Abstract
Febrile seizures (FSs) constitute the most prevalent seizure type during childhood. Whether prolonged FSs alter limbic excitability, leading to spontaneous seizures (temporal lobe epilepsy) during adulthood, has been controversial. Recent data indicate that, in the immature rat model, prolonged FSs induce transient structural changes of some hippocampal pyramidal neurons and long-term functional changes of hippocampal circuitry. However, whether these neuroanatomical and electrophysiological changes promote hippocampal excitability and lead to epilepsy has remained unknown. By using in vivo and in vitro approaches, we determined that prolonged hyperthermia-induced seizures in immature rats caused long-term enhanced susceptibility to limbic convulsants that lasted to adulthood. Thus, extensive hippocampal electroencephalographic and behavioral monitoring failed to demonstrate spontaneous seizures in adult rats that had experienced hyperthermic seizures during infancy. However, 100% of animals developed hippocampal seizures after systemic administration of a low dose of kainate, and most progressed to status epilepticus. Conversely, a minority of normothermic and hyperthermic controls had (brief) seizures, none developing status epilepticus. In vitro, spontaneous epileptiform discharges were not observed in hippocampal-entorhinal cortex slices derived from either control or experimental groups. However, Schaeffer collateral stimulation induced prolonged, self-sustaining, status epilepticus-like discharges exclusively in slices from experimental rats. These data indicate that hyperthermic seizures in the immature rat model of FSs do not cause spontaneous limbic seizures during adulthood. However, they reduce thresholds to chemical convulsants in vivo and electrical stimulation in vitro, indicating persistent enhancement of limbic excitability that may facilitate the development of epilepsy.
Febrile seizures are common, affecting 3% to 5% of infants and young children.1–3 The relationship of childhood febrile seizures to adult temporal lobe epilepsy (TLE) has remained a focus of intense discussion4–6; ie, although prospective epidemiological studies have not shown a progression of febrile seizures to TLE,2,7,8 retrospective analyses of adults with TLE document a high prevalence (30–50%) of a history of febrile seizures during early childhood, suggesting a causative role for these seizures in the development of TLE.9–11 Specifically, neuronal damage induced by febrile seizures has been suggested as a mechanism for the development of mesial temporal sclerosis, the pathological hallmark of TLE.12–14 An alternative mechanism for the correlation of febrile seizures and TLE involves preexisting neuronal injury triggering both the febrile seizures and the subsequent TLE.4,6,15
These critical questions regarding the causal relationship of febrile seizures and TLE are difficult to resolve in human studies. However, animal models permit induction of febrile seizures, prospective studies, and interventions addressed at dissecting out their mechanisms and consequences.16,17 Therefore, a model of febrile seizures in the immature rat has been developed and characterized,18–20 using animals during a brain development age generally equivalent to that of the human infant and young child.21 This model has permitted acute and long-term prospective analysis of the consequences of hyperthermic seizures on neuronal integrity and survival. Thus, hyperthermic seizures, but not hyperthermia alone, were shown to result in structural alterations of select hippocampal and amygdala neurons.19 These cells developed affinity to silver stains (argyrophilia) that persisted for at least 2 weeks; but, by 4 weeks after the seizures, neuronal counts in heavily involved limbic regions of control and experimental animals were similar, indicating that significant neuronal death had not occurred. However, whether the apparently transient alterations of neuronal structure induced by hyperthermic seizures were associated with functional disruption sufficient to influence the excitation/inhibition balance in the involved circuits has remained unclear.
Indeed, persistent functional modulation of hippocampal circuitry in this immature rat model of febrile seizures has recently been demonstrated.20 Hyperthermia-induced seizures (but not hyperthermia alone) caused a selective presynaptic increase of inhibitory synaptic transmission in the hippocampus that lasted into adulthood. These in vitro electrophysiological data indicated long-lasting modifications of the balance of excitation and inhibition in neuronal micro-circuits within the limbic system. However, the relationship between these alterations of synaptic communication and the development of limbic epilepsy has not been resolved. Therefore, the goals of the study reported here were to determine whether (1) febrile seizures in the immature rat model caused spontaneous behavioral and electrophysiological seizures during adulthood, and (2) these early life seizures altered seizure threshold to chemical convulsants in vivo and to electrical stimulation in vitro, indicating persistent enhancement of limbic excitability that may facilitate the development of epilepsy.
Subjects and Methods
Animals
Sprague–Dawley–derived rats (total n = 45) were born and maintained in quiet facilities with controlled temperature (21–22°C), 12-hour light schedule and unlimited access to food and water.22 After hyperthermic seizures (or hyperthermia) on postnatal day 10 or 11, experimental rats were kept with littermate controls in home cages. In vitro experiments were conducted on days 17 and 18, whereas for in vivo experiments, rats were weaned on postnatal day 22 and housed 2 or 3 per cage. Only males were used, to avoid potential confounding effects of variable estrogen levels on neuronal excitability.23
Design: In Vivo Experiments
The overall strategy was to determine the presence of spontaneous seizures and of electroencephalograhic (EEG) abnormalities in adult rats that had hyperthermia-induced seizures early in life, compared with littermate normothermic and hyperthermic controls. In addition, to investigate whether these early-life seizures conferred susceptibility to seizures during adulthood, the convulsant potency of kainate for both EEG and behavioral seizures was determined in all three groups.
