administration of METH at varying temperatures (Kiyatkin and Sharma, 2009).
These are just a few examples of the many external factors that may impact HIV-1 neuroinvasion. This area of research is important and will require further investigation to facilitate the identification of new targets for neuroprotective therapies to counter the detrimental effects of substance abuse during the course of HIV-1 disease. Specific concerns with research in this area are concentration and duration of treatment and route of administration. These conditions need to be kept consistent with naturally occurring values in order for the research to be relevant to the natural history of the disease and more relevant to development of treatment and prevention strategies.
6. HIV-1 proteins in the CNS alter BBB permeability
Throughout the course of HIV disease, penetration of the virus into the CNS likely occurs periodically with subsequent replication and pathogenesis. Once virus is introduced into the CNS, cells other than resident macrophages can be infected. Astrocytes have functional CXCR4 and CCR5 coreceptors (Klein et al., 1999) and have been shown to be infected with HIV-1. Infection of astrocytes is characterized by expression of Tat, high expression of Nef (Ranki et al., 1995), and increased secretion of proinflammatory cytokines, including TNF-alpha and IL-6. Nef is a 27–35-kDa protein that is myristoylated at the N-terminus, anchoring it to the host cell membrane; this localization is thought to be essential for proper function of the protein (Kirchhoff et al., 2008). The established roles of Nef in viral replication and pathogenicity have been reviewed (Kirchhoff et al., 2008).
Nef expression has been correlated with AIDS-associated dementia through evidence from brain tissue samples derived from patients with AIDS who died with moderate to severe dementia. Six of eight tissue samples stained positive for Nef, specifically in astrocytes (Ranki et al., 1995). This finding was compared to only one of six patients with no clinical signs of dementia having a similar Nef distribution pattern (Ranki et al., 1995). Additionally, Nef expression in astrocytes was correlated with a higher level of activation, as measured by elevated glial fibrillary acidic protein and CD88 surface expression (Kohleisen et al., 1999). These correlations suggested a link between Nef and the functional breakdown of the BBB. Defining the mechanisms associated with Nef activity in this regard is currently an important area of research.
In response to HIV infection, astrocytes have also been shown to overexpress chemokines, resulting in the recruitment of more monocytes to the site of infection. MCP-1/CCL2, a potent chemotactic factor for monocytes (Gendelman et al., 2009), was overexpressed up to 80-fold in response to treatment with Tat (Pu et al., 2003). Astrocytes transfected with a Nef-expressing vector secreted CCL2 in levels sufficient to induce monocyte migration (Lehmann et al., 2006). Additional screening of these astrocyte supernatants revealed increased protein levels of IL-8, IL-2, and TNF-alpha and decreased protein levels of stromal cell-derived factor-1, IL-6, and IL-10 (Lehmann et al., 2006). Nef was also shown to increase mRNA levels of CCL2 and IL-8, and all of these effects were dependent on myristoylation of Nef (Lehmann et al., 2006). Myristoylation not only anchors Nef in the cell membrane but allows for the protein to interact with calmodulin (Matsubara et al., 2005). Blocking calmodulin in Nef-expressing astrocytes prevented any increase in CCL2 mRNA or protein (Lehmann et al., 2006). The mechanism behind Nef-induced increase in CCL2 mRNA is not known. Its affect could be pre-or post-transcriptional, and the exact role of calmodulin in this process has not been determined. Importantly, release of CCL2 by astrocytes resulted in decreased TEER and decreased levels of occludin, claudin-5, ZO-1, and ZO-2 proteins (Stamatovic et al., 2005), further breaking down TJs and increasing BBB permeability (Fig. 2C), allowing for recruited monocytes to pass into the CNS with much greater ease.
Infected astrocytes induce apoptosis of uninfected, neighboring astrocytes in a gap junction-dependent manner (Eugenin and Berman, 2007). The mechanism of apoptotic induction is unknown. It is an important mechanism to explore because the loss of uninfected astrocytes may result in a nearly complete lack of normally functioning astrocytes, causing breakdown of the biochemical environment necessary for BBB architectural and functional stability. Overall, infection in astrocytes will lead to further loss of BBB function, bringing the system closer to a stage of BBB damage beyond repair. At this point, passage into the CNS will be unregulated, and intra-CNS damage will be further increased when combined with the formation of a chemotactic gradient for monocytes. The monocytes entering the CNS may either already be infected or may become infected during or after CNS entry, leading to an increased level of neurocognitive dysfunction.
In addition to increased permeability due to TJ breakdown by viral proteins or drugs of abuse, transcellular trafficking is also impacted by dysregulation of BMECs. As mentioned, when small, lipophilic compounds diffuse into BMECs, there is an internal mechanism to shuttle these compounds out, preventing entry into the CNS. It is possible that these mechanisms are also disrupted, leading to enhanced transcellular diapedesis. In this situation, BMECs are either actively shuttling or passively allowing cells and proteins from the periphery to cross into the CNS. Knowing this, additional research concerning the mechanisms through which BMECs limit transcellular trafficking would be valuable with regard to the processes of dysregulation and breakdown of the BBB.
7. Discussion
Although many factors relating to the process of HIV-1 neuroinvasion are still unknown, a general framework of the mechanism has emerged (Fig. 3). The BBB is one of the major reasons why neuroinvasion and intra-CNS HIV disease represent unique pathological processes and do not always necessarily correlate with disease progression in the peripheral blood or other end organs. In a healthy individual, the BBB is effective in preventing uncontrolled entry of infectious agents, infected cells, and other abnormal physiological elements into the CNS. It has become clear that dysregulation of the BBB, including TJ disruption, is a key step in neuroinvasion. Initially, the BBB is most likely an effective barrier with respect to viral penetration, but early virus-associated pathogenic events facilitate some form of viral breach of the barrier. At this point, the BBB may be temporarily dysregulated or partially disrupted, but normal repair mechanisms remain functional and partially or completely reverse initial pathological damage. As disease progresses, peaks in viral replication and viral protein production, and increased levels of inflammatory cytokines impact the permeability of the BBB, providing free virus and virus-infected cells an opportunity to enter the CNS. The cyclic nature of chronic infection, the control of virus replication with antiretroviral therapy, and the resultant change in the production levels of viral proteins and inflammatory cytokines provide intervals for repair of the BBB. However, during late stage disease, when viral replication is consistently high and infection in the CNS has also progressed, the BBB is continuously weakened beyond repair by pathological events occurring on both sides of the BBB, and entry into the CNS becomes unregulated and eventually irreversible.
