Abstract
Background and Rationale
Infantile spasms (IS) are an age-specific seizure disorder occurring in 1:2,000 infants and associated with mental retardation in ~90% of affected individuals. The costs of IS in terms of loss of lifetime productivity and emotional and financial burdens on families are enormous. It is generally agreed that the seizures associated with IS respond poorly to most conventional anticonvulsants. In addition, in the majority of patients, a treatment course with high-dose corticotropin (ACTH) arrests the seizures completely within days, often without recurrence on discontinuation of the hormone. However, the severe side effects of ACTH require development of better treatments for IS. Based on the rapid, all-or-none and irreversible effects of ACTH and on the established physiological actions of this hormone, it was hypothesized that ACTH eliminated IS via an established neuroendocrine feedback mechanism involving suppression of the age-specific endogenous convulsant neuropeptide corticotropin-releasing hormone (CRH). Indeed, IS typically occur in the setting of injury or insult that activate the CNS stress system, of which CRH is a major component. CRH levels may be elevated in the IS brain, and the neuropeptide is known to cause seizures in infant rats, as well as neuronal death in brain regions involved in learning and memory. If ‘excess’ CRH is involved in the pathogenesis of IS, then blocking CRH receptors should eliminate both seizures and the excitotoxicity of CRH-receptor-rich neurons subserving learning and memory.
Patients and Methods
With FDA approval, α-helical CRH, a competitive antagonist of the peptide, was given as a phase I trial to 6 infants with IS who have either failed conventional treatment or who have suffered a recurrence. The study was performed at the Clinical Research Center of the Childrens Hospital, Los Angeles. The effects of α-helical CRH on autonomic parameters (blood pressure, pulse, temperature, respiration) were determined. In addition, immediate and short-term effects on ACTH and cortisol and on electrolytes and glucose were examined. The potential efficacy of α-helical CRH for IS was studied, using clinical diaries and video EEG.
Results
α-Helical CRH, a peptide, did not alter autonomic or biochemical parameters. Blocking peripheral CRH receptors was evident from a transient reduction in plasma ACTH and cortisol. No evidence for the compound’s penetration of the blood-brain barrier was found, since no central effects on arousal, activity or seizures and EEG patterns were observed. In addition, a striking resistance of the patients’ plasma ACTH to the second infusion of α-helical CRH was noted.
Conclusions
Peptide analogs of CRH do not cross the blood-brain barrier, and their effects on peripheral stress hormones are transient and benign. Nonpeptide compouds that reach CNS receptors are required to test the hypothesis that blocking CRH receptors may ameliorate IS and its cognitive consequencs.
Keywords: Infantile spasms, Corticotropin-releasing hormone receptor
Introduction
Infantile Spasms
Infantile spasms (IS) are a seizure disorder restricted primarily to the first year of life [1, 2]. It is relatively common, occurring in 1:2,000–2,400 births, and has been known to respond to corticotropin (ACTH) since 1958 [3]. Although ACTH eliminates the seizures in the large majority of cases, it may not alter the long-term intellectual deficits of affected infants: IS are associated with moderate to severe mental retardation in 85–95% of patients [1, 2]. Infants with IS have evidence of highly abnormal neuronal activity, reflected by a chaotic EEG pattern called hypsarrhythmia. It is generally considered that this ongoing abnormal neuronal activity may contribute to the poor cognitive outcome of IS. Therefore, the optimal, effective treatment of IS should eliminate the seizures, suppress the hypsarrhythmia and improve cognitive outcome.
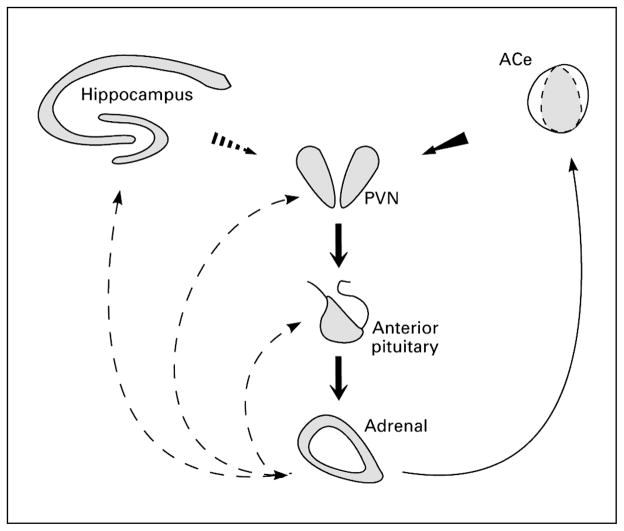
Although the response to ACTH is generally recognized, the mechanisms of action of ACTH are entirely unknown. The response pattern is not consistent with a conventional anticonvulsant effect: In responders, the seizures completely disappear (‘all-or-none’), with a median response time of 2 days [2, 4]. In addition, after a course of 2–4 weeks, discontinuation of ACTH generally does not result in recurrence of the spasms [1–4]. This response pattern suggests that ACTH may act via a neuroendocrine mechanism consistent with this hormone’s established effects: a negative feed-back action on corticotropin-releasing hormone (CRH), as is illustrated in figure 1. CRH is a stress neurohormone that is up-regulated by a variety of stress signals [5–9] (see below). It has been proposed that the common denominator for the large number of prenatal or perinatal insults predisposing to IS involves perturbation of normal neuronal environment (i.e. they are ‘stressful’, activating the CNS stress response) during a critical neurodevelopmental period [2].