Febrile Seizures Model
The hyperthermic seizure paradigm has been described in detail previously.18,19 In brief, on postnatal day 10 or 11, the temperature of pups (n = 19) was raised by using a regulated stream of moderately heated air, aiming for a core temperature of approximately 41°C (as during high fever). Rats were placed in a 3-L glass container and the air stream directed approximately 50 cm above them. Core temperatures were measured at baseline, at 2-minute intervals, and at the onset of hyperthermic seizures. Hyperthermia was induced for 30 minutes, and the presence and duration of seizures for each animal were noted at 2-minute intervals (Table 1). The behavioral seizures in this paradigm are stereotyped, consisting of arrest of the heat-induced hyperkinesis, body flexion, and biting of an extremity, occasionally followed by clonus. Controls included a normothermic group (n = 11; rectal temperature, 33–34°C) and a group subjected to the same degree and duration of hyperthermia (n = 6; see Table 1), but in which seizures were prevented by using a short-acting barbiturate (pentobarbital, 30 mg/kg) intraperitoneally. Hyperthermic animals were placed on a cool surface, monitored for 15 minutes, and returned to home cages for rehydration. Those sedated because of pentobarbital treatment were hydrated orally and returned to cages when their behavior normalized (typically, <1 hour).
Table 1.
Parameters of Hyperthermia and of Seizures in the Immature Rat Febrile Seizure Model
| Group/Parameter | Hyperthermia Duration (min) | Maximal Temperature (°C) | Threshold Temperature for Seizures (°C) | Seizure Duration (min) |
|---|---|---|---|---|
| Hyperthermic seizures | 28.2 ± 0.7 | 41.96 ± 0.14 | 40.88 ± 0.3 | 21.5 ± 0.9 |
| Hyperthermic controls | 28.7 ± 0.6 | 41.91 ± 0.11 | — | — |
Hyperthermia duration is defined as the time during which core temperature exceeded 39°C. In the hyperthermic control group, seizures were prevented by using a short-acting barbiturate. Data are mean ± SEM values. Differences between duration and magnitude (maximal temperature) of hyperthermia in control and experimental groups are not significant.
To verify the correlation of behavioral and electrophysiological hyperthermic seizures in immature rats and to determine with certainty that pentobarbital treatment indeed prevented EEG seizures, a separate group of immature rats (n = 6) was implanted on postnatal day 10 with bipolar electrodes directed at the dorsal hippocampus, using methods and coordinates described in detail previously.18,24–26 On the following day, baseline hippocampal EEGs were performed in the presence or absence of pentobarbital, and hyperthermia-induced seizures, as well as the anticonvulsant effects of the barbiturate, were documented. Correct electrode placement was verified in all animals.
Analysis for Spontaneous Seizures in Adult Rats That Experienced Hyperthermic Seizures Early in Life
BEHAVIORAL SEIZURES
Two months after hyperthermic seizures (ie, on postnatal day 71.4 ± 5.1), rats were monitored for the presence of spontaneous behavioral seizures. Specifically, structured observation periods were conducted that lasted 3 hours (2–5 pm) daily for 7 days, during which they were scored for established behavioral measures of limbic seizures. These included automatisms (also head nodding and bobbing), prolonged immobility with staring, rhythmic unilateral or bilateral clonic movements (exclusive of grooming), wet-dog shakes, and rearing and loss of balance.25–31
EEG SEIZURES
After the week of monitoring for spontaneous behavioral seizures, rats were implanted unilaterally with bipolar twisted wire hippocampal electrodes (Plastics One, Roanoke, VA), as described previously.18,24–26 In brief, electrodes were inserted through a burr hole into the dorsal hippocampus, using the coordinates of AP −3.7, L 2.9, V −3.7 mm with reference to the bregma. The assembly was embedded and anchored with dental cement and five stainless steel screws. Animals were allowed a 1-week recovery period, then subjected to hippocampal EEGs. Thus, hippocampal EEGs were performed 10 to 11 weeks after the hyperthermic seizures, to examine for the possibility of limbic EEG seizures not associated with behavioral manifestations, to determine the epileptic nature of any observed behaviors, and to localize their substrate. After a 10-minute habituation period, hippocampal recordings were performed via long flexible cables in freely moving rats, using a Grass 78E polygraph (Grass Instruments, Quincy, MA) for 1 hour on 7 consecutive days.
Determination of Threshold to Kainate-Induced Limbic Seizures in Adult Rats That Had Hyperthermia-Induced Seizures Early in Life
To determine whether early-life hyperthermic seizures influenced susceptibility to limbic convulsants, control and experimental adult animals were subjected to subthreshold doses of kainate. In a pilot study, using age- and weight-matched control animals (n = 10), 5 mg/kg of the limbic convulsant kainate, given intraperitoneally, was determined to be a sub-threshold dose in naive adult rats of the colony used here; this dose modified behavior minimally and did not lead to overt seizures. Kainate was administered to hippocampal electrode–carrying adult rats on postnatal days 95.7 ± 5.1 (3 months after hyperthermic seizures) and EEGs and behavior monitored as above, with attention to latencies and duration of kainate-induced seizures. If kainate did not provoke EEG and/or behavioral seizures, monitoring continued for 3 hours. If prolonged seizures or status epilepticus (SE) resulted, animals were killed after 1 hour to prevent death and to permit collection of the brains. Kainate-induced EEG seizures in these adult rats were defined based on EEG criteria (see Fig 2), consisting of trains of rhythmic spikes or spike waves with increasing amplitude and decreasing frequency, in a sample free of movement artifacts. SE was defined as a prolonged (>30 minute) series of seizures, with minimal interictal intervals.
Fig 2.
Hippocampal electroencephalograms (EEGs) from adult rats before (left) and 45 minutes after administration of low-dose (5 mg/kg) kainate (right). (Left) Normal EEGs in rats that had sustained hyperthermic seizures early in life (H-seiz), in hyperthermic controls in which early-life seizures were prevented (H-ctl), and in normothermic controls (N-ctl). (Right) Differential effects of kainate in these groups. Prolonged train of spikes (top), leading to status epilepticus developed in H-seiz animals (8 of 11), whereas the EEG remained normal in the large majority of hyperthermic and normothermic controls. Calibration: vertical, 1 mV; horizontal, 1 second.
