Abstract
Aims
The objective of this study was to investigate whether vascular endothelial growth factor (VEGF) secreted by mesenchymal stem cells (MSC) improves myocardial survival and the engraftment of implanted MSC in infarcted hearts and promotes recruitment of stem cells through paracrine release of myocardial stromal cell-derived factor-1α (SDF-1α).
Methods and results
VEGF-expressing MSC (VEGFMSC)-conditioned medium enhanced SDF-1α expression in heart slices and H9C2 cardiomyoblast cells via VEGF and the vascular endothelial growth factor receptor (VEGFR). The VEGFMSC-conditioned medium markedly promoted cardiac stem cell (CSC) migration at least in part via the SDF-1α/CXCR4 pathway and involved binding to VEGFR-1 and VEGFR-3. In vivo, VEGFMSC-stimulated SDF-1α expression in infarcted hearts resulted in massive mobilization and homing of bone marrow stem cells and CSC. Moreover, VEGF-induced SDF-1α guided the exogenously introduced CSC in the atrioventricular groove to migrate to the infarcted area, leading to a reduction in infarct size. Functional studies showed that VEGFMSC transplantation stimulated extensive angiomyogenesis in infarcted hearts as indicated by the expression of cardiac troponin T, CD31, and von Willebrand factor and improved the left ventricular performance, whereas blockade of SDF-1α or its receptor by RNAi or antagonist significantly diminished the beneficial effects of VEGFMSC.
Conclusion
Exogenously expressed VEGF promotes myocardial repair at least in part through SDF-1α/CXCR4-mediated recruitment of CSC.
Keywords: Myocardial infarction, VEGF, SDF-1α, Cardiac stem cell, Migration
1. Introduction
Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) are crucial regulators of the growth, development, and differentiation of heart and blood vessels.1 The therapeutic effect of VEGF is signified by the improvement of heart structure and function.2,3 VEGF gene delivery together with other growth factors and cell therapy (a part of the multimodal therapy approach) has been shown to promote the survival, engraftment, and differentiation of donor cells.4,5 The role of VEGF in engaging cardiac stem cells (CSCs) during the cardiac repair process, however, remains to be determined.
Mesenchymal stem cells (MSC) are multipotential progenitor cells secreting a plethora of angiogenic and mitogenic cytokines and growth factors in normoxic culture condition; the secretion of these factors is increased significantly in response to anoxia.6 Secretion of paracrine factors by other cell types, such as endothelial progenitor cells and cardiomyocytes (CMs), has also been reported.7,8 The expression of these cytokines provides an alternative and/or supportive mechanism for cell transplantation in tissue repair.9 The paracrine pathway in CMs is important to maintain normal cardiac function due to VEGF release.8 However, the mechanisms underlying the alteration of paracrine action in CMs after MSC transplantation are still unknown. We hypothesize that MSC play a pivotal role in recruiting CSC to the injured heart via the release of CM-derived SDF-1α, a potent chemoattractant of stem cells.
2. Methods
The present study was conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. All animal protocols were approved by the Institutional Animal Care and Use Committee of Hubei University of Medicine.
2.1. Western blot and ELISA
Western blot was carried out with rabbit polyclonal antibody raised against SDF-1α (Santa Cruz) and mouse polyclonal antibody raised against VEGF (Santa Cruz) to identify the protein expression of VEGF and SDF-1α.
Rat left ventricles (LVs) were used for comparison among all groups 7 days after treatment. These samples were homogenized on ice in RIPA buffer containing protease inhibitors. Fifty micrograms of proteins was resolved in 12% SDS–PAGE gel and transferred onto a nitrocellulose membrane (Millipore). After being blocked with 5% non-fat milk, the membrane was incubated with primary antibody (1:1000 of dilution) for 90 min followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (anti-rabbit IgG and anti-mouse IgG, Santa Cruz). Protein expression was visualized by enhanced chemiluminescence reaction (Amersham Pharmacia Biotech) and measured by densitometry. Cardiac tissue and serum contents of hVEGF, rVEGF (Neobioscience, China), rSDF-1α (Ever System Biology Laboratory, Inc., USA), and hSDF-1α (R&D Systems, Minneapolis, MN, USA) were quantitatively measured by ELISA.10
2.2. Immunostaining
Heart tissues were fixed in 4% paraformaldehyde and embedded in optimum cutting temperature compound (Fisher Scientific). Serial transverse sections (5 μm) were cut across the longitude axis of the heart and mounted on slides. After a brief wash in phosphate-buffered saline (PBS), heart sections were incubated in a blocking buffer [PBS containing 1% foetal calf serum (FCS) and 0.1% Triton X-100] at room temperature for 1 h. Incubations in antibodies (diluted 1:250 in blocking buffer) were carried out at 4°C overnight for primary antibodies, and room temperature for 2 h for secondary antibodies. The primary antibodies used were: mouse anti-rat CD31 (Abcam), rabbit anti-rat von Willebrand factor (VWF, Santa Cruz), rabbit anti-c-kit (Santa Cruz), rabbit anti-rat MDR1 (Santa Cruz), mouse anti- cardiac troponin T (cTnt, NeoMarkers), mouse anti-Flk-1 (Santa Cruz), goat anti-Flt-1 (Santa Cruz), and rabbit anti-rat CXCR4 (Santa Cruz). The secondary antibodies were TRITC-conjugated anti-rabbit IgG, TRITC-conjugated anti-mouse IgG, FITC-conjugated anti-rabbit IgG, FITC-conjugated anti-mouse IgG, and FITC-conjugated anti-goat IgG (Santa Cruz).10
2.3. Characterization of hVEGF165-modified human MSC
Bone marrow-derived MSC were isolated and cultured as described previously.11 Control (Ad-LacZ) and human VEGF165 (Ad-VEGF) adenoviral vector have been reported in our previous studies.10 The replication-deficient vectors were propagated in 293 cells cultured in DMEM supplemented with 15% foetal calf serum (FCS, Hyclone, USA). MSC were infected with Ad-LacZ or Ad-VEGF at a multiplicity of infection of 100 for 2 days to produce MSC expressing LacZ (LacZMSC) or VEGF165 (VEGFMSC). MSC and supernatants were collected for examining the transduction efficiency and VEGF secretion, respectively, by ELISA and western blotting (see Supplementary material online, Data 1).