Fig. 1.
The stress-activated neuroendocrine CRH-ACTH-glucocorticoid axis. Stress-conveying signals rapidly activate immediate early genes in CRH-expressing neurons of the central nucleus of the amygdala (ACe) and in the hypothalamic paraventricular nucleus (PVN). Concurrent rapid CRH release from terminals of PVN neurons into the hypothalamic-pituitary-portal system induces ACTH and gluco-corticoid (cortisol) secretion from the pituitary and adrenal, respectively. Glucocorticoids exert a negative feedback on the PVN (directly and via the hippocampus), yet activate CRH gene expression in the amygdala, potentially promoting further CRH release in this region. Continuous and dashed arrows denote established or putative potentiating and inhibitory actions, respectively. Arrows do not imply monosynaptic connections.
The Developmental Neurobiology of CRH
CRH is a neuropeptide with both neuroendocrine and neurotransmitter properties [5–12]. The peptide, isolated originally from the hypothalamus [5], is the primary modulator of the release of ACTH from the pituitary in response to stress [5, 6] (fig. 1). Activation of CRH-producing neurons is seen with physiological types of stress (immobilization, hypothermia, hemorrhage), as well as with ‘emotional’ stress stimuli such as social defeat or fear. CRH functions as a neurotransmitter in a number of limbic and autonomic brain circuits [10–13]. Both the peptide [14, 15] and CRH receptors [16–20] are widely but specifically distributed in the CNS. For example, in human brain, CRH is found in cortical interneurons of layers II and III while receptors predominate in layers I and IV [21, 22]. Abnormal CRH levels in the brain and spinal fluid, and of CRH receptors in the cortex, have been demonstrated in Alzheimer disease [22], and abnormal CRH activity has been implicated in anxiety, depression and epilepsy [2, 23–25].
CRH-producing neurons in the hypothalamus [6, 26], amygdala [27, 28] and hippocampus [29] release the peptide, which acts on postsynaptic target neurons possessing CRH receptors. Two members (and several splice variants) of the CRH receptor family are currently known and consist of membrane-spanning G-protein-coupled molecules [17–19]. The first discovered receptor, CRF1, is found in the CNS, immune cells and the pituitary, and is thus the candidate mediator of the endocrine effects of CRH. This receptor type has recently been shown to mediate the excitatory effects of CRH [30].
During development, the synthesis and levels of both CRH and CRF1 are tightly regulated. CRH gene expression commences during late gestation in the rat [31, 32], and CRH messenger RNA (mRNA) levels remain high until the day prior to birth. During the neonatal and infancy periods in the rat (the first and second weeks of life, respectively), however, CRH gene expression is low, and robust synthesis resumes during a period equivalent to early childhood in the human [33]. Receptor levels and CRF1 RNA abundance display a different developmental profile [20, 34]. In the brain as a whole, and particularly in excitable limbic regions such as the hippocampus and amygdala, both CRF1 mRNA levels and available binding sites for CRH are maximal during infancy [20, 34, 35]. Interestingly, no such developmental profile has been found for the second CRH receptor (CRF2) whose activation does not promote neuronal excitability [36]. It also has been demonstrated that stress during infancy (but not neonatally) increases CRH synthesis [8, 9, 26]. Therefore, insults or stressors during that developmental period, leading to increased CRH production, could lead to activation of the large numbers of unoccupied CRF1 receptors and consequent enhancement of neuronal excitability along key limbic circuits.
CRH Excess Hypothesis – Animal and Human Evidence
CRH has been shown to act as a convulsant [37–39] and an excitotoxin [40, 41] in the infant brain. Thus, it has been proposed that stress-induced enhancement of CRH-mediated neurotransmission would result in seizures and worsening cognitive deficits associated with IS [2, 25, 38]. The efficacy of ACTH has been attributed to its suppression of the synthesis or secretion of CRH in key limbic regions [2]. Indeed, CRH was found to be an age-specific convulsant with a 200-fold greater potency in the infant rat as compared to the adult [37, 38]. As mentioned above, CRH receptors and CRF1 mRNA were found to peak during infancy in limbic regions of the rat [20, 34], and stressful signals such as hypothermia have been shown to increase CRH synthesis in the infant rat [8, 9, 26]. Further, repeated administration of CRH, inducing prolonged seizures, was found to result in the death of neurons in limbic brain regions subserving learning and memory, in immature, but not in adult brain [40, 41].