After the rats were killed, brains were examined for electrode placement as well as for preexisting lesions or those potentially resulting from the surgical procedures. Because such lesions may influence EEGs or the susceptibility to the convulsant effects of kainate, 4 animals in which lesions were observed (3 with hemorrhagic infarcts and 1 with hydrocephalus) were excluded from analysis.
In Vitro Electrophysiology
SLICE PREPARATION
Hippocampal-entorhinal cortical (HEnC) slices were prepared as described previously32–34 (also see Walther and colleagues35 and Jones and Heinemann36). In brief, on postnatal day 17 or 18 (a week after hyperthermic seizures, an interval shown to permit development of long-term electrophysiological alterations in this model20), experimental rats and their littermate controls were anesthetized with halothane and decapitated. Brains were removed and cooled in 4°C oxygenated (95% O2/5% CO2) sucrose-artificial cerebrospinal fluid (ACSF),32–34 containing (in mM) 200 sucrose, 3 KCl, 1.25 Na2PO4, 26 NaHCO3, 10 glucose, 0.9 MgCl2, and 2 CaCl2. After a 2-minute incubation, brain slices (450 mm) were sectioned with a Vibratome tissue slicer (Lancer 1000; Polysciences, Warrington, PA) along a 12° inclined transverse plane, usually yielding one to three HEnC slices per rat. Slices were then preincu-bated, submerged in 32°C oxygenated ACSF containing 130 NaCl, 3 KCl, 26 NaHCO3, 2 CaCl2, 0.5 MgCl2, 1.25 NaH2PO4, and 10 glucose, for at least 1 hour (the reduced magnesium concentration was used to promote polysynaptic interactions33,34).
SPONTANEOUS AND EVOKED RECORDINGS
After incubation, individual slices were placed on filter paper stabilized with platinum wire weights, in a recording chamber perfused with warmed, oxygenated ACSF (35°C). Recordings were performed with an Axopatch-200A amplifier (Axon Instruments, Foster City, CA), and digitized at 88 kHz (Neuro-corder, Neuro Data, PA) before being stored in pulse code modulated form on videotape. Recording electrode pipettes were pulled from borosilicate (KG-33) glass capillary tubing (1.5 mm outer diameter; Garner Glass, Claremont, CA), with a Narishige (Tokyo, Japan) PP-83 two-stage electrode puller filled with ACSF, and placed in the CA1 pyramidal cell layer. A bipolar 90-mm tungsten stimulating electrode was placed in the CA1 stratum radiatum. The stimulation protocol33,34 consisted of a 2-second, 60-Hz train of stimuli applied to the Schaeffer collaterals (pulse width = 100 μs) and stimulus intensities were adjusted to four times the minimal intensity required to evoke a population spike of 0.5 mV in amplitude,33 usually 3.5 to 5 mA. Ten stimulating trains with a 10-minute interval were applied to each slice, unless sustained epileptiform activity developed (interval length was chosen to avoid interference by a postictal refractory period33).
Data Analysis
Data are presented as mean ± SEM. For in vivo data, the significance of observed differences among experimental and control groups was evaluated by using analysis of variance or Student’s t test with Welch’s correction, as appropriate. For in vitro data, “n” denotes the number of recorded slices. Because the generation of long-duration epileptiform activity was virtually an all-or-none event (lasting either >30 or <1.5 minutes), data derived from this experiment were not normally distributed.33,34 Therefore, nonparametric analysis (Mann-Whitney rank sum test) was used to assess the significance of differences between mean values. For all data, the significance level was set at p < 0.05.
Results
Hyperthermia, at Temperatures Consistent with Human Fever, Leads to Behavioral and EEG Hippocampal Seizures in the Immature Rat; These Seizures Are Prevented by Barbiturate Administration
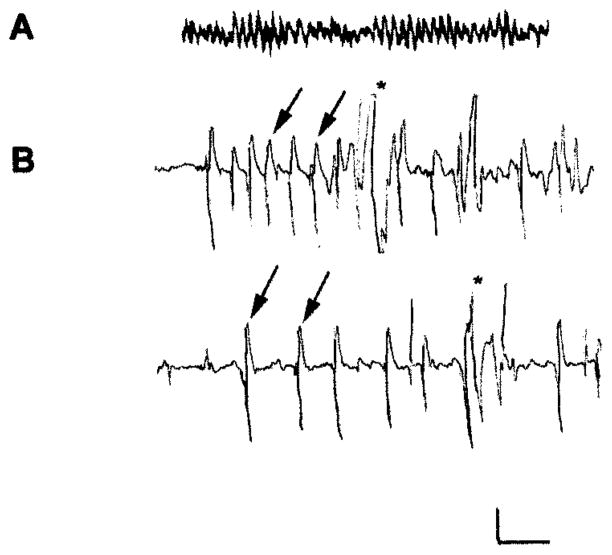
Table 1 demonstrates that the duration and magnitude of hyperthermia applied early in life did not differ in the group experiencing seizures compared with pentobarbital-pretreated hyperthermic controls. Figure 1 shows hippocampal EEGs from 11-day-old rats and the effects of hyperthermia. The top tracing (A) illustrates the baseline theta hippocampal activity in normothermic animals. Hyperthermia resulted in rhythmic, epileptiform hippocampal spike trains (arrows), signifying seizure (B). The behavioral correlate of this EEG seizure was a tonic, motionless posture. Pentobarbital administration altered the background rhythm, inducing a fast, β-like lower-amplitude trace, and prevented hyperthermia-induced EEG and behavioral seizures (not shown).
Fig 1.