2.4. Isolation and culture of c-kit+ cells from rat hearts
CSCs were isolated from the hearts of 3-day-old Sprague–Dawley (SD) rats by a method described previously.10 Briefly, hearts were removed under aseptic conditions from rats that had been overdosed with sodium pentobarbital. Isolated myocardial tissue was cut into 1–2 mm3 pieces and washed with Ca2+ and Mg2+-free PBS to remove blood. Heart pieces were then digested with 0.2% trypsin (Invitrogen) and 0.1% collagenase IV (Sigma) at 37°C for three times (5 min each digestion). At the end of enzymatic digestion, heart tissue was minced. Cell suspension was collected and filtered through a 0.22 µm filter (Becton Dickson). Cells were then incubated with a rabbit anti-c-kit antibody (Santa Cruz) and separated using sheep anti-rabbit immunomagnetic microbeads (Miltenyi Biotec). Small round cells containing most of the c-kit+ population were collected. Newly isolated cardiac c-kit+ cells were seeded in 25 cm2 flasks coated with 200 µg/mL fibronectin (Sigma) and grown in DMEM containing 15% FCS, 10 ng/mL basic fibroblast growth factor (bFGF), and 10 ng/mL leukaemia inhibitory factor at 37°C in an atmosphere of 5% CO2 for 3 days. To induce differentiation, CSCs were cultured in VEGFMSC-conditioned medium in 24-well plates for 7 days.
2.5. In vitro CSC migration assay with VEGFMSC-conditioned medium
VEGFMSC-conditioned medium (VEGFCM) was produced following the protocol described in Supplementary material online, Data 2. To observe the effect of VEGFCM on CSC migration, CSC were collected and seeded in the top well of a transwell insert (Millipore, USA) at a density of 2 × 104 cells/well in 200 µL DMEM containing 2% FCS. CtrlCM, LacZCM, or VEGFCM (600 µL) were added to the bottom wells of the transwell plates (pore size: 8 μm). DMEM containing 2% FCS was used as a migration control. For inhibition experiments, CSC were preincubated with CXCR4 antagonist AMD3100 (10 μg/mL, Sigma), VEGFR1 inhibitor AMG706 (AMG, 2 nM, selleckchem), VEGFR2 kinase inhibitor (VEGFR2-I, 70 nM, Merk), or VEGFR3 inhibitor MAZ51 (MAZ, 10 μM, Merk) for 30 min before seeding. CSC were then cultured at 37°C in a humidified atmosphere of 5% CO2 for 12 h. Transwell inserts were then removed, and migration activity was evaluated by the mean number of cells migrating to the bottom wells from five high-power fields (200×) per chamber as observed by phase contrast microscopy. The migration index was calculated to express stimulated migration using the following equation: Migration index = Stimulated migration/Random migration. Each assay was carried out in triplicate wells.
2.6. Cell labelling
For transplantation studies, MSC were labelled with PKH26 cell tracker dye using a PKH26 Red Fluorescent Cell Linker Kit (Sigma) according to the manufacturer's instructions. The efficiency of PKH26 cell labelling was evaluated by flow cytometry (see Supplementary material online, Data 3).
2.7. Myocardial infarction and cell implantation
Myocardial infarction (MI) was achieved by ligation of the left anterior descending coronary artery (LAD) as previously described.10 Briefly, SD rats (280–300 g) were anaesthetized with ketamine (50 mg/kg, ip) and xylazine (10 mg/kg, ip). Tracheal ventilation with room air was carried out by using a Colombus ventilator (HX-300, Taimeng Instruments, China). Following left lateral thoracotomy at the fourth intercostal space, the LAD was ligated. Before chest closure, infarction was confirmed by observation of injury demarcation with blanching of the myocardium as well as electrocardiography. Rats were grouped randomly.
One week after MI induction, the rat thorax was reopened, and suspensions of LacZMSC or VEGFMSC in serum-free medium (5 × 106 in 0.2 mL) were injected with a 30-gauge tuberculin syringe into four sites (0.05 mL per site) for each MI heart in the implantation groups. Two injection sites were in the myocardium bordering the ischaemic area, and two within the ischaemic area. Vehicle (H2O) or AMD3100 (AMD) was continuously injected each per day for 6 days (1 mg/kg, ip) in VEGFMSC (VEGF), LacZMSC (LacZ), and control (Ctrl) groups 1 day after cell implantation. Adenovirus expressing control (shCtrl) or SDF-1α shRNA (shSDF) was simultaneously injected with VEGFMSC implantation (see Supplementary material online, Data 4). In sham-operation group, rats underwent identical surgery but without ligation of the coronary artery. Penicillin (150 000 U/mL, iv) was given before each procedure. Buprenorphine hydrochloride (0.05 mg/kg, sc) was administered twice a day for the first 48 h after the procedure.
2.8. In vivo CSC migration assay with implantation of VEGFMSC
For intramyocardial implantation, cultured CSC were labelled with PKH26 following the manufacturer's instructions. 2 × 105 PKH26-labelled CSC at a volume of 50 µL were injected into the myocardium at the atrioventricular (AV) groove of infarcted hearts with implantation of VEGFMSC. In order to determine whether VEGF induces CSC migration through SDF1α/CXCR4, we used AMD3100 (10 μg/mL) to block CXCR4 activity prior to CSC injection. shSDF was used to confirm the specificity of AMD3100 inhibition. shSDF and VEGFMSC were simultaneously injected into four sites as described already.
