Abstract
Bone morphogenetic protein-2 (BMP-2) induces bone formation and regeneration in adult vertebrates and regulates important developmental processes in all animals. BMP-2 is a homodimeric cysteine knot protein that, as a member of the transforming growth factor-β (TGF-β) superfamily, signals by oligomerizing type I and type II receptor serine-kinases in the cell membrane. The binding epitopes of BMP-2 for BMPR-IA (type I) and BMPR-II or ActR-II (type II) were characterized using BMP-2 mutant proteins for analysis of interactions with receptor ectodomains. A large epitope 1 for high-affinity BMPR-IA binding was detected spanning the interface of the BMP-2 dimer. A smaller epitope 2 for the low-affinity binding of BMPR-II was found to be assembled by determinants of a single monomer. Symmetry-related pairs of the two juxtaposed epitopes occur near the BMP-2 poles. Mutations in both epitopes yielded variants with reduced biological activity in C2C12 cells; however, only epitope 2 variants behaved as antagonists partially or completely inhibiting BMP-2 activity. These findings provide a framework for the molecular description of receptor recognition and activation in the BMP/TGF-β superfamily.
Keywords: binding epitope/bone morphogenetic protein/receptor signalling/TGF-β superfamily
Introduction
BMP-2, as the prototype of the bone morphogenetic proteins, induces bone regeneration and ectopic bone formation in adult vertebrates (see Reddi, 1997, 1998) and determines important steps during early embryogenesis and limb/wing development in animals (see Hogan, 1996). Accordingly, BMP-2 and its close relative BMP-4 have been studied intensively in developmental biology, and these proteins are of interest for therapeutic applications.
The backbone fold of the homodimeric BMP-2 (Scheufler et al., 1999) is very similar to that of BMP-7 (Griffith et al., 1996) and the transforming growth factor-βs (TGF-βs) (Daopin et al., 1992; Schlunegger and Grutter, 1992; Mittl et al., 1996), despite the low similarities in amino acid sequence (see for example McPherron et al., 1997). As a member of the large group of TGF-β-like protein factors, BMP-2 signals through cellular receptors composed of two types of serine-kinase receptor chains (see Heldin, 1995; Massague, 1998). The type I chain is defined by the cytoplasmic GS box and a serine kinase that activates the cellular SMAD 1 and 5 signalling proteins (Heldin et al., 1997; Nishimura et al., 1998). The type II chain activates a type I receptor serine kinase by transphosphorylating the GS box segment. The small receptor ectodomains of 120–150 residues show only limited similarities between the type I and type II chains and also between the various chains of the same type. The crystal structure of the ActR-II ectodomain (Greenwald et al., 1999) revealed a typical pattern of four disulfide bridges that seem to occur in all the known receptor chains of the TGF-β superfamily. Additional disulfide bridges and the positions of a few amino acid residues seem to be characteristic for either the type I or the type II receptor proteins.
The binding of BMP-2 to its cellular receptors follows a mechanism differing from that established for TGF-βs or activins (Massague, 1998). The ordered sequential binding mechanism established for TGF-β involves, firstly, the binding of external ligand to the type II receptor chain (TR-II) and, secondly, within the membrane, the recruitment of the type I receptor (TR-I) into the complex (ten Dijke et al., 1994a; Wrana et al., 1994). Accordingly, the type II chain represents the high-affinity receptor for TGF-β. The interaction of activins with their receptors seems to follow a comparable route. In contrast, the high-affinity receptors for BMP-2 (Koenig et al., 1994; ten Dijke et al., 1994b; Macias-Silva et al., 1998) are the type I chains BMPR-IA, BMPR-IB and possibly also ActR-I. Type II chains on their own also bind solute BMP-2 but with lower affinity. Free BMPR-II or ActR-IIA in whole cells can be cross-linked with low efficiency to BMP-2, and the binding is found to be enhanced by co-expression of type I chains (Kawabata et al., 1995; Liu et al., 1995; Nohno et al., 1995; Rosenzweig et al., 1995; Yamashita et al., 1995). Thus, the sequential order of type I and type II receptor interaction with BMP-2 may be reversed compared with that of the TGF-βs, and a measurable but low affinity of type II BMP receptor chains for free BMP-2 exists. Compared with the TGF-β systems, the polarization in high- and low-affinity receptor types is less pronounced in the BMP-2 receptor systems. In contrast to observations on TGF-β type I and type II receptors, which were found to be fully homodimeric in the absence of ligand (Luo and Lodish, 1996, 1997; Weis Garcia and Massague, 1996), the BMP-2 receptors showed a more flexible oligomerization pattern. In live cells, a certain level of pre-existing heteromeric and homodimeric receptors has been detected in the absence of the BMP-2 ligand. Both hetero- and homo-oligomerization increased upon ligand binding (Gilboa et al., 2000). Because homo- as well as hetero-oligomerization has been shown to be crucial for activation and signalling of the TGF-β family of receptors, a more flexible oligomerization pattern might be of functional importance for the multiple biological activities and ligand–receptor interactions in the BMP receptor systems (see Gilboa et al., 2000).
For none of the type I or type II receptor interactions has a BMP-2 epitope been localized and characterized. On the basis of the three-dimensional structure (Scheufler et al., 1999), loops and cavities in the homodimeric BMP-2 protein have been proposed to represent possible binding sites. Mutations of dpp, the homologue of Drosophila BMP-2/-4, and of Xenopus or zebrafish BMP-2 and -4 have been identified, leading to inactive or in some instances dominant-negative proteins (Matzuk et al., 1995; Hammerschmidt et al., 1996; Chen et al., 1998). Peptides have been designed representing the loop regions of BMP-2 that inhibit BMP-2 activity (Kurita et al., 1995). Recently, for TGF-β1 (Huang et al., 1999) and activin A (Wuytens et al., 1999), mutant proteins that exhibit altered biological activity and receptor binding affinity have been constructed and analysed.
During the present study employing a collection of purified BMP-2 mutant proteins, two different non-overlapping epitopes of BMP-2 have been identified determining biological activity and binding to BMPR-IA or to the BMPR-II and ActR-II receptor chains. Most informative was the finding that antagonist/partial agonist activity of a subset of BMP-2 variants was correlated with reduced affinity for the BMPR-II receptor chain. A large epitope involved in BMPR-IA binding comprised elements from both monomers previously not suspected to be involved in receptor interaction. The results on epitope 1 are in excellent agreement with complementary data from the crystal structure determination of a BMP-2/–BMPR-IA ectodomain complex (Wuytens et al., 1999
Results
Collection of BMP-2 variants
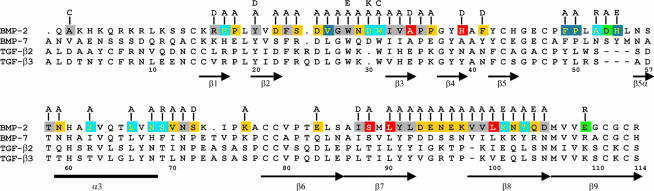
In order to identify functionally important amino acid side chains and receptor-binding epitopes in the mature part of human BMP-2, the 57 residues at the positions coloured in Figure 1 were substituted singly by in vitro mutagenesis. The mutant proteins were expressed in Escherichia coli (Ruppert et al., 1996) and a set of 42 variants substituted at 40 different positions could be isolated as dimeric proteins with a purity >95% and in a yield sufficient for a subsequent analysis of biological activity and receptor binding.