Electrophysiological characteristics of hyperthermia-induced seizures in immature rats and their prevention by pentobarbital. Records performed via bipolar hippocampal depth electrodes in freely moving 11-day-old rats. (A) Baseline tracing of hippocampal activity during normothermia, showing a nonrhythmic pattern in the theta range. (B) Three minutes after hyperthermia onset in the same rat, hippocampal rhythmic epileptiform spikes (arrows) are shown indicating a seizure; during this electroencephalographic (EEG) seizure, the rat was motionless except for myoclonic jerks indicated by asterisks. In pentobarbital-treated rats, hyperthermia failed to induce EEG and behavioral seizures (not shown). Calibration: vertical, 100 μV; horizontal; 1 second.
Spontaneous Seizures Are Not Revealed by In Vivo Electrophysiology and Behavioral Analysis in Adult Rats That Had Experienced Hyperthermic Seizures Early in Life
Adult rats (71.4 ± 5.1 days) subjected to structured monitoring sessions for spontaneous behavioral seizures during a week did not demonstrate limbic or other seizures, regardless of their treatment early in life. In addition, hippocampal EEGs from all three groups of adult rats were indistinguishable and devoid of significant abnormalities. Thus, hyperthermic seizures early in life permitted normal hippocampal EEGs, including theta rhythm (Fig 2, left traces).
Enhanced Susceptibility to Limbic Seizures in Adult Rats Experiencing Early-Life Hyperthermic Seizures
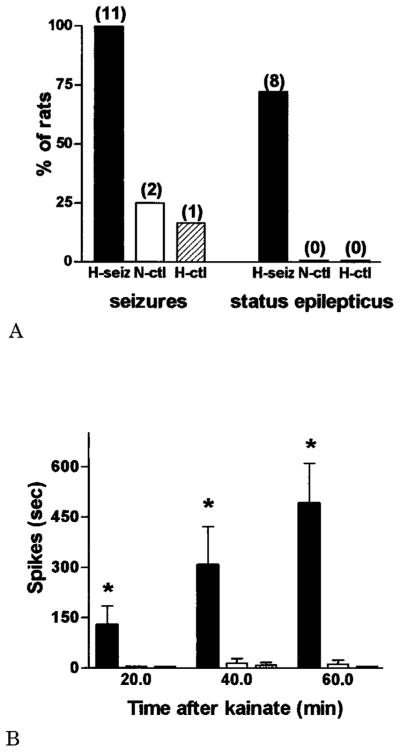
A subthreshold dose of kainate37,38 (5 mg/kg, on postnatal days 95.7 ± 5.1) administered intraperitoneally did not induce behavioral or EEG seizures in the large majority of adult rats that were normothermic or hyperthermic controls early in life (see Fig 2, right panels). Behaviorally, control rats responded to kainate by transient hyperactivity interspersed with immobility, starting approximately 5 minutes after kainate injection and disappearing in most animals within 80 minutes (Table 2). No electrographic or behavioral seizures developed during the 3 hours after kainate administration in 75% of normothermic and 84% of hyperthermic controls. Two normothermic controls had two brief (<1 minute) seizures each, occurring in rapid succession, 31 and 46 minutes after kainate administration, respectively (see Table 2). A single hyperthermic control rat developed a less than 30-second seizure 53 minutes after kainate administration. In contrast, 100% of adult rats experiencing hyperthermia-induced seizures early in life developed limbic seizures, and most progressed to SE (Fig 3A; see Fig 2 and Table 2). All rats in this group developed, in rapid succession, hyperactivity, immobility, and frank EEG hippocampal seizures (see Fig 2). It is interesting that the latencies to the development of these EEG seizures, whether or not they progressed to SE, were short (15.4 ± 3.9 and 14.8 ± 3.1 minutes, respectively; see Table 2), in comparison with latencies to the rare seizures of the control groups (36, 41, and 53 minutes). In addition, although seizure duration in 3 animals in this group was short, most (8 of 11; 73%) developed SE, which persisted to the time they were killed.
Table 2.
Differential Effects of Low-Dose Kainate on Adult Rats as a Function of Treatment Early in Life
| Group/Parameter | Seizure Latency (min) (n Affected) | Seizure Duration (min) | SE Latency (min) | SE Duration (min) |
|---|---|---|---|---|
| Hyperthermic seizures | 15.4 ± 3.9 (11/11) | 0.9 ± 0.2a | 14.8 ± 3.1c | 45.2 ± 3.0c |
| Hyperthermic controls | 53 (1/6) | 0.83 | — | — |
| Normothermic controls | 36, 41 (2/8) | 0.9 ± 0.1b | — | — |
Adult, hippocampal electrode–carrying rats given 5 mg/kg kainate intraperitoneally were monitored for electroencephalographic and behavioral seizures. Seizures occurred in all animals who had experienced early-life “febrile seizures” (hyperthermic seizures group), but only in 16.6% and 25% of hyperthermic and normothermic controls.
Seizure duration is shown for the 3 of 11 animals who did not develop status epilepticus (SE); 8 of 11 of this group progressed to SE.
Two normothermic controls had two short seizures each; a single hyperthermic control had one seizure.
Latency and duration of SE are shown for the 8 of 11 rats who developed it; because SE was terminated 1 hour after kainate administration, the duration shown is an underestimate.
Fig 3.
Differential induction of seizures and of status epilepticus (SE) in adult rats by low-dose kainate as a function of prolonged hyperthermic seizures early in life. Three groups of adult rats were investigated: those experiencing prolonged hyperthermic seizures on postnatal day 10 to 11 (H-seiz; n = 11), those subjected to similar hyperthermia, but in which seizures were blocked (H-ctl; n = 6), and a group kept normothermic (N-ctl; n = 8). (A) Kainate led to seizures in all adult H-seiz animals, and to SE in most, whereas 2 of 8 and 1 of 6 of N-ctl and H-ctl groups, respectively, developed brief seizures. (B) Quantitative analysis of kainate-induced, epileptic discharge duration. For all epochs, a significantly longer spike discharge duration (*p < 0.05) occurred in the H-seiz group compared with either control group (one-way analysis of variance with Bonferroni’s multiple comparison post hoc test).
