Abstract
Massive infantile spasms (MIS), a seizure disorder unique to infants, is considered an age-dependent response of the immature brain to various insults and stressors. The seizures improve with ACTH and glucocorticoids, both major components of the brain-adrenal axis. We hypothesized that CNS levels of these hormones are abnormal in infants with MIS and studied CSF from 14 infants with MIS and 13 age-matched controls by analysis for corticotropin-releasing hormone (CRH), ACTH, cortisol, and interleukin-1-beta. ACTH levels in CSF of patients were significantly lower than those of controls, but differences in cortisol levels between patients and controls were not statistically significant. CRH levels in both groups were similar and fluctuated diurnally. These results indicate an alteration of specific CNS components of the brain-adrenal axis in MIS.
Massive infantile spasms (MIS), or West syndrome, consists of myoclonic seizures, hypsarrhythmic EEG, and subsequent mental retardation.1–4 MIS is age-specific: onset of spasms is usually between the third and ninth postnatal month, and rarely after the first year.1–3 Since MIS occurs in one of 2,400 to 4,000 live births and the majority of patients have long-term cognitive sequelae, the entity is an important cause of mental retardation.1,5
MIS has been associated with structural abnormalities, neurocutaneous syndromes, intrauterine infections, metabolic aberrations, meningitis, encephalitis, and cerebral infarcts.1–4 Infants with MIS and other neurologic abnormalities (symptomatic) are distinguished from those with no known etiology for the seizures (cryptogenic). The cryptogenic group is further subdivided by the neurologic examination (normal or delayed).
The pathophysiology of MIS is unclear. The common denominator of infants with symptomatic MIS is the past occurrence of a major CNS insult or stress.6 Thus, MIS has been considered a unique, age-dependent manifestation of stress or injury to the immature brain.6,7
The therapy of MIS remains unsatisfactory. Conventional anticonvulsants, with the possible exception of benzodiazepines, are generally ineffective.1–4 Benzodiazepines, including nitrazepam, may be less effective than corticotropin (ACTH) and glucocorticoids (GC).1,8 Since the original administration of ACTH by Sorel in the late 1950s, ACTH and steroids have been the mainstay of therapy.1,9 Numerous uncontrolled studies, as well as a double-blinded and controlled one,10 have shown their efficacy to be 60 to 70%. Control of the spasms themselves, moreover, may not affect the overall outcome: mental retardation occurs in 80 to 90% of patients and subsequent epilepsy in approximately 50%.1–4 A better understanding of the pathophysiology of MIS may lead to better treatment for both the seizures and the intellectual and neurodevelopmental sequelae of this entity.
We hypothesize that ACTH and GC are efficacious in MIS because they counteract an abnormality of the brain-adrenal axis. Such abnormalities might include increased abundance of corticotropin-releasing hormone (CRH) or a reduction of ACTH.
Methods
Patient population
Infants presenting to the neurology service of Childrens Hospital of Los Angeles (CHLA) between July 1988 and March 1991 with a clinical diagnosis of MIS and a hypsarrhythmic EEG4 constituted the patient group. They underwent a lumbar puncture as part of an evaluation for the etiology of the syndrome. No patient was on ACTH or GC therapy at the time of the procedure. Age-matched controls were infants undergoing lumbar puncture for various reasons in CHLA or the emergency room. The use of CSF for the study was approved by the Internal Review Board for Human Studies of CHLA. The clinical characteristics of both groups of patients are delineated in the two tables.
Cerebrospinal fluid methodology
CSF was obtained into ice-chilled tubes and transferred immediately to −80 °C. Samples were aliquoted and, after the addition of 500 KIU/ml aprotinin, were kept at −80 °C. For CRH radioimmunoassay (RIA), 0.4 ml of CSF was lyophilized and reconstituted in 0.1 ml of assay buffer. For ACTH and cortisol analyses, 0.3 ml of CSF was lyophilized. Because of the limited volume of CSF available, not all hormonal tests were performed on each sample, as indicated in the tables.