2.9. Measurement of angiomyogenesis
CM was produced following the protocol described in Supplementary material online, Data 9. VEGFCM-H9C2 (VEGFMSC-conditioned medium-cultured H9C2-conditioned medium) or VEGFCM-HS [VEGFMSC-conditioned medium-cultured heart slices (HS)-conditioned medium] were used to stimulate CSC differentiation to endothelial cells in vitro, which was detected by the expression of CD31. VEGFMSC-induced angiogenesis in vivo was determined by the expression of a mature endothelial cell marker, vwFVIII.
2.10. Measurement of haemodynamic parameters
Measurements of haemodynamic parameters, histological, and morphometric analysis of hearts were carried out 28 days after the treatments as previously described.10 Rats were anaesthetized with pentobarbital sodium (60 mg/kg, ip). The carotid artery and femoral artery were isolated. Two catheters that were filled with heparinized (10 U/mL) saline solution and connected to a Statham pressure transducer (Gould, Saddle Brook, NJ, USA) were planted into the carotid artery and femoral arteries. The carotid arterial catheter was advanced into the LV to record ventricular pressure. The femoral artery catheter was inserted into an isolated femoral artery and used to monitor mean arterial pressure (BP) and heart rate. These haemodynamic parameters including left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), and rate of rise and fall of ventricular pressure (+dP/dtmax and –dP/dtmax) were monitored simultaneously and recorded on both a thermal pen-writing recorder (RJG-4122, Nihon Kohden, Japan) and an FM magnetic tape recorder (RM-7000, Sony, Tokyo, Japan). The heart was rapidly removed after the measurements were taken (n = 10).10
2.11. Statistical analyses
Data are given as mean ± SD. Statistical significance between two groups was determined by paired or unpaired Student's t-test. Results for more than two experimental groups were evaluated by one-way ANOVA to specify differences between groups. P <0.05 was considered as significant difference.
3. Results
3.1. Transplantation of VEGFMSC stimulated SDF-1α expression in infarcted myocardium
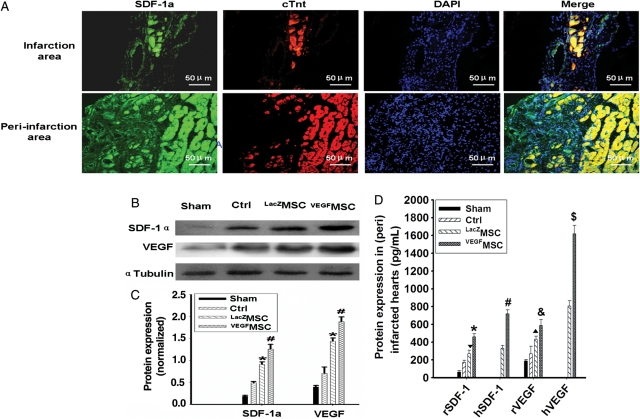
Immunostaining showed that SDF-1α expression in the infarcted areas and peri-infarction areas were highly expressed in VEGFMSC-transplanted hearts (Figure 1A). Quantitative analysis indicated that the expression of both VEGF and SDF-1α protein was significantly elevated in the (peri) infarcted areas (Figure 1B and C), remote tissues of hearts and serum (see Supplementary material online, Data 5) of VEGFMSC-transplanted hearts when compared with the control and LacZMSC-transplanted hearts. It appeared that VEGF-induced SDF-1α expression from VEGFMSC accounted for 60%, while VEGF-induced SDF-1α from the (peri) infarcted tissues accounted for 40% of the total SDF-1α expressed in the (peri) infarcted areas (Figure 1D).
Figure 1.
Transplantation of VEGFMSC enhanced SDF-1α expression in infarcted hearts. (A) Immunostaining of SDF-1α. The expression of SDF-1α and cardiomyocyte marker cTnt was examined in both infarction and peri-infarction areas from VEGFMSC-transplanted hearts. DAPI stained nuclei. Bar represents 50 µm. (B) VEGF induced SDF-1α expression in infarcted heart tissues. Sham-operated and infarcted heart tissues without (Ctrl) or with LacZMSC or VEGFMSC transplantation were isolated 7 days after treatment. Total proteins were extracted followed by western blotting to detect the expression of SDF-1α and VEGF as indicated. α-Tubulin served as an internal control (n= 5). (C) Quantitative analysis of SDF-1α and VEGF expression (n = 9). *P < 0.01 vs. Ctrl; #P < 0.01 vs. LacZ. (D) Comparison of VEGF-induced expression of SDF-1α from the (peri) infarcted tissue and transplanted VEGFMSC. SDF-1α and VEGF expression from rat (peri) infarcted heart and transplanted VEGFMSC was examined by ELISA (n = 5). For rat SDF-1α (rSDF-1α) expression, ▾P < 0.01 vs. Ctrl; *P < 0.05 vs. LacZ. For VEGFMSC-expressed human SDF-1α (hSDF-1α) expression, #P <0.01 vs. LacZ. For Rat VEGF (rVEGF) expression, ▴P < 0.01 vs. Ctrl; &P < 0.01 vs. LacZ. For VEGFMSC secreted human VEGF (hVEGF) expression, $P < 0.01vs. LacZ.
In addition, VEGFCM increased SDF-1α protein expression in HSs and cardiomyoblast cells H9C2. The SDF-1α protein expression was further enhanced by oxygen deficit and FCS-starvation. The increased expression of SDF-1α in VEGFCM-HS and VEGFCM-H9C2 was blocked by VEGFR1, VEGFR-2, and VEGFR3 inhibitors (see Supplementary material online, Data 2).
