Abstract
The activity of protein phosphatase 2A (PP2A) is compromised and believed to be the cause of the abnormal hyperphosphorylation of tau in Alzheimer’s disease (AD) brain. Activity of PP2A is regulated by two endogeneous inhibitor proteins, called as I1PP2A and I2PP2A. Previously, we reported that: (i) I1PP2A and I2PP2A are upregulated with cleavage of I2PP2A holoprotein and translocation of its amino terminal fragment from the nucleus to the cytoplasm in neuronal cells in AD brains; and (ii) translocated I2PP2A colocalized not only with the PP2A catalytic subunit, but also with phosphorylated tau in neuronal cytoplasm. Furthermore, according to preliminary data, the cleavage site of I2PP2A is located between amino acids 175 and 176 of the I2PP2A sequence. Because the sequence from amino acids 168 to 181 on I2PP2A presumably functions as a nuclear localization signal (NLS), inhibition of break down of the NLS in I2PP2A is expected to be a novel therapeutic target for the treatment of Alzheimer’s disease.
Keywords: Alzheimer’s disease, I2PP2A, neurofibrillary tangle, protein phosphatase 2A, tau
INTRODUCTION
In recent years, there has been a rapid increase in the elderly population worldwide; consequently, the number of patients with dementia has also increased. At present there are approximately 1 700 000 patients with dementia in Japan, and it is estimated that this figure will increase to 2 500 000 in 2015 and to 3 230 000 in 2025.1 Thus, the aging society is one of the most important issues not only from a social viewpoint, but also from a psychogeriatric medical point of view. Behavioral and psychiatric symptoms of dementia (BPSD) are one of the major issues for care and therapy,2,3 and therapeutic approaches to the treatment of BPSD have been developed.4–8 Although treatment of BPSD is important from a social point of view, therapy for the pathological process of dementia, namely neurodegenerative disease, is essential from the medical point of view.
Alzheimer’s disease (AD) is a major neurodegenerative disease and the underlying pathological mechanisms are under intense investigation worldwide. Alzheimer’s disease is characterized by progressive neurodegeneration and there are two types of abnormal deposits that are observed pathologically in AD brains, namely neurofibrillary tangles (NFT) and extracellualr amyloid plaques, composed of hyperphosphorylated tau forming paired helical filaments (PHF) and amyloid β (Aβ) peptide.9,10 As pathological components of AD, Aβ and tau have been investigated in considerable and the results of this research applied for the early diagnosis and therapy of AD.11–14
At present, the main therapeutic target in AD is Aβ and several therapeutic approaches have been developed accordingly, including β/γ secretase inhibitor(s)/modulator(s) and Aβ vaccines. However, alternative or supplemental therapies are required.
In the present review, one of the possible therapeutic targets for neurofibrillary degeneration, especially targeting the hyperphosphorylation of tau, in AD brain is discussed.
FUNCTION OF TAU PROTEIN AND ITS COMPROMISE IN AD BRAIN
Tau protein is one of the major microtubule-associated proteins that is mainly localized in neuronal axons, as well as in neuronal cell bodies, dendrites, and non-neuronal cells, such as astrocytes and oligodendrocytes. The major biological function of tau is to promote microtubule assembly and maintain the stability of the previously formed microtubules, which are essential for the axonal transport of the neurons.
When tau is hyperphosphorylated, several crucial events occur, including: (i) loss of the ability to promote microtubule assembly; (ii) binding with and sequestration of normal unphosphorylated tau from microtubules; and (iii) promotion of the self-assembly of normal unphosphorylated tau into PHF.
REGULATION OF TAU PHOSPHORYLATION AND ROLE OF PROTEIN PHOSPHATASES IN AD BRAIN
Hyperphosphorylation of tau is thought to be the result of an imbalance in the regulation of protein kinases and protein phosphatases (PP). Recently, it was reported that tyrosine 18 or tyrosine 394 on tau are phosphorylated by the src family tyrosine kinase fyn or non-receptor tyrosine kinase c-Abl.15,16 However, known functions associated with phosphorylation sites on tau have been reported for serine and threonine residues only, suggesting a crucial role for serine and threonine kinases and PP in AD.
