Abstract
The role of blood-cerebrospinal fluid barrier (BCB) dysfunction in Alzheimer’s disease (AD) has been addressed but not yet established. We evaluated the BCB integrity in 179 samples of cerebrospinal fluid (CSF) retrospectively collected from AD patients and control cases using both CSF/serum albumin ratio (QAlb) and CSF secretory Ca2+-dependent phospholipase A2 (sPLA2) activity. These analyses were supplemented with the measurement of total tau, amyloid-β1–42 (Aβ1–42), and ubiquitin CSF levels. We found that due to its higher sensitivity, CSF sPLA2 activity could 1) discriminate AD from healthy controls and 2) showed BCB impairment in neurological control cases while QAlb could not. Moreover, the CSF sPLA2 activity measurement showed that around half of the AD patients were characterized by a BCB impairment. The BCB dysfunction observed in AD was independent from Mini-Mental State Examination score as well as CSF levels of total tau, Aβ1–42, and ubiquitin. Finally, the BCB dysfunction was not limited to any of the CSF biomarkers-based previously identified subgroups of AD. These results suggest that the BCB damage occurs independent of and probably precedes both Aβ and tau pathologies in a restricted subgroup of AD patients.
Keywords: Albumin, Alzheimer’s disease, cerebrospinal fluid, secretory phospholipase A2
INTRODUCTION
The blood-neural barriers (BNB) exist between the peripheral circulation and neural tissues and include among others, the blood-brain barrier (BBB) and the blood-cerebrospinal fluid barrier (BCB). While the BBB is diffusely distributed throughout the brain capillaries, the BCB is found at the choroid plexus [1]. The main functions of these barriers are to protect the brain from potentially harmful substances from blood, such as cytokines, which may be deletorious to the fragile brain microenvironment and at the same time to ensure the supply of nutrients as well as the removal of brain-borne substances. These essential roles make their integrity, which is in part achieved by the well-known tight junctions, critical for the maintenance of brain homeostasis [1]. Thus, any disruption of the BNB architecture is likely to contribute to a brain disorder.
The notion that BNB impairment might play an important role in the pathogenesis of Alzheimer’s disease (AD) has been extensively considered [2–4] but, not yet established. Indeed, in spite of many histopathological, neuroimaging and biochemical studies, it remains unclear whether a BNB disruption is a part of the pathophysiology of AD and in this instance, which particular BNB is impaired.
Imaging studies seem to suggest an absence of BBB dysfunction in AD. Indeed, studies using the most robust approaches to assess specifically BBB impairment, i.e., Gadolinium enhancement in T1-weighted magnetic resonance imaging scans, failed to demonstrate a BBB permeability defect in AD [5–7]. In addition, a normal function of BBB in AD is also supported by other non-invasive imaging studies using positron emission tomography and computed tomography scans [8, 9].
Inconsistent data have been reported on BCB integrity in AD as revealed by the meta-analysis performed from a systematic review of the literature from 1966 to 2006 [4]. Indeed, while several studies showed a BCB permeability increase in AD compared to age-matched control [4, 10–12], others did not detect any difference [4, 13–17]. The common and well-established method used to assess these conflicting results consisted in measuring the level of a blood-specific protein, i.e., albumin, in cerebrospinal fluid (CSF) and serum and reporting the CSF/serum albumin ratio (QAlb) [18–20]. Recently, we discovered a new approach to evaluate the BCB dysfunction, i.e., the measurement of the CSF activity of a well-known inflammatory enzyme, the secretory Ca2+-dependent phospholipase A2 (sPLA2) [21]. While we demonstrated that the sPLA2 activity assay was a more sensitive method than QAlb for measuring the BCB permeability [21], we also reported an increase of CSF sPLA2 activity in a small AD cohort [22].
In the present study, using 179 cases which included 139 AD and 40 controls, we compared for the first time in AD the two biochemical approaches, i.e., QAlb and CSF sPLA2 activity to assess the degree of BCB impairment. We also investigated the relationship of the BCB permeability level to Mini-Mental State Examination (MMSE) score as well as to CSF levels of total tau, amyloid-β (Aβ1–42), and ubiquitin, the three molecular markers of the AD histopathological changes.
MATERIALS AND METHODS
Study participants
We studied retrospectively collected lumbar CSFs and sera available for research purposes. The study was approved by the ethics committees of Eastern Norway and the University of Gothenburg and by the Institutional Review Board of the New York State Institute for Basic Research in Developmental Disabilities. Consent was obtained from all participants in accordance with the provisions of the Helsinki Declaration. The healthy control group (H-Control45–75 years) were from the Department of Psychiatry at Mölndal Hospital (Mölndal, Sweden) and consisted of 21 individuals (59.4± 9.2 years), without any history, symptoms, or signs of psychiatric or neurological disorders, malignant disease, or systemic disorders (e.g., rheumatoid arthritis, infectious disease). None of the subjects had cognitive symptoms. For individuals over 60 years of age, the cognitive status was examined using the MMSE and individuals with scores below 28 were not included. The “neurological” control or non-demented group (ND-Control45–75 years) were from the Department of Neurology at Boden Hospital (Boden, Sweden) and consisted of 19 individuals (59.5± 7.0 years) with mild neurological symptoms such as headache and vertigo, where the medical examination did not reveal any organic brain disorder. None of the patients had cognitive symptoms, and all patients were also followed through medical records for 10 years and none developed dementia. The AD group consisted of 139 AD patients diagnosed with probable AD according to criteria established by the work group of the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) [23]. MMSE scores were documented for most AD patients. The demographics and clinical features of the patients involved in this study are summarized in Tables 1 and 3.
Table 1.
Demographics, clinical features, CSF/serum albumin ratio (QAlb) and CSF levels of total tau, Aβ1–42, ubiquitin and sPLA2 activity of AD and control cases
| H-Control45–75 years (n = 21)A |
ND-Control45–75 years (n = 19) |
AD45–75 years (n = 54) |
AD>75 years (n = 85)B |
Statistical analysis Statistical value |
p value | |
|---|---|---|---|---|---|---|
| Gender (male) | 12 (57%) | 13 (68%) | 29 (54%) | 61 (72%) | χ2 (df =3) = 5.30 | 0.151 |
| Age (years) | 59.4±9.2 | 59.5±7.0 | 69.3±4.3 | 80.4±3.3** | H(df=3) = 142.60 | <0.0001 |
| MMSE | 29.5±0.5† | 27.7±3.4† | 22.4±6.0‡ | 22.4±4.9‡ | H(df=3) = 40.33 | <0.0001 |
| ApoE4 carrier | 0 (0%)§ | 6 (32%)§ | 40 (74%) | 58 (68%) | χ2 (df=3) = 28.12 | <0.0001 |
| T-tau (pg/mL) | 317.2±133.0† | 282.2±123.4 | 763.5±301.4‡ | 716.7±397.0‡ | H(df=3) = 57.86 | <0.0001 |
| Aβ1−42 (pg/mL) | 921.8±205.6† | 914.2±267.6† | 582.0±246.1‡ | 514.3±169.4‡ | H(df=3) = 44.04 | <0.0001 |
| Ubiquitin (ng/mL) | 94.4±37.1 | 117.5±53.9 | 133.4±67.8 | 144.2±96.0 | F(3,163) = 1.78 | 0.154 |
| QAlb | 5.3±2.0 | 4.8±1.8 | 4.9±1.5 | 5.4±1.6 | F(3,173) = 2.08 | 0.105 |
| sPLA2 activity (ΔFI/min) | 3.9±0.8* | 5.4±1.5 | 5.2±1.7 | 5.4±1.6 | F(3,175) = 6.36 | 0.0004 |
Categorical data (gender and ApoE4 carrier) are expressed as number of subject (%) and differences between groups were assessed using χ2 test. Continuous variables are expressed as mean ± SD and differences between groups were assessed using Kruskal-Wallis’s test followed by Dunn’s multiple comparison test (age, MMSE, T-tau, Aβ1−42) or one-way ANOVA after logarithmic transformation followed by Tukey-Kramer post-hoc analysis (Ubiquitin, QAlb, sPLA2 activity).