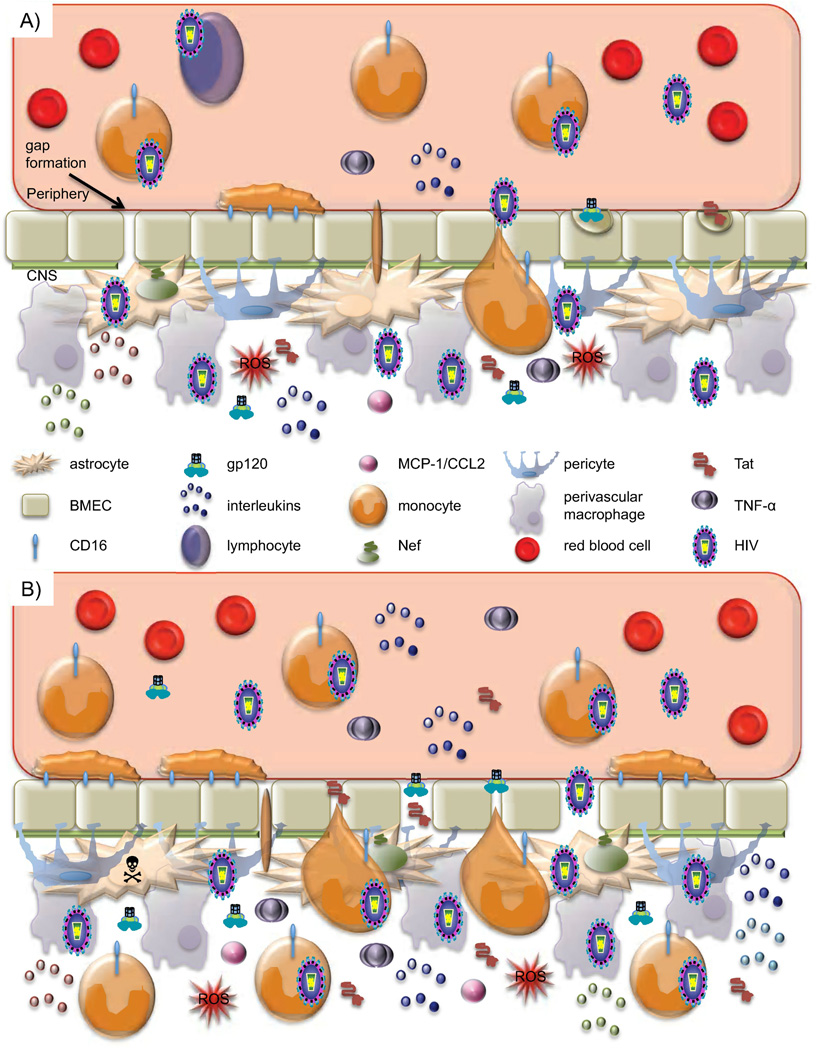
Fig. 3. Tipping point: A model of BBB dysregulation and loss of homeostasis.
A) During acute infection, an initial viral entry event into the CNS occurs. This is believed to be through entry of an infected cell of the monocyte-macrophage lineage. Throughout chronic infection, increased generation of or differentiation to a specific monocyte subset, CD14low/CD16+ that traffics preferentially to the BBB and constitutively secretes IL-1 and TNF-alpha is observed in patients with HIV. Once at the BBB, infected cells secrete IL-1, TNF-alpha, Tat, gp120 and other viral proteins causing altered TJ protein expression and localization and BMEC activation leading to increased passage across the BBB. Crosstalk between monocytes and BMECs in this inflammatory state leads to reciprocal activation of the two cell types, allowing for diapedesis of, likely infected, monocytes. Tat and gp120 are able to enter BMECs through adsorptive endocytosis, altering TJ regulation and delaying TJ closure, creating an opportunity for free virus and viral proteins to enter the CNS through non-specific passage mechanisms. In addition, gap formation has been observed under these conditions, however, the endothelium is able to repair. In the CNS, other cells including astrocytes, pericytes, and perivascular macrophages can be infected and will further secrete Tat, gp120, and proinflammatory cytokines leading to further BMEC activation. Additionally, astrocytes will express high levels of Nef, and secrete MCP-1/CCL2, IL-2, 6, and 8. This process greatly facilitates viral entry into the CNS. Additionally, Tat has been shown to be chemotactic for monocytes, bringing more monocytes to the site of infection. While homeostasis is strained, as long as the barrier is able to recover and repair balance can be restored. This state of enhanced passage may correlate with early HAND development. B) After prolonged exposure to viral proteins, proinflammatory cytokines, and infectious virus, BBB dysregulation exceeds the threshold of what can be restored. Constant BMEC activation leads to greatly increased monocyte firm adhesion and diapedesis, further spreading infection throughout the CNS. High levels of Tat and gp120 entering BMECs altering TJ protein expression and stability cause further delays in TJ closing, and there is more leakiness in passage. Gap formation is also enhanced, pushing repair mechanisms to a limit. Astrocyte death is also observed, often in cells neighboring those that are infected. Disturbance of the homeostasis between the periphery and the CNS, along with peaks in inflammatory cytokines, neurotoxic viral proteins, and infectious virions suggest a correlation with the onset of more severe neurocognitive symptoms.
This increase in BBB permeability facilitates entry into the CNS of virus and virus-infected cells, specifically an enhanced subset of circulating CD14low/CD16+ monocytes. Once virus has been introduced into the CNS, it begins to spread to resident cells, including astrocytes. Infection of astrocytes is another key step in this process because astrocyte function is an integral component of BBB stability. As astrocyte function is disrupted, the BBB becomes further weakened, allowing more virus and virus-infected cells to cross the barrier. Chemotactic factors are also secreted from infected astrocytes at an elevated level, promoting recruitment of more monocytes to the site of infection.
The role of viral proteins in this breakdown is an important area of investigation. Early evidence shows the impact not only of Tat but also of gp120 and Nef on BBB stability. Viral protein gp120 has been linked to ROS generation in BMECs, and Nef protein has been linked to influencing transcription of MMPs at the BBB. This finding brings to light the fact that many of the proteins activated in BMECs by viral proteins play roles in other organ systems during HIV-1 disease progression. Elevated levels of MMPs in the myocardium and subsequently in the circulation are associated with an increased risk of heart disease, as is HIV-1 infection itself. Few studies have been done that cross organ systems, but investigators must remain conscious of the ubiquitous nature of HIV-1 infection. Many of the factors that play a role in neuroinvasion are likely to play a role peripherally. These connections must be identified as pathways are elucidated in order to develop additional neuroprotective therapeutic strategies.