There are more than 10 serine/threonine protein kinases, such as extracellular signal-regulated kinase (ERK) 1/2, cell division cycle (cdc)-2 kinase, cyclin-dependent kinase (cdk)-2, cdk-5, calcium–calmodulin-dependent protein kinase II (CaMKII), protein kinase (PK) A, PKC, casein kinase I/II, and P110MARK, that have been shown to phosphorylate tau in vitro. Of these, glycogen synthase kinase (GSK) 3β is the most likely to be involved in the abnormal hyperphosphorylation of tau in AD brain.17
It has been reported that the PPs that participate in dephosphorylation of tau in AD are PP1, PP2A, PP2B, and PP5, but not PP2C.18 Of these, PP2A is the most effective phosphatase in dephosphorylating hyperphosphorylated tau isolated from AD brains.19 It has been shown that PP2A accounts for approximately 71% of the total tau phophatase activity in the human brain, with the activity of PP2A being significantly decreased in AD brains.20,21
REGULATION OF PP2A ACTIVITY IN VIVO
The PP2A holoenzyme consists of two common subunits, namely catalytic subunit C (PP2Ac) and a structural scaffolding subunit A, together with various a regulatory B subunits.
The activity of PP2A is regulated by several post-translational modifications, including phosphorylation of tyrosines22 or threonines,23 which inactivate PP2A, and methylation of the carboxyl-termminal leucine Leu309, which activates PP2A.24
In addition, PP2A activity is regulated by two inhibitor proteins, termed I1PP2A and I2PP2A. Both inhibit PP2A in a non-competitive manner and exhibit apparent Ki values in the nanomolar range.25 Recently, I1PP2A and I2PP2A were cloned from the human brain and it was demonstrated that overexpression of I1PP2A and I2PP2A led to the hyperphosphorylation of tau via inhibition of PP2A activity in vitro.26
INCREASED I1PP2A AND I2PP2A mRNA LEVELS IN AD BRAIN
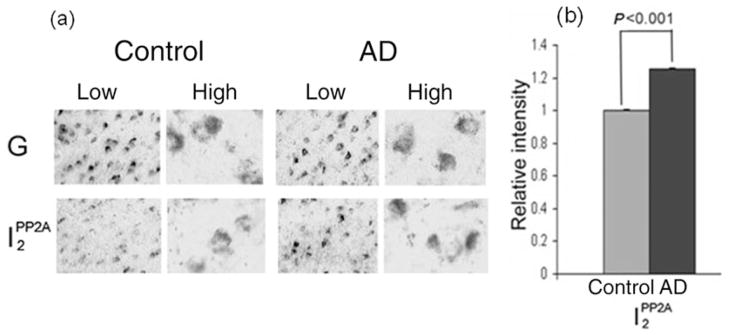
We investigated the involvement of the PP2A inhibitors I1PP2A and I2PP2A, particularly I2PP2A, in neurofibrillary pathology in AD.27 The expression of I1PP2A and I2PP2A was determined by digoxigenin-labeled in situ hybridization histochemistry in AD and control brains. Comparison of the levels of I1PP2A and I2PP2A between AD and control subjects revealed a disease-associated increase of neuronal I1PP2A and I2PP2A mRNA in the temporal cortex of AD brains (Fig. 1a). The relative expression of both I1PP2A and I2PP2A mRNAs after normalizataion against GAPDH mRNA was approximately 25% higher (P < 0.001) in the temporal cortex of AD brains compared with control (Fig. 1b; data not shown for I1PP2A).
Figure 1.
Expression of I2PP2A mRNA in Alzheimer disease (AD) and control brain.27 (a) The I2PP2A signal was significantly elevated in AD brain (temporal cortex) compared with control brain (P < 0.001), whereas the GAPDH signal (G) was similar between the two. Differences between AD and control brains were analyzed statistically by Student’s t-test. High, high magnification (original magnification ×630); Low, low magnification (original magnification ×200). (b) The I2PP2A signals from five AD and five control cases were quantified and normalized against the GAPDH signal. Data show the mean ± SEM. Modified and reproduced with the permission from the American Society for Investigative Pathology from Tanimukai et al.27
REDISTRIBUTION OF I2PP2A FROM THE NUCLEUS TO THE CYTOPLASM OF NEURONS FROM AD BRAIN
The subcellular localization of I2PP2A has been reported in various cultured cells. For example, I2PP2A, which is the same as SET/template-activating factor (TAF)-1β, is mainly localized in the nucleus.28–31 However, TAF1β has been shown to be cleaved and the amino terminal cleaved half, which has PP2A inhibitory activity similar to the full-length of I2PP2A, is localized in the cytoplasm.30
The subcellular distribution of I2PP2A was investigated in AD and control brains by immunohistochemistry using a specific polyclonal antibody (R-42187) that recognizes the amino terminal region of I2PP2A.27 Surprisingly, I2PP2A was translocated from the nucleus to the cytoplasm in many neuronal cells in the temporal cortex of AD brains (Fig. 2a). The number of neurons in the temporal cortex of exhibiting translocation of I2PP2A from the nucleus to the cytoplasm was counted and compared between AD and control brains. It was found that the ratio of the number of neurons with immunonegative nuclei to those with nuclei immunopositive for I2PP2A was more than sixfold greater in AD than control brains (Fig. 2b; P < 0.05). In the cerebellum, the subcellular localization of I2PP2A was similar between AD and control brains (Fig. 2c).