In vivo electrophysiology illustrated the differential effects of the low dose of systemic kainate on the hippocampal circuit in the three groups. The right-hand tracings in Figure 2 demonstrate the typical appearance of kainate-induced hippocampal spike trains in adult rats that had hyperthermia-induced seizures early in life, compared with the unremarkable EEGs from the control groups. Furthermore, the duration of these spike discharges increased progressively over consecutive 20-minute epochs (see Fig 3B) and was significantly longer in the experimental versus either of the control groups (effect of treatment, p = 0.019, analysis of variance; Bonferroni’s multiple comparison post hoc test: hyperthermic seizures vs normothermic controls, p < 0.05; hyperthermic seizures vs hyperthermic controls, p < 0.05; normothermic vs hyperthermic controls, p > 0.05).
The Lower Threshold to a Chemical Convulsant In Vivo after Early-Life Hyperthermic Seizures Is Supported by In Vitro Data That Show Self-Sustaining Excitability after Electrical Stimulation
In vitro electrophysiology was performed in HEnC slices of rats that had sustained hyperthermia-induced seizures compared with controls. Slices were prepared 1 week after hyperthermia, based on previous data showing that by this time, long-term electrophysiological alterations in this model were already present and did not decline subsequently.20 Immediately after stimulation of Schaeffer collaterals with a 2-second, 60-Hz train, a short (20–70 second) afterdischarge was triggered in HEnC slices from both control and experimental rats. However, control slices did not develop longer duration, self-sustaining activity even after repeated stimulus trains (Fig 4). In contrast, slices prepared from rats that experienced hyperthermic seizures showed significantly enhanced epileptogenesis when compared with those from control littermates. In these slices, in addition to the stimulus train–induced short afterdischarge (see Fig 4A), a long-duration (>30 minutes), self-sustaining epileptic activity33,34 developed rapidly with subsequent stimulus trains (sometimes even after the first stimulus train, as in the example shown in Fig 4A). Once the self-sustaining epileptiform field discharge activity developed, it could be readily observed in CA1 (see Fig 4), as well as in other regions of the HEnC slice (in agreement with Rafiq and associates33), including the CA3 and dentate gyrus (not shown).
Fig 4.
Recurrent, self-sustaining epileptiform activity triggered by repeated stimulus trains in hippocampal-entorhinal cortex (HEnC) slices from animals experiencing hyperthermic seizures a week earlier (H-seiz) but not in slices from control litter-mates. (A) Representative traces recorded from the CA1 pyramidal cell layer from slices of a control and a H-seiz rat show that the first stimulus train failed to elicit spontaneous ictal-like epileptiform activity after the immediate afterdischarge in the control slice, but induced recurrent, spontaneous, self-sustaining field discharges in the H-seiz slice (segments of recordings at time points indicated by asterisks below the traces are also shown in an expanded time scale on the right). This self-sustaining activity had a characteristic temporal development, progressively increasing in amplitude as well as in frequency.33 (B) Plot of Schaeffer collateral stimulation train number versus duration of the afterdischarge induced by the train, showing enhanced epileptogenesis in the HEnC slices from H-seiz rats, compared with controls. Because recordings were terminated if the sustained epileptic activity lasted for more than 30 minutes, the maximal duration indicated on the y scale is 1,800 seconds (Control, n = 4; H-seiz, n = 5).
Discussion
The major findings of this study were as follows: (1) Febrile seizures in the immature rat model did not lead to spontaneous limbic seizures in the adult, as determined by both electrophysiological and behavioral analyses. However, (2) they reduced seizure threshold to excitatory input both in vivo (kainate) and in vitro (electrical stimulation). These long-term alterations in excitability of the hippocampal network may thus be considered proepileptogenic.
This study, using an animal model for prolonged febrile seizures in a controlled, prospective manner, provides both in vivo and in vitro evidence for long-term alterations of limbic excitability induced by these seizures. It is noteworthy that these alterations result in overall increased susceptibility to the generation of limbic seizures. Specifically, febrile seizures in this immature rat model did not provoke spontaneous seizures or change the “baseline” EEG. Thus, these early-life seizures did not result in spontaneous limbic epilepsy during adulthood. However, these seizures increased the probability that subsequent events enhancing excitation in the limbic circuit, via glutamate receptor activation and/or depolarization, would lead to prolonged limbic seizures and SE.
It is noteworthy that the mechanism of enhanced susceptibility to limbic seizures, as found in this study, required the presence of prolonged hyperthermic seizures and was not caused by the hyperthermia per se. The hyperthermic control group, ie, adults rats exposed to hyperthermia early in life but in which seizures were prevented by using barbiturates, demonstrated a sensitivity to the low kainate dose (16%) in the range of that of the normothermic control group (25%). This requirement for hyperthermia-induced seizures (versus hyperthermia per se) has also been demonstrated for the in vitro electrophysiological modulations of the limbic circuit established in this model.20 Although potential neuroprotective effects of pentobarbital cannot be entirely excluded, the short-time action of this specific barbiturate made such effect less likely.