Fig. 1. BMP-2 residues substituted in this study. Variant BMP-2 proteins with reduced binding affinity for the type II receptor BMPR-II are indicated by the red colour of the substituted position. Altered binding affinities for the type I receptor BMPR-IA due to either a decreased association rate or an increased dissociation rate constant are indicated by dark and light blue of the respective substituted positions. Green indicates positions determining superagonist activity. Yellow positions indicate no measurable alterations in function of the respective variants. Variants substituted at the positions coloured grey could not be isolated in a purity or in amounts sufficient for functional analysis. The structure-based amino acid sequence alignment of BMP-2, BMP-7, TGF-β1 and TGF-β2 as well as the location of secondary structure elements as β-sheets (β1–β9) and α-helix (α3) was adapted from Scheufler et al. (1999). The numbering is according to the BMP-2 sequence.
For a first round of mutagenesis, 20 residues with surface-exposed side chains were chosen that cover the whole surface of the BMP-2 molecule in a net-like fashion (Scheufler et al., 1999). After obtaining variants with promising phenotypes, juxtaposed surface residues were exchanged systematically. Initially, residues were substituted by alanine in order to estimate the contribution of the substituted side chains to binding energy. Later on, charged residues were introduced as well, since it was expected that potentially disruptive substitutions cause a more severe phenotype. Finally, the additivity of single mutant phenotypes was studied by combining two amino acid substitutions in double mutant BMP-2 proteins.
Biological activity of BMP-2 variants
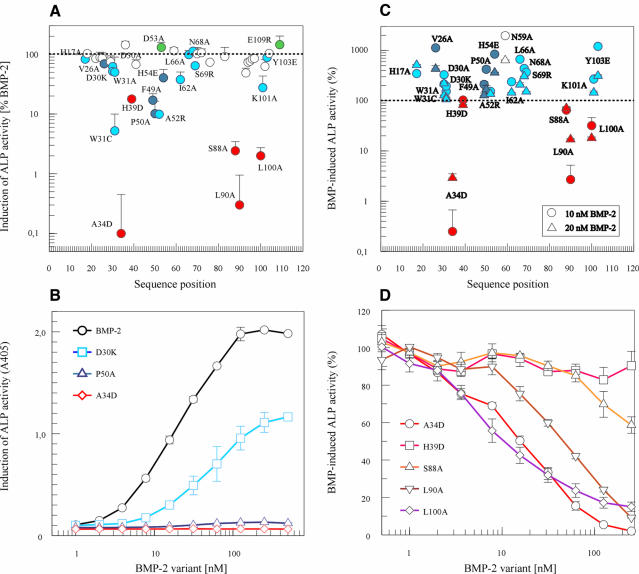
The expression of alkaline phosphatase (ALP) activity in C2C12 cells used for a quantitative assay of the biological activity of BMP-2 variants allowed rather small alterations to be established reproducibly. BMP-2 induces a high ALP activity with an ED50 of 20 ± 10 nM in a dose-dependent manner (Figure 2B). The occurrence and functional importance of BMPR-IA for the osteoinductive effects of BMP-2 in C2C12 cells has been reported (Namiki et al., 1997; Chalaux et al., 1998). The BMPR-IB receptor is detected only at very low levels and is unlikely to play a functional role during this cellular response. Type II receptors BMPR-II and ActR-II are present in C2C12 cells and can be cross-linked to the BMP-2 ligand in the absence, and more efficiently in the presence, of BMPR-IA. It has not been established thoroughly, however, if both type II receptors mediate BMP-2 responses in these cells.
Fig. 2. Biological activity and inhibitory properties of BMP-2 variants. (A) Alkaline phosphatase (ALP) activity was measured after incubation with 250 nM BMP-2 or BMP-2 variant. The response elicited by each variant was calculated as a percentage of the BMP-2 response. Values represent the mean value (±SD) of four measurements. Variants with red symbols are altered in BMPR-II interaction, as shown in Figure 3. Dark or light blue symbols indicate variants with altered association or dissociation rate constants, respectively, for binding to the BMPR-IA receptor chain. (B) Dose-dependent induction of ALP activity in serum-starved C2C12 cells is shown for BMP-2 (circles, black) and for BMP-2 variants D30K (squares, light blue), P50A (triangles, dark blue) and A34D (diamonds, red). (The background absorption at 405 nm of 0.08 ± 0.02 was not subtracted to indicate the signal-to-noise ratio.) (C) The inhibition of induction of ALP activity was determined in serum-starved C2C12 cells after incubation with 250 nM BMP-2 variant in the presence of 10 (circles) or 20 nM (triangles) BMP-2. The response obtained in the presence of BMP-2 alone is indicated by the dotted line and was taken as 100%. Data points represent the mean ± SD of four measurements. Variants with red symbols are altered in BMPR-II interaction. Light or dark blue symbols indicate variants with altered association or dissociation rate constants, respectively for the binding to the BMPR-IA receptor chain. (D) Inhibition of BMP-2 activity (10 nM BMP-2) by increasing doses of putative antagonistic/partial agonistic BMP-2 variants in serum-starved C2C12 cells. Dose–response curves of the variants A34D (circles), H39D (squares), S88A (upright triangles), L90A (inverted triangles) and L100A (diamonds) in the presence of 10 nM BMP-2 were obtained after incubation of the cells for 3 days and analysis of the induced ALP activity.
Several BMP-2 variants exhibited a clearly reduced activity when assayed at a concentration of 250 nM (Figure 2A). Variants A34D and L90A induced no significant response at all. Some other mutant proteins showed a reduced activity ranging from 2 to 30% of the BMP-2 activity. The majority of the variants exhibited a normal BMP-2-like response or an only slightly reduced response. Because the BMP-2 proteins precipitated at concentrations >500 nM in the cell culture medium, doses above 250 nM were not analysed. It therefore remained unknown whether the variants might still elicit a maximal response comparable with BMP-2 at higher concentrations. The symbols in the figures indicating the activity of the individual proteins at 250 nM are coloured according to the results of the receptor interaction analysis (see below). Red symbols indicate a reduced affinity for the BMPR-II ectodomain and blue colours a changed interaction with the BMPR-IA ectodomain.
Two variants behaved as ‘superagonists’. Variant D53A exhibited an ED50 of ∼6 nM. This means that it acts at 3-fold lower concentrations than BMP-2. Variant E109R consistently induced a 30% higher maximal response than BMP-2.
Antagonist activity
Surprisingly, some of the variants were able to inhibit BMP-2 activity. When C2C12 cells were stimulated with 10 nM BMP-2 in the presence of 250 nM variant, the induction of ALP activity was found to be reduced to <0.5% by A34D, to ∼3% by L90A, to 32% by L100A and to 65% by S88A of the value induced by BMP-2 in the absence of the mutant proteins (Figure 2C). At 20 nM BMP-2, an equal or somewhat weaker inhibition occurred. Variants W31C and H39D exerted no measurable effect on the BMP-2 response. The inhibitory activity of the latter variant is probably too low to be detected. The experimental set up of the C2C12 assay would not detect partial antagonist activities <30–50%. (Variant W31C is most probably inactive due to structural alterations, since the W31A variant showed a reduced but significant stimulatory activity.) In the presence of all other variants, the 10 and 20 nM BMP-2 responses were found to be increased to different extents.