3.2. VEGFMSC-conditioned medium induced CXCR4 expression and promoted CSC migration in vitro
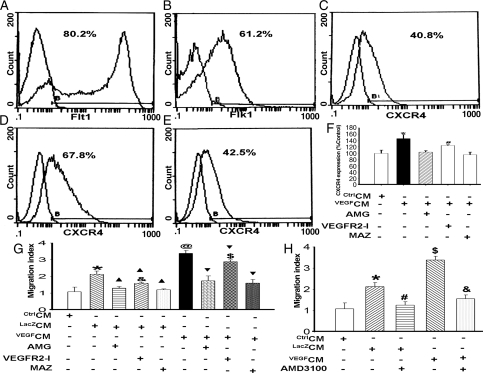
SDF-1α exerts biological activities through binding to its receptor CXCR4. To determine whether VEGF secreted from MSC induces CXCR4 expression in cardiac CSC, we isolated CSC from rat hearts (see Supplementary material online, Data 6).10 Flow cytometric analysis showed that majority of c-kit+ CSC expressed VEGF receptors Flt-1 and Flk-1 (Figure 2A and B). Forty-one per cent of CSC expressed CXCR4 (Figure 2C). However, VEGFMSC-conditioned medium (VEGFCM) significantly increased the number of CSC that expressed CXCR4 (Figure 2D). Importantly, as shown in Figure 2E–F, all three VEGF receptors appeared to be involved in VEGF-induced CXCR4 expression although VEGFR1 and VEGFR3 had a greater effect than VEGFR2.
Figure 2.
VEGFCM stimulated CXCR4 expression, leading to increased migration of CSC. (A and B) Flow cytometric analysis showed that untreated CSC expressed VEGF receptors Flt-1 and Flk-1. (C–E) VEGFCM stimulated CXCR4 expression in CSC 24 h after VEGFCM treatment. CSC were untreated (Control, C), or treated with VEGFMSC-conditioned medium (VEGFCM, D) or VEGFCM and VEGFR1 inhibitor AMG (E) followed by flow cytometry. AMG inhibited the expression of VEGFCM-induced CXCR4. (F) Quantitative analysis of CXCR4 expression by flow cytometry. CSC were treated with CtrlCM or VEGFCM with or without VEGFR specific inhibitors for VEGFR1 (AMG), VEGFR2 (VEGFR2-I), and VEGFR3 (MAZ). *P < 0.05 vs. control, AMG, VEGFR2-I. and MAZ; #P < 0.05 vs. AMG and MAZ (n = 5). (G) VEGFCM promoted CSC migration. *P < 0.05 vs. CtrlCM; ▴P <0.01 vs. CtrlCM; &P < 0.05 vs. CtrlCM+AMG and CtrlCM+MAZ (n = 15); @P < 0.01 vs. CtrlCM; ▾P <0.01 vs. CtrlCM; $P < 0.05 vs. VEGFCM+AMG and VEGFCM+MAZ. (H) VEGFCM-mediated CSC migration was blocked by CXCR4 inhibitor AMD3100. CTLCM was used as a migration control (n = 15). *P <0.01vs. CtrlCM; #P <0.01 vs. LacZCM; $P <0.01 vs. CtrlCM and LacZCM; &P <0.01 vs. VEGFCM.
As shown in Figure 2G, the migratory response of CSC to VEGFCM was increased two-fold compared with control. All VEGFR inhibitors inhibited VEGFCM-mediated CSC migration, although VEGFR1 and VEGFR3 inhibitors had a greater effect than VEGFR2 inhibitor, consistent with their effect on CXCR4 expression. Importantly, the increased migration was blocked by CXCR4 antagonist AMD3100 (Figure 2H), suggesting that VEGF enhanced CSC migration at least in part through SDF-1α/CXCR4 pathway. However, a direct effect of VEGF on CSC migration via binding VEGFR-1 and VEGFR-3 cannot be excluded at this point.
3.3. Transplanted VEGFMSC induced mobilization of cardiac stem cells through CXCR4 in infarcted myocardium
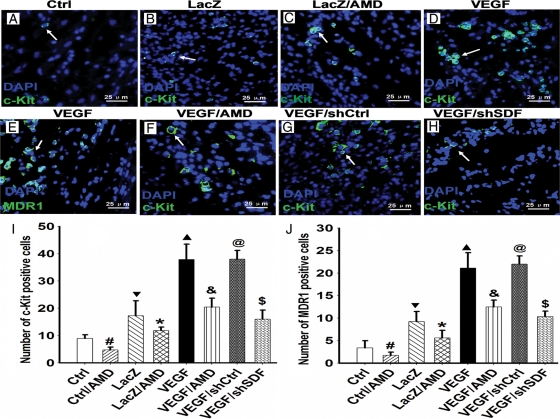
To determine whether VEGF secreted from MSC is able to stimulate the mobilization of CSC in infarcted hearts, we implanted PKH26-labelled VEGFMSC in the infarcted areas, isolated the infarcted myocardium 7 days after the infarction, and detected the presence of CSC by immunostaining of stem cell markers c-kit and MDR1. A large number of c-Kit+ and MDR1+ cells were observed in the VEGFMSC-transplanted myocardium (Figure 3D and E). To determine whether VEGF stimulated CSC mobilization through SDF-1α/CXCR4 pathway, we used AMD3100 (AMD) to block CXCR4 activity, and found that the number of c-Kit+ cells in the VEGFMSC engrafted areas was significantly reduced by AMD3100 treatment (Figure 3C and F) compared with the vehicle-treated infarcted hearts (Figure 3B and D). The dependence of VEGF in inducing CSC mobilization on SDF-1α/CXCR4 was confirmed by SDF-1α shRNA knockdown assay (Figure 3H). The reduction in c-kit+ and MDR1+ cells by AMD3100 and SDF-1α shRNA clearly demonstrated that VEGFMSC stimulated CSC mobilization through SDF1α/CXCR4 pathway (Figure 3I and J).