Uncovering more precise components of the overall mechanisms associated with the disruption of the BBB, initial neuroinvasion, further BBB breakdown, and recruitment to the CNS is the key to fully understanding HIV-1 neuroinvasion and the development of new therapeutic strategies. This review highlights the advancements that have been made in identifying the players in the breakdown of the BBB; however, the connections between these pathological components need further refinement. The pathological end points reached involving TJ protein degradation or mislocalization of newly synthesized TJ proteins are achieved based on the interactions of both host and viral proteins, but it has not yet been determined if these end points are reached through the same or distinct mechanisms. Uncovering these mechanisms will be important for identifying host and viral protein synergies that may serve as targets for neuroprotective therapies. It must be determined which factors trigger parallel pathways, thereby amplifying the same effect, and which factors act on the same pathway. Specifically, do Tat and gp120 lead to TJ protein degradation through the same or distinct mechanisms? Studies have yet to be done with Tat and gp120 in combination using a BBB model or in vivo. Viral proteins are not likely to be present on an individual basis in the serum of infected patients. Therefore, researchers should move toward experimental approaches that use combinations. Interactions and synergies among host factors, viral proteins, and drugs of abuse also require further exploration. The mechanisms through which individual drugs of abuse degrade BBB function may be distinct and/or more complex with intricate interconnected pathological effects on the physiology and integrity of BBB with the combinatorial exposure to drugs and viral proteins triggering alternative signaling cascades in BMECs. Defining the connections between the already established pathway components will be the next critical step in elucidating the pathological process of HIV-1 BBB neuroinvasion.
Finally, the introduction of HAART has resulted in significantly lower mortality rates, culminating in an epidemic that increasingly affects older adults. It is estimated that by 2015, approximately half of those infected in the United States will be over the age of 50. As such, it is of paramount importance to understand the risk factors associated with aging and HIV-1 infection (Smith, 2006) including BBB deregulation and disruption. The combined effects of aging and HIV-1 infection make the CNS extremely vulnerable. Changes in brain structure (Ernst and Chang, 2004; Jernigan et al., 2005) and brain function (Larussa et al., 2006; Valcour et al., 2004a) have been observed and are suggestive of neurocognitive consequences of HIV infection in the older population. Data from the Hawaii Aging and HIV Cohort have shown that the frequency of dementia among HIV-positive patients 50 years and older is approximately double that seen in HIV-positive patients between the ages of 20 and 39 (Valcour et al., 2004a; Valcour et al., 2004b). Additionally, it was shown that, after controlling for educational level, race, depression, substance abuse, viral load, CD4 T cell count, and antiretroviral therapy status, the odds of having dementia are 3.26 times greater for older HIV-positive adults compared to younger HIV-positive adults (Valcour and Paul, 2006; Valcour et al., 2004b). Several potential mechanisms could explain the cognitive decline seen in the older compared to the younger HIV-positive population. One mechanism is the possible accompaniment of cardiovascular and cerebrovascular copathology that occurs frequently within the aging HIV-positive population, especially when these patients are on a HAART regimen that includes a protease inhibitor, which has been shown to increase cholesterol and potentially compromise cardiovascular and cerebrovascular health (Connor et al., 2000). The reduction in a patient’s CD4 T cell count may also play a role, as could increased proliferation and activation of the macrophage population. Additionally, an increase in inflammatory glial activation has been shown within the frontal white matter of older HIV-positive patients beyond that seen in normal aging or in younger HIV-positive patients (Ernst and Chang, 2004). Another potential risk factor is represented by the expression of apolipoprotein E4. A relationship between apolipoprotein E4 and Alzheimer’s disease and HIV-associated dementia has already been demonstrated (Valcour et al., 2004a). Current research also suggests a possible relationship between coinfection with hepatitis C and increased neurocognitive deficits in aging HIV-positive adults. Lastly, immune failure commonly occurs with both aging and HIV infection, though for different reasons. Common immunologic perturbations in both HIV-infected individuals and aging adults include inversion of the normal CD4:CD8 ratio, smaller numbers of naïve CD4 T cells, changes in cytokine profiles including a decrease in IL-2 production accompanied by an increase in interferon-gamma production, telomere shortening in CD4 and CD8 T cells, lower T-cell proliferation potentially as a consequence of decreased IL-2 production, and increases in late differentiated CD4 and CD8 T cells. The combined effects of HIV and aging on the immune system may accelerate immune activation and immunosenescence (Appay and Sauce, 2008; Cao et al., 2009; Gandhi et al., 2006; Kirk and Goetz, 2009). Given these observations, as research elucidates BBB dysregulation due to HIV-1 infection and the HIV-1-infected population ages, new models and mechanisms of action will need to be explored.