In the human, cerebrospinal fluid analysis of untreated infants with IS revealed abnormally low ACTH and cortisol levels [42, 43], consistent with a down-regulation of CRH receptors due to excess brain levels of CRH [44]. The hypothetical basis for IS may involve a stress-enhanced availability of CRH at the receptors during the developmental period when receptors are present in large numbers in the brain. ACTH would be effective because it decreases CRH synthesis long enough for the CRH receptor number to decrease, according to their established developmental profile [20]. If IS result from increased neurotransmission of CRH at its receptors, then receptor antagonists [30, 45] should block the seizures, as well as potential adverse cognitive effects of the peptide. Therefore, these compounds are candidate drugs for the effective treatment of IS.
Animal and Human Studies of CRH Antagonists
Most available information regarding toxicity, side effects and effective doses of peptide CRH receptor blockers is based on animal experiments and on predicted potential effects of blocking the functions of CRH. The doses used in animals and the side effects found are summarized in tables 1 and 2. Essentially, the studies of Brown et al. [46], Corder et al. [48] and Lyons et al. [50] documented the lack of adverse autonomic effects of α-helical CRH using continuous monitoring of heart rate (HR) and mean arterial pressure even by the maximal antagonist doses used. In addition, Lyons et al. [50] documented that 1,000 μg/kg of antagonist given directly into the cerebral ventricles did not alter plasma glucose (124 ± 6 vs. 120 ± 6 mg/dl in controls). Thus, in the rodent, α-helical CRH did not alter blood pressure (BP) or HR in doses up to 500 μg/kg i.c.v. or 250 μg/kg i.v. While the antagonist did not prevent changes in BP or HR induced by stress, it effectively blocked hypotension caused by CRH itself [48]. In the monkey, CRH antagonists, given intracerebroventricularly, reduced ‘anxiety’-like behaviors and had no autonomic effects [49]. Thus, CRH receptor blockers given once were essentially free of side effects in animals.
Table 1.
Summary of animal studies using CRH antagonists
| Study | Species | Age | Dose, μg/kg | Route | Results | Side effects |
|---|---|---|---|---|---|---|
| Rivier et al. [45] | rat | adult | 800–2,400 | i.v. | block CRH, stress | none |
| Brown et al. [46] | rat | adult | 250 | i.v. | block stress | none |
| Fisher et al. [47] | rat | adult | 180–3,000 | i.v. | block CRH, stress | none |
| Corder et al. [48] | rat | adult | 1,000 | i.v. | block CRH | none |
| Winslow et al. [49] | monkey | adult | 10 | i.c.v. | block CRH | none |
| Lyons et al. [50] | rat | adult | 1,000 | i.c.v. | block stress | none |
Fisher et al. evaluated several doses, the results of which are given in table 2. i.v. = Intravenous; i.c.v. = into the lateral cerebral ventricle.
Table 2.
Effects of CRH antagonists on autonomic and hormonal parameters in the rodent
| Parameter | Vehicle | Helical CRH (9–41) | Dose, μg/kg |
|---|---|---|---|
| MAP, mm Hg | 103±1 | 104±2 | 180 i.v. |
| HR, beats/min | 384±4 | 382±4 | 180 i.v. |
| Norepinephrine, pg/ml | 309±20 | 248±11 | 360 i.c.v. |
| Epinephrine, pg/ml | 58±30 | 42±7 | 360 i.c.v. |
| ACTH, pg/ml | 40±7 | 37±4 | 3,000 i.v. |
| β-Endorphin, pg/ml | <300 | <300 | 3,000 i.v. |
MAP = Mean arterial pressure; i.v. = intravenous; i.c.v. = into the lateral cerebral ventricle.
A single, human study approved by the Food and Drug Agency (FDA) has been reported, looking at the hormonal, autonomic and neurological effects of α-helical CRH in normal young adults [51]. In that study, 6 volunteers received 2 intravenous infusions of α-helical CRH during 2 consecutive days, 50 μg/kg on the first day and 100 μg/ kg on the second. Neither adverse effects nor any influences on CNS were found. Transient hormonal alterations consistent with blocking of CRH receptors in the pituitary were observed [51].
In summary, a rationale for using CRH antagonists in human infants affected with infantile spasms has been established. Here we report on the administration of peptide CRH inhibitors to 6 human infants with IS.
Materials and Methods
Agent
α-Helical CRH (9–41) was synthesized according to Good Manufacturing Procedures by Bachem (Torrance, Calif., USA). The final product underwent rigorous quality assurance and purity analyses, received an IND from the FDA and was provided in a sterile and pyrogen-free preparation [51]. After dilution, the peptide was stored frozen in premeasured aliquots and thawed (once only) immediately prior to administration.
Bioavailability and Potency Tests
The bioavailability and potency of each peptide batch was verified after infusion to the experimental subjects using a bioassay approach: Leftover diluted compound remaining in the infusion bags was refrozen, lyophilized and reconstituted. It was then administered to rats 30 min prior to infusion of CRH. The ability of known amounts of antagonist to prevent CRH-induced seizures was documented. Residual potency of the preparation averaged 30–60%.
Experimental Design
A phase I study was approved by the FDA (IND 45969) and by the Institutional Review Board of the Childrens Hospital, Los Angeles. The subjects were 6 infants who had failed previous therapy or who have had a recurrence of their IS. Subject characteristics are summarized in table 3.
Table 3.