Figure 3.
VEGFMSC induced endogenous CSC mobilization through SDF-1α in infarcted hearts. PKH26-labelled VEGFMSC were implanted in the ischaemic myocardium. Seven days later, the mobilization of endogenous CSC to the heart region with VEGFMSC engraftment was examined by immunostaining of stem cell markers c-kit and MDR1 as shown in green. Mobilized cells were shown as green (white arrow). DAPI stained nuclei (blue) (400×). (A) c-Kit+ stem cells in the infarcted hearts after MI. (B and C) c-Kit+ stem cells (green) induced by LacZMSC was significantly reduced by AMD3100 treatment (C) when compared with the vehicle-treated group (B). (D–F) c-Kit+(D) and MDR1+(E) stem cells (green) induced by VEGFMSC was significantly reduced by AMD3100 treatment (F) when compared with the vehicle-treated groups. (G) Control shRNA (shCtrl) had no inhibition effect on the mobilization of CSC induced by VEGFMSC. (H) SDF-1α shRNA (shSDF) significantly inhibited the mobilization of CSC induced by VEGFMSC. (I and J) The numbers of the c-kit+ and MDR1+ cells in infarcted hearts were counted and statistical analysis was performed (n = 5). Bar represents 25 µm. #P =0.0001 vs. Ctrl (no implantation); ▾P <0.01 vs. Ctrl; *P <0.05 and ▴P <0.01 vs. LacZ; &P <0.01, and $P <0.01 vs. VEGF; @P >0.01 vs. VEGF.
The stem cells present in infarcted myocardium were further confirmed by RT–PCR of other stem cell markers CD31 and CD34 (see Supplementary material online, Data 7). The number of CD31+ and CD34+ cells was significantly higher in VEGFMSC- than in LacZMSC-implanted hearts. AMD3100 or shSDF-1 reduced the number of VEGFMSC-induced CD31+ and CD34+ cells in infarcted hearts in a dose-dependent manner (see Supplementary material online, Data 8).
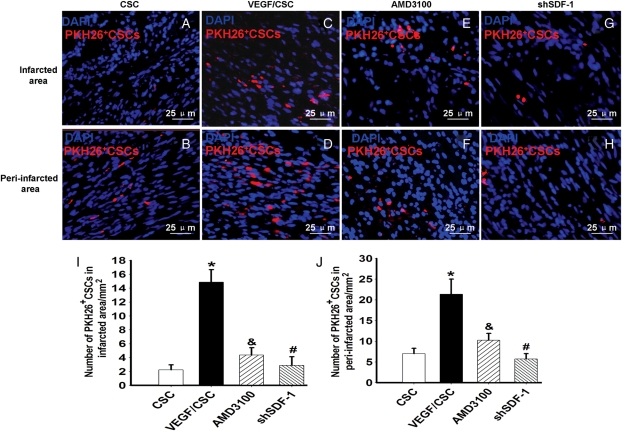
3.4. VEGFMSC stimulated the migration of exogenously-introduced CSC in infarcted hearts
To confirm the role of VEGF in inducing CSC mobilization in infarcted heart, we labelled c-kit+ cells isolated from rat hearts with PKH26. The cells incubated with or without AMD3100 were implanted in the AV groove of infarcted hearts. The VEGFMSC were simultaneously implanted into the infarcted and peri-infarcted areas. We found that PKH26-labelled CSC migrated to the peri-infarction area (Figure 4A and B). VEGFMSC implantation significantly increased the numbers of migrated CSC (Figure 4C and D). However, AMD3100 (Figure 4E and F) and shSDF-1 (Figure 4G and H) inhibited VEGFMSC-induced migration of CSC. Quantitative analysis showed that VEGFMSC enhanced the CSC migration to both infarction and peri-infarction areas. AMD3100 blocked the VEGFMSC effects. Specific blockade of SDF-1α by RNAi also inhibited VEGFMSC-induced CSC migration (Figure 4I and J). Additional studies indicate that conditioned medium from H9C2 or HSs treated with VEGFCM also promoted CSC migration, and AMD3100 blocked the VEGFCM-H9C2 effects in vitro (see Supplementary material online, Data 9). These data further demonstrated that VEGF secreted from VEGFMSC induced the mobilization and migration of CSC through SDF-1α/CXCR4 pathway.
Figure 4.
VEGFMSC stimulated exogenous CSC migration in vivo. (A–D): VEGFMSC stimulated CSC migration via CXCR4. PKH26-labelled CSC were implanted into the atrioventricular groove of the infarcted hearts (A and B). VEGFMSC were transplanted into the infarcted and peri-infarction areas of the hearts that was simultaneously implanted with CSC untreated (C and D) and treated (E and F) by AMD or VEGFMSC with SDF-1α shRNA (shSDF-1) (G and H) were transplanted into the infarcted and peri-infarction areas of the hearts that was simultaneously implanted with CSC. (I and J) Quantitative analysis of the migration of PKH26-labelled CSC to the infarcted and peri-infarction areas (n = 5). Bar represents 25 µm. *P <0.01 vs. CSC-implanted group; &<0.01 and #P <0.01 vs. VEGF/CSC-implanted groups.