In addition, the duration of the hyperthermic seizures in this study (21.5 ± 0.9 minutes) was relatively long compared with that of most febrile seizures in humans; ie, most febrile seizures last less than 10 minutes, and only 6% to 7% last longer than 15 minutes.7,39 Thus, seizures in this model may be considered prolonged or complex.40 Indeed, it is these prolonged seizures in humans that have been associated with subsequent TLE.10,11,14,41
The nature of the modulation of the hippocampal circuit in this model has recently been clarified.20 Single-cell electrophysiological studies in vitro revealed that hippocampi from animals experiencing hyperthermic seizures on postnatal days 10 to 11 were distinguished by a selective presynaptic increase in inhibitory synaptic transmission. These changes were clearly established by a week after the seizures and lasted into adulthood.20 These results are quite striking, because they indicate a γ-aminobutyric acid (GABA)ergic mechanism for the altered inhibition/excitation balance resulting from early-life hyperthermic seizures.20 In addition, these changes are, to date, unique and specific to this febrile seizures model, thus holding a promise for targeted interventions.19,20 It is interesting that, although the single-cell recording indicated enhanced release of the inhibitory neurotransmitter GABA after the hyperthermic seizures, the overall effect on limbic circuits, as shown here both in vitro and in vivo, was that of enhanced excitability. These data suggest that mechanisms of altered excitability in the hippocampal/limbic circuit involve complex regulatory loops, so that enhanced GABA release, perhaps via synchronizing the firing of hippocampal excitatory neurons,42,43 may paradoxically increase excitability, accounting for the findings reported here. In contrast, the enhanced presynaptic GABA release may be a “compensatory” mechanism in response to the overall increased excitability of the hippocampal network demonstrated in the current study.43 Finally, the data presented here suggest that both in vitro and in vivo studies may be required to fully delineate not only the cellular or multisynaptic modulations of hippocampal neurons, but the net sum effect of such changes on limbic excitability.
The present study used an HEnC slice preparation that encompasses the principal components of the limbic circuit,32,33 to show that prolonged hyperthermia-induced seizures in the immature rat promote self-sustaining SE-like activity in response to electrical stimulation. These results correlated well with the enhanced potency of chemical stimulation (activation of glutamate receptors by kainate) demonstrated in vivo. Several measures of the convulsant potency of kainate were increased in adult rats that had experienced hyperthermia-induced seizures early in life. Latency to the development of kainate-induced seizures (or SE) was significantly shortened compared with controls (see Table 2). In addition, the duration of the resulting seizures was much longer, manifest as SE (ie, seizures longer than 30 minutes) in 8 of 11 animals. These findings are in general agreement with findings after hypoxic44 and chemical seizure models31,45,46 in the immature rat.
Taken together, these in vivo and in vitro results indicate that prolonged febrile seizures in the immature rat model increase the probability of hippocampal seizure induction later in life. However, because spontaneous seizures did not develop, we conclude that these seizures did not result in epilepsy. Indeed it may be interpreted that febrile seizures in this model constitute a required but insufficient step in a multistage process by which such seizures contribute to the development of an epileptic limbic circuit. The extrapolation, to humans, of results achieved in rodent models is undertaken with great caution; in addition, we recognize that hyperthermia may not fully replicate the setting of a febrile illness in an infant or child. Nevertheless, application of these results may provide insight into mechanisms of human epilepsy. Thus, we suggest that human prolonged febrile seizures may facilitate the development of epilepsy in the event of additional excitatory insults (the counterpart of kainate or electrical stimulation in the model). Furthermore, in the setting of a previously injured or genetically compromised central nervous system, when a previous proexcitant event had already occurred, prolonged febrile seizures may provide a sufficient epileptogenic stimulus.
This interpretation is consistent with, and helps to reconcile, conflicting data from prospective and retrospective human research into the important clinical question of the consequences of febrile seizures and, in particular, into the relationship between prolonged febrile seizures and TLE. Patients with intractable TLE, reported in retrospective risk factor analyses, have a high frequency (30–60%) of a history of febrile seizures.9–11 These numbers far exceed the 3% to 5% frequency of febrile seizures in the general population. This correlation has been interpreted as causal, ie, that febrile seizures produce temporal lobe injury and spontaneous seizures.5,12,47 This body of retrospective human research has stood in direct conflict with a substantial number of prospective epidemiological studies, demonstrating that febrile seizures are not associated with an increased likelihood of TLE.2,7,8 However, both retrospective and prospective studies have singled out prolonged febrile seizures as more likely to be associated with subsequent TLE.2,10,47,48 Thus, long-term prospective follow-up studies suggested increased frequency of TLE in adulthood after early-life prolonged febrile seizures.49
Although it has been proposed that prolonged febrile seizures predispose to TLE via seizure-induced injury to temporal lobe structures (“mesial temporal sclerosis”), an alternative hypothesis suggests that a preexisting lesion, structural or functional, genetic or acquired, may be the cause of both the prolonged febrile seizure and the subsequent TLE.4,6,50 Expressed differently, prolonged febrile seizures in the human may be a marker of already existing TLE that is triggered by fever.2,4,6 In support of this supposition, the increased likelihood of TLE after prolonged febrile seizures is far more evident in individuals with preexisting brain abnormalities compared with those with normal neurological status and neuroimaging studies.51 The data presented here are consistent with a third, more complex, mechanism for the association of prolonged febrile seizures and TLE. They support the notion that the functional alterations induced by these seizures create a neuronal system with a lower seizure threshold to excitatory input, ie, a “seizure-prone” transition between the normal and epileptic states.
In summary, this study introduces in vivo and in vitro evidence for long-term alterations in limbic excitability caused by prolonged early-life seizures in the immature rat model of febrile seizures. Although spontaneous seizures did not occur during adulthood, and spontaneous neuronal activity in hippocampal EEGs or in slice recordings were unaltered, these persistent changes resulted in an overall increased propensity to the generation of limbic seizures.
Acknowledgments
This research was supported by NIH grants NS28912 and NS35439 (to T.Z.B.) and NS38580 (to I.S.), by the UC Systemwide Biotechnology Research and Education Program (BREP-98-02 to T.Z.B., K.C., and I.S.), and by an Epilepsy Foundation of America predoctoral award (to C.D.).