Characteristics of infants with IS receiving α-helical CRH
| Patient | Etiology | Age months | Sex | Seizure type | Clusters | EEG | Prior ACTH |
|---|---|---|---|---|---|---|---|
| 1 | none/delayed | 36 | F | myoclonic | + | modified hyps. | +/responded |
| 2 | none | 9 | M | classic spasms | + | hyps. | low dose/responded |
| 3 | none/delayed | 24 | M | classic spasms | + | multifocal | +/responded |
| 4 | asphyxia | 19 | F | classic spasms | – | modified hyps. | – |
| 5 | none/delayed | 26 | F | drop and myoclonic | – | multifocal | – |
| 6 | none/delayed | 22 | M | classic spasms | + | n.a. | +/no response |
hyps. = Hypsarrhythmia; n.a. = not assessed.
The total duration of the study was 4 days. On the morning of the first day, infants were admitted to the clinical research center, an intravenous line was inserted and video EEG initiated to obtain baseline EEG and seizure counts, in addition to those supplied by the parents. On day 2 (7 a.m.) plasma ACTH, cortisol, glucose and electrolytes were sampled through the intravenous line, to obviate the stress response to venipuncture. The CRH antagonist was administered at a dose of 50 μg/kg i.v. in 5% glucose in 0.25 N saline (50 μg/ ml). The solution was infused at a rate of 1 ml/kg over 2 h. Vital signs were monitored as described below. On termination of the infusion and 12 h later, plasma was sampled for hormonal and biochemical analysis. On day 3, plasma was again sampled, followed by an infusion of 100 μg/kg of α-helical CRH (9–41) in a volume of 2 ml/kg over 2 h. During and subsequent to the infusion, vital signs and biochemical/hormonal parameters were monitored as described for day 2. On day 4, video EEG was continued, to monitor potential changes in EEG pattern and seizure frequency. In order to distinguish between decreases in plasma ACTH and cortisol due to blocking of CRH receptors in the pituitary and between potential declines due to the circadian rhythm of these hormones, plasma samples were obtained at 7 and 9 a.m. also on day 4, when no CRH antagonist was given. Subjects were discharged from the clinical research center on the evening of day 4.
Parameters Monitored
Autonomic parameters included BP and HR, which were monitored prior to infusion onset and continuously throughout it. Values were recorded at 15-min intervals, at the end of the infusion and hourly for the subsequent 12 h. Core (rectal, oral or otic) temperature was measured prior to the infusion, at 15-min intervals during the infusion and hourly thereafter for the subsequent 12 h. Biochemical parameters, i.e. plasma electrolytes, glucose, cortisol and ACTH, were obtained prior to infusion and at its end. All values were also obtained in the evening (nadir) and at two time points, 2 h apart, on the morning of day 4.
Hormone Assays
Plasma hormone levels were analyzed using commercial radioimmunoassays (Endocrine Sciences, Calabasas Hills, Calif., USA). The sensitivity of the ACTH assay was 5 pg/ml and that of the cortisol assay 1 μg/dl. Some samples were subjected to repeat assays, and interassay variability averaged 8%.
Potential adverse effects of the CRH antagonist were considered. These included temperature drop (or surge), drop of BP and increases in HR. Hydrocortisone (intravenous preparation, 20 mg/ m2) was prepared by the bedside, as well as 10% glucose solution, to be administered as appropriate.
Analysis
Outcome criteria for this study were mainly adverse effects of α-helical CRH. However, to avoid missing potential therapeutic effects of the antagonist, continuous video EEG was carried out. The potential for a rapid (2-day) response time was based on the fact that the median response time of IS to ACTH is 2 days [4]. Thus, on theoretical grounds, efficacy of the CRH antagonist could be expected. The significance of differences between groups was determined using the two-tailed paired Wilcoxon signed-rank test without assumptions regarding value distribution.
Results
Patient Characteristics
The characteristics of the 6 infants participating in the current study are described in table 3. Briefly, 3 males and 3 females were aged 22.6 ± 3.4 months at the time of entry into the study. All had had IS and hypsarrhythmia was present at some point in their course. At study entry, EEGs were characterized as described in table 3 (1 classic and 2 modified hypsarrhythmias, 2 multifocal spikes and 2 unavailable). Four had received ACTH previously, and 3 had responded with subsequent recrudescence of spasms. Etiologies were available in 1 patient only, but 4 others were developmentally delayed.
Hormonal Changes Induced by the CRH Antagonist
The effects of α-helical CRH administration on plasma ACTH and cortisol are shown in figures 2 and 3, which represent individual values immediately prior to and at the end of each 2-hour infusion. Prior to the first infusion, ACTH levels averaged 26.8 ± 7.7 pg/ml; mean plasma ACTH 2 h later, at the end of the first CRH antagonist infusion, was 17.4 ± 3.2 pg/ml. Cortisol levels at the end of the first infusion averaged 12.4 ± 2 μg/dl, compared with 19.0 ± 5 μg/dl at its onset (p < 0.05). These results indicate that the first α-helical CRH infusion resulted in decreased hypothalamic-pituitary-adrenal (HPA) plasma hormone levels in the infants.
Fig. 2.