Acknowledgements
Drs. Brian Wigdahl and Michael Nonnemacher are supported in part by funds from the Public Health Service, National Institutes of Health through grants from the National Institute of Neurological Disorders and Stroke, NS32092 and NS46263, the National Institute of Drug Abuse, DA19807 (Dr. Brian Wigdahl, Principal Investigator). Dr. Michael Nonnemacher was also supported by faculty development funds provided by the Department of Microbiology and Immunology and the Institute for Molecular Medicine and Infectious Disease. Marianne Strazza is supported by a Ruth L. Kirschstein National Research Service Award (5T32MH079785). The contents of this review are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- BBB
blood-brain barrier
- BMECs
brain microvascular endothelial cells
- CNS
central nervous system
- COX
cyclooxygenase
- HIV-1
human immunodeficiency virus type 1
- HAD
HIV-1-associated dementia
- HAND
HIV-associated neurocognitive disease
- HIVE
HIV encephalitis
- ICAM
intercellular adhesion molecule
- IL
interleukin
- MMP
matrix metalloproteinase
- METH
methamphetamine
- MCP-1/CCL2
monocyte chemoattractant protein
- NF-kappaB
nuclear factor kappa-light-chain-enhancer of activated B cells
- ROS
reactive oxygen species
- TJ
tight junction
- TEER
transendothelial electrical resistance
- TNF-alpha
tumor necrosis factor alpha
- VCAM
vascular cell adhesion molecule
- ZO-1
zonula occludens 1
- ZO-2
zonula occludens 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Key for codes used in place of non-keyboard
Alpha for greek letter alpha
Kappa for greek letter kappa
References
- Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Zhang X, Manda KR, Banks WA, Ercal N. HIV proteins (gp120 and Tat) and methamphetamine in oxidative stress-induced damage in the brain: potential role of the thiol antioxidant N-acetylcysteine amide. Free Radic Biol Med. 2010;48:1388–1398. doi: 10.1016/j.freeradbiomed.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Akerstrom V. HIV-1 protein gp120 crosses the blood-brain barrier: role of adsorptive endocytosis. Life Sci. 1997;61:PL119–PL125. doi: 10.1016/s0024-3205(97)00597-3. [DOI] [PubMed] [Google Scholar]
- Banks WA, Akerstrom V, Kastin AJ. Adsorptive endocytosis mediates the passage of HIV-1 across the blood-brain barrier: evidence for a post-internalization coreceptor. J Cell Sci. 1998;111(Pt 4):533–540. doi: 10.1242/jcs.111.4.533. [DOI] [PubMed] [Google Scholar]
- Banks WA, Robinson SM, Nath A. Permeability of the blood-brain barrier to HIV-1 Tat. Exp Neurol. 2005;193:218–227. doi: 10.1016/j.expneurol.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Barillari G, Sgadari C, Fiorelli V, Samaniego F, Colombini S, Manzari V, Modesti A, Nair BC, Cafaro A, Sturzl M, Ensoli B. The Tat protein of human immunodeficiency virus type-1 promotes vascular cell growth and locomotion by engaging the alpha5beta1 and alphavbeta3 integrins and by mobilizing sequestered basic fibroblast growth factor. Blood. 1999;94:663–672. [PubMed] [Google Scholar]
- Bo L, Peterson JW, Mork S, Hoffman PA, Gallatin WM, Ransohoff RM, Trapp BD. Distribution of immunoglobulin superfamily members ICAM-1, -2, -3, and the beta 2 integrin LFA-1 in multiple sclerosis lesions. J Neuropathol Exp Neurol. 1996;55:1060–1072. [PubMed] [Google Scholar]
- Bonkowski D, Katyshev V, Balabanov RD, Borisov A, Dore-Duffy P. The CNS microvascular pericyte: pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS. 2011;8:8. doi: 10.1186/2045-8118-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro L, Barillari G, Chang HK, Bohan CA, Kao V, Morgan R, Gallo RC, Ensoli B. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J Virol. 1992;66:7159–7167. doi: 10.1128/jvi.66.12.7159-7167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, Alvarez X, Kuroda MJ, Williams KC. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Jamieson BD, Hultin LE, Hultin PM, Effros RB, Detels R. Premature aging of T cells is associated with faster HIV-1 disease progression. J Acquir Immune Defic Syndr. 2009;50:137–147. doi: 10.1097/QAI.0b013e3181926c28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547–556. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman CV. Mechanisms for transcellular diapedesis: probing and pathfinding by 'invadosome-like protrusions'. J Cell Sci. 2009;122:3025–3035. doi: 10.1242/jcs.047522. [DOI] [PubMed] [Google Scholar]
- Carrithers MD, Visintin I, Kang SJ, Janeway CA., Jr. Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain. 2000;123(Pt 6):1092–1101. doi: 10.1093/brain/123.6.1092. [DOI] [PubMed] [Google Scholar]
- Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell Jt, Bixby D, Savona MR, Collins KL. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16:446–451. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh LL, Bonasio R, Mazo IB, Halin C, Cheng G, van der Velden AW, Cariappa A, Chase C, Russell P, Starnbach MN, Koni PA, Pillai S, Weninger W, von Andrian UH. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nat Immunol. 2005;6:1029–1037. doi: 10.1038/ni1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, Haqqani AS, Kreymborg K, Krug S, Moumdjian R, Bouthillier A, Becher B, Arbour N, David S, Stanimirovic D, Prat A. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- Chene G, Binquet C, Moreau JF, Neau D, Pellegrin I, Malvy D, Ceccaldi J, Lacoste D, Dabis F. Changes in CD4+ cell count and the risk of opportunistic infection or death after highly active antiretroviral treatment. Groupe d'Epidemiologie Clinique du SIDA en Aquitaine. AIDS. 1998;12:2313–2320. doi: 10.1097/00002030-199817000-00013. [DOI] [PubMed] [Google Scholar]
- Churcher MJ, Lamont C, Hamy F, Dingwall C, Green SM, Lowe AD, Butler JG, Gait MJ, Karn J. High affinity binding of TAR RNA by the human immunodeficiency virus type-1 tat protein requires base-pairs in the RNA stem and amino acid residues flanking the basic region. J Mol Biol. 1993;230:90–110. doi: 10.1006/jmbi.1993.1128. [DOI] [PubMed] [Google Scholar]
- Cioni C, Annunziata P. Circulating gp120 alters the blood-brain barrier permeability in HIV-1 gp120 transgenic mice. Neurosci Lett. 2002;330:299–301. doi: 10.1016/s0304-3940(02)00814-5. [DOI] [PubMed] [Google Scholar]
- Connor MD, Lammie GA, Bell JE, Warlow CP, Simmonds P, Brettle RD. Cerebral infarction in adult AIDS patients: observations from the Edinburgh HIV Autopsy Cohort. Stroke. 2000;31:2117–2126. doi: 10.1161/01.str.31.9.2117. [DOI] [PubMed] [Google Scholar]