For 11 cases MMSE, ApoE4 carrier and levels of T-tau, Aβ1−42, and Ubiquitin were unknown and for 2 cases QAlb was unknown;
For 2 cases MMSE was unknown and for 1 case level of Ubiquitin was unknown.
Statistically different from the other groups (p < 0.01);
Statistically different from the other groups (p < 0.001);
Statistically different from both AD groups (p < 0.001);
Statistically different from both control groups (p < 0.001);
Statistically different from the grand mean (p < 0.01);
H-Control, healthy control; ND-Control, non-demented control; AD, Alzheimer disease; T-tau, total tau‡
Table 3.
Demographics, clinical features, CSF/serum albumin ratio (QAlb) and CSF levels of total tau, Aβ1–42, ubiquitin and sPLA2 activity of different subgroups of AD
| HARO | ATEO | AELO | LEBALO | Statistical Analysis |
||
|---|---|---|---|---|---|---|
| (n = 11) | (n = 24) | (n = 71)A,B | (n = 32)A | Statistical value | p value | |
| Gender (male) | 6 (55%) | 13 (54%) | 51 (72%) | 19 (59%) | χ2(df=3) = 3.76 | 0.289 |
| Age (years) | 74.3±5.8 | 74.3±7.9 | 76.6±5.9 | 77.2±6.8 | H(df=3) = 3.456 | 0.327 |
| MMSE | 24.5±5.8 | 20.7±5.6 | 22.9±4.9 | 21.9±5.7 | H(df=3) = 5.041 | 0.169 |
| ApoE4 carrier | 4 (36%)§ | 13 (54%) | 56 (79%) | 25 (78%) | χ2(df=3) = 12.88 | 0.005 |
| T-tau (pg/mL) | 684.5±174.8** | 1279.3±517.0† | 706.9±109.4 | 408.9±87.0† | H(df=3) = 105.1 | <0.0001 |
| Aβ1–42 (pg/mL) | 1089.9±150.6‡ | 468.8±74.1 | 507.9±129.5 | 470.0±127.0 | H(df=3) = 32.12 | <0.0001 |
| Ubiquitin (ng/mL) | 145.3±59.4 | 162.2±144.7 | 148.1±73.2 | 103.8±46.3* | F(3,133) = 3.716 | 0.013 |
| QAlb | 5.3±1.3 | 5.5±1.8 | 5.1±1.5 | 5.0±1.4 | H(df=3) = 1.512 | 0.680 |
| sPLA2 activity (ΔFI/min) | 5.8±1.1 | 5.6±1.7 | 5.3±1.7 | 4.9±1.3 | F(3,134) = 1.240 | 0.298 |
Categorical data (gender and ApoE4 carrier) are expressed as number of subject (%) and differences between groups were assessed using χ2 test. Continuous variables are expressed as mean ± SD and differences between groups were assessed using Kruskal-Wallis’s test followed by Dunn’s multiple comparison test (age, MMSE, T-tau, Aβ1–42, QAlb) or one-way ANOVA after logarithmic transformation followed by Tukey-Kramer post-hoc analysis (Ubiquitin, sPLA2 activity). For this analysis, only patients without BCB impairment were selected: 1 patient from AELO subgroup was removed.
For one case MMSE was unknown;
For one case CSF level of Ubiquitin was unknown.
Statistically different from AELO subgroup (p < 0.05);
Statistically different from ATEO and LEBALO subgroups (p < 0.01);
Statistically different from the other subgroups (p < 0.01);
Statistically different from the other subgroups (p < 0.001);
Statistically different from the grand mean (p < 0.05); T-tau, total tau.
CSF/Serum sampling and biochemical analyses
CSF samples were taken between 11 and 12 am by lumbar puncture from the L3/L4 or L4/L5 inter-vertebral space. The first 12 mL portion of CSF was collected in polypropylene tubes then centrifuged at 2000 ×g for 10 min at 4°C in order to eliminate cells and other insoluble material. Before freezing at −80°C, the CSF samples were aliquoted in 1mL polypropylene tubes with screw cap. In addition, venipuncture was performed to obtain serum. Finally, all samples were sent in dry ice from both Mölndal and Boden Hospitals to New York State Institute for Basic Research, and kept at −80°C until used.
The ApoE isoform was determined by isoelectric focusing/immunoblotting using serum samples [24]. QAlb was calculated as CSF albumin (mg/L)/serum albumin (g/L) [19]. CSF levels of total tau and Aβ1–42 were measured by sandwich enzyme-linked immunosorbent assay [25] using INNOTEST® hTAU Ag and INNOTEST™ β-amyloid (1–42) commercial kits (Innogenetics, Ghent, Belgium). Conjugated ubiquitin levels were assayed by competitive inhibition enzyme-linked immunosorbent assay [25] using the monoclonal 5–25 antibody as primary antibody (Signet Labs, Dedham, MA) [25, 26].
The CSF sPLA2 activity was measured using a newly developed continuous fluorescent assay [22]. In a 96-well microplate, 5 µL lumbar CSF was diluted in 90 µL sPLA2 assay buffer (10 mM Tris-HCl, pH 7.4, 100 mM KCl, 5 mM CaCl2, 1 mM DTT). Then, 5 µL liposomes made from 0.4 mg/mL 100% DOPC and labeled with 100 µM Bis-BODIPY® FL C11-PC were added to each well and the microplate was immediately placed in a temperature controlled (30°C) cytofluor multi-well plate reader series 4000 (PerSeptive Biosystems, Foster City, CA, USA). The fluorescence intensity was recorded over 90min (91 cycles of 60 s each) at 485 nm excitation and 530 nm emission. Finally, sPLA2 activity was evaluated using linear curve fitting with Graph Prism 3.0 (GraphPad, San Diego, CA, USA). Except for CSF/serum albumin ratio (QAlb) and sPLA2 activity, data from biochemical analyses (ApoE isoform, total tau, Aβ1–42 and ubiquitin) have been previously reported [25].