The inhibitory property of these antagonist/partial agonist variants was confirmed by determining dose–inhibition curves as presented in Figure 2D. The mutant proteins A34D, L90A and L100A inhibited half-maximally at concentrations of 20–40 nM. This IC50 value is similar to the concentration of 10 nM BMP-2 present during the assay. Accordingly, these inhibitory variants work at concentrations similar to BMP-2, most probably by competing for a common receptor-binding site.
The detection of BMP-2 variants with antagonist/partial agonist properties indicates that specific alterations had been produced by the respective amino acid substitutions that affect the potency of the BMP-2 proteins but leave the receptor binding affinity largely unaffected (‘emasculated hormone’; Black, 1989).
Interaction of BMP-2 variants with receptor ectodomains
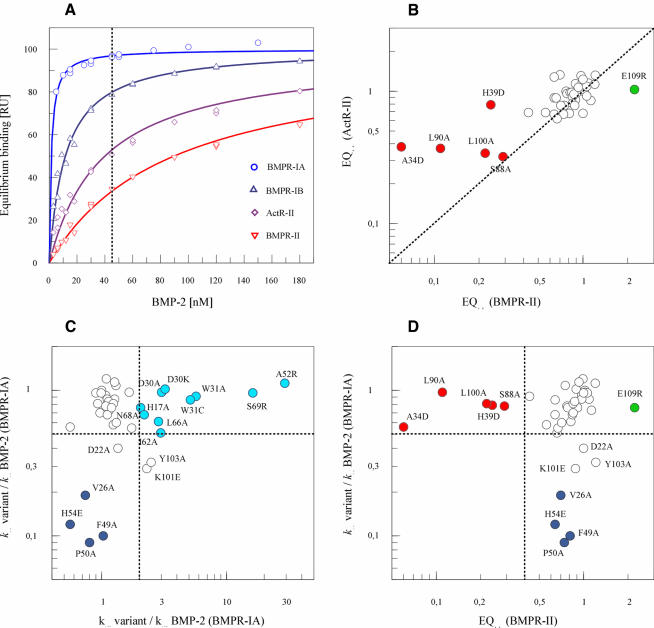
The alterations in biological activity observed with several of the BMP-2 variants are most likely to result from specific alterations in the BMP-2 epitopes for the binding of type I or type II receptor chains. This possibility was explored in detail by interaction analysis employing recombinant ectodomains of BMPR-IA, BMPR-II and ActR-II. Quantitative radioligand binding experiments with BMP-2 variants and whole cells would have posed serious experimental problems, since the BMP-2 protein binds tightly to the glycosaminoglycans present in the extracellular matrix and on cell surfaces (see for example Koenig et al., 1994; Reddi, 1994; Ruppert et al., 1996). The vast number of non-receptor BMP-2-binding sites in situ is probably responsible for the relatively high ED50 of 20 nM for BMP-2 during cellular responses (see above). Employing immobilized BMPR-IA ectodomain, differences in the rate constants of complex formation (kon) and dissociation (koff) with BMP-2 variants could be analysed readily by means of a biosensor system. The concentration dependence of BMP-2 equilibrium binding as evaluated in Figure 3A yields an apparent Kd of ∼1 nM. This affinity is in the range of the high-affinity binding observed, for example, between hGH and hGHbp or interleukin-4 (IL-4) and IL-4 receptor α (IL-4Rα) (Cunningham and Wells, 1993; Shen et al., 1996), and results mainly from the very slow dissociation rate of the ligand (apparent koff ∼4 × 10–4/s), suggesting a half-life of the complex of ∼0.5 h. It could not be decided if this exceedingly long half-life was caused by a simultaneous interaction of BMP-2 with two immobilized receptors or if it results from a true 1:1 interaction. The association rate (apparent kon ∼6 × 105/M/s) is comparable with that of other receptors (see for example Northrup and Erickson, 1992). BMPR-IB ectodomain, which exhibited an apparent Kd of 11 nM during dose-dependent equilibrium binding, was not analysed further in the present study, since this receptor chain is present in serum-starved C2C12 cells at very low levels only (Namiki et al., 1997; Chalaux et al., 1998).
Fig. 3. Interaction of BMP-2 variants with type I (BMPR-IA) or type II (BMPR-II, ActR-II) receptor ectodomains. The association (kon) and dissociation rate constants (koff) for the interaction of a BMP-2 variant at 15, 30 and 45 nM with immobilized BMPR-IA receptor ectodomain were evaluated. Equilibrium binding (EQ45) to the immobilized BMPR-II or ActR-II receptor ectodomain was measured at 45 nM variant. All values were normalized by taking the koff, kon and EQ45 values of BMP-2 as unity. (A) Equilibrium binding at increasing concentrations of BMP-2 to the type I receptors BMPR-IA (circles, light blue) and BMPR-IB (upright triangles, dark blue) as well as to the type II receptors ActR-II (diamonds, magenta) and BMPR-II (inverted triangles, red). (B) Binding affinity of BMP-2 variants for type II receptors BMPR-II or ActR-II. Equilibrium binding during biosensor analysis of 45 nM variant (EQ45) to BMPR-II is plotted versus the binding to ActR-II. (C) Plot of the association rate constants (kon) versus the dissociation rate constants (koff) for the interaction of BMP-2 variants with the BMPR-IA receptor. Variants specifically altered in their kon rates are indicated by dark blue symbols, and those with specifically altered koff by light blue symbols. (D) Plot of the association rate constant (kon) for BMPR-IA binding versus the equilibrium binding (EQ45) to BMPR-II of the same set of variants as in (C). Because both the association rate constant and equilibrium binding are dependent on the concentration of the BMP-2 variant, specific (and concentration-independent) alterations become apparent. (see for example K101E and Y103A for correlation of koff and EQ45). Variants with a specific decrease in equilibrium binding are marked in red.
A subset of variants exhibited specifically an increased dissociation rate of the complex with BMPR-IA, with koff values up to 30-fold higher than those of BMP-2 (Figure 3C; light blue symbols). The two variants modified at position D30 (D30A and D30K) and two variants at position W31 (W31A and W31C) belong to this subset. The most pronounced loss in binding affinity for BMPR-IA due to an increased koff value was established for charge variants A52R and S69R. Another subset of four variants exhibited decreased rate constants for the association with the BMPR-IA ectodomain, with kon values 5- to 10-fold lower than that of BMP-2 (Figure 3C; dark blue symbols). The reduced kon values of V26A, F49A, P50A and H54D were observed only for BMPR-IA but nor for BMPR-II or ActR-II interaction and, therefore, cannot result from instability or impurity, i.e. a lower effective concentration of these variants. Two variants (K101E and Y103A) showed 2-fold alterations in both kon and koff. The kinetics of the interaction with BMPR-IA of the other BMP-2 variants did not differ markedly from those of BMP-2.
Binding of BMP-2 and BMP-2 variants to the BMPR-II ectodomain could also be recorded (Figure 3B), despite the low affinity of this interaction. An apparent Kd of ∼100 nM was evaluated from the concentration dependence of equilibrium binding as shown in Figure 3A. The affinity of BMP-2 for the ActR-II ectodomain was found to be marginally stronger (Kd ∼50 nM). Remarkably, the apparent Kd for activin A binding to ActR-II ectodomain was reported to be 2–7 nM (Donaldson et al., 1999). The kinetic constants for the interaction between BMP-2 and the type II receptors are quite high (kon >106/M/s; koff >10–2/s). This prevented a reliable evaluation of the kon or koff values. A specific subset of the variants representing substitutions at five different positions showed an equilibrium binding (EQ45) to BMPR-II ectodomain that was up to 15 times lower than that of BMP-2 (Figure 3B and D, filled red symbols). These alterations were specific for the BMPR-II interaction. The kon values of these variants for BMPR-IA binding were inconspicuous. It is notable that the variants with decreased affinity for BMPR-II, and only these variants, behaved as competitive inhibitors of BMP-2 during the C2C12 assay.