Hormone assays
Amounts of CRH in CSF samples (CSF-CRH) were determined by RIA according to Vale, who generously provided the CRH-antiserum.11 Radio-iodinated CRH was obtained from ICN Biomedical (Costa Mesa, CA). RIA sensitivity was 1 pg per tube (2.5 pg/ml). All samples were analyzed together, and the assay was repeated. ACTH was determined as previously described,12 using antiserum and radio-iodinated ACTH obtained from ICN Biomedical. Sensitivity (10% difference from background) was 15 pg/ml CSF; interassay variability was <20% and was monitored by the determination of the same patient samples (at least three) in all assays. Cortisol was analyzed using an RIA kit obtained from Radioassay Systems Laboratories (Carson, CA). Assay sensitivity was 25 pg/0.5 ml.12 All samples were analyzed for cortisol in the same assay. Human interleukin-l (IL-l)-beta was determined using an enzyme-linked immunosorbent assay (ELISA) kit (Cistron Biotechnology, Pine Brook, NJ). We found the sensitivity of the ELISA to be 50 pg/ml CSF.
Statistical analysis
Groups were compared using the Wilcoxon rank sum test (SAS program, Scientific Inst., Cary, NC). Correlation of variables was determined without assumption of normality or linearity using Kendall’s rank correlation coefficient (SAS).
Results
As evident from the tables, there were no significant differences between patient and control mean age (p = 0.56) or between the times of CSF sampling. Three of the control infants were febrile at sampling time, but no significant effect of fever was seen on ACTH levels: mean ± standard errors were 49.0 ± 12.3 and 62.7 ± 8.0 pg/ml in febrile and afebrile patients, respectively. CSF-cortisol in two febrile controls (2.0 and 3.0 ng/ml) did not differ from those of afebrile controls (2.17 ± 0.15; n = 7). Only one CRH value of a febrile infant was available.
ACTH levels in CSF of infants with MIS (31.4 ± 3.35 pg/ml) were significantly decreased compared with age-matched controls (58.1 ± 6.68; p = 0.003; Wilcoxon test). Actual ACTH values are shown in figure 1. Cortisol levels in MIS patients (1.27 ± 0.11 ng/ml) were somewhat lower than in controls (2.24 ± 0.49), but this difference did not reach statistical significance (p = 0.08; Wilcoxon test). IL-1-beta levels were low or undetectable in all samples analyzed (six patients and three controls). CRH levels did not differ between MIS and control infants. Concentrations of CRH in infant CSF underwent diurnal variation. Since no samples were obtained between 10 PM and 8 AM, samples were divided into “early” and “late,” before or after 3 PM. “Early” CSF-CRH levels were significantly lower than those obtained later in the day: 35.4 ± 3.8 versus 55.5 ± 3.8 pg/ml (p = 0.002). The diurnal variability in CSF-CRH was preserved in infants with MIS (36.0 ± 5.4 versus 51.8 ± 6.0 pg/ml; p = 0.079), although to a lesser degree than in controls (34.8 ± 6.4 versus 60.0 ± 4.3 pg/ml; p = 0.011). A graphic depiction of the diurnal fluctuation of CSF-CRH is shown in figure 2. Since no difference was found between CRH levels of patients and controls, the group was analyzed as a whole.
Figure 1.
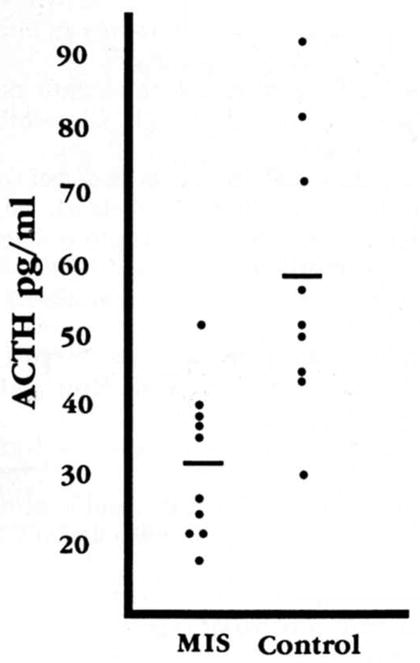
Distribution of individual ACTH values in CSF samples of infants with massive infantile spasms and age-matched controls. The means of the two groups are significantly different (p = 0.002, Student’s t test corrected for unequal variance.
Figure 2.