Plasma ACTH levels in 6 infants treated with α-helical CRH. Plasma samples were drawn from an existing intravenous line, minimizing stress to the infants. Pre1 and pre2 indicate levels prior to the first and second CRH antagonist infusions, respectively. Post1 and post2 show values after the infusions. While the expected reduction of ACTH plasma levels upon blocking of CRH receptors in the pituitary is evident after the first infusion, this reduction is absent after the second CRH antagonist administration. Following the second infusion, plasma ACTH levels are actually significantly higher (p = 0.03, paired t test).
Fig. 3.
Plasma cortisol levels in 6 infants treated with α-helical CRH. Plasma samples were drawn from an existing intravenous line, minimizing stress to the infants. Pre1 and pre2 indicate levels prior to the first and second CRH antagonist infusion, respectively. Post1 and post2 show values after the infusions.
The second infusion of the CRH antagonist, however, caused strikingly different results: ACTH levels were 14.9 ± 3.8 pg/ml prior to the infusion and 26.5 ± 6 pg/ml at its termination. These values suggest a significant increase in ACTH (p = 0.03, paired t test), indicating a resistance to the effect of the antagonist. Cortisol levels decreased modestly, from 22.2 ± 3 to 13.0 ± 3 μg/dl. These data are consistent with inhibition of ACTH secretion via blocking of pituitary CRH receptors by α-helical CRH during the first infusion, as has been demonstrated in adults, and a resistance to the effects of the second antagonist infusion in these infants.
In order to distinguish between an effect of the antagonist on CRH receptors and the diurnal decline in plasma ACTH seen normally in the morning hours, plasma ACTH and cortisol levels were also measured on the morning of day 4, when no antagonist was infused. ACTH levels measured at 7.30 a.m. averaged 24.8 ± 6 pg/ml, highly similar to values obtained on each of the previous mornings, and validating the relative lack of stress associated with plasma drawing from the intravenous line. Two hours later (with no antagonist infusion), values for ACTH were 23.2 ± 6 pg/ml, not significantly different (p = 0.19. Wilcoxon signed-rank sum). Corresponding cortisol levels were 14.2 ± 3 and 11.7 ± 2 μg/dl (p > 0.1 from preinfusion levels). Therefore, it was concluded that the infusion of α-helical CRH was responsible for the hormonal changes observed after the first infusion.
Effect of CRH Antagonist on Autonomic Parameters
Before, during and after the infusions of α-helical CRH, the ‘subjects’ autonomic and physiological parameters, including BP, HR and temperature, were measured. As found previously for adults, values during and after infusion of the CRH antagonist did not differ significantly from those obtained prior to onset of the infusion, nor were any trends evident (not shown). In addition, little consistent effect of the infusion on infants’ behavior, sleep and feeding was noted, with one exception (see below).
Effect of α-Helical CRH on IS
The number of both individual seizures and of the number of clusters in the patients revealed remarkable daily variability. For each patient, the average daily cluster number, determined from parent diaries and from the observation on the first day of the study, fluctuated widely. No significant changes in daily cluster number during or following CRH antagonist infusions were noted in 5 of the 6 infants. A single infant had complete cessation of clusters (with increased sleep) during the hospitalization and for several days later.
Discussion
The major findings of this study were: (1) α-helical CRH given intravenously blocked peripheral CRH receptors transiently; (2) 12 infusions of the agent to infants did not reveal adverse effects; (3) a resistance to the hormonal effects of the CRH antagonist emerged in IS infants; (4) transient blocking of peripheral CRH receptors does not alter EEG or seizure parameters in IS.
The hormonal changes observed in this study are concordant with those found in adults subjected to the same regimen of α-helical CRH [51]: When the agent was administered to 6 normal adults, no toxicity was observed at doses of 50 and 100 μg/kg, given intravenously over 2 h – the regimen used here. Selective cognitive, motor and cerebellar measures examined in these subjects were not affected. In addition, the subjects did not report any subjective feelings which might be related to central effects of the CRH antagonist.
The lack of CNS effects in both infants and adults cannot be due to loss of potency or bioavailability. In both groups, α-helical CRH reduced ACTH and cortisol levels, suggesting that it was bioactive under the administration conditions. In addition, the residual agent in the infusion bags was tested in immature rats that were given CRH to produce characteristic seizures. Using this bioassay – in which the antagonist was infused directly into the cerebral ventricles – the residual CRH antagonist retained 30–60% of its potency. This is quite striking considering that the peptide was at room temperature for the duration of the infusions, was exposed to large bag and tubing surface areas that promote adherence and subsequently underwent a lyophilization step. Thus, although peptidases are always a concern in the intravenous administration of peptides, these results suggest that the α-helical CRH was bioavailable and reached peripheral CRH receptors. It may be noted that the antagonist comprises only a fragment (amino acids 9–41) of the native CRH, and is expected to form a rigid, α-helix three-dimensional structure [45] that may protect it from enzymatic degradation [45, 47].