- Couty JP, Rampon C, Leveque M, Laran-Chich MP, Bourdoulous S, Greenwood J, Couraud PO. PECAM-1 engagement counteracts ICAM-1-induced signaling in brain vascular endothelial cells. J Neurochem. 2007;103:793–801. doi: 10.1111/j.1471-4159.2007.04782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Vaida F, Letendre S, Gibson S, Cherner M, Woods SP, McCutchan JA, Heaton RK, Ellis RJ. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73:342–348. doi: 10.1212/WNL.0b013e3181ab2b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, Achim CL. Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1915–1927. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon NK, Peng F, Bokhari S, Callen S, Shin SH, Zhu X, Kim KJ, Buch SJ. Cocaine-mediated alteration in tight junction protein expression and modulation of CCL2/CCR2 axis across the blood-brain barrier: implications for HIV-dementia. J Neuroimmune Pharmacol. 2008;3:52–56. doi: 10.1007/s11481-007-9091-1. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Peterson M, Catalanotto F, Marlow S, Ho SY, Ostrom M, Weinstein A. Zinc profiles in rheumatoid arthritis. Clin Exp Rheumatol. 1990;8:541–546. [PubMed] [Google Scholar]
- Dorfel MJ, Westphal JK, Huber O. Differential phosphorylation of occludin and tricellulin by CK2 and CK1. Ann N Y Acad Sci. 2009;1165:69–73. doi: 10.1111/j.1749-6632.2009.04043.x. [DOI] [PubMed] [Google Scholar]
- Elali A, Hermann DM. ATP-Binding Cassette Transporters and Their Roles in Protecting the Brain. Neuroscientist. 2011 doi: 10.1177/1073858410391270. [DOI] [PubMed] [Google Scholar]
- Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Wolburg H. Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004;34:2955–2963. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L. Effect of aging on brain metabolism in antiretroviral-naive HIV patients. AIDS. 2004;18 Suppl 1:S61–S67. [PubMed] [Google Scholar]
- Eugenin EA, Berman JW. Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J Neurosci. 2007;27:12844–12850. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Sule Z, Toth-Szuki V, Matyas A, Antal P, Farkas IG, Mihaly A, Bari F. Tumor necrosis factor-alpha increases cerebral blood flow and ultrastructural capillary damage through the release of nitric oxide in the rat brain. Microvasc Res. 2006;72:113–119. doi: 10.1016/j.mvr.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Fiala M, Gan XH, Zhang L, House SD, Newton T, Graves MC, Shapshak P, Stins M, Kim KS, Witte M, Chang SL. Cocaine enhances monocyte migration across the blood-brain barrier. Cocaine's connection to AIDS dementia and vasculitis? Adv Exp Med Biol. 1998;437:199–205. doi: 10.1007/978-1-4615-5347-2_22. [DOI] [PubMed] [Google Scholar]
- Fiala M, Eshleman AJ, Cashman J, Lin J, Lossinsky AS, Suarez V, Yang W, Zhang J, Popik W, Singer E, Chiappelli F, Carro E, Weinand M, Witte M, Arthos J. Cocaine increases human immunodeficiency virus type 1 neuroinvasion through remodeling brain microvascular endothelial cells. J Neurovirol. 2005;11:281–291. doi: 10.1080/13550280590952835. [DOI] [PubMed] [Google Scholar]
- Finco O, Nuti S, De Magistris MT, Mangiavacchi L, Aiuti A, Forte P, Fantoni A, van der Putten H, Abrignani S. Induction of CD4+ T cell depletion in mice doubly transgenic for HIV gp120 and human CD4. Eur J Immunol. 1997;27:1319–1324. doi: 10.1002/eji.1830270604. [DOI] [PubMed] [Google Scholar]
- Fiorelli V, Barillari G, Toschi E, Sgadari C, Monini P, Sturzl M, Ensoli B. IFN-gamma induces endothelial cells to proliferate and to invade the extracellular matrix in response to the HIV-1 Tat protein: implications for AIDS-Kaposi's sarcoma pathogenesis. J Immunol. 1999;162:1165–1170. [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L'Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Adeniyi A, Rybicka K, Morgello S, Khalili K, Rappaport J. Macrophage/microglial accumulation and proliferating cell nuclear antigen expression in the central nervous system in human immunodeficiency virus encephalopathy. Am J Pathol. 2004;164:2089–2099. doi: 10.1016/S0002-9440(10)63767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris S, Ruuls SR, Wierinckx A, van der Pol SM, Dopp E, van der Meide PH, Dijkstra CD, De Vries HE. Interferon-beta directly influences monocyte infiltration into the central nervous system. J Neuroimmunol. 2002;127:69–79. doi: 10.1016/s0165-5728(02)00098-x. [DOI] [PubMed] [Google Scholar]
- Folks TM, Kessler SW, Orenstein JM, Justement JS, Jaffe ES, Fauci AS. Infection and replication of HIV-1 in purified progenitor cells of normal human bone marrow. Science. 1988;242:919–922. doi: 10.1126/science.2460922. [DOI] [PubMed] [Google Scholar]
- Gan X, Zhang L, Newton T, Chang SL, Ling W, Kermani V, Berger O, Graves MC, Fiala M. Cocaine infusion increases interferon-gamma and decreases interleukin-10 in cocaine-dependent subjects. Clin Immunol Immunopathol. 1998;89:181–190. doi: 10.1006/clin.1998.4607. [DOI] [PubMed] [Google Scholar]
- Gan X, Zhang L, Berger O, Stins MF, Way D, Taub DD, Chang SL, Kim KS, House SD, Weinand M, Witte M, Graves MC, Fiala M. Cocaine enhances brain endothelial adhesion molecules and leukocyte migration. Clin Immunol. 1999;91:68–76. doi: 10.1006/clim.1998.4683. [DOI] [PubMed] [Google Scholar]
- Gandhi N, Saiyed ZM, Napuri J, Samikkannu T, Reddy PV, Agudelo M, Khatavkar P, Saxena SK, Nair MP. Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: implications for HIV-1-associated neurocognitive disorder. J Neurovirol. 2010;16:294–305. doi: 10.3109/13550284.2010.499891. [DOI] [PubMed] [Google Scholar]
- Gandhi RT, Spritzler J, Chan E, Asmuth DM, Rodriguez B, Merigan TC, Hirsch MS, Shafer RW, Robbins GK, Pollard RB. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr. 2006;42:426–434. doi: 10.1097/01.qai.0000226789.51992.3f. [DOI] [PubMed] [Google Scholar]
- Gendelman HE. The neurology of AIDS, Vol. Oxford ; New York: Oxford University Press; 2005. [Google Scholar]
- Gendelman HE, Ding S, Gong N, Liu J, Ramirez SH, Persidsky Y, Mosley RL, Wang T, Volsky DJ, Xiong H. Monocyte chemotactic protein-1 regulates voltage-gated K+ channels and macrophage transmigration. J Neuroimmune Pharmacol. 2009;4:47–59. doi: 10.1007/s11481-008-9135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002;109:383–392. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham NM, Zeger SL, Park LP, Vermund SH, Detels R, Rinaldo CR, Phair JP. The effects on survival of early treatment of human immunodeficiency virus infection. N Engl J Med. 1992;326:1037–1042. doi: 10.1056/NEJM199204163261601. [DOI] [PubMed] [Google Scholar]
- Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- Hamm S, Dehouck B, Kraus J, Wolburg-Buchholz K, Wolburg H, Risau W, Cecchelli R, Engelhardt B, Dehouck MP. Astrocyte mediated modulation of blood-brain barrier permeability does not correlate with a loss of tight junction proteins from the cellular contacts. Cell Tissue Res. 2004;315:157–166. doi: 10.1007/s00441-003-0825-y. [DOI] [PubMed] [Google Scholar]
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, Eron JJ, Jr., Feinberg JE, Balfour HH, Jr., Deyton LR, Chodakewitz JA, Fischl MA. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- Hasegawa A, Liu H, Ling B, Borda JT, Alvarez X, Sugimoto C, Vinet-Oliphant H, Kim WK, Williams KC, Ribeiro RM, Lackner AA, Veazey RS, Kuroda MJ. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009;114:2917–2925. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve F, Ghinea N, Scherrmann JM. CNS delivery via adsorptive transcytosis. AAPS J. 2008;10:455–472. doi: 10.1208/s12248-008-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman DW, Klein RS, Ransohoff RM. The blood-brain barrier, chemokines and multiple sclerosis. Biochim Biophys Acta. 2011;1812:220–230. doi: 10.1016/j.bbadis.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PW, Deeks SG, Rodriguez B, Valdez H, Shade SB, Abrams DI, Kitahata MM, Krone M, Neilands TB, Brand RJ, Lederman MM, Martin JN. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17:1907–1915. doi: 10.1097/00002030-200309050-00009. [DOI] [PubMed] [Google Scholar]
- Ivey NS, MacLean AG, Lackner AA. Acquired immunodeficiency syndrome and the blood-brain barrier. J Neurovirol. 2009;15:111–122. doi: 10.1080/13550280902769764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang KT, Xiao H, Rich EA. Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J Biol Chem. 1999;274:28837–28840. doi: 10.1074/jbc.274.41.28837. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS/World Health Organization) AIDS epidemic update - November 2009. 2009 doi: 10.1016/S0968-8080(09)34466-3. [DOI] [PubMed]
- Ju SM, Song HY, Lee JA, Lee SJ, Choi SY, Park J. Extracellular HIV-1 Tat up-regulates expression of matrix metalloproteinase-9 via a MAPK-NF-kappaB dependent pathway in human astrocytes. Exp Mol Med. 2009;41:86–93. doi: 10.3858/emm.2009.41.2.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmogne GD, Primeaux C, Grammas P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol. 2005;64:498–505. doi: 10.1093/jnen/64.6.498. [DOI] [PubMed] [Google Scholar]
- Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab. 2007;27:123–134. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoh H, Sato H, Tsunezuka Y, Takino T, Kawashima A, Okada Y, Seiki M. MT-MMP, the cell surface activator of proMMP-2 (pro-gelatinase A), is expressed with its substrate in mouse tissue during embryogenesis. J Cell Sci. 1996;109(Pt 5):953–959. doi: 10.1242/jcs.109.5.953. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F, Schindler M, Specht A, Arhel N, Munch J. Role of Nef in primate lentiviral immunopathogenesis. Cell Mol Life Sci. 2008;65:2621–2636. doi: 10.1007/s00018-008-8094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk JB, Goetz MB. Human immunodeficiency virus in an aging population, a complication of success. J Am Geriatr Soc. 2009;57:2129–2138. doi: 10.1111/j.1532-5415.2009.02494.x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Sharma HS. Acute methamphetamine intoxication brain hyperthermia, blood-brain barrier, brain edema, and morphological cell abnormalities. Int Rev Neurobiol. 2009;88:65–100. doi: 10.1016/S0074-7742(09)88004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Williams KC, Alvarez-Hernandez X, Westmoreland S, Force T, Lackner AA, Luster AD. Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J Immunol. 1999;163:1636–1646. [PubMed] [Google Scholar]
- Kohleisen B, Shumay E, Sutter G, Foerster R, Brack-Werner R, Nuesse M, Erfle V. Stable expression of HIV-1 Nef induces changes in growth properties and activation state of human astrocytes. AIDS. 1999;13:2331–2341. doi: 10.1097/00002030-199912030-00004. [DOI] [PubMed] [Google Scholar]
- Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisakk P, Ransohoff RM, Hofbauer M, Farina C, Derfuss T, Hartle C, Newcombe J, Hohlfeld R, Meinl E. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129:200–211. doi: 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- Lai CH, Kuo KH. The critical component to establish in vitro BBB model: Pericyte. Brain Res Brain Res Rev. 2005;50:258–265. doi: 10.1016/j.brainresrev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Lal-Nag M, Morin PJ. The claudins. Genome Biol. 2009;10:235. doi: 10.1186/gb-2009-10-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larussa D, Lorenzini P, Cingolani A, Bossolasco S, Grisetti S, Bongiovanni M, Moretti F, Uccella I, Zannoni P, Foresti S, Mazzarello G, Arcidiacono MI, Pedale R, Ammassari A, Tozzi V, Perno CF, Monforte AD, Cinque P, Antinori A. Highly active antiretroviral therapy reduces the age-associated risk of dementia in a cohort of older HIV-1-infected patients. AIDS Res Hum Retroviruses. 2006;22:386–392. doi: 10.1089/aid.2006.22.386. [DOI] [PubMed] [Google Scholar]
- Lehmann MH, Masanetz S, Kramer S, Erfle V. HIV-1 Nef upregulates CCL2/MCP-1 expression in astrocytes in a myristoylation- and calmodulin-dependent manner. J Cell Sci. 2006;119:4520–4530. doi: 10.1242/jcs.03231. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Li JC, Lee DC, Cheung BK, Lau AS. Mechanisms for HIV Tat upregulation of IL-10 and other cytokine expression: kinase signaling and PKR-mediated immune response. FEBS Lett. 2005;579:3055–3062. doi: 10.1016/j.febslet.2005.04.060. [DOI] [PubMed] [Google Scholar]
- Li TS, Tubiana R, Katlama C, Calvez V, Ait Mohand H, Autran B. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet. 1998;351:1682–1686. doi: 10.1016/s0140-6736(97)10291-4. [DOI] [PubMed] [Google Scholar]
- Lopez-Huertas MR, Callejas S, Abia D, Mateos E, Dopazo A, Alcami J, Coiras M. Modifications in host cell cytoskeleton structure and function mediated by intracellular HIV-1 Tat protein are greatly dependent on the second coding exon. Nucleic Acids Res. 2010;38:3287–3307. doi: 10.1093/nar/gkq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review. Histol Histopathol. 2004;19:535–564. doi: 10.14670/HH-19.535. [DOI] [PubMed] [Google Scholar]