Statistical analysis
Statistical analyses were performed using Statgraphics Centurion XV (StatPoint, Herndon, VA, USA) and Graph Prism 3.0 (GraphPad software, San Diego, CA, USA). MedCalc 10 (MedCalc Software, Mariakerke, Belgium) was used to compare receiver operating characteristic (ROC) curves. A failure in the normal distribution of a variable was considered when values of Skewness and Kurtosis were outside of the range −2 to +2. If a variable was not normally distributed, a parametric test following a logarithmic transformation or a non-parametric test was performed. Frequency distributions were compared with Fisher’s test or Chi-square test followed by an analysis of means. Differences between two means were assessed with unpaired, two-tailed Student’s t-test or Mann-Whitney’s test. To assess difference between more than 2 means, one-way ANOVA or Kruskal-Wallis’s test followed by a Tukey-Kramer or a Dunn’s post-hoc tests, respectively, was used. Correlations were analyzed statistically using Pearson’s correlation test or Spearman’s rank correlation test. Bland-Altman analysis [27] was used for comparison between QAlb and CSF sPLA2 activity. ROC analysis was used to calculate sensitivity, specificity and cutoff values of considered biomarkers. The optimal cutoff value was defined at the optimal combination of sensitivity and specificity. The level of significance was defined as p < 0.05.
RESULTS
BCB status in AD and control patients
In order to understand the involvement of BCB dysfunction in the pathophysiology of AD, we assessed BCB permeability by measuring both QAlb and sPLA2 activity in CSFs from 139 AD patients and 40 control cases (Table 1). The control groups were made up of healthy elderly individuals without any history or symptoms of cognitive disturbance or signs of neurological or psychiatric disorders, who were actively recruited (Healthy Controls or H-Control45–75 years, n = 21) or of patients with mild neurological symptoms (Non-Demented Controls or ND-Control45–75 years, n = 19). Moreover, due to the large age range of the AD group relative to both control groups and in order to evaluate sPLA2 activity and QAlb independently of the age, two AD age groups, AD45–75 years (n = 54) and AD>75 years (n = 85),were created using the median age of the population as a cutoff, i.e., 75 years.
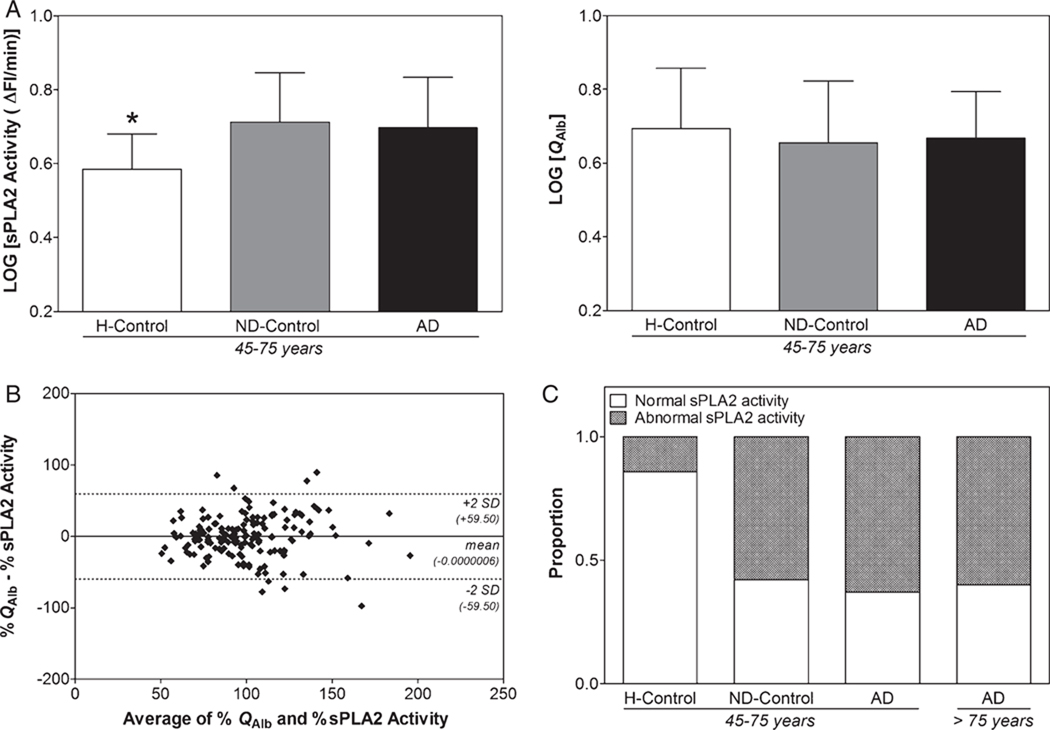
We found a significant increase in sPLA2 activity in both ND-Control45–75 years and AD45–75 years groups compared with H-Control45–75 years, while no significant difference was observed for QAlb among these groups (Fig. 1A). Moreover, no significant difference was observed between AD45–75 years and AD>75 years groups in either QAlb or CSF sPLA2 activity (Table 1). These findings were confirmed by the correlation analysis between sPLA2 activity and QAlb. While significant and positive correlations between sPLA2 activity and QAlb in ND-Control45–75 years (r = 0.796; p < 0.0001; 95% CI: 0.535–0.918), AD45–75 years (r = 0.407; p = 0.002; 95% CI: 0.157–0.609) and AD>75 years (r = 0.510; p < 0.0001; 95% CI: 0.334–0.653) groups were observed, no such significant correlation was noticed for H-Control45–75 years (r = 0.414; p = 0.078; 95% CI: −0.050–0.731). The analysis for agreement between QAlb and CSF sPLA2 activity using the Bland-Altman method revealed similar results. Indeed, when considering H-Control45–75 years, ND-Control45–75 years, AD45–75 years as well as AD>75 years groups, the two BCB assay methods did not agree fairly since 7.26% of the patients had values that differed by >2 SD (data not shown). But, once the H-Control45–75 years group was not considered, only 5.06% of the patients had values that differed by >2 SD (Fig. 1B). These differences between sPLA2 activity and QAlb can be attributed to the higher sensitivity of sPLA2 activity in estimating BCB impairment. In agreement with this hypothesis, it should be noticed that according to the upper reference limit for QAlb (10.2 for individuals over 45 years of age [20]), only one out of the 179 cases independent of their clinical diagnoses was found to have a BCB impairment.
Fig. 1.
Comparison of sPLA2 activity with QAlb in AD and control groups. A) There was a significant difference in sPLA2 activity (F(2,91) = 6.788; p = 0.002) but not in QAlb (F(2,89) = 0.369; p = 0.693) between H-Control45–75 years group and ND-Control45–75 years or AD45–75 year group. Data are shown as mean±SD; B) Bland-Altman analysis showing the agreement between QAlb and sPLA2 activity for the measurement of BCB impairment in 158 clinically diagnosed AD or non-demented cases. QAlb and CSF sPLA2 activity are normalized to the average of all values of the corresponding parameters and converted to percentage. The mean difference±2 SDs are represented by horizontal solid and dotted lines, respectively. C) Proportion of patients with normal and abnormally increased sPLA2 activity in H-Control45–75 years (n = 21), ND-Control45–75 years (n = 19), AD45–75 years (n = 54) and AD>75 years (n = 85) using the cutoff value of 4.82 ΔFI/min.
We estimated the proportion of patients with normal and abnormally increased sPLA2 activity in H-Control45–75 years, ND-Control45–75 years, AD45–75 years and AD>75 years. In order to differentiate normal from abnormally increased sPLA2 activity, we used the cutoff value, i.e., 4.82 ΔFI/min, obtained from the ROC analysis (see below) between H-Control45– 75 years and AD45–75 years groups. We found BCB impairment in around half of AD cases as well as in some non-demented patients (Fig. 1C). This result corroborates the lack of integrity of the BCB restricted to a subgroup of AD petients and thereof clarifies the inconsistency in the literature about the involvement of BCB impairment in AD. In this study on a total number of 139 AD cases, 61% and 0.7% of them irrespective of the age, i.e., <75 or >75, showed BCB impairment as revealed by the CSF sPLA2 activity and QAlb, respectively.