Figure 2.
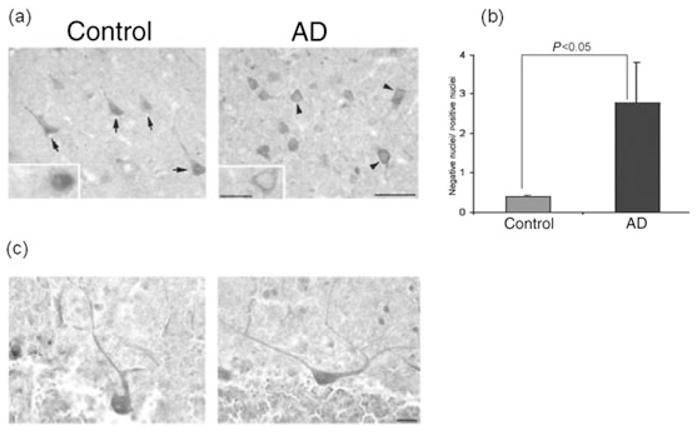
Subcellular localization of I2PP2A in Alzheimer disease (AD) and control brains.27 (a) I2PP2A was predominantly expressed in the nucleus (arrows) of neurons in the temporal cortex from control brain, but was translocated from the nucleus to cytosol (arrowheads) in AD brain. (b) Ratio (mean ± SEM) of neurons with immunonegative to immunopositive nuclei. In AD brains, the number of neurons in the temporal cortex showing the translocation of I2PP2A from the nucleus to a cytoplasmic localization increased markedly (P < 0.05). Differences between AD and control cases were analyzed statistically by Student’s t-test. (c) In contrast, the subcellular localization of I2PP2A in the cerebellum was restricted to the nucleus in both AD and control brain. Modified and reproduced with the permission from the American Society for Investigative Pathology from Tanimukai et al.27
CLEAVAGE OF I2PP2A IN THE TEMPORAL CORTEX OF AD BRAIN
To biochemically confirm the results of immunohistochemical analysis, western blots were preformed using nuclear and cytosol fractions prepared from temporal cortices of seven AD and seven control brains.27 Consistent with the immunohistochemical findings, the signal for I2PP2A in the nuclear fraction was reduced (P < 0.05) in AD compared with control brains. In the cytosol, the 39 kDa I2PP2A was cleaved and fragment levels were higher in samples from AD brains compared with control. The signal for the 39 kDa band in the cytosolic fraction was decreased in AD brains (P < 0.05). A major cleavage product, the approximately 20 kDa I2PP2A polypeptide, which was seen in the cytosolic but not the nuclear fraction, appeared in few control samples but was found in most samples from AD brain (Fig. 3). Levels of the 20 kDa polypeptide were significantly higher in AD cytosol compared with control (P < 0.05).
Figure 3.