The findings that the peptide CRH antagonist failed to penetrate not only the normal adult blood-brain barrier (BBB) but also that of infants with IS is remarkable. This finding is consistent with a significant maturity of the BBB at the ages studied and also challenges theories postulating that IS may be associated with an inflammatory process, as the latter would be expected to alter BBB permeability. A similar lack of BBB penetration of peptide CRH antagonists was observed also in the ‘infant’ rat [30, 52].
An unanticipated finding of this study was the emergence of resistance to the second infusion of α-helical CRH. In a previous study in normal adults, the second administration of the antagonists suppressed ACTH levels to the same extent as the first [51]. However, in the current study of infants with IS, the second infusion not only failed to decrease, but resulted in elevation of plasma ACTH levels. This is unlikely to be a spurious effect of – for example –stress, since it was observed neither during the first administration nor during the last study day, when no infusion was given. Other procedures were similar throughout these 3 days. In addition, the antagonist was aliquoted prior to the study, and a fresh batch was thawed each morning. Infants were not studied concurrently so that a common, undetected storage or administration problem can be excluded. Also, the bioassay activity of all tested batches was rather homogeneous. In addition, it is unlikely that previous ACTH treatment influenced the unusual hormonal response of these infants: ACTH had been given to 4 of the 6 infants a minimum of 2 months earlier. However, any long-term effects of ACTH on the infants’ hypothalamic-pituitary-adrenal axis would have emerged upon the first CRH antagonist infusion.
The mechanism for the apparent resistance of the patients to the second α-helical CRH infusion is therefore obscure. A potential explanation may involve a rapid up-regulation of CRH receptors in IS infants by the first administration of the antagonist, i.e. by the transient absence of the ligand, CRH.
The implications of this perturbation of CRH receptor regulation for the pathophysiology of IS are unclear but are in support of other abnormalities of the CNS CRH-ACTH axis demonstrated in these infants [2, 42, 43, 53–55].
In summary, administration of α-helical CRH to infants with IS demonstrated that peptide analogs of CRH do not cross the BBB and their effects on peripheral stress hormones are transient and benign. Nonpeptide compounds that reach CNS receptors are required to test the hypothesis that blocking CRH receptors may ameliorate IS and their cognitive consequences.
Acknowledgments
Supported by an Innovative Clinical Research grant of the Childrens Hospital, Los Angeles, Research Institute (T.Z.B.) and by the NIH NCRR GCRC grant MO 1 RR-43. T.Z.B. was supported in part by NIH NS 28912. The authors are grateful to Drs. R. Parkman, K. Weinberg and I.T. Lott for their encouragement and to the clinical research center staff for their excellent assistance.
References
- 1.Aicardi J. Infantile spasms and related syndromes. In: Aicardi J, editor. Epilepsy in Children. New York: Raven Press; 1986. pp. 17–38. [Google Scholar]
- 2.Baram TZ. Pathophysiology of massive infantile spasms (MIS): Perspective on the role of the brian adrenal axis. Ann Neurol. 1993;33:231–237. doi: 10.1002/ana.410330302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorel L, Dusaucy-Bauloye E. A propos de 21 cas d’hypsarythmia de Gibbs: son traitement spectaculaire par l’ACTH. Acta Neurol Psychiatr Belg. 1958;58:130–141. [PubMed] [Google Scholar]
- 4.Baram TZ, Mitchell WG, Tournay A, Snead OC, Hanson RA, Horton EJ. High dose ACTH versus prednisone for massive infantile spasms: A prospective randomized blinded study. Pediatrics. 1996;97:375–379. [PMC free article] [PubMed] [Google Scholar]
- 5.Vale W, Rivier C, Brown MR, Spiess J, Koob G, Swanson L, Bilezikjian L, Bloom F, Rivier J. Chemical and biological characterization of corticotropin releasing factor. Rec Prog Horm Res. 1983;39:245–270. doi: 10.1016/b978-0-12-571139-5.50010-0. [DOI] [PubMed] [Google Scholar]
- 6.Lightman SL, Harbuz MS. Expression of corticotropin releasing factor mRNA in response to stress. Corticotropin Releasing Factor. CIBA Symposium; Chichester, Wiley. 1993. pp. 172pp. 173–198. [DOI] [PubMed] [Google Scholar]
- 7.Kalin NH, Takahashi LK, Chen FL. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 1994;656:182–186. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- 8.Hatalski CG, Avishai-Eliner S, Eghbal-Ahmadi M, Baram TZ. Induction of corticotropin releasing factor heteronuclear messenger RNA by a acute cold stress in the developing rat hypothalamus (abstract 516, unpublished) [Google Scholar]