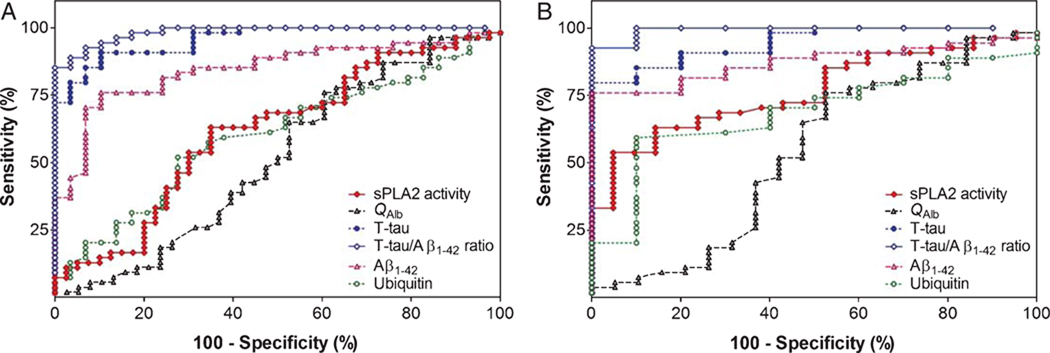
We also evaluated the discrimination power of sPLA2 activity in AD diagnosis by performing ROC analyses between total Control45–75 years and AD45–75 years groups (Fig. 2A, Table 2) as well as between H-Control45–75 years and AD45–75 years groups (Fig. 2B, Table 2). While no significant difference was observed between sPLA2 activity and QAlb AUC to discriminate Control45–75 years and AD45–75 years (z = 0.727; p = 0.467), a significant difference was observed between these AUCs (z = 2.006, p = 0.045) when only healthy elderly control patients were considered to form the control group. It is important to note that even though level of sPLA2 activity discriminates AD45–75 years from Control45–75 years much better than QAlb, it is still not powerful enough to be used alone for diagnosing AD. Indeed, with a cutoff value of 4.824 ΔFI/min, sPLA2 activity sensitivity and specificity were only 63%and 86%, respectively. The reason for such an observation can be due to the fact that BCB impairment is restricted to a subgroup of AD patients.
Fig. 2.
Area under the receiver operating characteristic curve indicating the discriminating ability of CSF measurements of sPLA2 activity, QAlb, T-tau, Aβ1–42, Ubiquitin and T-tau/Aβ1–42 ratio A) in patients with AD45–75 years (n = 54) versus controls (n = 40), and B) in patients with AD45–75 years (n = 54) versus H-Control45–75 years (n = 21). The optimal cutoff levels, sensitivity, specificity and AUC are shown in Table 2. T-tau, total tau.
Table 2.
Performance of CSF biomarkers (sPLA2 Activity, QAlb, total tau, Aβ1–42, Ubiquitin and total tau/Aβ1–42 ratio) in the discriminating AD cases from healthy/non-demented controls and healthy controls
| sPLA2 Activity | QAlb | T-tau | Aβ1–42 | Ubiquitin | T-tau/Aβ1–42 | |
|---|---|---|---|---|---|---|
| Total control45–75 years vs. AD45–75 years | ||||||
| CutoffA | 4.82 | 5.03 | 437 | 608 | 117 | 0.59 |
| % Sens (95% CI) | 63 (49–76) | 65 (51–77) | 91 (80–97) | 76 (62–87) | 57 (43–71) | 93 (82–98) |
| % Spec (95% CI) | 65 (48–79) | 47 (31–64) | 90 (73–98) | 90 (73–98) | 66 (46–82) | 93 (77–99) |
| AUC (95% CI) | 0.61 (0.50–0.73) | 0.51 (0.39–0.64) | 0.96 (0.92–0.99) | 0.85 (0.76–0.93) | 0.60 (0.47–0.72) | 0.98 (0.97–1.00) |
| Healthy control45–75 years vs. AD45–75 years | ||||||
| CutoffA | 4.82 | 5.54 | 500 | 622 | 114 | 0.59 |
| % Sens (95% CI) | 63 (49–76) | 76 (62–87) | 80 (66–89) | 76 (62–87) | 59 (45–72) | 93 (82–98) |
| % Spec (95% CI) | 86 (64–97) | 47 (24–71) | 100 (69–100) | 100 (69–100) | 90 (56–100) | 100 (69–100) |
| AUC (95% CI) | 0.77 (0.66–0.87) | 0.54 (0.37–0.71) | 0.94 (0.89–1.00) | 0.87 (0.79–0.96) | 0.69 (0.53–0.84) | 0.99 (0.98–1.01) |
| Healthy control45–75 years vs. Non-demented control 45–75 years | ||||||
| CutoffA | 4.71 | 5.20 | 345 | 872 | 114 | 0.33 |
| % Sens (95% CI) | 68 (43–87) | 68 (43–87) | 74 (49–91) | 47 (24–71) | 53 (29–76) | 63 (38–84) |
| % Spec (95% CI) | 81 (58–95) | 53 (29–76) | 50 (19–81) | 70 (35–93) | 90 (56–100) | 60 (26–88) |
| AUC (95% CI) | 0.78 (0.63–0.93) | 0.55 (0.36–0.74) | 0.60 (0.37–0.83) | 0.51 (0.29–0.73) | 0.65 (0.45–0.86) | 0.56 (0.34–0.77) |
Cutoff for sPLA2 activity is in ΔFI/min; cutoffs for T-tau and Aβ1–42 are in pg/mL; cutoff for ubiquitin is in ng/mL and cutoffs for QAlb and T-tau/Aβ1–42 ratio are dimensionless. Sens: Sensitivity; Spec: Specificity; T-tau, total tau.
Since we found that patients with mild neurological symptoms had elevated level of CSF sPLA2 as compared with the healthy control cases, we evaluated the discrimination power of total tau, Aβ1–42, ubiquitin and total tau/Aβ1–42 ratio in AD diagnosis by performing ROC analyses between total Control45–75 years and AD45–75 years groups (Fig. 2A, Table 2) as well as between H-Control45–75 years and AD45–75 years groups (Fig. 2B Table 2). For each of these CSF biomarkers, no significant difference was found concerning their discrimination power between the ROC analyses performed on total Control45–75 years versus AD45–75 years groups and on H-Control45–75 years versus AD45–75 years groups (Data not shown). These findings 1) indicated that patients with mild neurological symptoms recruited to constitute a control group seem not to affect the differential diagnosis between control and AD and 2) suggest that the BCB impairment observed in both neurological controls and AD patients apparently occurs independently of both Aβ and tau pathologies.