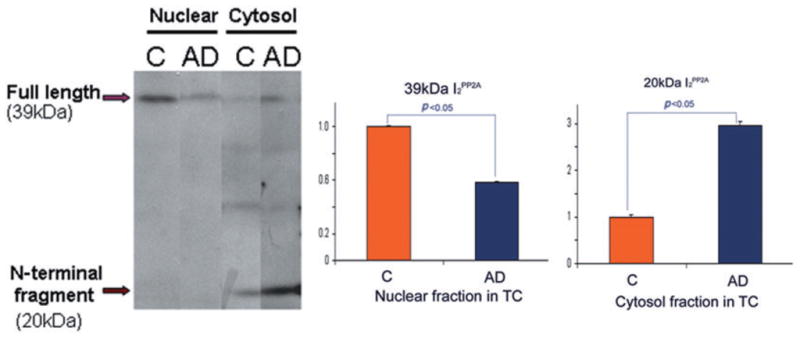
Cleavage and distribution of I2PP2A in nuclear and cytosolic fractions of the temporal cortex (TC) in Alzheimer disease (AD) and control (C) brains.27 Levels of I2PP2A in the nuclear fraction were decreased in AD compared with control brain. In contrast, the 39 kDa I2PP2A in the cytosolic fraction was decreased in AD brain, but the approximately 20 kDa fragment of I2PP2A was significantly increased in AD compared with control brain (*P < 0.05). Differences between AD and control brains were analyzed statistically by Student’s t-test. Modified and reproduced with the permission from the American Society for Investigative Pathology from Tanimukai et al.27
The same study was performed using nuclear and cytosol fractions from the cerebellum.27 In the cerebellum, there was no significant difference in the expression of I2PP2A between AD and control brain (data not shown), suggesting that this cleavage of I2PP2A was limited to areas of the brain that develop neurofibrillary pathology.
COLOCALIZATION OF PP2A INHIBITORS WITH PP2A CATALYTIC SUBUNITS AND WITH HYPERPHOSPHORYLATED TAU IN NEURONAL CYTOPLASM
The increased levels of I2PP2A mRNA, cleavage of I2PP2A protein, and its translocation from the nuclear to the cytoplasmic compartment in neurons in AD brain prompted us to investigate whether PP2A inhibitors were involved in the hyperphophorylation of tau in AD. We performed double-labeled immunohistochemical studies using specific antibodies against I2PP2A (R-42187), PP2A catalytic subunit, and tau abnormally hyperphosphorylated at Ser262/356 (12E8).27 We found that I2PP2A was colocalized with the PP2A catalytic subunit in the neuronal cytoplasm in AD brains (Fig. 4a). In addition, we found that I2PP2A was colocalized in early or middle-stage neurofibrillary tangles of abnormally hyperphosphorylated tau in the neuronal cytoplasm (Fig. 4b). The neurons that expressed I2PP2A mainly in the nucleus did not exhibit phosphorylated tau immunoreactivity, indicating that the I2PP2A in the cytoplasm was probably responsible for tau hyperphosphorylation.
Figure 4.
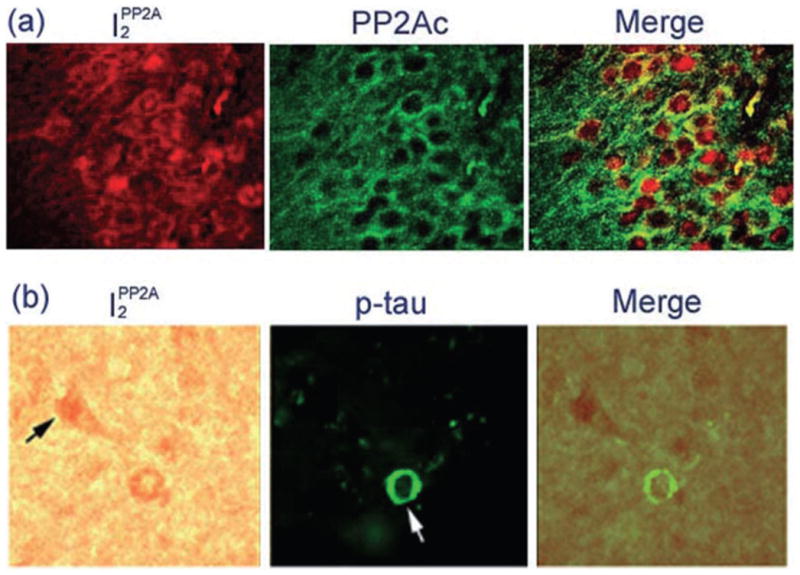
Colocalization of I2PP2A with protein phosphatase 2A (PP2A) and with phosphorylated tau in Alzheimer disease (AD) brain.27 (a) Subcellular localizations of I2PP2A and the PP2A catalytic subunit in AD hippocampus. I2PP2A was colocalized with PP2A in the neuronal cytoplasm. (b) I2PP2A was colocalized with phosphorylated tau in mostly early stage (white arrow) to middle stage (white arrowhead), where neurofibrillary changes were seen using a phosphorylation-dependent antibody to abnormally hyperphosphorylated (p-) tau in the AD temporal cortex. Neurons that expressed I2PP2A mainly in the nucleus did not colocalize with p-tau (black arrows). Modified and reproduced with the permission from the American Society for Investigative Pathology from Tanimukai et al.27
WHY IS THE EXPRESSION OF I2PP2A INCREASED IN AD BRAIN AND HOW DOES I2PP2A TRANSLOCATE FROM THE NEURONAL NUCLEUS TO THE CYTOPLASM IN AD BRAIN?