References
- 1.Shinnar S. Febrile seizures. In: Johnson RT, editor. Current therapy in neurological disease. New York: Marcel Dekker; 1990. pp. 29–32. [Google Scholar]
- 2.Verity CM, Golding J. Risk of epilepsy after febrile convulsions: a national cohort study. BMJ. 1991;303:1373–1376. doi: 10.1136/bmj.303.6814.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35(Suppl 2):S1–S6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- 4.Shinnar S. Prolonged febrile seizures and medial temporal sclerosis. Ann Neurol. 1998;43:411–412. doi: 10.1002/ana.410430402. [DOI] [PubMed] [Google Scholar]
- 5.Sloviter RS, Pedley TA. Subtle hippocampal malformation: importance in febrile seizures and development of epilepsy. Neurology. 1998;50:846–849. doi: 10.1212/wnl.50.4.846. [DOI] [PubMed] [Google Scholar]
- 6.Lewis DV. Febrile convulsions and mesial temporal sclerosis. Curr Opin Neurol. 1999;12:197–201. doi: 10.1097/00019052-199904000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Nelson KB, Ellenberg JH. Predictors of epilepsy in children who have experienced febrile seizures. N Engl J Med. 1976;295:1029–1033. doi: 10.1056/NEJM197611042951901. [DOI] [PubMed] [Google Scholar]
- 8.Berg AT, Shinnar S. Do seizures beget seizures? Assessment of the clinical evidence in humans. J Clin Neurophysiol. 1997;14:102–110. doi: 10.1097/00004691-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Gloor P. Mesial temporal sclerosis: historical background and an overview from a modern perspective. In: Luders HO, editor. Epilepsy surgery. New York: Raven Press; 1991. pp. 689–703. [Google Scholar]
- 10.French JA, Williamson PD, Thadani VM, et al. Characteristics of medial temporal lobe epilepsy. I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 11.Cendes F, Andermann F, Dubeau F, et al. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures, and temporal lobe epilepsy: an MRI volumetric study. Neurology. 1993;43:1083–1087. doi: 10.1212/wnl.43.6.1083. [DOI] [PubMed] [Google Scholar]
- 12.Falconer MA, Serafetinides EA, Corsellis JAN. Etiology and pathogenesis of temporal lobe epilepsy. Arch Neurol. 1964;10:233–248. doi: 10.1001/archneur.1964.00460150003001. [DOI] [PubMed] [Google Scholar]
- 13.Sagar HJ, Oxbury JM. Hippocampal neuron loss in temporal lobe epilepsy: correlation with early childhood convulsions. Ann Neurol. 1987;22:334–340. doi: 10.1002/ana.410220309. [DOI] [PubMed] [Google Scholar]
- 14.Theodore WH, Bhatia S, Hatta J, et al. Hippocampal atrophy, epilepsy duration, and febrile seizures in patients with partial seizures. Neurology. 1999;52:132–136. doi: 10.1212/wnl.52.1.132. [DOI] [PubMed] [Google Scholar]
- 15.Davies KG, Hermann BP, Dohan FC, Jr, et al. Relationship of hippocampal sclerosis to duration and age of onset of epilepsy, and childhood febrile seizures in temporal lobectomy patients. Epilepsy Res. 1996;24:119–126. doi: 10.1016/0920-1211(96)00008-3. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 17.Sperber EF, Stanton PK, Haas K, et al. Developmental differences in the neurobiology of epileptic brain damage. In: Engel J, Wasterlain C, Cavelheiro EA, et al., editors. Molecular neurobiology of epilepsy. Amsterdam: Elsevier; 1992. pp. 67–81. [PubMed] [Google Scholar]
- 18.Baram TZ, Gerth A, Schultz L. Febrile seizures: an age appropriate model. Dev Brain Res. 1997;246:134–143. doi: 10.1016/s0165-3806(96)00190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toth Z, Yan XX, Haftoglou S, et al. Seizure-induced neuronal injury: vulnerability to febrile seizures in immature rat model. J Neurosci. 1998;18:4285–4294. doi: 10.1523/JNEUROSCI.18-11-04285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen K, Baram TZ, Soltesz I. Febrile seizures in the immature brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888–894. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottlieb A, Keydar Y, Epstein HT. Rodent brain growth stages: an analytical review. Biol Neonate. 1977;32:166–176. doi: 10.1159/000241012. [DOI] [PubMed] [Google Scholar]
- 22.Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Regulation of the expression of corticotropin releasing factor receptor type 2 (CRF2) in the hypothalamus and amygdala of the immature rat. J Neurosci. 1999;19:3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunson K, Schultz L, Baram TZ. The in vivo proconvulsant effects of corticotropin releasing hormone in the developing rat are independent of ionotropic glutamate receptor activation. Brain Res. 1998;111:119–128. doi: 10.1016/s0165-3806(98)00130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baram TZ, Hirsch E, Snead OC, III, Schultz L. Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala. Ann Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baram TZ, Hirsch E, Schultz L. Short-interval amygdala kindling in neonatal rats. Dev Brain Res. 1993;73:79–83. doi: 10.1016/0165-3806(93)90048-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben-Ari Y, Tremblay E, Riche D, et al. Electrographic, clinical and pathological alterations following systemic administration of kainic acid, bicuculline or pentetrazole: metabolic mapping using the deoxyglucose method with special reference to the pathology of epilepsy. Neuroscience. 1981;6:1361–1391. doi: 10.1016/0306-4522(81)90193-7. [DOI] [PubMed] [Google Scholar]