BCB impairment in AD subgroups
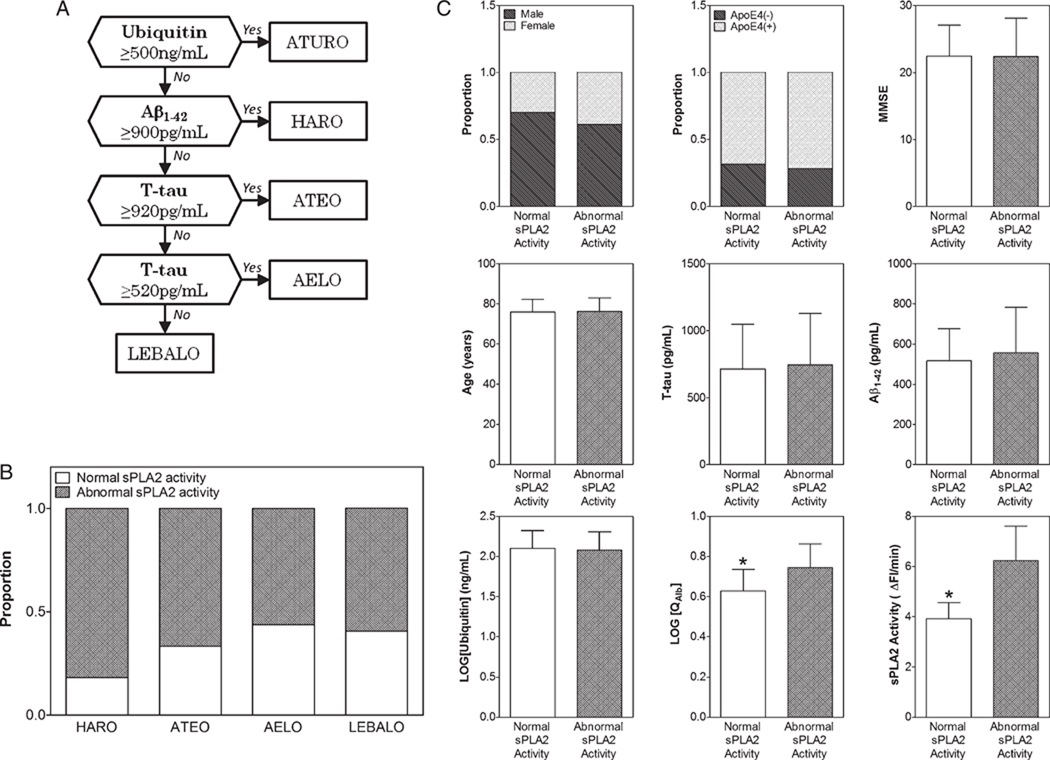
Previously, we identified five subgroups of AD based on the CSF levels of total tau, Aβ1–42, and ubiquitin [25]. The identification of these various and distinct patterns of neurodegeneration in AD reflect the multifactorial nature and heterogeneity of this pathology. We investigated whether one or several of these AD subgroups were also, on top of total tau, Aβ1–42, and ubiquitin CSF levels, characterized by QAlb and/or level of CSF sPLA2 activity. The 139 AD CSFs included in the present study were subclassified using the Iqbal’s Decision Tree [25] shown in Fig. 3A. As shown in Table 3, we identified 4 of the 5 subgroups from the AD cases studied: a) the HARO subgroup (n = 11) which was characterized by high level of Aβ1–42 and recent onset; b) the ATEO subgroup (n = 24) which was characterized by high level of total tau as well as low level of Aβ1–42 and early onset; c) the AELO subgroup (n = 72) which was characterized by low level ofAβ1–42, high incidence of ApoE4 genotype and late onset; and d) the LEBALO subgroup (n = 32) which was characterized by low level of Aβ1–42, high incidence of Lewy bodies and late onset. As noticed previously and according to the upper reference limit for QAlb (10.2 for individuals over 45 years of age [20]), only one out of the 139 AD cases was found to have a BCB impairment and therefore was excluded from the AD subgroup analyses.
Fig. 3.
sPLA2 activity in AD subgroups and effects of gender, age, MMSE, ApoE4 genotype, total tau, Aβ1–42, and ubiquitin on this activity. A) Iqbal’s Decision Tree [25] employed for identifying various AD subgroups based on CSF levels of T-tau, Aβ1–42 and ubiquitin; B) Proportion of patients with normal and abnormally increased sPLA2 activity in HARO (n = 11), ATEO (n = 24), AELO (n = 71) and LEBALO (n = 32) AD subgroups using the cutoff value of 4.82 ΔFI/min. C) Gender and ApoE4 proportion as well as MMSE, Age, and CSF levels of T-tau, Aβ1–42, Ubiquitin, QAlb, and sPLA2 activity in AD patients with normal (n = 92) and abnormally increased (n = 46) sPLA2 activity. Categorical data were assessed using Fisher’s test. Continuous variables are expressed as mean±SD. Differences were assessed using Mann-Whitney’s test (MMSE, age, T-tau, Aβ1–42, and Ubiquitin) or Student’s t-test (QAlb and sPLA2 activity). Statistical differences are indicated by an asterisk. T-tau, total tau.
While we found no significant difference for both QAlb and CSF sPLA2 activity between the four subgroups (Table 3), a positive correlation between QAlb and CSF sPLA2 activity was observed in ATEO (r = 0.539, p = 0.007, 95%CI: 0.173–0.774) and AELO (r = 0.501, p < 0.0001, 95% CI: 0.304–0.658) subgroups as well as in the total AD group (r = 0.453, p < 0.0001, 95% CI: 0.309–0.576). Thus, the strong and positive correlation between QAlb and sPLA2 activity is dependent of the AD subgroup considered. We postulate that these differences are due to different nature of the BCB impairment in AELO and ATEO versus LEBALO and HARO AD subgroups. We estimated the proportion of patients with normal and abnormally increased sPLA2 activity in HARO, ATEO, AELO and LEBALO subgroups (Fig. 3B). As above, in order to differentiate normal from abnormally increased sPLA2 activity, we used the cutoff value, i.e., 4.82 ΔFI/min, obtained from the ROC analysis between H-Control45–75 years and AD45–75 years groups. As shown in Fig. 3B, the degree of BCB impairment, measured by sPLA2 activity, is not characteristic of anyone AD subgroup (χ2(df=3) = 3.007, p = 0.391) and points out the fact that a defective BCB characterizes around half of the cases in each AD subgroup studied. A higher proportion of cases with abnormal sPLA2 activity seems to occur in the HARO subgroup suggesting an increase of Aβ1–42 in CSF might associate to an increase of BCB permeability. However, in the small number of HARO cases in the present study, this tendency was found to be insignificant.
Finaly, we investigated if there existed any difference in gender or ApoE4 proportion as well as MMSE score, age or CSF level of total tau, Aβ1–42, ubiquitin and QAlb between AD patients with normal and abnormally increased sPLA2 activity (Fig. 3C). No significant difference was found between the normal and abnormally increased sPLA2 cases for all considered parameters except QAlb level which was found to be lower in AD patients with normal sPLA2 activity (t(df = 137) = 5.792, p < 0.0001).
DISCUSSION
QAlb analyses have shown inconsistent data on BCB integrity in AD [4]. The present study shows that this discrepancy could have been due to the low sensitivity of QAlb in the assessment of BCB permeability and partly because the BCB is altered only in around half of the AD cases. Due to its higher sensitivity, the CSF sPLA2 activity assay allowed us to observe a significant difference in BCB permeability between AD and healthy controls and to underline the importance of recruiting normal healthy individuals as controls because patients with mild neurological symptoms show BCB impairment. We also demonstrated that BCB impairment appears to be independent of and probably precedes the AD histopathology.