- 9.Hatalski CG, Guirguis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J Neuroendocrinol. 1998;10:663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox EA, Gruol DL. CRF suppresses the after-hyperpolarization in cerebellar Purkinje neurons. Neurosci Lett. 1993;49:103–107. doi: 10.1016/0304-3940(93)90358-r. [DOI] [PubMed] [Google Scholar]
- 11.Rainnie DG, Fernhout BJ, Shinnick-Gallagher P. Differential actions of corticotropin releasing factor on basolateral and central amygdaloid neurons in vitro. J Pharmacol Exp Ther. 1992;263:846–858. [PubMed] [Google Scholar]
- 12.Curtis AL, Pavcovich D, Grigoriadis DE, Valentino RJ. Previous stress alters corticotropin releasing factor neurotransmission in the locus ceruleus. Neuroscience. 1995;65:541–550. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- 13.Hollrigel G, Baram TZ, Soltesz I. Corticotropin releasing hormone increases excitatory synaptic transmission in the hippocampus of infant rats. Neuroscience. 1998;84:71–79. doi: 10.1016/s0306-4522(97)00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young WS., III . Distribution and regulation of CRF-mRNA in brain using in situ hybridization histochemistry. In: De Souza EB, Nemeroff CB, editors. Corticotropin Releasing Factor: Basic and Clinical Studies of a Neuropeptide. Boca Raton: CRC; 1990. pp. 213–220. [Google Scholar]
- 15.Yan XX, Toth Z, Schultz L, Ribak CE, Baram TZ. Corticotropin releasing hormone (CRH)-containing neurons in the hippocampal formation: Morphological and neurochemical characterization. Hippocampus. 1998;8:1–13. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Souza EB, Kuhar MJ. Corticotropin-releasing factor receptors: Autoradiographic identification. In: Martin JB, Barchas JD, editors. Neuropeptides in Neurologic and Psychiatric Disease. New York: Raven Press; 1986. pp. 179–198. [PubMed] [Google Scholar]
- 17.Wong ML, Licinio J, Gold PW. Localization of CRH receptor mRNA in adult rat by in situ hybridization. Endocrinology. 1994;135:2275–2278. doi: 10.1210/endo.135.5.7956950. [DOI] [PubMed] [Google Scholar]
- 18.Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale WW. Distribution of CRF-receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci USA. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avishai-Eliner S, Yi SJ, Baram TZ. Developmental profile of CRH-receptor messenger RNA in the rat limbic system. Dev Brain Res. 1996;91:159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bissette G, Reynolds GP, Kilts CD, Widerlov E, Nemeroff CB. Corticotropin releasing factor like immunoreactivity in senile dementia of the Alzheimer type. JAMA. 1985;254:3067–3069. [PubMed] [Google Scholar]
- 22.Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer’s disease. Nature. 1995;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- 23.Heim C, Owens MJ, Plotsky PM, Nemeroff CB. Persistent changes in corticotropin-releasing factor systems due to early life stress. Relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacol Bull. 1997;33:185–192. [PubMed] [Google Scholar]
- 24.Tsigos C, Chrousos GP. Physiology of the hypothalamic pituitary adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinol Metab Clin North Am. 1994;23:451–466. [PubMed] [Google Scholar]
- 25.Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: A key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi SJ, Baram TZ. Corticotropin releasing factor mediates the response to cold stress in the neonatal rat, without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pich EM, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merali Z, McIntosh J, Kent P, Michaud D, Ansiman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci. 1998;18:4758–4766. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan XX, Brunson KL, Gerth A, Baram TZ. Stress-induced reduction of corticotropin releasing hormone (CRH) immunoreactive hippocampal interneurons. Soc Neurosci Abstr. 1998;243:5. [Google Scholar]
- 30.Baram TZ, Chalmers DT, Chen C, Kotsoukos Y, De Souza EB. The CRF1 receptor mediates the excitatory actions of corticotropin releasing factor in the developing rat brain – in vivo evidence using a novel, selective non-peptide CRF receptor antagonist. Brain Res. 1997;770:89–95. doi: 10.1016/s0006-8993(97)00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grino MW, Young WS, 3rd, Burgundier JM. Ontogeny of expression of the CRF gene in the hypothalamic paraventricular nucleus and of the proopiomelanocortin gene in rat pituitary. Endocrinology. 1989;124:60–68. doi: 10.1210/endo-124-1-60. [DOI] [PubMed] [Google Scholar]
- 32.Baram TZ, Lerner SP. Corticotropin releasing hormone – Ontogeny of gene expression in rat hypothalamus. Int J Dev Neurosci. 1991;9:473–478. doi: 10.1016/0736-5748(91)90033-i. [DOI] [PubMed] [Google Scholar]
- 33.Gottlieb A, Keydar I, Epstein HT. Rodent brain growth stages: An analytical review. Biol Neonate. 1977;32:166–176. doi: 10.1159/000241012. [DOI] [PubMed] [Google Scholar]
- 34.Insel TR. Brain corticotropin releasing factor and development. In: De Souza EB, Nemeroff CB, editors. Corticotropin Releasing Factor: Basic and Clinical Studies of a Neuropeptide. Boca Raton: CRC; 1990. pp. 91–106. [Google Scholar]