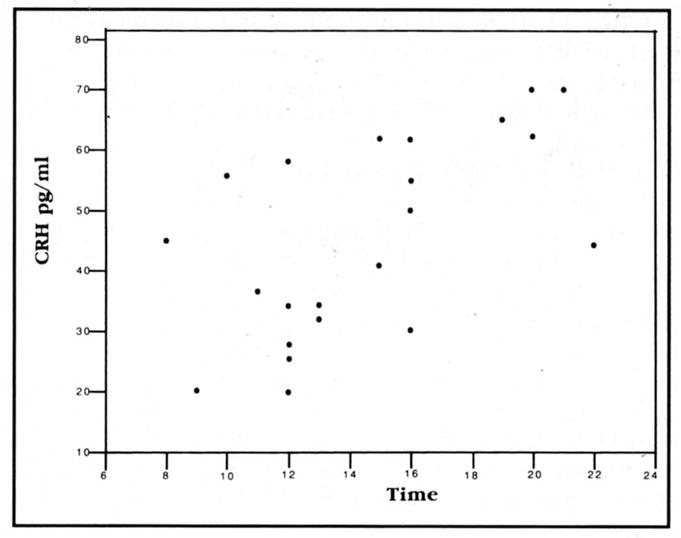
Diurnal variation of CRH levels in infant CSF. CRH units are pg/ml. Kendall’s rank correlation coefficient is significant (0.005).
CRH levels in infant CSF were not significantly age-dependent (Kendall’s rank correlation coefficient = 0.21). Cortisol levels in all infants’ CSF correlated inversely with CRH levels (Spearman correlation coefficient = 0.08).
Discussion
The role of the brain-adrenal axis in MIS is unknown. A functional abnormality of the CRH-ACTH-GC regulatory loop is suggested by two lines of evidence: first, MIS, which responds poorly to conventional anticonvulsants, improves with ACTH and GC.1–4,9,10 Second, CRH, which is regulated negatively by ACTH and GC, has been shown to be a convulsant in laboratory animals.13–15
Hypothetical mechanisms for MIS may involve a decrease in ACTH or cortisol in the CNS, which is corrected by the administration of ACTH or GC. Alternatively, the latter may improve MIS by suppressing CRH synthesis or secretion.16–18 CRH synthesis is increased by stress.19,20 Stress during a critical perinatal period can result in long-term effects: handling neonatal rats resulted in an increase in hippocampal GC receptors and preserved ability to perform learned tasks during senescence.21 We hypothesize that early stress is the pathophysiologic denominator for MIS6,7 via a long-term derangement of the brain-adrenal axis of these infants.
MIS is restricted to infancy. We have recently shown that the convulsant effects of CRH are age-dependent as well: CRH is a far more rapid and potent convulsant in infant, as compared with adult, rats.22,23 The “CRH excess” hypothesis predicts increased CNS levels of this neuropeptide. CRH levels were similar in MIS infants and in controls. The lack of increase in CSF-CRH levels in infants with MIS may be due to the nonhypothalamic origin of CSF-CRH.24,25 Whether CRH levels in specific brain regions in infants with MIS differ from those of controls is unknown. Since the spasms disappear spontaneously, usually within 1 year,1–4 autopsy studies of children with a history of MIS may be futile.
CRH levels in primate CSF follow a diurnal pattern, with a maximum at 7 to 10 PM and a nadir at 6 to 10 AM.24,26 Our data suggest that such diurnal variability is also found in human infants and is preserved in infants with MIS. A single study of CSF-CRH in children examined the CSF of 22 subjects aged 6 days to 15 years and found an inverse correlation of CSF-CRH with age.27 In our population of infants aged 1 to 18 months, CRH did not co-vary with age. Different age ranges (only eight patients were 1 to 18 months old) and inclusion of patients with CNS barrier damage by these authors may account for the different results.
We have found markedly decreased amounts of ACTH in the CSF of infants with MIS. Similar results were reported by Nalin et al,28 who did not study cortisol and CRH and found no difference in beta-endorphin levels between patients and controls. No correlation with age or diurnal variability was investigated.
Decreased CSF-ACTH in infants with MIS may reflect reduced brain synthesis, which is ameliorated by the exogenous ACTH used as therapy. Conversely, reduced amounts of CSF-ACTH may reflect an increased utilization of the neuropeptide in brains of infants with MIS, in analogy to other neurotransmitters.29 Several authors postulate an intrinsic role for ACTH in the therapy of MIS independent of its elevation of plasma GC.1,9,30 Our study, showing a far greater decrease in CSF-ACTH than in CSF-cortisol, is compatible with this view.