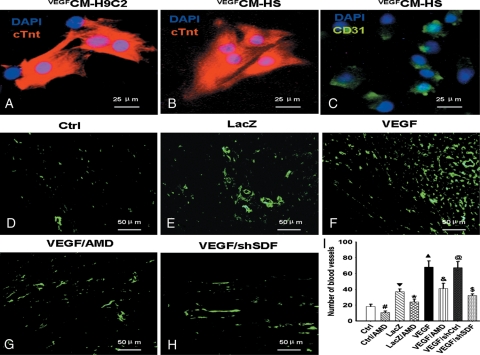
3.5. VEGFMSC promoted angiogenesis in vivo
VEGFCM-HS or VEGFCM-H9C2 promoted the differentiation of CSC into cardiomyocytes (Figure 5A and B) and endothelial cells (Figure 5C) in vitro. To determine whether VEGFMSC induce angiogenesis in infarcted myocardium and if VEGFMSC function through SDF1α/CXCR4, LacZMSC (LacZ), VEGFMSC (VEGF), VEGFMSC with AMD3100 (VEGF/AMD), or VEGFMSC transduced with adenovirus expressing SDF-1α shRNA (VEGF/shSDF) were implanted in the infarcted myocardium. The infarcted tissues were removed 28 days after the implantation. The angiogenesis was evaluated by measuring the microvessel density as shown by the expression of vwFVIII, a mature endothelial cell marker (Figure 5D–H). The number of blood vessels were counted for each treatment and analysed for their statistical difference (Figure 5I). We found that VEGFMSC significantly increased the blood vessel formation (Figure 5F and I). However, both AMD3100 and SDF-1α shRNA blocked the effect of VEGFMSC (Figure 5G–I). These data demonstrate that VEGF overexpressed from implanted MSC enhanced angiogenesis in infarcted myocardium.
Figure 5.
VEGFMSC promoted angiomyogenesis in infarcted myocaridum. (A–C) VEGFCM-H9C2 or VEGFCM-HS promoted the differentiation of CSC into cardiomyocytes and endothelial cells in vitro. CSC were cultured in conditioned medium of H9C2 cells (A) or HS (B and C) that were cultured in VEGFMSC-conditioned medium. The differentiation of cardiomyocytes and endothelial cells was detected by the expression of cTnt (A and B) and endothelial marker CD31 (C), respectively. Bar represents 25 µm. (D–H) VEGFMSC induced angiogenesis through SDF-1α/CXCR4 in vivo. LacZMSC (LacZ), VEGFMSC (VEGF), VEGFMSC with AMD3100 (VEGF/AMD) or VEGFMSC transduced with adenovirus expressing SDF-1α shRNA (VEGF/shSDF) were implanted in the infarcted myocardium. 28 days later, the myocardium was harvested and the angiogenesis in infarcted areas was detected by the expression of vwFVIII with immunostaining (green). Bar represents 50 µm. (I) Quantitative analysis of blood vessel density in infarcted areas (n = 10). #P <0.01 vs. Ctrl; ▾P <0.01 vs. Ctrl; *P <0.01 vs. LacZ; ▴P <0.01 vs. LacZ; &P <0.01 vs. VEGF; $P <0.01 vs. VEGF; @P >0.05 vs. VEGF.
In addition, we found that transplanted MSC and mobilized cells can also be differentiated to cardiomyocytes (cTnt-positive, green) at the site of the cell graft in vivo (see Supplementary material online, Data 10), suggesting that the implantation of VEGFMSC has dual functions in cardiac repair after MI.
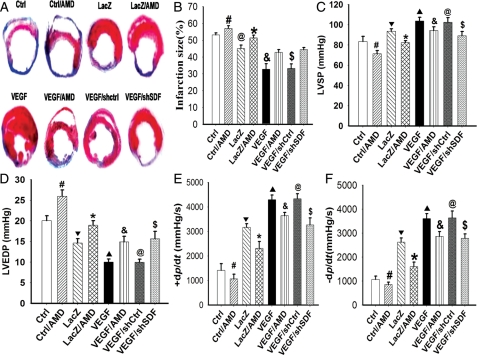
3.6. Transplanted VEGFMSC reduced infarction size and improved cardiac function
To determine whether VEGFMSC repair cardiac damage and functional impairment caused by MI, LacZMSC (LacZ), VEGFMSC (VEGF), VEGFMSC with AMD3100 (VEGF/AMD), or VEGFMSC transduced with adenovirus expressing SDF-1α shRNA (VEGF/shSDF) were implanted in the infarcted myocardium of ischaemic hearts. Masson's trichrome staining of whole hearts revealed that 28 days post-MI, more collagen accumulation was observed in infarction and peri-infarction areas in control (Ctrl), Ctrl/AMD, LacZMSC, LacZ/AMD, VEGF/shSDF, and VEGF/AMD (LacZ) groups compared with VEGFMSC (VEGF) and VEGF/shCtrl groups (Figure 6A). Semi-quantitative analysis indicated that the collagen volume fraction in the infarcted area was significantly decreased in VEGFMSC and VEGF/shCtrl groups compared with the other groups. A marked increase in the average LV wall thickness was also observed in the VEGFMSC hearts (see Supplementary material online, Data 11). Moreover, animals receiving VEGFMSC had a significantly reduced infarct size (Figure 6B) and ventricular dilation (see Supplementary material online, Data 11) in comparison with the other groups. AMD3100 treatment or SDF-1α shRNA abolished the beneficial effect of VEGFMSC.
Figure 6.
VEGFMSC transplantation improved haemodynamics. (A) VEGFMSC partially restored structural damage of myocardium caused by ischaemia. 28 days after infarction, hearts were removed and Masson's trichrome staining was performed. (B–E) VEGFMSC reduced infarction size and improved the left ventricular (LV) function of infarcted hearts. LacZMSC (LacZ), VEGFMSC (VEGF), or VEGFMSC transduced with adenovirus expressing SDF-1α shRNA (VEGF/shSDF) were implanted in the infarcted myocardium. AMD3100 treatment was described in Methods. LV function of infarcted hearts was measured under baseline resting conditions 4 weeks after treatment. (B) Infarction size (n = 10). (C) Left ventricular systolic pressure (LVSP) (n = 10). (D) Left ventricular end-diastolic pressure (LVEDP). (E and F) Rate of rise and fall of ventricular pressure [+dP/dtmax (E) and –dP/dtmax (F)] (n = 10), #P <0.05 vs. Ctrl; ▾P <0.05 vs. Ctrl; *P <0.05 vs. LacZ; ▴P< 0.05 vs. LacZ; &P < 0.05 vs. VEGF; $P < 0.05 vs. VEGF; @P>0.05 vs. VEGF.