The loss in ActR-II ectodomain binding was at most 3-fold with the alanine variants A34D, L90A, L100A and S88A. Thus, the binding residues of BMP-2 determining affinity for BMPR-II or ActR-II interaction differ quantitatively and qualitatively. Such differential effects of amino acid substitutions for binding to different receptors might allow the construction of selective agonists activating one or the other receptor chain preferentially.
Additive effects of combined amino acid substitutions in BMP-2 double mutants
Simultaneous substitution of two residues of BMP-2 aggravated the alterations in receptor binding (Table I). Interestingly, the type of alteration produced in a combination mutant was qualitatively the same as in the respective single mutants. The D30A/W31A variant exhibited a 30-fold increased rate constant specifically for the dissociation of the complex with BMPR-IA. Another double variant, F49A/P50A, combining single mutations leading to a reduced rate constant for the association with BMPR-IA, showed a 50-fold reduced relative kon value. Double mutants H39D/S88A and H39D/L100A, combining single substitutions H39D, S88A, and L100A, which have been discussed above for their at most 5-fold reduced binding to BMPR-II type II receptors, showed a 10- and 50-fold decrease, respectively, in equilibrium binding to BMPR-II. The roughly additive effects of these substitutions support the conclusion that the side chains analysed and also the two epitopes bind independently. The observation of a specific alteration in either the kon or koff rate constant for BMPR-IA interaction also in the double mutants indicates that the affected side chains contribute to the binding affinity of BMP-2 by different mechanisms. For unknown reasons, the binding affinity for ActR-II was decreased in the double mutants by only ∼3-fold, similar to several of the variants with single substitutions.
Table I. Combination of amino acid substitutions in BMP-2 double mutant proteins.
| Variant | Epitope | BMPR-IA |
BMPR-II |
ActR-II |
ALP(250) |
|||
|---|---|---|---|---|---|---|---|---|
|
koff |
kon |
EQ45 |
|
+10 nM BMP-2 |
+20 nM BMP-2 |
|||
| (var)/(wt) | (%BMP-2) | |||||||
| BMP-2 | 1.0 | 1.0 | 0.99 | 1.0 | 100 | 320 | 220 | |
| D30A | 1 | 3.0 | 0.97 | 1.0 | 1.0 | 62 | 330 | 220 |
| W31A | 1 | 5.7 | 0.91 | 0.43 | 0.69 | 50 | 150 | 120 |
| D30A/W31A | 1/1 | 30 | 0.92 | 0.49 | 1.0 | <0.5 | 88 | 99 |
| F49A | 1 | 1.0 | 0.10 | 0.81 | 0.68 | 17 | 210 | 130 |
| P50A | 1 | 0.80 | 0.09 | 0.74 | 0.67 | 10 | 420 | 170 |
| F49A/P50A | 1/1 | 2.6 | 0.02 | 0.84 | 0.60 | <0.5 | 130 | 120 |
| H39D | 2 | 1.1 | 0.79 | 0.24 | 0.79 | 18 | 100 | 82 |
| S88A | 2 | 1.1 | 0.78 | 0.29 | 0.32 | 2.4 | 65 | 71 |
| L100A | 2 | 1.2 | 0.81 | 0.22 | 0.34 | 2.0 | 32 | 18 |
| H39D/S88A | 2/2 | 1.2 | 0.88 | 0.09 | 0.35 | <0.5 | 34 | 50 |
| H39D/L100A | 2/2 | 1.1 | 0.90 | 0.02 | 0.33 | 0.6 | 2.6 | 4.2 |
| A34D | 2 | 0.56 | 0.56 | 0.06 | 0.38 | <0.5 | <0.5 | 2.9 |
| D53A | 1 | 1.1 | 1.2 | 0.99 | 1.2 | 130 | ||
| E109R | 2 | 1.2 | 0.76 | 2.2 | 1.0 | 150 | ||
| D30A/A34D | 1/2 | 1.9 | 0.85 | 0.02 | 0.31 | <0.5 | 2.2 | 7.4 |
| A34D/D53A | 2/1 | 1.1 | 1.5 | <0.02 | 0.31 | <0.5 | <0.5 | 0.7 |
| D53A/E109R | 1/2 | 1.4 | 1.3 | 2.7 | 1.1 | 130 | ||
Additive effects of kinetic rate constants kon and koff for association and dissociation of the complex with BMPR-IA ectodomain as well as of equilibrium binding at 45 nM concentrations, EQ45, to BMPR-II and ActR-II ectodomain. ALP activity at 250 nM variant, ALP(250), was measured in the absence and presence of 10 or 20 nM BMP-2.
Additive effects were also observed for double substitutions in trans comprising residues of both epitopes (Table I). Variant D30A/A34D had a higher relative koff for BMPR-IA, whereas the A34D/D53A mutant showed a slight increase in kon for BMP-IA. The equilibrium binding to BMPR-II was similarly low in the combination mutants and in variant A34D. Thus, the respective double variants retained the properties of both single variants. Another double variant, D53A/E109R, integrating substitutions for superagonist activities in both epitopes, exhibited both 2- to 3-fold increased equilibrium binding to BMPR-II and a slightly increased kon for BMPR-IA. This protein, however, also showed some increase in koff for BMPR- IA (see also F49A/P50A), suggesting that structural perturbations exist in this mutant protein.
Additive effects of substitutions were also found for the biological activity of the combination mutants (Table I). ALP induction in C2C12 cells was reduced below detectable levels in the variants D30A/W31A, F49A/P50A and H39D/S88A. Variant D53A/E109R, combining two superagonist substitutions, induced a higher ALP activity in C2C12 cells than BMP-2. The inhibitory activity of double mutants was increased in variants H39D/S88A and H39D/L100A compared with that of the single mutant proteins. The inhibitory activity of D30A/A34D was lower than that of the A34D single mutant. The A34D/D53A combination mutant in the presence of 20 nM BMP-2 showed the highest antagonist activity of all variants tested (Table I). This might result from the D53A substitution, which in the single mutant caused an increase in BMPR-IA affinity as well as in biological activity.
Location of binding epitopes
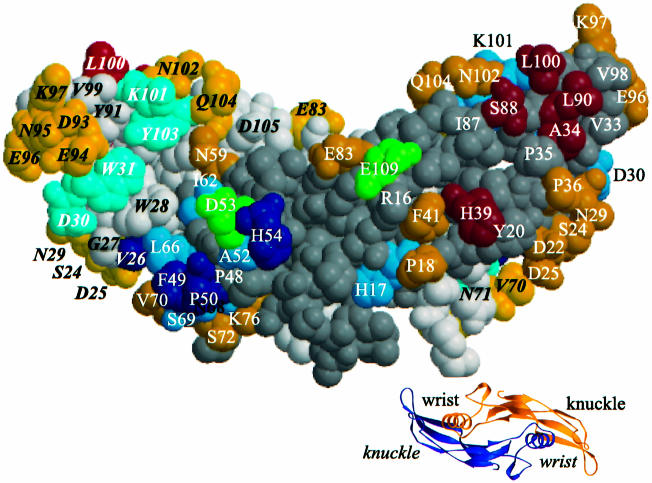
The amino acid positions of BMP-2 determining the binding affinity for either the BMPR-IA or BMPR-II receptor chains represent two non-overlapping subsets. As depicted in Figure 1, these determinants are distributed along the whole sequence of BMP-2. The space-filling model (Scheufler et al., 1999) in Figure 4 indicates, however, that the functional residues form two separate epitopes on the surface of the homodimeric BMP-2 molecule.