The secretory Ca2+-dependent phospholipases A2 belong to the phospholipase A2 family and catalyze the hydrolysis of the sn-2 ester bond of glycerophospholipids, resulting in the production of free fatty acids (e.g., arachidonic acid, docosahexaenoic acid) and lysophospholipids, both of which are precursors for the synthesis of proinflammatory mediators such as eicosanoids [28]. In spite of its well-known role in neuroinflammation, the in vivo biological functions in physiological as well as pathological conditions of CSF sPLA2 remain unknown. In the present study, we have shown that while CSF sPLA2 activity could discriminate healthy controls from both neurological controls and AD cases, QAlb could not. These results suggest a higher sensitivity of CSF sPLA2 activity compared to QAlb in order to assess the BCB integrity, as previously reported [21]. In addition, while significant and positive correlations between CSF sPLA2 activity and QAlb were observed in ND-Control45–75 years, AD45–75 years, AD>75 years groups as well as in ATEO and AELO subgroups, no correlation was noticed for other groups/subgroups. This inconsistency corroborates the difference in sensitivity between CSF sPLA2 activity and QAlb and suggests their diverse involvement in the BCB impairment process. While an increase in QAlb results more likely and directly of albumin transit from blood to CSF across an impair BCB [18–20], an increase of CSF sPLA2 activity might reflect phenomena taking place directly at the BCB interface [21]. Thus, while CSF sPLA2 activity seems to be involved early in the BCB impairment process, QAlb increase results from it and this difference probably accounts for the difference of sensitivity between these two parameters. However, longitudinal study should be highly considered in order to confirm such assumption. Supporting this hypothesis, Hampel and colleagues suggested that an altered BCB function in neurodegenerative disorders may result from immune mediated events initiated, for example by increased levels of circulating inflammatory mediators [14]. In return, these inflammatory cytokines can induce the secretory PLA2 expression [29] followed by an increase of arachidonic acid synthesis. Then, this polyunsaturated fatty acid may increase the BCB permeability [30].
CSF sPLA2 activity underlined a lack of BCB integrity in patients with mild neurological symptoms such as headache and vertigo which corroborates recent reports suggesting blood-neural barrier damage in patients with migraine [31, 32]. The implication of blood-neural barrier in the pathophysiology of migraine is not completely understood. To our knowledge no one has ever reported any increase in QAlb in migraineurs. We hypothesize that this knowledge deficiency regarding the involvement of blood-neural barriers impairment in mild neurological symptoms can be in part due to the lack of sensitive tools available. Thus, we believe that CSF sPLA2 activity can facilitate the identification of conditions with blood-neural barrier impairment.
While CSF sPLA2 activity could discriminate healthy controls from both neurological controls and AD cases, it could not differentiate neurological controls from AD cases. A maximum of detection for CSF sPLA2 activity could be the reason for such observation. Indeed, we previously have shown that the values of CSF sPLA2 activity are higher and lower than the values of QAlb at low and high levels of QAlb, respectively, suggesting that: 1) due to its higher sensitivity, CSF sPLA2 activity can reveal a low BCB impairment while QAlb cannot and 2) due to the reach of a detection plateau, CSF sPLA2 activity cannot reveal a high BCB impairment while QAlb can [21]. Thus, it was unexpected that QAlb could neither discriminate healthy controls from both neurological controls and AD cases or neurological controls from AD cases. The reason for such observation can be attributed to a low-to-mild level of BCB impairment in patients enrolled in the present study. Indeed, according to the upper reference limit for QAlb (10.2 for individuals over 45 years of age [20]), only 1 out of the 179 cases independent of their clinical diagnoses was found to have a BCB impairment.
In addition, the absence of BCB impairment between neurological controls and AD cases, as measured by both CSF sPLA2 activity and QAlb, can also be due to the inconsistency of BCB impairment among AD patients. Corroborating such hypothesis, we found that 61% of AD patients included in this study had an abnormally increased CSF sPLA2 activity, which implies that a lack of BCB integrity concerns only a subpopulation of AD. Such results are in concordance with previous reports estimating that more than 30%of AD cases exhibit cerebrovascular pathology associated with blood-brain barrier impairment [33, 34].
To date, it is not clear to what extent, changes in BCB integrity in AD subpopulation are a causal contributor to the disease or whether its dysfunction is a consequence and reinforce the primary pathological process within the brain. It has previously been suggested by others that blood-neural barrier leakage may be a primary event in the pathogenesis of AD [35–37]. Our results seem to corroborate this hypothesis as shown by a significant difference observed between healthy control and patients with mild neurological symptoms. In agreement with this assumption, Skoog et al. reported that non-demented women with a high QAlb are at increased risk of developing dementia within three years [12]. Thus, from the present study, changes in BCB permeability appear to be a cause more than a consequence in the pathophysiology of a subgroup of AD cases suggesting that BCB dysfunction is an early event.
Based on the levels of total tau, Aβ1–42, and ubiquitin in CSF, we previously reported that AD can be divided into five subgroups [25]. The identification of these various and distinct patterns of neurodegeneration in AD reflect the heterogeneity of this pathology. Assuming that BCB integrity is affected differently from one AD patient to another, we investigated whether one or several of these AD subgroups were also, on top of total tau, Aβ1–42, and ubiquitin CSF levels, characterized by a BCB impairment. Our analyses did not reveal any significant difference between the four AD subgroups concerning QAlb or CSF sPLA2 activity, suggesting that the AD patients with an abnormally increased sPLA2 activity do not belong mainly to any one particular of the four subgroups HARO, ATEO, AELO, or LEBALO. Moreover, we found no significant difference between AD patients with normal and abnormally increased sPLA2 activity for all considered parameters, i.e., gender, ApoE4 genotype, MMSE scores, age, CSF levels of total tau, Aβ1–42, or ubiquitin. The BCB impairment probably occurs prior to AD histopathology and does not affect total tau, Aβ1–42, or ubiquitin CSF levels. We hypothesize a co- and independent development of both cerebrovascular and neurodegenerative lesions in the pathophysiology of AD. Moreover, since clinical studies have demonstrated blood-neural barrier dysfunction in AD patients who exhibit peripheral vascular abnormalities such as hypertension, cardiovascular disease and diabetes [38, 39], the investigation of a correlation between these vascular factors and an increase in CSF sPLA2 activity should be considered. In addition, the association of BCB impairment with other microvascular cerebral damage should also be investigated [40].
To date, several techniques other than CSF sPLA2 activity measurement are available to assess blood-neural barrier permeability and their safety, specificity, or sensitivity have been recently reconsidered. To date, only CSF/Serum albumin ratio (QAlb) remains extensively used and widely accepted to assess BCB and/or BBB function [18–20]. Although the CSF sPLA2 activity assay is advantageous over existing methods of measurement of BNB in its ease of use, the nature of BNB measured remains unknown. But, based on the strong correlation between QAlb and CSF sPLA2 activity [21], it seems that the latter mainly measures the assessment of BCB impairment instead of BBB. Indeed, even if it is sometimes assumed that an increase of albumin concentration in CSF is an estimation of a BBB permeability, it seems more accurate to associate such assessment to a BCB breakdown. The reason for such a conclusion is due to the fact that a QAlb increase more likely and directly reflects albumin release across the BCB than a transport from blood to brain across an impaired BBB followed by diffusion across the permeable barrier between brain interstitial fluid and CSF. In this context we also considered the possibility that changes in CSF sPLA2 activity reflect mainly changes in brain metabolism rather than altered BCB function. It is conceivable that this increase in CSF sPLA2 activity is due to a transport from the brain interstitial fluid, which readily communicates (by relatively unrestricted diffusion) with the CSF. But this assumption is contradicted by: 1) the ability of CSF sPLA2 activity to discriminate patients with blood-neural barrier leakage from patients without any impairment [21] and 2) a lack of CSF sPLA2 activity increase in MS patients [21] while a key role for sPLA2 in the pathophysiology of this disorder has been previously reported [41, 42].