Functional analysis showed that LV function, including LVSP, LVEDP, +dP/dtmax, and –dP/dtmax, was significantly improved in VEGFMSC-implanted hearts when compared with the control or LacZMSC-implanted animals (Figure 6C–F). AMD3100 and SDF-1α shRNA, however, reduced VEGFMSC-improved LV function (Figure 6C–F). Additional studies indicate that AMD3100 attenuated the improvement of LV function by VEGFMSC transplantation in a dose-dependent manner (see Supplementary material online, Data 12).
4. Discussion
MSC therapy is currently a promising strategy in repairing MI. The beneficial effects of MSC implantation and mechanisms underlying MSC function, however, remain largely unknown. Modified MSC with overexpression of therapeutic genes of interest are likely to have better effects than unmodified MSC.12 In this study, we found that VEGF-gene-modified MSC markedly reduced infarction size and improved the function of infarcted heart. The beneficial effects of VEGFMSC-implantation appeared to be mediated by the SDF-1α/CXCR4 activation, which induced the mobilization and migration of CSC.
MSC secrete numerous cytokines including VEGF, SDF-1, bFGF, IGF-1, and HGF.12,13 These paracrine factors, especially VEGF, are important for maintaining or improving normal cardiac function in MSC-mediated cardiac repair.8,14,15 The functions of MSC are not only due to its potential to differentiate into cardiomyocytes and endothelial cells,16,17 but also depend on the paracrine effects, including enhanced survival of implanted MSC,18 mobilization and migration of stem cells,7,19–21 which contribute to the overall repair of MI (see Supplementary material online, Data 13). Given the crucial role of SDF-1 in stem cell mobilization and retention,22 SDF-1 was also produced in response to myocardial ischaemia.23 Previous studies demonstrate that overexpression of SDF-1 in the heart provides a cue for stem cells to mobilize and home into the infarcted heart. Consequently, interactions between SDF-1 and CXCR4 contribute to ischaemic tissue repair.23–25 The present studies provide further support for the paracrine function of MSC in remodelling of infarcted myocardium. We found that VEGF-modified MSC, i.e. VEGFMSC-stimulated release of SDF-1α and expression of its receptor CXCR4 in CSC represent an important mechanism directing MSC-mediated repair of MI. Interestingly, overexpression of VEGF by VEGFMSC induced the release of SDF-1α in both the transplanted MSC and adjacent/remote myocardium. This is likely due to the diffusion of VEGF from the injection sites, resulting in the migration of CSCs to the remote myocardium. Other possibilities may be the migration of MSC from the injected sites to the remote myocardium or less likely the inaccuracy of cell transplantation to the remote areas.
VEGFMSC appear to repair MI by regulating a series of sequential molecular and cellular events.26,27 VEGF overexpressed from VEGFMSC first activates SDF-1α/CXCR4 pathway. Activated SDF-1α then induces mobilization and migration of CSC into infarcted areas. By the stimulation of VEGF and SDF-1α, CSC differentiates to endothelial cells, leading to the enhanced angiogenesis in infarcted myocardium. Meanwhile, CSC and MSC can differentiate to cardiomyocytes,10,28 and MSC can stimulate CSC proliferation and differentiation.29 The newly formed blood vessels and stem cells-differentiated cardiomyocytes may work together to restore the damaged myocardial tissues and improve the function of injured heart.
Interestingly, CXCR4 antagonist AMD3100 not only inhibits cardiac function of VEGFMSC-implanted animals, but also reduces cardiac function of the control animals. AMD3100 effect on control animals is consistent with a recent report that long-term or chronic usage of AMD3100 has an anomalous effect on heart function.30 AMD3100 appears to deteriorate cardiac function by inducing cardiac myocyte apoptosis.30 In our study, AMD3100 reduces VEGFMSC-improved LV function with continuously intraperitoneal injections for 7 days after MI, suggesting that AMD3100 may have impaired heart function through both the induction of cardiac myocyte apoptosis and the blockade of CXCR4 receptor. The specific effect of SDF-1α/CXCR4 pathway on cardiac function is further demonstrated by knocking down SDF-1α expression using shRNA (Figure 6).
Recent studies have shown that paracrine release of SDF-1α/CXCR4 by Akt or IGF-1-modified MSC promotes myocardial repair.18,20,31 IGF-1 has been reported to stimulate VEGF expression in endothelial cells.32 Akt signalling appears to be critical for VEGF-induced postnatal angiogenesis.33 We found that VEGF also stimulated Akt phosphorylation in MSC (see Supplementary material online, Data 14). Therefore, VEGF, IGF-1, Akt, and SDF-1α are likely to form an integrated system in the regulation of MSC functions in the cardiac repair after the infarction.
4.1. Clinical relevance
Although MSC are superior to angiogenic growth factors for improving myocardial performance with acute MI,34 MSC therapy has limitations: age and other risk factors for cardiovascular diseases reduce MSC availability and limit its potential for differentiation and proliferation.35 VEGF is a crucial paracrine factor in defining the age threshold in adult and neonatal stem cell function.36 Expression of VEGF via VEGFMSC not only helps improve the function of transplanted MSC, including paracrine secretion of cytokines, survival, migration, and proliferation, but also contributes to the sustainable expression of VEGF gene.35,36 Since VEGF delivered in protein and gene form did not gain impressive results in clinical trials,37 VEGFMSC implantation may be a potentially effective approach in enhancing cell therapy for cardiovascular diseases, especially for cardiac repair after MI. The underlying mechanism is that VEGF induces stem cells trafficking via activation of SDF-1/CXCR4 signalling pathway.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflicts of interest: none declared
Funding
This study was supported by grants from the National Natural Science Foundation of China (30700306, 30570674), Hubei Natural Foundation (2005ABA079, JX3B29, Q200524003, JX5B24) and National Institutes of Health (HL093429).