Fig. 4. Residues determining type I (wrist epitope) or type II receptor binding (knuckle epitope) are indicated on the space-filling model of BMP-2. Residues coloured red determine BMPR-II ectodomain binding. Substitutions of residues coloured blue result in altered BMPR-IA receptor binding either by increasing the dissociation rate constant koff (light blue) or by decreasing the association rate constant kon (dark blue). Residues coloured green determine enhanced receptor binding. In the space-filling model, the 2-fold axis of BMP-2 runs in the plane of the figure. The residues of one of the two monomers are indicated by bold italic letters. The residues of the other monomer are coloured with a darker hue. The small ribbon model presents a view of BMP-2 along the 2-fold axis and shows the location of the wrist and knuckle epitopes.
The determinants for BMPR-IA interaction co-localize in an epitope 1 that comprises residues from subunits 1 and 2. One monomer provides residues V26, D30 and W31 from the long loop connecting strands β2 and β3 as well as the weak determinants K101 and Y103 localized in strand β8. The other monomer contributes residues I62, L66, N68 and S69 in helix α3 as well as residues F49, P50, A52 and H54 in the long loop in front of helix α3. Remarkably, the latter loop residues yielded variants with reduced association rate constants. This might be related to the observation that residues in this loop showed the highest B-factors of BMP-2 (Scheufler et al., 1999). An even higher mobility of this loop in the variants could decrease the probability of a productive encounter with BMPR-IA and therefore decelerate association. Residue D53, which after alanine substitution yielded a variant with increased biological activity, is located in epitope 1. The weak determinant H17 seems to be separated from the other functional epitope 1 residues. It is possible that BMP-2 residues that have not yet been analysed and that are located between H17 and H54 may represent further contact residues for BMPR-IA.
Epitope 2 of BMP-2 involved in binding of BMPR-II is constituted by residues of one subunit only. Residues A34 and H39 occur in strands β3 and β4, respectively, residues S88 and L90 in β7, and residue L100 in β8. Residue E109, that after an arginine substitution was found to yield a variant with increased affinity for BMPR-II, might be a further contact residue. Epitope 2 seems to be smaller than epitope 1, since many residues at the boundary of epitope 2 have been modified without detectable effects on receptor binding or biological activity. It cannot be excluded, however, that epitope 2 comprises further functional residues, since substitutions of some residues in this region yielded proteins that could not be refolded and isolated at a functional state (e.g. P35, I87 and V98).
The homodimeric BMP-2 protein has a 2-fold symmetry axis that in Figure 4 lies in the plane of the paper and runs from top to bottom. Thus, on the back of the protein, a second pair made up of epitope 1 and epitope 2 exists. Some of the symmetry-related residues can be recognized at the borders of the model.
As a puzzling and even irritating result, no main determinants or ‘hot spots of binding’ as in other receptor systems (see for example Cunningham and Wells, 1993) have been detected during the present alanine-scanning mutational analysis of BMP-2. At most a 5- to 10-fold alteration in kinetic or equilibrium constants could be observed in some of the variants. It is a distinct possibility that some of the main determinants are not represented in the present collection of BMP-2 variants. The possibly missing determinants may be among the residues that after substitution yielded proteins that could not be recovered. Residues G27 and W28 might provide such examples. Another more likely possibility, however, might be that hydrogen bridges involving the N or O atoms of the main chain peptide bonds interact with the receptor(s) and therefore contribute to the binding affinity.
Discussion
The identification and characterization of two distinct binding epitopes in human BMP-2 as well as the detection of antagonistic BMP-2 variants provide new insights into the primary steps and mechanism of BMP receptor activation. Receptor-binding epitopes have not been described before for any of the closely related members of the TGF-β superfamily that signal via type I and type II receptor serine-kinases. All TGF-β-like proteins are dimers, usually homodimers, where the monomers have been compared with an open hand (Griffith et al., 1996; Mittl et al., 1996), with the central α-helix (α3) being the wrist or heel and the two aligned two-stranded β-sheets representing the four fingers, with loop 1 and loop 2 at the tips of each pair of fingers. The N-terminal segment exists at the position of the thumb. Consequently, the epitope 1 assembled around the central α-helix is called in the following the ‘wrist epitope’ and epitope 2 located at the back of the hand near the outer finger segments is called the ‘knuckle epitope’.
The wrist epitope has dimensions of ∼20 × 25–30 Å (500–600 Å2). This large area would be compatible with the function as a high-affinity interaction site. The knuckle epitope seems to be smaller with dimensions of 10 × 20–25 Å (200–250 Å2) in accordance with the lower affinity of the interaction with BMPR-II at this site. The wrist epitope is highly discontinuous (see Figure 1) and it comprises different elements of both monomers. In heterodimers, e.g. of BMP-4 and BMP-7 (see for example Iemura et al., 1998) or of the inhibin/activin-type factors (see for example Wuytens et al., 1999), this has the interesting consequence that the two symmetry-related epitopes are no longer equivalent and may therefore exhibit different receptor-binding properties. In the knuckle epitope, the binding residues are provided by one monomer only and are located in sheets β3, β4, β7 and β8 and possibly also in β9 (E109). An epitope with similar topology has been established in glial cell-derived neurotrophic factor (GDNF) (Eketjall et al., 1999), a distant member of the TGF-β superfamily. The GDNF epitope determining high-affinity binding to its accessory receptor GFRα1 extends, however, more to the poles of the dimer and comprises side chains of the finger 2 loop and the knuckle of finger 1 that are not critical for BMPR-II binding in BMP-2.
The juxtaposed knuckle and wrist epitopes are only 10–15 Å apart. The distances between the two type I (∼40 Å) or the two type II chains (∼55 Å), that possibly are assembled at BMP-2, are much larger. This appears to be especially relevant for the receptor serine-kinases. Their small ectodomain is connected to the membrane-spanning segment by a short linker of <12 residues. In the receptor complex with BMP-2, this short distance between epitopes 1 and 2 might facilitate the interaction of the type I and type II receptor serine-kinases.
The identification and detailed molecular characterization of the two binding epitopes in BMP-2 have implications for an understanding of binding affinity and specificity in other BMP subfamilies and probably also in other members of the TGF-β superfamily. BMP-7 and GDF-5 have been found to be able to bind to a distinct set of receptor chains that partially overlaps with the receptor chains mediating the signals of the BMP-2/BMP-4 subfamily (ten Dijke et al., 1994b; Liu et al., 1995; Rosenzweig et al., 1995; Nishitoh et al., 1996). They also exhibit a high affinity for the type I receptors and bind weakly to the type II chains. Their binding affinity for the complex formed by type I and type II receptors is stronger than the binding to either receptor type alone (cooperative binding mode). Thus it is conceivable that the binding epitopes are located in all these proteins at the same sites as in BMP-2. It is interesting to note that most residues existing in the BMP-2 wrist epitope are invariant or replaced by isofunctional side chains in BMP-7 (10/15) and GDF-5 (12/15). These similarities suggest that specificity in the wrist epitope is determined by only a small subset of residues, whereas the binding affinity may be provided by the same set of residues in the high-affinity epitope of all BMP/GDF proteins. A similar specification into affinity- or specificity-determining residues seems to exist in the BMP-2 knuckle epitope. Most binding residues in the second epitope of BMP-2 are invariant in BMP-7 (4/6) and GDF-5 (5/6). It is noteworthy that also in the BMP-2/-4 subfamily the few differences between the amino acid sequences of BMP-2 and BMP-4 are not in the wrist epitope and only one difference exists (H39/Q39) in the knuckle epitope.