- Lv S, Song HL, Zhou Y, Li LX, Cui W, Wang W, Liu P. Tumour necrosis factor-alpha affects blood-brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver Int. 2010;30:1198–1210. doi: 10.1111/j.1478-3231.2010.02211.x. [DOI] [PubMed] [Google Scholar]
- MacLean AG, Rasmussen TA, Bieniemy D, Lackner AA. Activation of the blood-brain barrier by SIV (simian immunodeficiency virus) requires cell-associated virus and is not restricted to endothelial cell activation. Biochem Soc Trans. 2004;32:750–752. doi: 10.1042/BST0320750. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, Nath A. Human immunodeficiency virus type 1 tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity. Ann Neurol. 1995;37:373–380. doi: 10.1002/ana.410370314. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Fernandez SF, Chawda R, Shanahan TC, Schwartz SA. Tight junction regulation by morphine and HIV-1 tat modulates blood-brain barrier permeability. J Clin Immunol. 2008;28:528–541. doi: 10.1007/s10875-008-9208-1. [DOI] [PubMed] [Google Scholar]
- Mahlknecht U, Dichamp I, Varin A, Van Lint C, Herbein G. NF-kappaB-dependent control of HIV-1 transcription by the second coding exon of Tat in T cells. J Leukoc Biol. 2008;83:718–727. doi: 10.1189/jlb.0607405. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Mucke L. Pathogenesis of HIV-1 associated neurodegeneration. Crit Rev Neurobiol. 1996;10:57–67. doi: 10.1615/critrevneurobiol.v10.i1.30. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Jing T, Kawamura K, Shimojo N, Titani K, Hashimoto K, Hayashi N. Myristoyl moiety of HIV Nef is involved in regulation of the interaction with calmodulin in vivo. Protein Sci. 2005;14:494–503. doi: 10.1110/ps.04969605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:8053–8064. doi: 10.4049/jimmunol.177.11.8053. [DOI] [PubMed] [Google Scholar]
- Miller V, Staszewski S, Nisius G, Lepri AC, Sabin C, Phillips AN. Risk of new AIDS diseases in people on triple therapy. Lancet. 1999;353:463. doi: 10.1016/S0140-6736(98)04954-X. [DOI] [PubMed] [Google Scholar]
- Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers G, Korber BT, Foley BT, Jeang K-T, Mellors JW, Wain-Hobson S. Human Retroviruses and AIDS: A Compilation and Analysis of Nucleic Acid and Amino Acid Sequences. Los Alamos, NM: Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; 1996. pp. III-11–III-26. [Google Scholar]
- Nair MP, Schwartz SA, Mahajan SD, Tsiao C, Chawda RP, Whitney R, Don Sykes BB, Hewitt R. Drug abuse and neuropathogenesis of HIV infection: role of DC-SIGN and IDO. J Neuroimmunol. 2004;157:56–60. doi: 10.1016/j.jneuroim.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Toborek M. Human Immunodeficiency Virus Type I (HIV-1) Infects Human Brain Pericytes in Vitro. Journal of Neuroimmune Pharmacology. 2011;6:S38. [Google Scholar]
- Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, Kido H. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J Neurovirol. 2008;14:186–195. doi: 10.1080/13550280801993630. [DOI] [PubMed] [Google Scholar]
- Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, McArthur J. Evolution of HIV dementia with HIV infection. Int Rev Psychiatry. 2008;20:25–31. doi: 10.1080/09540260701861930. [DOI] [PubMed] [Google Scholar]
- Neuveut C, Scoggins RM, Camerini D, Markham RB, Jeang KT. Requirement for the second coding exon of Tat in the optimal replication of macrophage-tropic HIV-1. J Biomed Sci. 2003;10:651–660. doi: 10.1159/000073531. [DOI] [PubMed] [Google Scholar]
- Newman DK, Hamilton C, Newman PJ. Inhibition of antigen-receptor signaling by Platelet Endothelial Cell Adhesion Molecule-1 (CD31) requires functional ITIMs, SHP-2, and p56(lck) Blood. 2001;97:2351–2357. doi: 10.1182/blood.v97.8.2351. [DOI] [PubMed] [Google Scholar]
- Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- Oh SK, Cruikshank WW, Raina J, Blanchard GC, Adler WH, Walker J, Kornfeld H. Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients. J Acquir Immune Defic Syndr. 1992;5:251–256. [PubMed] [Google Scholar]
- Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood-brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res. 2007;24:1745–1758. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart L, Gendelman HE. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1599–1611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plateel M, Dehouck MP, Torpier G, Cecchelli R, Teissier E. Hypoxia increases the susceptibility to oxidant stress and the permeability of the blood-brain barrier endothelial cell monolayer. J Neurochem. 1995;65:2138–2145. doi: 10.1046/j.1471-4159.1995.65052138.x. [DOI] [PubMed] [Google Scholar]
- Price TO, Ercal N, Nakaoke R, Banks WA. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005;1045:57–63. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Price TO, Uras F, Banks WA, Ercal N. A novel antioxidant N-acetylcysteine amide prevents gp120- and Tat-induced oxidative stress in brain endothelial cells. Exp Neurol. 2006;201:193–202. doi: 10.1016/j.expneurol.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Pu H, Tian J, Flora G, Lee YW, Nath A, Hennig B, Toborek M. HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Mol Cell Neurosci. 2003;24:224–237. doi: 10.1016/s1044-7431(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Pu H, Tian J, Andras IE, Hayashi K, Flora G, Hennig B, Toborek M. HIV-1 Tat protein-induced alterations of ZO-1 expression are mediated by redox-regulated ERK 1/2 activation. J Cereb Blood Flow Metab. 2005;25:1325–1335. doi: 10.1038/sj.jcbfm.9600125. [DOI] [PubMed] [Google Scholar]
- Pu H, Hayashi K, Andras IE, Eum SY, Hennig B, Toborek M. Limited role of COX-2 in HIV Tat-induced alterations of tight junction protein expression and disruption of the blood-brain barrier. Brain Res. 2007;1184:333–344. doi: 10.1016/j.brainres.2007.09.063. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Sun B, Rempel H. Invasive chronic inflammatory monocyte phenotype in subjects with high HIV-1 viral load. J Neuroimmunol. 2004;157:93–98. doi: 10.1016/j.jneuroim.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Raine CS, Cannella B, Duijvestijn AM, Cross AH. Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab Invest. 1990;63:476–489. [PubMed] [Google Scholar]