Possible mechanisms and a new therapeutic target for AD
In an unpublished study (H. Tanimukai et al., unpubl. data, 2004, 2007), we have investigated why the expression of I2PP2A is increased in AD brain and how I2PP2A translocates from neuronal nucleus to the cytoplasm in AD brain. To address the first question, SH-SY5Y neuroblastoma cells were stimulated with 3 μmol/L Aβ(1–42) for 0, 2, 4, 24, and 48 h. After stimulation with Aβ(1–42), cell lysates were collected and the expression of I2PP2A was investigated by western blot. The preliminary evidence indicates that the expression of the I2PP2A holoprotein (molecular weight 39 kDa) was increased in a time-dependent manner (Fig. 5; H. Tanimukai et al., unpubl. data, 2007), suggesting that Aβ(1–42) stimulation is probably responsible for the increase in I2PP2A expression.
Figure 5.
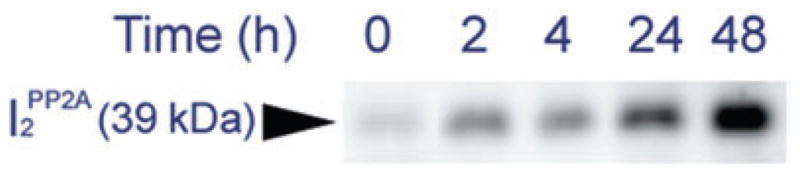
Expression of I2PP2A SH-SY5Y neuroblastoma cells after stimulation with 3 μmol/L Aβ(1–42) for 0, 2, 4, 24, and 48 h. The induction of the expression of I2PP2A by 3 μmol/L Aβ(1–42) was time dependent.
The activity of PP2A against tau protein is not likely to be compromised simply as a result of increased I2PP2A expression in the neuronal nucleus because an interaction between I2PP2A and the PP2A catalytic subunit is needed for a reduction in PP2A activity in the neuronal cytoplasm, where the PP2A catalytic subunit and tau mainly localize. So, we speculated that the translocation of I2PP2A from the neuronal nucleus to the cytoplasm was the most crucial event in the hyperphosphorylation of tau following on from a reduction in PP2A activity.
To address the second question, recombinant I2PP2A 26 was digested with brain extract from AD cases and the digested product (approximately 20 kDa), which was similar to that seen in AD brain, was analyzed by mass spectrometry and amino terminal sequencing. As expected, the digested product was from the amino terminal half or greater and the digestion site was located between amino acids 175 and 176 on I2PP2A (H. Tanimukai et al., unpubl. data, 2004). Because the sequence between amino acids 168 and 181 (KRxxxxxxxxxRKR) on I2PP2A is considered a plausible nuclear localization signal (NLS),32 we speculate that the translocation of I2PP2A from the nucleus to cytoplasm is due to a break in the NLS located on the I2PP2A sequence by an as yet unidentified protease(s) that we will temporarily call protease X. Previously, we reported an increase in the I2PP2A cleavage activity by protease X in AD brain compared with control brain.27 In addition, Chohan et al. reported that overexpression of I2PP2A resulted in abnormal hyperphosphorylation of tau in cultured cells and that this was observed only when a subcellular shift of I2PP2A occurred from the nucleus to cytoplasm that was accompanied by cleavage of I2PP2A into the 20 kDa fragment.33
Taken together, these data indicate that a potential novel therapeutic target would be to inhibit the translocation of I2PP2A from the neuronal nucleus to the cytoplasm (Fig. 6). Although further investigation is required to identify protease X, the activity of which is elevated in AD brain, inhibitors of protease X may also turn out to be new therapeutic drugs for AD.
Figure 6.