Supplementary Material
References
- 1.Wagner M, Siddiqui MA. Signal transduction in early heart development (II): ventricular chamber specification, trabeculation, and heart valve formation. Exp Biol Med (Maywood) 2007;232:866–880. [PubMed] [Google Scholar]
- 2.Yockman JW, Choi D, Whitten MG, Chang CW, Kastenmeier A, Erickson H, et al. Polymeric gene delivery of ischemia-inducible VEGF significantly attenuates infarct size and apoptosis following myocardial infarct. Gene Ther. 2009;16:127–135. doi: 10.1038/gt.2008.146. [DOI] [PubMed] [Google Scholar]
- 3.Hao X, Månsson-Broberg A, Grinnemo KH, Siddiqui AJ, Dellgren G, Brodin LA, et al. Myocardial angiogenesis after plasmid or adenoviral VEGF-A(165) gene transfer in rat myocardial infarction model. Cardiovasc Res. 2007;73:481–487. doi: 10.1016/j.cardiores.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto R, Omura T, Yoshiyama M, Hayashi T, Inamoto S, Koh KR, et al. Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2005;25:1168–1173. doi: 10.1161/01.ATV.0000165696.25680.ce. [DOI] [PubMed] [Google Scholar]
- 5.Pons J, Huang Y, Arakawa-Hoyt J, Washko D, Takagawa J, Ye J, et al. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun. 2008;76:419–422. doi: 10.1016/j.bbrc.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 7.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Giordano FJ, Gerber HP, Williams SP, VanBruggen N, Bunting S, Ruiz-Lozano P, et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci U S A. 2001;98:5780–5785. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH, Joo SY, et al. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70:530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Tang J, Wang J, Kong X, Yang J, Guo L, Zheng F, et al. Vascular endothelial growth factor promotes cardiac stem cell migration via the PI3K/Akt pathway. Exp Cell Res. 2009;315:3521–3531. doi: 10.1016/j.yexcr.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 12.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, et al. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res. 2005;66:543–551. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Zisa D, Shabbir A, Suzuki G, Lee T. Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair. Biochem Biophys Res Commun. 2009;390:834–838. doi: 10.1016/j.bbrc.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zisa D, Shabbir A, Mastri M, Suzuki G, Lee T. Intramuscular VEGF repairs the failing heart: role of host-derived growth factors and mobilization of progenitor cells. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1503–R1515. doi: 10.1152/ajpregu.00227.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pijnappels DA, Schalij MJ, Ramkisoensing AA, van TJ, de Vries AA, van der LA, et al. Forced alignment of mesenchymal stem cells undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circ Res. 2008;103:167–176. doi: 10.1161/CIRCRESAHA.108.176131. [DOI] [PubMed] [Google Scholar]
- 17.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 18.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–378. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 19.Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296:H1888–H1897. doi: 10.1152/ajpheart.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haider HKh, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 21.Nakanishi C, Yamagishi M, Yamahara K, Hagino I, Mori H, Sawa Y, et al. Activation of cardiac progenitor cells through paracrine effects of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;374:11–16. doi: 10.1016/j.bbrc.2008.06.074. [DOI] [PubMed] [Google Scholar]
- 22.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 23.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 24.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, et al. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ball SG, Shuttleworth CA, Kielty CM. Mesenchymal stem cells and neovascularization: role of platelet-derived growth factor receptors. J Cell Mol Med. 2007;11:1012–1030. doi: 10.1111/j.1582-4934.2007.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz de Almodovar C, Luttun A, Carmeliet P. An SDF-1 trap for myeloid cells stimulates angiogenesis. Cell. 2006;124:18–21. doi: 10.1016/j.cell.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 29.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai S, Yuan F, Mu J, Li C, Chen N, Guo S, et al. Chronic AMD3100 antagonism of SDF-1alpha-CXCR4 exacerbates cardiac dysfunction and remodeling after myocardial infarction. J Mol Cell Cardiol. 2010;49:587–597. doi: 10.1016/j.yjmcc.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 32.Miele C, Rochford JJ, Filippa N, Giorgetti-Peraldi S, Van Obberghen E. Insulin and insulin-like growth factor-I induce vascular endothelial growth factor mRNA expression via different signaling pathways. J Biol Chem. 2000;275:21695–21702. doi: 10.1074/jbc.M000805200. [DOI] [PubMed] [Google Scholar]
- 33.Kitamura T, Asai N, Enomoto A, Maeda K, Kato T, Ishida M, et al. Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat Cell Biol. 2008;10:329–337. doi: 10.1038/ncb1695. [DOI] [PubMed] [Google Scholar]
- 34.Shyu KG, Wang BW, Hung HF, Chang CC, Shih DT. Mesenchymal stem cells are superior to angiogenic growth factor genes for improving myocardial performance in the mouse model of acute myocardial infarction. J Biomed Sci. 2006;13:47–58. doi: 10.1007/s11373-005-9038-6. [DOI] [PubMed] [Google Scholar]
- 35.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markel TA, Wang Y, Herrmann JL, Crisostomo PR, Wang M, Novotny NM, et al. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am J Physiol Heart Circ Physiol. 2008;295:H2308–H2314. doi: 10.1152/ajpheart.00565.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart DJ, Kutryk MJ, Fitchett D, Freeman M, Camack N, Su Y, et al. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol Ther. 2009;17:1109–1115. doi: 10.1038/mt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.