In the small number of CSF samples analyzed, IL-1 levels were very low in both infants with MIS and age-matched controls. In the rodent, IL-1 has been shown to increase the synthesis and the secretion of CRH and thus activate the brain-adrenal axis.31–33 Hypothalamic neurons containing IL-1-beta have been demonstrated in humans.34 The effect of this cytokine on hypothalamic or CSF-CRH in humans has not been reported.
In conclusion, we find altered CSF levels of brain-adrenal axis hormones in untreated infants with MIS. Put together with the therapeutic efficacy of ACTH and GC in MIS and with the age-specific convulsant potency of CRH, these findings suggest the involvement of the CRH-ACTH-GC regulatory loop in this infantile seizure disorder.
Table 1.
Characteristics of patients with MIS
| No. | Age/Sex (mo) | Etiology | Time (hr) | CRH (pg/ml) | ACTH (pg/ml) | Cortisol (ng/ml) | IL-1 (pg/ml) |
|---|---|---|---|---|---|---|---|
| 1 | 3.5/M | Congenital CMV | 4 PM | 30 | — | 1.04 | — |
| 2 | 10/M | Cryptogenic (DD) | 9 PM | 70 | — | 0.96 | 16 |
| 3 | 8/M | Cryptogenic (NL) | 10 AM | 56 | — | 1.24 | — |
| 4 | 5.5/F | Cryptogenic (NL) | 7 PM | 65 | 40 | — | — |
| 5 | 7/F | Meningitis | 11 AM | 37 | 51 | 1.28 | 15 |
| 6 | 6/F | Congenital malformation | 8 AM | 45 | 27 | 1.30 | ND |
| 7 | 6/M | Down’s syndrome | 12 PM | 26 | 18 | 1.76 | ND |
| 8 | 3/M | Cryptogenic (DD) | 4 PM | 55 | 25 | 0.96 | — |
| 9 | 5/F | Neurodegenerative disease | 4 PM | 50 | 21 | 2.00 | — |
| 10 | 6/F | Cryptogenic (DD) | 3 PM | 41 | 38 | 1.72 | — |
| 11 | 12/M | Hydrocephalus | 1 PM | 32 | 21 | 1.36 | — |
| 12 | 4/F | Cryptogenic (NL) | 9 AM | 20 | — | — | ND |
| 13 | 5/F | Congenital hydrocephalus | 6 PM | — | 36 | 1.15 | ND |
| 14 | 11/M | Congenital malformation | 10 PM | — | 37 | 0.50 | — |
DD Developmentally delayed.
NL Normal development.
ND Nondetectable.
Table 2.
Characteristics of control patients
| No. | Age/Sex (mo) | Etiology | Time (hr) | Fever (>38 °C) | CRH (pg/ml) | ACTH (pg/ml) | Cortisol (ng/ml) | IL-1 (pg/ml) |
|---|---|---|---|---|---|---|---|---|
| 1 | 9/F | Congenital toxoplasmosis | 3 PM | — | 62 | — | 0.4 | — |
| 2 | 4/F | Congenital malformation | 12 PM | — | 20 | — | — | 35 |
| 3 | 5/M | FTT | 10 PM | — | 44 | — | — | — |
| 4 | 3.5/M | R/O sepsis | 1 PM | + | 34 | 72 | — | — |
| 5 | 2/M | Respiratory virus | 12 PM | — | 58 | 57 | — | — |
| 6 | 2/F | R/O sepsis | 12 PM | + | 28 | 30 | 2.0 | — |
| 7 | 10/F | Seizure, MR | 4 PM | — | 62 | — | 1.2 | — |
| 8 | 1/M | Seizure | 8 PM | — | 70 | 44 | 1.3 | 17 |
| 9 | 18/M | Ataxia | 8 PM | — | 62 | 81 | 2.0 | ND |
| 10 | 5/M | Viral syndrome | 12 PM | — | 34 | 50 | 5.0 | — |
| 11 | 10/M | Febrile seizure | 4 PM | + | — | 45 | 3.0 | — |
| 12 | 3/M | Seizures | 7 PM | — | — | 51 | 1.3 | — |
| 13 | 1/M | Seizures | 3 PM | — | — | 93 | 4.0 | ND |
FTT Failure to thrive.