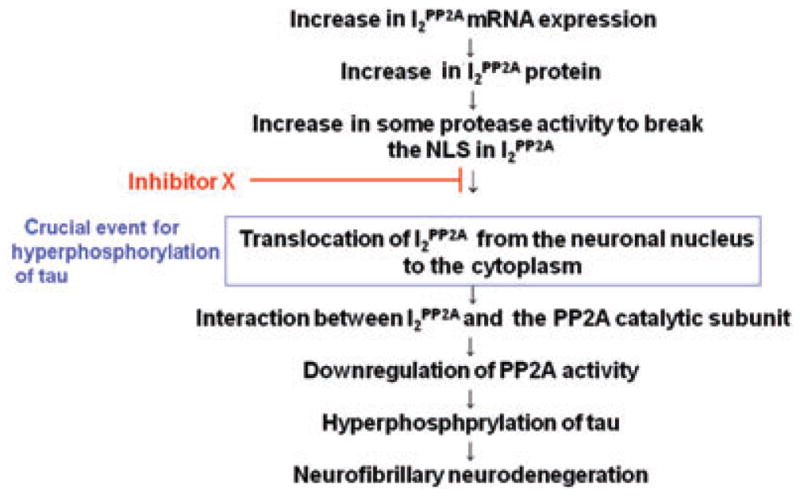
Schematic of a proposed therapeutic strategy for the neurofibrillary neurodegeneration in Alzheimer’s disease. NLS, nuclear localization signal; PP2A, protein phosphatase 2A.
Footnotes
This review article was presented by the author in Symposium of the 23rd annual meeting of Japanese Psychogeriatric Society in Kobe, 27–28 June 2008.
References
- 1.Ministry of Health, Labour and Welfare. Working Group of Care for Elderly (on-line) 2003 Available from http://www.mhlw.go.jp/topics/kaigo/kentou/15kourei/3c.html [10 October, 2008] (in Japanese)
- 2.Hamuro A, Isono H, Sugai Y, et al. Behavioral and psychological symptoms of dementia in untreated Alzheimer’s disease patient. Psychogeriatrics. 2007;7:4–7. [Google Scholar]
- 3.Hamuro A, Isono H, Sugai Y, Mimura M, Kamijima K. Characteristics of behavioral and psychological symptoms of dementia in untreated oldest old Alzheimer’s disease. Psychogeriatrics. 2008;8:8–11. [Google Scholar]
- 4.Tanaka T, Kazui H, Morihara T, Sadik G, Kudo T, Takeda M. Post-marketing survey of donepezil hydrochloride in Japanese patients with Alzheimer’s disease with BPSD. Psychogeriatrics. 2008;8:114–123. [Google Scholar]
- 5.Kosaka K. Behavioral and psychological symptoms of dementia (BPSD) in dementia with Lewy bodies. Psychogeriatrics. 2008;8:134–136. [Google Scholar]
- 6.Mizukami K. Kampo therapy as an alternative to pharmacotherapy using antipsychotic medicines for BPSD. Psychogeriatrics. 2008;8:137–141. [Google Scholar]
- 7.Kinoshita T. Role of the home visit medical service for patients with BPSD living in the community. Psychogeriatrics. 2008;8:142–147. [Google Scholar]
- 8.Takita M. How to treat BPSD: Do not treat patients exhibiting symptoms like BPSD with neuroleptics from the beginning. Psychogeriatrics. 2008;8:148–150. [Google Scholar]
- 9.Yamaguchi H. Alzheimer pathology during the past 100 years. Psychogeriatrics. 2007;7:109–113. [Google Scholar]
- 10.Kudo T, Tanii H, Takeda M. Neurodegenerative dementias involving aberrant protein aggregation. Psychogeriatrics. 2007;7:114–117. [Google Scholar]
- 11.Tanaka T, Tomioka M, Sadik G, Takeda M. 13th Congress of the International Psychogeriatric Association and recent expansion of research into psychogeriatrics. Psychogeriatrics. 2008;8:1–3. [Google Scholar]
- 12.Takeda M, Tanaka T, Arai H, et al. Basic and clinical studies on the measurement of β-amyloid (1–42) in cerebrospinal fluid as a diagnoctic marker for Alzheimer’s disease and related disorders: Multi study in Japan. Psychogeriatrics. 2001;1:56–63. [Google Scholar]
- 13.Matsuda H. Progress in neuroimaglng of Alzheimer’s disease. Psychogeriatrics. 2007;7:118–124. [Google Scholar]
- 14.Shinotoh H, Suhara T. Beyond PIB: The next generation of amyloid-imaging ligands. Psychogeriatrics. 2008;8:105–107. [Google Scholar]
- 15.Lee G, Thangavel R, Sharma VM, et al. Phosphorylation of tau by fyn: Implications for Alzheimer’s disease. J Neurosci. 2004;24:2304–2312. doi: 10.1523/JNEUROSCI.4162-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derkinderen P, Scales TM, Hanger DP, et al. Tyrosine 394 is phosphorylated in Alzheimer’s paired helical filament tau and in fetal tau with c-Abl as the candidate tyrosine kinase. J Neurosci. 2005;25:6584–6593. doi: 10.1523/JNEUROSCI.1487-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F, Liang Z, Gong CX. Hyperphosphorylation of tau and protein phosphatases in Alzheimer disease. Panminerva Med. 2006;48:97–108. [PubMed] [Google Scholar]