We previously suggested that an increase of CSF sPLA2 activity might reflect phenomena taking place directly at the choroid plexus (CP) epithelium, a primary component of the blood-cerebrospinal fluid barrier [21]. The hypothesis advanced regarding the role of CSF sPLA2 in BCB dysfunction was the activation of the arachidonic acid pathway. Another mechanism generally advanced in order to explain BCB impairment in AD, is an Aβ accumulation at CP epithelium which alters the CP function [43] as it has been observed at the BBB interface [33, 44, 45], and may in return increase both the clearance from CSF of CSF-derived protein, for instance Aβ [46] and the accumulation in CSF of blood-derived compounds, e.g., amino acids [47]. Would an increase of BCB impairment be the mechanism involved in the decrease of Aβ level in AD CSF? The present study contradicts this hypothesis by showing: 1) an absence of negative correlation between CSF sPLA2 and Aβ1–42; 2) a significant increase of CSF sPLA2 activity between healthy controls and patients with mild neurological symptoms while no change was observed in Aβ1–42 level; and 3) a significant increase of Aβ1–42 level between HARO and other AD subgroups while no change was observed in CSF sPLA2 activity. Obviously additional investigations are required in order to understand the mechanism involved in the BCB leakage observed in AD [48]. We believe that, due to its high sensitivity against BCB damage, CSF sPLA2 activity should facilitate such studies.
In conclusion, the present study confirms the higher sensitivity of CSF sPLA2 activity compared to QAlb to assess BCB integrity and shows that BCB impairment is restricted to a subgroup of AD patients. Our previous analyses [25] suggested the multifactorial nature of AD. The present study is consistent with more than one etiopathogenic mechanism of this disease. Once established, therapeutic strategies will have to be planned for each individual AD subgroup. For example, the AD subgroup characterized by a high CSF sPLA2 activity due to a BCB impairment, might benefit differently than other AD subgroups to an anti-amyloid immunoglobulin therapy.
ACKNOWLEDGMENTS
We are grateful to Janet Murphy for secretarial assistance. This work was supported in part by the New York State Office of People with Developmental Disabilities, and research grants from the National Institutes of Health – National Institute on Aging [AG028538], Alzheimer’s Association [IIRG-06-25836] and the Swedish Research Council.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=789).
REFERENCES
- 1.Choi YK, Kim KW. Blood-neural barrier: its diversity and coordinated cell-to-cell communication. BMB Rep. 2008;41:345–352. doi: 10.5483/bmbrep.2008.41.5.345. [DOI] [PubMed] [Google Scholar]
- 2.Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology. 2007;68:1809–1814. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stolp HB, Dziegielewska KM. Review: Role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. Neuropathol Appl Neurobiol. 2009;35:132–146. doi: 10.1111/j.1365-2990.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- 4.Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease – systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Golob EJ, Su MY. Vascular volume and blood-brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls. J Magn Reson Imaging. 2006;24:695–700. doi: 10.1002/jmri.20669. [DOI] [PubMed] [Google Scholar]
- 6.Wahlund LO, Bronge L. Contrast-enhanced MRI of white matter lesions in patients with blood-brain barrier dysfunction. Ann N Y Acad Sci. 2000;903:477–481. doi: 10.1111/j.1749-6632.2000.tb06402.x. [DOI] [PubMed] [Google Scholar]
- 7.Bronge L. Magnetic resonance imaging in dementia. A study of brain white matter changes. Acta Radiol Suppl. 2002:1–32. doi: 10.1034/j.1600-0455.43.s.428.1.x. [DOI] [PubMed] [Google Scholar]
- 8.Schlageter NL, Carson RE, Rapoport SI. Examination of blood-brain barrier permeability in dementia of the Alzheimer type with [68Ga]EDTA and positron emission tomography. J Cereb Blood Flow Metab. 1987;7:1–8. doi: 10.1038/jcbfm.1987.1. [DOI] [PubMed] [Google Scholar]
- 9.Caserta MT, Caccioppo D, Lapin GD, Ragin A, Groothuis DR. Blood-brain barrier integrity in Alzheimer’s disease patients and elderly control subjects. J Neuropsychiatry Clin Neurosci. 1998;10:78–84. doi: 10.1176/jnp.10.1.78. [DOI] [PubMed] [Google Scholar]
- 10.Wada H. Blood-brain barrier permeability of the demented elderly as studied by cerebrospinal fluid-serum albumin ratio. Intern Med. 1998;37:509–513. doi: 10.2169/internalmedicine.37.509. [DOI] [PubMed] [Google Scholar]
- 11.Blennow K, Wallin A, Chong JK. Cerebrospinal fluid ’neuronal thread protein’ comes from serum by passage over the blood-brain barrier. Neurodegeneration. 1995;4:187–193. doi: 10.1006/neur.1995.0023. [DOI] [PubMed] [Google Scholar]
- 12.Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, Gottfries CG, Blennow K. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer’s disease and vascular dementia. Neurology. 1998;50:966–971. doi: 10.1212/wnl.50.4.966. [DOI] [PubMed] [Google Scholar]
- 13.Hagnelius NO, Wahlund LO, Nilsson TK. CSF/serum folate gradient: physiology and determinants with special reference to dementia. Dement Geriatr Cogn Disord. 2008;25:516–523. doi: 10.1159/000129696. [DOI] [PubMed] [Google Scholar]
- 14.Hampel H, Kotter HU, Moller HJ. Blood-cerebrospinal fluid barrier dysfunction for high molecular weight proteins in Alzheimer disease and major depression: indication for disease subsets. Alzheimer Dis Assoc Disord. 1997;11:78–87. doi: 10.1097/00002093-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Sun YX, Minthon L, Wallmark A, Warkentin S, Blennow K, Janciauskiene S. Inflammatory markers in matched plasma and cerebrospinal fluid frompatientswithAlzheimer’s disease. Dement Geriatr Cogn Disord. 2003;16:136–144. doi: 10.1159/000071001. [DOI] [PubMed] [Google Scholar]
- 16.Hampel H, Kotter HU, Padberg F, Korschenhausen DA, Moller HJ. Oligoclonal bands and blood – cerebrospinal-fluid barrier dysfunction in a subset of patients with Alzheimer disease: comparison with vascular dementia, major depression, and multiple sclerosis. Alzheimer Dis Assoc Disord. 1999;13:9–19. doi: 10.1097/00002093-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Janciauskiene SM, Erikson C, Warkentin S. A link between sICAM-1, ACE and parietal blood flow in the aging brain. Neurobiol Aging. 2009;30:1504–1511. doi: 10.1016/j.neurobiolaging.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Ganrot K, Laurell CB. Measurement of IgG and albumin content of cerebrospinal fluid, and its interpretation. Clin Chem. 1974;20:571–573. [PubMed] [Google Scholar]