- 28.Cherubini E, De Feo MR, Mecarelli O, Ricci GF. Behavioral and electrographic patterns induced by systemic administration of kainic acid in developing rats. Brain Res. 1983;285:69–77. doi: 10.1016/0165-3806(83)90110-4. [DOI] [PubMed] [Google Scholar]
- 29.Albala BJ, Moshe SL, Okada R. Kainic acid-induced seizures: a developmental study. Brain Res. 1984;315:139–148. doi: 10.1016/0165-3806(84)90085-3. [DOI] [PubMed] [Google Scholar]
- 30.Haas KZ, Sperber EF, Moshe SL. Kindling in developing animals: expression of severe seizures and enhanced development of bilateral foci. Dev Brain Res. 1990;56:275–280. doi: 10.1016/0165-3806(90)90093-e. [DOI] [PubMed] [Google Scholar]
- 31.Holmes GL, Thompson JL. Effects of kainic acid on seizure susceptibility in the developing brain. Dev Brain Res. 1988;39:51–59. doi: 10.1016/0165-3806(88)90066-1. [DOI] [PubMed] [Google Scholar]
- 32.Rafiq A, DeLorenzo RJ, Coulter DA. Generation and propagation of epileptiform discharges in a combined entorhinal cortex/ hippocampal slice. J Neurophysiol. 1993;70:1962–1974. doi: 10.1152/jn.1993.70.5.1962. [DOI] [PubMed] [Google Scholar]
- 33.Rafiq A, Zhang YF, DeLorenzo RJ, Coulter DA. Long-duration self-sustained epileptiform activity in the hippocampal-parahippocampal slice: a model of status epilepticus. J Neurophysiol. 1995;74:2028–2042. doi: 10.1152/jn.1995.74.5.2028. [DOI] [PubMed] [Google Scholar]
- 34.Coulter DA, Rafiq A, Shumate M, et al. Brain injury-induced enhanced limbic epileptogenesis: anatomical and physiological parallels to an animal model of temporal lobe epilepsy. Epilepsy Res. 1996;26:81–91. doi: 10.1016/s0920-1211(96)00044-7. [DOI] [PubMed] [Google Scholar]
- 35.Walther H, Lambert JD, Jones RS, et al. Epileptiform activity in combined slices of the hippocampus, subiculum and entorhinal cortex during perfusion with low magnesium medium. Neurosci Lett. 1986;69:156–161. doi: 10.1016/0304-3940(86)90595-1. [DOI] [PubMed] [Google Scholar]
- 36.Jones RS, Heinemann U. Synaptic and intrinsic responses of medial entorhinal cortical cells in normal and magnesium-free medium in vitro. J Neurophysiol. 1988;59:1476–1496. doi: 10.1152/jn.1988.59.5.1476. [DOI] [PubMed] [Google Scholar]
- 37.Sperk G, Lassmann H, Baran H, et al. Kainic acid-induced seizures: neurochemical and histopathological changes. Neuroscience. 1983;10:1301–1315. doi: 10.1016/0306-4522(83)90113-6. [DOI] [PubMed] [Google Scholar]
- 38.Cronin J, Dudek FE. Chronic seizures and collateral spouting of dentate mossy fibers after kainic acid treatment in rats. Brain Res. 1988;474:181–184. doi: 10.1016/0006-8993(88)90681-6. [DOI] [PubMed] [Google Scholar]
- 39.Offringa M, Bossuyt PMM, Lubsen J, et al. Risk factors for seizure recurrence in children with febrile seizures: a pooled analysis of individual patient data from five studies. J Pediatr. 1994;124:574–584. doi: 10.1016/s0022-3476(05)83136-1. [DOI] [PubMed] [Google Scholar]
- 40.Commission on Classification and Terminology of the International League Against Epilepsy: proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;33:661–666. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 41.Harvey AS, Grattan-Smith JD, Desmond PM, et al. Febrile seizures and hippocampal sclerosis: frequent and related findings in intractable temporal lobe epilepsy of childhood. Pediatr Neurol. 1995;12:201–206. doi: 10.1016/0887-8994(95)00022-8. [DOI] [PubMed] [Google Scholar]
- 42.Cobb SR, Buhl E, Halasy K, et al. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 43.Walker MC, Kullman DM. Febrile convulsions: a “benign” condition? Nat Med. 1999;5:871–872. doi: 10.1038/11308. [DOI] [PubMed] [Google Scholar]
- 44.Jensen FE, Wang C. Hypoxia-induced hyperexcitability in vivo and in vitro in the immature hippocampus. Epilepsy Res. 1996:131–140. doi: 10.1016/s0920-1211(96)00049-6. [DOI] [PubMed] [Google Scholar]
- 45.Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- 46.Stafstrom CE, Thompson JL, Holmes GL. Kainic acid seizures in the developing brain: status epilepticus and spontaneous recurrent seizures. Dev Brain Res. 1992;65:227–236. doi: 10.1016/0165-3806(92)90184-x. [DOI] [PubMed] [Google Scholar]
- 47.Maher J, McLachlan RS. Febrile convulsions: is seizure duration the most important predictor of temporal lobe epilepsy? Brain. 1995;118:1521–1528. doi: 10.1093/brain/118.6.1521. [DOI] [PubMed] [Google Scholar]
- 48.Berg AT, Shinnar S. Unprovoked seizures in children with febrile seizures: short term outcome. Neurology. 1996;47:562–568. doi: 10.1212/wnl.47.2.562. [DOI] [PubMed] [Google Scholar]
- 49.Annegers JF, Hauser WA, Shirts SB, et al. Factors prognostic of unprovoked seizures after febrile convulsions. N Engl J Med. 1987;316:493–498. doi: 10.1056/NEJM198702263160901. [DOI] [PubMed] [Google Scholar]
- 50.Germano IM, Zhang YF, Sperber EF, Moshe SL. Neuronal migration disorders increase susceptibility to hyperthermia induced seizures in developing rats. Epilepsia. 1996;37:902–910. doi: 10.1111/j.1528-1157.1996.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 51.Maytal J, Shinnar S. Febrile status epilepticus. Pediatrics. 1990;86:611–616. [PubMed] [Google Scholar]