- 19.Wang JZ, Gong CX, Zaidi T, Grundke-Iqbal I, Iqbal I. Dephosphorylation of Alzheimer paired helical filaments by protein phosphatase-2A and -2B. J Biol Chem. 1995;270:4854–4860. doi: 10.1074/jbc.270.9.4854. [DOI] [PubMed] [Google Scholar]
- 20.Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem. 1993;61:921–927. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- 21.Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal I. Phosphatase activity toward abnormally phosphorylated tau: Decrease in Alzheimer desease brain. J Neurochem. 1995;65:732–738. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Martin BL, Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257:1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- 23.Guo H, Damuni Z. Autophosphorylation-activated protein kinase phosphorylates and inactivates protein phosphatase 2A. Proc Natl Acad Sci USA. 1993;90:2500–2504. doi: 10.1073/pnas.90.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Favre B, Zolnierowicz S, Turowski P, Hemmings BA. The catalytic subunit of protein phosphatase 2A is carboxyl-methylated in vivo. J Biol Chem. 1994;269:16 311–16 317. [PubMed] [Google Scholar]
- 25.Li M, Guo H, Damuni Z. Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry. 1995;34:1988–1996. doi: 10.1021/bi00006a020. [DOI] [PubMed] [Google Scholar]
- 26.Tsujio I, Zaidi T, Xu J, Kotula L, Grundke-Iqbal I, Iqbal K. Inhibitors of protein phosphatase-2A from brain structures, immunocytological localization and activities towards dephosphorylation of the Alzheimer type hyperphosphorylated tau. FEBS Lett. 2005;579:363–372. doi: 10.1016/j.febslet.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 27.Tanimukai H, Grundke-Iqbal I, Iqbal K. Up-regulation of inhibitors of protein phosphatase-2A in Alzheimers desease. Am J Pathol. 2005;166:1761–1771. doi: 10.1016/S0002-9440(10)62486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Linden M, van Baal S, Wiegant J, Raap A, Hagemeijer A, Grosveld G. Can, a putative oncogene associated with myeloid leukemogenesis, may be activated by fusion of its 3′ half to different genes: Characterization of the set gene. Mol Cell Biol. 1992;12:3346–3355. doi: 10.1128/mcb.12.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adachi Y, Pavlakis GN, Copeland TD. Identification and characterization of SET, a nuclear phosphoprotein encoded by the translocation break point in acute undifferentiated leukemia. J Biol Chem. 1994;269:2258–2262. [PubMed] [Google Scholar]
- 30.Nagata K, Saito S, Okuwaki M, et al. Cellular localization and expression of template-activating factor I in different cell types. Exp Cell Res. 1998;240:274–281. doi: 10.1006/excr.1997.3930. [DOI] [PubMed] [Google Scholar]
- 31.Tanimukai H, Grundke-Iqbal I, Iqbal K. Inhibitors of protein phosphatase 2A: Topology and subcellular localization. Mol Brain Res. 2004;126:146–156. doi: 10.1016/j.molbrainres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Robbins J, Dilworth SM, Laskey RA, Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: Identification of a class of bipartite nuclear targeting sequence. Cell. 1991;64:615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- 33.Chohan MO, Khatoon S, Grundke-Iqbal I, Iqbal K. Involvement of I2PP2A in the abnormal hyperphosphorylation of tau and its reversal by memantine. FEBS Lett. 2006;580:3973–3979. doi: 10.1016/j.febslet.2006.06.021. [DOI] [PubMed] [Google Scholar]