- 19.Tibbling G, Link H, Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest. 1977;37:385–390. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- 20.Blennow K, Fredman P, Wallin A, Gottfries CG, Karlsson I, Langstrom G, Skoog I, Svennerholm L, Wikkelso C. Protein analysis in cerebrospinal fluid. II. Reference values derived from healthy individuals 18–88 years of age. Eur Neurol. 1993;33:129–133. doi: 10.1159/000116919. [DOI] [PubMed] [Google Scholar]
- 21.Chalbot S, Zetterberg H, Blennow K, Fladby T, Grundke-Iqbal I, Iqbal K. Cerebrospinal fluid secretory Ca2+-dependent phospholipase A2 activity: a biomarker of blood-cerebrospinal fluid barrier permeability. Neurosci Lett. 2010;478:179–183. doi: 10.1016/j.neulet.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalbot S, Zetterberg H, Blennow K, Fladby T, Grundke-Iqbal I, Iqbal K. Cerebrospinal fluid secretory Ca2+-dependent phospholipase A2 activity is increased in Alzheimer disease. Clin Chem. 2009;55:2171–2179. doi: 10.1373/clinchem.2009.130286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 24.Kataoka S, Paidi M, Howard BV. Simplified isoelectric focusing/immunoblotting determination of apoprotein E phenotype. Clin Chem. 1994;40:11–13. [PubMed] [Google Scholar]
- 25.Iqbal K, Flory M, Khatoon S, Soininen H, Pirttila T, Lehtovirta M, Alafuzoff I, Blennow K, Andreasen N, Vanmechelen E, Grundke-Iqbal I. Subgroups of Alzheimer’s disease based on cerebrospinal fluid molecular markers. Ann Neurol. 2005;58:748–757. doi: 10.1002/ana.20639. [DOI] [PubMed] [Google Scholar]
- 26.Kudo T, Iqbal K, Ravid R, Swaab DF, Grundke-Iqbal I. Ubiquitin in cerebrospinal fluid: a rapid competitive enzyme-linked immunoflow assay. Neuroreport. 1994;5:1522–1524. doi: 10.1097/00001756-199407000-00028. [DOI] [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 28.Farooqui AA, Horrocks LA. Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist. 2006;12:245–260. doi: 10.1177/1073858405285923. [DOI] [PubMed] [Google Scholar]
- 29.Samuelsson M, Fisher L, Iverfeldt K. beta-Amyloid and interleukin-1beta induce persistent NF-kappaB activation in rat primary glial cells. Int J Mol Med. 2005;16:449–453. [PubMed] [Google Scholar]
- 30.Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teepker M, Munk K, Mylius V, Haag A, Moller JC, Oertel WH, Schepelmann K. Serum concentrations of s100b and NSE in migraine. Headache. 2009;49:245–252. doi: 10.1111/j.1526-4610.2008.01228.x. [DOI] [PubMed] [Google Scholar]
- 32.Gao HM, Li L, Zhang KL, Chen XH, Tian SQ, Zhang ZL. Impact of migraine attacks on the blood-brain barrier. Chin Med J (Engl) 2010;123:2559–2561. [PubMed] [Google Scholar]
- 33.Kalaria RN. The blood-brain barrier and cerebrovascular pathology in Alzheimer’s disease. Ann N Y Acad Sci. 1999;893:113–125. doi: 10.1111/j.1749-6632.1999.tb07821.x. [DOI] [PubMed] [Google Scholar]
- 34.Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813–836. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- 35.Berzin TM, Zipser BD, Rafii MS, Kuo-LeBlanc V, Yancopoulos GD, Glass DJ, Fallon JR, Stopa EG. Agrin and microvascular damage in Alzheimer’s disease. Neurobiol Aging. 2000;21:349–355. doi: 10.1016/s0197-4580(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 36.Donahue JE, Berzin TM, Rafii MS, Glass DJ, Yancopoulos GD, Fallon JR, Stopa EG. Agrin in Alzheimer’s disease: altered solubility and abnormal distribution within microvasculature and brain parenchyma. Proc Natl Acad Sci U S A. 1999;96:6468–6472. doi: 10.1073/pnas.96.11.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de la Torre JC. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 38.Blennow K, Wallin A, Uhlemann C, Gottfries CG. White-matter lesions on CT in Alzheimer patients: relation to clinical symptomatology and vascular factors. Acta Neurol Scand. 1991;83:187–193. doi: 10.1111/j.1600-0404.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 39.Blennow K, Wallin A, Fredman P, Karlsson I, Gottfries CG, Svennerholm L. Blood-brain barrier disturbance in patients with Alzheimer’s disease is related to vascular factors. Acta Neurol Scand. 1990;81:323–326. doi: 10.1111/j.1600-0404.1990.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 40.Ewers M, Mielke MM, Hampel H. Blood-based biomarkers of microvascular pathology in Alzheimer’s disease. Exp Gerontol. 2010;45:75–79. doi: 10.1016/j.exger.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinto F, Brenner T, Dan P, Krimsky M, Yedgar S. Extracellular phospholipase A2 inhibitors suppress central nervous system inflammation. Glia. 2003;44:275–282. doi: 10.1002/glia.10296. [DOI] [PubMed] [Google Scholar]
- 42.Cunningham TJ, Yao L, Oetinger M, Cort L, Blankenhorn EP, Greenstein JI. Secreted phospholipase A2 activity in experimental autoimmune encephalomyelitis and multiple sclerosis. J Neuroinflammation. 2006;3:26. doi: 10.1186/1742-2094-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dietrich MO, Spuch C, Antequera D, Rodal I, de Yebenes JG, Molina JA, Bermejo F, Carro E. Megalin mediates the transport of leptin across the blood-CSF barrier. Neurobiol Aging. 2008;29:902–912. doi: 10.1016/j.neurobiolaging.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 44.Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 45.Miyakawa T, Kimura T, Hirata S, Fujise N, Ono T, Ishizuka K, Nakabayashi J. Role of blood vessels in producing pathological changes in the brain with Alzheimer’s disease. Ann N Y Acad Sci. 2000;903:46–54. doi: 10.1111/j.1749-6632.2000.tb06349.x. [DOI] [PubMed] [Google Scholar]
- 46.Monro OR, Mackic JB, Yamada S, Segal MB, Ghiso J, Maurer C, Calero M, Frangione B, Zlokovic BV. Substitution at codon 22 reduces clearance of Alzheimer’s amyloid-beta peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol Aging. 2002;23:405–412. doi: 10.1016/s0197-4580(01)00317-7. [DOI] [PubMed] [Google Scholar]
- 47.Segal MB, Preston JE, Collis CS, Zlokovic BV. Kinetics and Na independence of amino acid uptake by blood side of perfused sheep choroid plexus. Am J Physiol. 1990;258:F1288–F1294. doi: 10.1152/ajprenal.1990.258.5.F1288. [DOI] [PubMed] [Google Scholar]
- 48.Serot JM, Bene MC, Faure GC. Choroid plexus, aging of the brain, and Alzheimer’s disease. Front Biosci. 2003;8:s515–s521. doi: 10.2741/1085. [DOI] [PubMed] [Google Scholar]