- 35.Pihoker C, Cain ST, Nemeroff CB. Postnatal development of regional binding of corticotropin-releasing factor and adenylate cyclase activity in the rat brain. Prog Neuropsychopharmacol Biol Psych. 1992;16:581–586. doi: 10.1016/0278-5846(92)90063-k. [DOI] [PubMed] [Google Scholar]
- 36.Eghbal-Ahmadi M, Hatalski CG, Lovenberg TW, Avishai-Eliner S, Baram TZ. The developmental profile of the corticotropin releasing factor receptor (CRF2) in rat brain predicts distinct age-specific functions. Brain Res. 1998;107:81–90. doi: 10.1016/s0165-3806(98)00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baram TZ, Schultz L. CRH is a rapid and potent convulsant in the infant rat. Dev Brain Res. 1991;61:97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baram TZ, Hirsch E, Snead OC, III, Schultz L. CRH induced seizures in the infant brain originate in the amygdala. Ann Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehlers CL, Henriksen SJ, Wang M, Rivier J, Vale W, Bloom FE. Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res. 1983;278:332–336. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- 40.Baram TZ, Ribak CE. Peptide-induced infant status epilepticus causes neuronal death and synaptic reorganization. Neuroreport. 1995;6:277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribak CE, Baram TZ. Selective death of hippocampal CA3 pyramidal cells with mossy fiber afferents after CRH-induced status epilepticus in infant rats. Dev Brain Res. 1996;91:245–251. doi: 10.1016/0165-3806(95)00183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baram TZ, Mitchell WG, Snead OC, III, Horton EJ, Saito M. Brain-adrenal axis hormones are altered in the cerebrospinal fluid of infants with massive infantile spasms. Neurology. 1992;42:1171–1175. doi: 10.1212/wnl.42.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baram TZ, Mitchell WG, Snead OC, III, Horton EJ. Corticotropin and cortisol are increased in the cerebrospinal fluid of infants with massive infantile spasms. Pediatr Neurol. 1995;13:108–110. doi: 10.1016/0887-8994(95)00121-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tizabi Y, Aguilera G. Desensitization of the HPA axis following prolonged administration of CRH or vasopressin. Neuroendocrinology. 1992;56:611–618. doi: 10.1159/000126283. [DOI] [PubMed] [Google Scholar]
- 45.Rivier J, Rivier C, Vale W. Synthetic competitive antagonists of corticotropin releasing factor: Effect of ACTH secretion in the rat. Science. 1984;224:889–891. doi: 10.1126/science.6326264. [DOI] [PubMed] [Google Scholar]
- 46.Brown MR, Gray TS, Fisher L. Corticotropin releasing factor receptor antagonist: Effects on the autonomic nervous system and cardiovascular function. Regul Pept. 1989;32:919–926. doi: 10.1016/0167-0115(86)90032-7. [DOI] [PubMed] [Google Scholar]
- 47.Fisher L, Rivier C, Rivier J, Brown M. Differential antagonist activity of helical CRF9–41 in 3 bioassay systems. Endocrinology. 1991;129:1312–1316. doi: 10.1210/endo-129-3-1312. [DOI] [PubMed] [Google Scholar]
- 48.Corder R, Turnhill D, Ling N, Gaillard RC. Attenuation of CRF-induced hypotension in anesthetized rats with the CRF antagonist alpha-helical CRF. Peptides. 1992;13:1–6. doi: 10.1016/0196-9781(92)90132-m. [DOI] [PubMed] [Google Scholar]
- 49.Winslow JT, Newman JD, Insel TR. CRH and alpha-helical CRH modulate behavioral measures of arousal in monkeys. Pharm Biochem Behav. 1989;32:919–926. doi: 10.1016/0091-3057(89)90059-2. [DOI] [PubMed] [Google Scholar]
- 50.Lyons MK, Anderson RE, Meyer F. CRF antagonist reduces ischemic hippocampal neuronal injury. Brain Res. 1991;545:339–342. doi: 10.1016/0006-8993(91)91310-w. [DOI] [PubMed] [Google Scholar]
- 51.Baram TZ, Mitchell WG, Haden E. Inhibition of pituitary-adrenal secretion by a corticotropin releasing hormone antagonist in humans. Mol Psychiatry. 1996;1:320–325. [PMC free article] [PubMed] [Google Scholar]
- 52.Baram TZ, Kotsoukos Y, Schultz L, Rivier J. The effect of ‘Astressin’, a novel antagonist of corticotropin releasing hormone (CRH), on CRH-induced seizures in the infant rat: Comparison with two other antagonists. Mol Psychiatry. 1996;1:223–226. [PMC free article] [PubMed] [Google Scholar]
- 53.Nalin A, Facchinetti F, Galli V, Petraglia F, Storchi R, Genazzani AR. Reduced ACTH content in cerebrospinal fluid of children affected by cryptogenic infantile spasms with hypsarrhythmia. Epilepsia. 1985;26:446–449. doi: 10.1111/j.1528-1157.1985.tb05678.x. [DOI] [PubMed] [Google Scholar]
- 54.Riikonen RS. How do cryptogenic and symptomatic infantile spasms differ? Review of biochemical studies in Finnish patients. J Child Neurol. 1996;11:383–388. doi: 10.1177/088307389601100508. [DOI] [PubMed] [Google Scholar]
- 55.Heiskala H. CSF ACTH and β-endorphin in infants with West syndrome and ACTH therapy. Brain Dev. 1997;19:339–342. doi: 10.1016/s0387-7604(97)00026-0. [DOI] [PubMed] [Google Scholar]