- Ramirez SH, Potula R, Fan S, Eidem T, Papugani A, Reichenbach N, Dykstra H, Weksler BB, Romero IA, Couraud PO, Persidsky Y. Methamphetamine disrupts blood-brain barrier function by induction of oxidative stress in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29:1933–1945. doi: 10.1038/jcbfm.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapasalo H, Krohn K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS. 1995;9:1001–1008. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- Sabatier JM, Vives E, Mabrouk K, Benjouad A, Rochat H, Duval A, Hue B, Bahraoui E. Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1. J Virol. 1991;65:961–967. doi: 10.1128/jvi.65.2.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Muresanu D, Sharma A, Patnaik R. Cocaine-induced breakdown of the blood-brain barrier and neurotoxicity. Int Rev Neurobiol. 2009;88:297–334. doi: 10.1016/S0074-7742(09)88011-2. [DOI] [PubMed] [Google Scholar]
- Shiu C, Barbier E, Di Cello F, Choi HJ, Stins M. HIV-1 gp120 as well as alcohol affect blood-brain barrier permeability and stress fiber formation: involvement of reactive oxygen species. Alcohol Clin Exp Res. 2007;31:130–137. doi: 10.1111/j.1530-0277.2006.00271.x. [DOI] [PubMed] [Google Scholar]
- Smith G. Aging hearing: HIV over fifty, exploring the new threat. Washington, DC: Senate Commitee on Aging; 2006. [Google Scholar]
- Smith SM, Pentlicky S, Klase Z, Singh M, Neuveut C, Lu CY, Reitz MS, Jr., Yarchoan R, Marx PA, Jeang KT. An in vivo replication-important function in the second coding exon of Tat is constrained against mutation despite cytotoxic T lymphocyte selection. J Biol Chem. 2003;278:44816–44825. doi: 10.1074/jbc.M307546200. [DOI] [PubMed] [Google Scholar]
- Sobel RA, Mitchell ME, Fondren G. Intercellular adhesion molecule-1 (ICAM-1) in cellular immune reactions in the human central nervous system. Am J Pathol. 1990;136:1309–1316. [PMC free article] [PubMed] [Google Scholar]
- Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell-cell junctions: how to "open" the blood brain barrier. Curr Neuropharmacol. 2008;6:179–192. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen BJ, Butcher EC, Engelhardt B. Evidence for involvement of ICAM-1 and VCAM-1 in lymphocyte interaction with endothelium in experimental autoimmune encephalomyelitis in the central nervous system in the SJL/J mouse. Am J Pathol. 1994;145:189–201. [PMC free article] [PubMed] [Google Scholar]
- Steiner O, Coisne C, Engelhardt B, Lyck R. Comparison of immortalized bEnd5 and primary mouse brain microvascular endothelial cells as in vitro blood-brain barrier models for the study of T cell extravasation. J Cereb Blood Flow Metab. 2011;31:315–327. doi: 10.1038/jcbfm.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayarani I, Chaudiere J, Lefauconnier JM, Bourre JM. Enzymatic protection against peroxidative damage in isolated brain capillaries. J Neurochem. 1987;48:1399–1402. doi: 10.1111/j.1471-4159.1987.tb05677.x. [DOI] [PubMed] [Google Scholar]
- Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- Toneatto S, Finco O, van der Putten H, Abrignani S, Annunziata P. Evidence of blood-brain barrier alteration and activation in HIV-1 gp120 transgenic mice. AIDS. 1999;13:2343–2348. doi: 10.1097/00002030-199912030-00005. [DOI] [PubMed] [Google Scholar]
- Ueno M, Nakagawa T, Wu B, Onodera M, Huang CL, Kusaka T, Araki N, Sakamoto H. Transporters in the brain endothelial barrier. Curr Med Chem. 2010;17:1125–1138. doi: 10.2174/092986710790827816. [DOI] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, Holck P, Grove J, Sacktor N. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004a;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Paul R. HIV infection and dementia in older adults. Clin Infect Dis. 2006;42:1449–1454. doi: 10.1086/503565. [DOI] [PubMed] [Google Scholar]
- Valcour VG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. AIDS. 2004b;18 Suppl 1:S79–S86. doi: 10.1097/00002030-200401001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–3940. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- Wang L, Lim EJ, Toborek M, Hennig B. The role of fatty acids and caveolin-1 in tumor necrosis factor alpha-induced endothelial cell activation. Metabolism. 2008;57:1328–1339. doi: 10.1016/j.metabol.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichold FF, Zella D, Barabitskaja O, Maciejewski JP, Dunn DE, Sloand EM, Young NS. Neither human immunodeficiency virus-1 (HIV-1) nor HIV-2 infects most-primitive human hematopoietic stem cells as assessed in long-term bone marrow cultures. Blood. 1998;91:907–915. [PubMed] [Google Scholar]
- Williams K, Schwartz A, Corey S, Orandle M, Kennedy W, Thompson B, Alvarez X, Brown C, Gartner S, Lackner A. Proliferating cellular nuclear antigen expression as a marker of perivascular macrophages in simian immunodeficiency virus encephalitis. Am J Pathol. 2002;161:575–585. doi: 10.1016/S0002-9440(10)64213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- Wong D, Dorovini-Zis K, Vincent SR. Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood-brain barrier. Exp Neurol. 2004;190:446–455. doi: 10.1016/j.expneurol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci U S A. 2000;97:11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol. 2008;172:521–533. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Yang Y, Kim KJ, Bethel-Brown C, Gong N, Funa K, Gendelman HE, Su TP, Wang JQ, Buch S. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115:4951–4962. doi: 10.1182/blood-2010-01-266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Duan M, Buch S. Cocaine-mediated induction of platelet-derived growth factor: implication for increased vascular permeability. Blood. 2011a;117:2538–2547. doi: 10.1182/blood-2010-10-313593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Kim K, Duan M, Hayashi T, Guo M, Morgello S, Prat A, Wang J, Su TP, Buch S. Cocaine Hijacks {sigma}1 Receptor to Initiate Induction of Activated Leukocyte Cell Adhesion Molecule: Implication for Increased Monocyte Adhesion and Migration in the CNS. J Neurosci. 2011b;31:5942–5955. doi: 10.1523/JNEUROSCI.5618-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998;21:75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- Zhao C, Ling Z, Newman MB, Bhatia A, Carvey PM. TNF-alpha knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice. Neurobiol Dis. 2007;26:36–46. doi: 10.1016/j.nbd.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Smart EJ, Weksler B, Couraud PO, Hennig B, Toborek M. Caveolin-1 regulates human immunodeficiency virus-1 Tat-induced alterations of tight junction protein expression via modulation of the Ras signaling. J Neurosci. 2008;28:7788–7796. doi: 10.1523/JNEUROSCI.0061-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]