The occurrence of the BMP-2 antagonists detected in this study is most likely to be a consequence of an ordered sequential binding mechanism operating during receptor activation. The antagonist blocks the high-affinity type I receptor chain via its intact wrist epitope, and the disrupted knuckle epitope prevents the subsequent interaction with the low-affinity type II chain(s). The similarly low IC50 of the antagonists as well as their efficient competition with BMP-2 for receptor binding could indicate that it is predominantly the type I chain(s) that adjust(s) the binding affinity of BMP-2 for the whole receptor complex. Interestingly, the velocity of complex formation and dissociation with BMPR-IA is equally critical, as revealed by the complete loss of biological activity of the respective double mutants (compare D30A/W31A and F49A/P50A in Table I). An intriguing finding is the dramatic loss of biological activity in variants of the knuckle epitope (see A34D and H39D/S88A), considering that binding to the ectodomains of the type II receptor chain(s) is reduced only 10- to 15-fold. It is possible that the simultaneous binding of two type II chains is necessary for an efficient receptor activation (Gilboa et al., 2000), and therefore a decrease in binding affinity becomes aggravated.
If other BMPs or GDFs activate their respective receptors by the same mechanism as BMP-2, i.e. via a high-affinity wrist epitope and a low-affinity knuckle epitope, then antagonist variants of these proteins might also be produced by amino acid substitutions in the knuckle epitope. For other members of the TGF-β superfamily, such as TGF-βs or activins, the location of the low-affinity epitope is still unclear. Recently, substitutions of two amino acid residues in TGF-β1 have been described (W52A, D55A) causing a 2- to 5-fold reduced activity of mutant proteins during a mink lung cell assay (Huang et al., 1999). As seen in the structure-based sequence comparison in Figure 1, the postulated binding residues of TGF-β1 occur in the N-terminal loop of helix α3 that in BMP-2 is four residues longer than in the TGF-βs and that contains at least five binding residues of the BMP-2 wrist epitope. This strongly suggests that TGF-β1 contains a binding epitope at a similar site to that in the wrist epitope in BMP-2. It is still unknown which of the TGF-β receptor types might interact with W52/D55 and whether these residues are part of a high- or low-affinity epitope. With activin A, two variants altered in ActR-II binding have been identified (Wuytens et al., 1999). Variant D27K was found to be active at lower concentrations than normal activin A (superagonist). Substitutions at a second position yielded variants K102R and K102A that both were ∼50-fold less potent than activin A. Both K102 and D27 alter affinity cross-linking to ActR-II expressed in COS cells. Sequence alignments suggest (for other interpretation see Wuytens et al., 1999) that K102 of activin A corresponds to L100 of BMP-2 or V98 (I98) of TGF-β3 (TGF-β2), which are all located on the edge of the convex side of the protein. BMP-2 residue L100 was identified as a binding determinant in the knuckle epitope. Activin D27 corresponds to BMP-2 D30 that was located at the border of the wrist epitope. Thus, the analysis of more activin mutant proteins has to be awaited before a clear assignment and comparison of the binding epitopes can be established. Because the low-affinity chain(s) are the type I receptors in these systems, available evidence does not help to discriminate if the wrist or the knuckle epitope determines binding according to the receptor type or the receptor affinity, respectively.
Members of the TGF-β superfamily have been associated with various disease states, such as, for example, BMP-2 with ectopic bone formation and periarticular ossification (see Reddi, 1998), BMP-6 with psoriasis (Blessing et al., 1996), GDF-8 with musculodegenerative syndroms (McPherron et al., 1997) and TGF-βs with scarring and fibrosis (Lawrence, 1996). The definition of binding epitopes in BMP-2 and the finding of BMP-2 antagonists is a first step towards the development of drugs targeted at receptors of the TGF-β superfamily.
Materials and methods
Preparation of recombinant receptor ectodomains
An extracellular domain of human BMPR-IA was expressed in E.coli and purified as described (Kirsch et al., 2000a). The extracellular domains of ActR-II (residues 19–126) (Matzuk and Bradley, 1992), BMPR-II (residues 27–151) (Rosenzweig et al., 1995) and BMPR-IB (residues 14–126) (Ide et al., 1997) were expressed with a C-terminal thrombin cleavage site (LVPRGS) plus a His6 tag in Sf9 insect cells according to the manufacturer’s instructions. The proteins were isolated from the culture medium of infected Sf-9 cells by standard procedures involving Ni-NTA–agarose (Qiagen) and BMP-2-affinity chromato graphy (Kirsch et al., 2000a). The purified receptor proteins were N-biotinylated by incubation with equimolar concentrations of sulfo- NHS-LC-biotin (Pierce) as described (Shen et al., 1996).
Preparation of BMP-2 variants
A BMP-2 cDNA representing residues 283–396 (Wozney et al., 1988) of the mature protein plus an N-terminal MA extension was submitted to in vitro cassette mutagenesis employing synthetic double-stranded oligonucleotides. The BMP-2 variants were expressed in E.coli, recovered as inclusion bodies, renatured and purified as detailed elsewhere (Ruppert et al., 1996).
C2C12 alkaline phosphatase assay
The promyoblast C2C12 cells (ATCC CRL-1772) were stimulated at a density of 3 × 104 cells/well in a 96-well microtitre plate for 3 days with 0.5–250 nM of each BMP-2 variant in 100 µl of Dulbecco’s modified Eagle’s medium (DMEM) containing 2% calf serum and antibiotics (100 U/ml penicillin G and 100 µg/ml streptomycin) at 37°C in a humidified atmosphere at 5% CO2 (Katagiri et al., 1994). Cells were washed with phosphate-buffered saline (PBS) and then lysed for 1 h with 100 µl of 1% NP-40 in ALP buffer (0.1 M glycine, pH 9.6, 1 mM MgCl2, 1 mM ZnCl2). ALP activity was determined by incubating lysed cells for 15 min with 100 µl of ALP buffer plus 1 mg/ml p-nitrophenylphosphate, and measuring the extinction at 405 nm. One A405 extinction unit corresponds to 1.5 nmol of p-nitrophenolate produced per min per 3 × 104 cells. Results were expressed as mean values measured during four independent experiments with a mean SD of ±39%. Inhibition experiments performed in the presence of 10 or 20 nM BMP-2 showed larger standard deviations, which are indicated in the respective figures.