MR Mental retardation.
ND Nondetectable.
Acknowledgments
Supported by NS01307 (Dr. Baram).
The assistance of L. Schultz, and the organizational skills of P. Killam, PNP, S. Kongelbeck, MN, RN, and M. Baron, MS, RN, are appreciated. We are indebted to N. Schonfeld, MD, and the CHLA emergency division.
References
- 1.Aicardi J. Infantile spasms: related syndromes. In: Aicardi J, editor. Epilepsy in children. New York: Raven Press; 1986. pp. 17–38. [Google Scholar]
- 2.Kellaway P, Frost JD, Hrachovy RA. Infantile spasms. In: Morselli PL, Pippenger CG, Penry JK, editors. Antiepileptic drug therapy in pediatrics. New York: Raven Press; 1983. pp. 115–136. [Google Scholar]
- 3.Jeavons PM, Bower BD. Infantile spasms. Clin Dev Med. 1964;15:1–82. [Google Scholar]
- 4.Holmes GL. Diagnosis and management of seizures in children. Philadelphia: WB Saunders; 1987. pp. 212–225. [Google Scholar]
- 5.Bellman M. Infantile spasms. In: Pedley TA, Meldrum BS, editors. Recent advances in epileptology. Edinburgh: Churchill-Livingstone; 1983. pp. 113–138. [Google Scholar]
- 6.Hrachovy RA, Frost JD., Jr . Infantile spasms: a disorder of the developing nervous system. In: Kellaway P, Noebels JL, editors. Problems and concepts in developmental neurophysiology. Baltimore: Johns Hopkins University Press; 1989. pp. 131–147. [Google Scholar]
- 7.Riikohen R. Infantile spasms: some new theoretical aspects. Epilepsia. 1983;24:159–168. doi: 10.1111/j.1528-1157.1983.tb04875.x. [DOI] [PubMed] [Google Scholar]
- 8.Volzke E, Doose H, Stephan E. The treatment of infantile spasms and hypsarrhythmia with Mogadon. Epilepsia. 1967;8:64–70. doi: 10.1111/j.1528-1157.1967.tb03821.x. [DOI] [PubMed] [Google Scholar]
- 9.Snead OC., III Treatment of infantile spasms. Pediatr Neurol. 1990;6:147–150. doi: 10.1016/0887-8994(90)90054-5. [DOI] [PubMed] [Google Scholar]
- 10.Hrachovy RA, Frost JD, Kellaway R, Zion TE. Double blind study of ACTH vs. prednisone therapy in infantile spasms. J Pediatr. 1983;103:641–645. doi: 10.1016/s0022-3476(83)80606-4. [DOI] [PubMed] [Google Scholar]
- 11.Vale W, Rivier C, Brown MR, et al. Chemical and biological characterization of corticotropin releasing factor. Recent Prog Horm Res. 1983;39:339–375. doi: 10.1016/b978-0-12-571139-5.50010-0. [DOI] [PubMed] [Google Scholar]
- 12.Baram TZ, Schultz L. Fetal and maternal plasma corticosterone and ACTH after pharmacological adrenalectomy. Life Sci. 1990:485–489. doi: 10.1016/0024-3205(90)90607-s. [DOI] [PubMed] [Google Scholar]
- 13.Ehlers CL, Henriksen SJ, Wang M, et al. Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res. 1983;278:332–336. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- 14.Weiss SRB, Post RM, Gold PW, et al. CRF-induced seizures and behavior: interaction with amygdala kindling. Brain Res. 1986;372:345–351. doi: 10.1016/0006-8993(86)91142-x. [DOI] [PubMed] [Google Scholar]
- 15.Marrosu F, Fratta W, Carcangiu P, et al. Localized epileptiform activity induced by murine CRF in rats. Epilepsia. 1988;29:369–373. doi: 10.1111/j.1528-1157.1988.tb03733.x. [DOI] [PubMed] [Google Scholar]
- 16.Sawchenko PE. Evidence for a local site of action for glucocorticoids in inhibiting CRF and vasopressin expression in the paraventricular nucleus. Brain Res. 1987;403:213–224. doi: 10.1016/0006-8993(87)90058-8. [DOI] [PubMed] [Google Scholar]
- 17.Kovacs KJ, Mezey E. Dexamethasone inhibits CRF gene expression in the rat PVN. Neuroendocrinology. 1987;46:365–368. doi: 10.1159/000124846. [DOI] [PubMed] [Google Scholar]