Biosensor interaction analysis
The BIA2000 system (BIAcore) was used to record the binding of BMP-2 variants to immobilized receptor ectodomains. The biotinylated proteins were fixed separately to streptavidin-coated matrix of biosensor CM5 in flow cells 2, 3 and 4 at a density of ∼200 resonance units (RU) corresponding to 200 pg of protein (∼15 fmol of receptor) per mm2. BMP-2 variants at 15–300 nM in HBS buffer (10 mM HEPES pH 7.4, 500 mM NaCl, 3.4 mM EDTA, 0.005% surfactant P20) were perfused over flow cells 1, 2, 3 and 4 in series at a flow rate of 10 µl/min at 25°C, and sensograms were recorded at a data sampling rate of 2.5 Hz. The association period was 20 min and the dissociation period was set to 6 min. Free receptors were regenerated by perfusion with 0.1 M acetic acid, 1 M NaCl for 2 min.
The background sensogram recorded for flow cell 1 (streptavidin control) was subtracted from the sensograms obtained for flow cells 2 (BMPR-II), 3 (ActR-II) and 4 (BMPR-IA). The differential sensograms were evaluated according to the fitting routine 2 provided by the BIA evaluation 2.2.4 software (BIAcore). Equilibrium binding of BMP-2 variants at 45 nM (EQ45) was measured twice in duplicate with a maximal SD of ±20%. The reported apparent rate constants kon for association and koff for dissociation for the interaction between BMPR-IA and BMP-2 variants are mean values of at least 12 measurements performed with at least three different concentrations of the ligands. Mean SDs with BMP-2 were 12% for kon and 20% for koff. Because the true stoichiometry of complex formation was not certain, all sensograms were evaluated on the basis of an unproven 1:1 association model, and therefore only apparent and not absolute constants are presented.
Supplementary data
Supplementary data to this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
The authors thank C.Söder and A.Will for excellent technical assistance, and P.Knaus and M.Dreyer for help and advice. This work was supported by the Deutsche Forschungsgemeinschaft (DFG), grant Se 435/3-3 and SFB 487 TP B1.
References
- Black J. (1989) Drugs from emasculated hormones: the principle of syntopic antagonism. Science, 245, 486–493. [DOI] [PubMed] [Google Scholar]
- Blessing M., Schirmacher,P. and Kaiser,S. (1996) Overexpression of bone morphogenetic protein-6 (BMP-6) in the epidermis of transgenic mice: inhibition or stimulation of proliferation depending on the pattern of transgene expression and formation of psoriatic lesions. J. Cell Biol., 135, 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalaux E., Lopez Rovira,T., Rosa,J.L., Bartrons,R. and Ventura,F. (1998) JunB is involved in the inhibition of myogenic differentiation by bone morphogenetic protein-2. J. Biol. Chem., 273, 537–543. [DOI] [PubMed] [Google Scholar]
- Chen Y., Riese,M.J., Killinger,M.A. and Hoffmann,F.M. (1998) A genetic screen for modifiers of Drosophila decapentaplegic signaling identifies mutations in punt, Mothers against dpp and the BMP-7 homologue, 60A. Development, 125, 1759–1768. [DOI] [PubMed] [Google Scholar]
- Cunningham B.C. and Wells,J.A. (1993) Comparison of a structural and a functional epitope [published erratum appears in J. Mol. Biol.,237, 513]. J. Mol. Biol., 234, 554–563. [DOI] [PubMed] [Google Scholar]
- Daopin S., Piez,K.A., Ogawa,Y. and Davies,D.R. (1992) Crystal structure of transforming growth factor-β2: an unusual fold for the superfamily. Science, 257, 369–373. [DOI] [PubMed] [Google Scholar]
- Donaldson C.J., Vaughan,J.M., Corrigan,A.Z., Fischer,W.H. and Vale,W.W. (1999) Activin and inhibin binding to the soluble extracellular domain of activin receptor II. Endocrinology, 140, 1760–1766. [DOI] [PubMed] [Google Scholar]
- Eketjall S., Fainzilber,M., Murray Rust,J. and Ibanez,C.F. (1999) Distinct structural elements in GDNF mediate binding to GFRα1 and activation of the GFRα1–c-Ret receptor complex. EMBO J., 18, 5901–5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L., Nohe,A., Geissendörfer,T., Sebald,W., Henis,Y.I. and Knaus,P. (2000) BMP receptor complexes on the surface of live cells: a new oligomerization mode for serine/threonine kinase receptors. Mol. Biol. Cell, 11, 1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald J., Fischer,W.H., Vale,W.W. and Choe,S. (1999) Three-finger toxin fold for the extracellular ligand-binding domain of the type II activin receptor serine kinase. Nature Struct. Biol., 6, 18–22. [DOI] [PubMed] [Google Scholar]
- Griffith D.L., Keck,P.C., Sampath,T.K., Rueger,D.C. and Carlson,W.D. (1996) Three-dimensional structure of recombinant human osteogenic protein 1: structural paradigm for the transforming growth factor beta superfamily. Proc. Natl Acad. Sci. USA, 93, 878–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt M., Serbedzija,G.N. and McMahon,A.P. (1996) Genetic analysis of dorsoventral pattern formation in the zebrafish: requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes Dev., 10, 2452–2461. [DOI] [PubMed] [Google Scholar]
- Heldin C.H. (1995) Dimerization of cell surface receptors in signal transduction. Cell, 80, 213–223. [DOI] [PubMed] [Google Scholar]
- Heldin C.H., Miyazono,K. and ten Dijke,P. (1997) TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature, 390, 465–471. [DOI] [PubMed] [Google Scholar]
- Hogan B.L. (1996) Bone morphogenetic proteins in development. Curr. Opin. Genet. Dev., 6, 432–438. [DOI] [PubMed] [Google Scholar]
- Huang S.S., Zhou,M., Johnson,F.E., Shieh,H.S. and Huang,J.S. (1999) An active site of transforming growth factor-β1 for growth inhibition and stimulation. J. Biol. Chem., 274, 27754–27758. [DOI] [PubMed] [Google Scholar]
- Ide H., Katoh,M., Sasaki,H., Yoshida,T., Aoki,K., Nawa,Y., Osada,Y., Sugimura,T. and Terada,M. (1997) Cloning of human bone morphogenetic protein type IB receptor (BMPR-IB) and its expression in prostate cancer in comparison with other BMPRs [published erratum appears in Oncogene, 15, 1121]. Oncogene, 14, 1377–1382. [DOI] [PubMed] [Google Scholar]
- Iemura S., Yamamoto,T.S., Takagi,C., Uchiyama,H., Natsume,T., Shimasaki,S., Sugino,H. and Ueno,N. (1998) Direct binding of follistatin to a complex of bone-morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryo. Proc. Natl Acad. Sci. USA, 95, 9337–9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T. et al. (1994) Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage [published erratum appears in J. Cell Biol., 128, following 713]. J. Cell Biol., 127, 1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata M., Chytil,A. and Moses,H.L. (1995) Cloning of a novel type II serine/threonine kinase receptor through interaction with the type I transforming growth factor-β receptor. J. Biol. Chem., 270, 5625–5630. [DOI] [PubMed] [Google Scholar]
- Kirsch T., Nickel,J. and Sebald,W. (2000a) Isolation of recombinant BMP receptor IA ectodomain and its 2:1 complex with BMP-2. FEBS Lett., 468, 215–219. [DOI] [PubMed] [Google Scholar]
- Kirsch T., Sebald,W. and Dreyer, M. (2000b) Crystal structure of the BMP-2-BRIA ectodomain complex. Nature Struct. Biol., 7, 492–496. [DOI] [PubMed] [Google Scholar]
- Koenig B.B. et al. (1994) Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol. Cell. Biol., 14, 5961–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T., Matsumoto,T., Kikuno,R., Otawara-Hamamoto,Y. and Breipohl,G. (1995) Dimeric bone morphogenetic proteins and fragments and analogs thereof and pharmaceutical compositions comprising them. European Patent Application, 0691 349 A2.
- Lawrence D.A. (1996) Transforming growth factor-β: a general review. Eur. Cytokine Network, 7, 363–374. [PubMed] [Google Scholar]
- Liu F., Ventura,F., Doody,J. and Massague,J. (1995) Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol. Cell. Biol., 15, 3479–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K. and Lodish,H.F. (1996) Signaling by chimeric erythropoietin-TGF-β receptors: homodimerization of the cytoplasmic domain of the type I TGF-β receptor and heterodimerization with the type II receptor are both required for intracellular signal transduction. EMBO J., 15, 4485–4496. [PMC free article] [PubMed] [Google Scholar]
- Luo K. and Lodish,H.F. (1997) Positive and negative regulation of type II TGF-β receptor signal transduction by autophosphorylation on multiple serine residues. EMBO J., 16, 1970–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias-Silva M., Hoodless,P.A., Tang,S.J., Buchwald,M. and Wrana,J.L. (1998) Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J. Biol. Chem., 273, 25628–25636. [DOI] [PubMed] [Google Scholar]
- Massague J. (1998) TGF-β signal transduction. Annu. Rev. Biochem., 67, 753–791. [DOI] [PubMed] [Google Scholar]
- Matzuk M.M. and Bradley,A. (1992) Cloning of the human activin receptor cDNA reveals high evolutionary conservation [published erratum appears in Biochim. Biophys. Acta, 1131, 243]. Biochim. Biophys. Acta, 1130, 105–108. [DOI] [PubMed] [Google Scholar]
- Matzuk M.M., Kumar,T.R., Vassalli,A., Bickenbach,J.R., Roop,D.R., Jaenisch,R. and Bradley,A. (1995) Functional analysis of activins during mammalian development. Nature, 374, 354–356. [DOI] [PubMed] [Google Scholar]
- McPherron A.C., Lawler,A.M. and Lee,S.J. (1997) Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature, 387, 83–90. [DOI] [PubMed] [Google Scholar]
- Mittl P.R., Priestle,J.P., Cox,D.A., McMaster,G., Cerletti,N. and Grutter,M.G. (1996) The crystal structure of TGF-β 3 and comparison to TGF-β 2: implications for receptor binding. Protein Sci., 5, 1261–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki M., Akiyama,S., Katagiri,T., Suzuki,A., Ueno,N., Yamaji,N., Rosen,V., Wozney,J.M. and Suda,T. (1997) A kinase domain-truncated type I receptor blocks bone morphogenetic protein-2-induced signal transduction in C2C12 myoblasts. J. Biol. Chem., 272, 22046–22052. [DOI] [PubMed] [Google Scholar]
- Nishimura R., Kato,Y., Chen,D., Harris,S.E., Mundy,G.R. and Yoneda,T. (1998) Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J. Biol. Chem., 273, 1872–1879. [DOI] [PubMed] [Google Scholar]
- Nishitoh H., Ichijo,H., Kimura,M., Matsumoto,T., Makishima,F., Yamaguchi,A., Yamashita,H., Enomoto,S. and Miyazono,K. (1996) Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J. Biol. Chem., 271, 21345–21352. [DOI] [PubMed] [Google Scholar]
- Nohno T., Ishikawa,T., Saito,T., Hosokawa,K., Noji,S., Wolsing,D.H. and Rosenbaum,J.S. (1995) Identification of a human type II receptor for bone morphogenetic protein-4 that forms differential heteromeric complexes with bone morphogenetic protein type I receptors. J. Biol. Chem., 270, 22522–22526. [DOI] [PubMed] [Google Scholar]
- Northrup S.H. and Erickson,H.P. (1992) Kinetics of protein–protein association explained by Brownian dynamics computer simulation. Proc. Natl Acad. Sci. USA, 89, 3338–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi A.H. (1994) Bone and cartilage differentiation. Curr. Opin. Genet. Dev., 4, 737–744. [DOI] [PubMed] [Google Scholar]
- Reddi A.H. (1997) Bone morphogenetic proteins: an unconventional approach to isolation of first mammalian morphogens. Cytokine Growth Factor Rev., 8, 11–20. [DOI] [PubMed] [Google Scholar]
- Reddi A.H. (1998) Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nature Biotechnol., 16, 247–252. [DOI] [PubMed] [Google Scholar]
- Rosenzweig B.L., Imamura,T., Okadome,T., Cox,G.N., Yamashita,H., ten Dijke,P., Heldin,C.H. and Miyazono,K. (1995) Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl Acad. Sci. USA, 92, 7632–7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert R., Hoffmann,E. and Sebald,W. (1996) Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur. J. Biochem., 237, 295–302. [DOI] [PubMed] [Google Scholar]
- Scheufler C., Sebald,W. and Hulsmeyer,M. (1999) Crystal structure of human bone morphogenetic protein-2 at 2.7 Å resolution. J. Mol. Biol., 287, 103–115. [DOI] [PubMed] [Google Scholar]
- Schlunegger M.P. and Grutter,M.G. (1992) An unusual feature revealed by the crystal structure at 2.2 Å resolution of human transforming growth factor-β2. Nature, 358, 430–434. [DOI] [PubMed] [Google Scholar]
- Shen B.J., Hage,T. and Sebald,W. (1996) Global and local determinants for the kinetics of interleukin-4/interleukin-4 receptor α chain interaction. A biosensor study employing recombinant interleukin-4-binding protein. Eur. J. Biochem., 240, 252–261. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Yamashita,H., Ichijo,H., Franzen,P., Laiho,M., Miyazono,K. and Heldin,C.H. (1994a) Characterization of type I receptors for transforming growth factor-β and activin. Science, 264, 101–104. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Yamashita,H., Sampath,T.K., Reddi,A.H., Estevez,M., Riddle,D.L., Ichijo,H., Heldin,C.H. and Miyazono,K. (1994b) Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J. Biol. Chem., 269, 16985–16988. [PubMed] [Google Scholar]
- Weis Garcia F. and Massague,J. (1996) Complementation between kinase-defective and activation-defective TGF-β receptors reveals a novel form of receptor cooperativity essential for signaling. EMBO J., 15, 276–289. [PMC free article] [PubMed] [Google Scholar]
- Wozney J.M., Rosen,V., Celeste,A.J., Mitsock,L.M., Whitters,M.J., Kriz,R.W., Hewick,R.M. and Wang,E.A. (1988) Novel regulators of bone formation: molecular clones and activities. Science, 242, 1528–1534. [DOI] [PubMed] [Google Scholar]
- Wrana J.L., Attisano,L., Wieser,R., Ventura,F. and Massague,J. (1994) Mechanism of activation of the TGF-β receptor. Nature, 370, 341–347. [DOI] [PubMed] [Google Scholar]
- Wuytens G. et al. (1999) Identification of two amino acids in activin A that are important for biological activity and binding to the activin type II receptors. J. Biol. Chem., 274, 9821–9827. [DOI] [PubMed] [Google Scholar]
- Yamashita H., ten Dijke,P., Huylebroeck,D., Sampath,T.K., Andries,M., Smith,J.C., Heldin,C.H. and Miyazono,K. (1995) Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J. Cell Biol., 130, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]