- 18.Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemistry study. J Comp Neurol. 1988;285:413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- 19.Lightman SL, Young WS., III Response of hypothalamic corticotropin releasing factor mRNA to stress, Opiates and opiate withdrawal. J Physiol (Lond) 1988;403:511–519. doi: 10.1113/jphysiol.1988.sp017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young WS., III . Distribution and regulation of corticotropin releasing factor mRNA using in situ hybridization. In: De Souza EB, Nemeroff CB, editors. Corticotropin-releasing factor: basic and clinical studies of a neuropeptide. Boca Raton: CRC Press; 1990. pp. 13–21. [Google Scholar]
- 21.Meaney MJ, Aitken DH, van Berkel C, et al. Effects of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- 22.Baram TZ, Schultz L. CRH is a rapid and potent convulsant in the infant rat. Dev Brain Res. 1991;61:97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baram TZ, Hirsch E, Snead OC, III, Schultz L. CRH induced seizures in infant rats originate in the amygdala. Ann Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalin NH, Shelton SE, Barksdale CM, Brownfield MS. A diurnal rhythm in cerebrospinal fluid corticotropin-releasing hormone different from the rhythm of pituitary-adrenal activity. Brain Res. 1987;426:385–393. doi: 10.1016/0006-8993(87)90894-8. [DOI] [PubMed] [Google Scholar]
- 25.Kalin NH. Behavioral and endocrine studies of corticotropin-releasing hormone in primates. In: De Souza EB, Nemeroff CB, editors. Corticotropin-releasing factor: basic and clinical studies of a neuropeptide. Boca Raton: CRC Press; 1990. pp. 275–289. [Google Scholar]
- 26.Garrick NA, Hill JA, Szele FG, et al. Corticotropin-releasing factor: a marked circadian rhythm in cerebrospinal fluid peaks in the evening and is inversely related to cortisol circadian rhythm. Endocrinology. 1987;121:1329–1333. doi: 10.1210/endo-121-4-1329. [DOI] [PubMed] [Google Scholar]
- 27.Hedner J, Hedner T, Lundell KH, et al. Cerebrospinal fluid concentrations of neurotensin and corticotropin-releasing factor in pediatric patients. Biol Neonate. 1989;55:260–267. doi: 10.1159/000242927. [DOI] [PubMed] [Google Scholar]
- 28.Nalin A, Facchinetti F, Galli V, et al. Reduced ACTH content in cerebrospinal fluid of children affected by cryptogenic infantile spasms with hypsarrhythmia. Epilepsia. 1985;26:446–449. doi: 10.1111/j.1528-1157.1985.tb05678.x. [DOI] [PubMed] [Google Scholar]
- 29.Butler IJ, Seifert WE, Ferkany JW, et al. Neurochemical analysis of rat cisternal cerebrospinal fluid. In: Wood JH, editor. Neurobiology of cerebrospinal fluid. Vol. 2. New York: Plenum Press; 1983. pp. 157–162. [Google Scholar]
- 30.Snead OC, III, Benton JW, Jr, Hosey LC, et al. Treatment of infantile spasms with high-dose ACTH: efficacy and plasma levels of ACTH and cortisol. Neurology. 1989;39:1027–1031. doi: 10.1212/wnl.39.8.1027. [DOI] [PubMed] [Google Scholar]
- 31.Berkenboxch F, van Oers J, del Rey A, et al. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 32.Uehara A, Gottschall PE, Dahl RR, Arimura A. Interleukin-1 stimulates ACTH release by an indirect action which requires endogenous corticotropin releasing factor. Endocrinology. 1987;121:1580–1582. doi: 10.1210/endo-121-4-1580. [DOI] [PubMed] [Google Scholar]
- 33.Suda T, Tozawa F, Ushiyama T, et al. Interleukin-1 stimulates corticotropin-releasing factor gene expression in rat hypothalamus. Endocrinology. 1990;126:1223–1228. doi: 10.1210/endo-126-2-1223. [DOI] [PubMed] [Google Scholar]
- 34.Breder CD, Dinarello CA, Saper CB. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988;240:321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]


