Abstract
Context
Disease variation can be substantial even in conditions with a single gene etiology such as cystic fibrosis (CF). Simultaneously studying the effects of genes and environment may provide insight into the causes of variation.
Objective
To determine whether secondhand smoke exposure is associated with lung function and other outcomes in individuals with CF, whether socioeconomic status affects the relationship between secondhand smoke exposure and lung disease severity, and whether specific gene-environment interactions influence the effect of secondhand smoke exposure on lung function.
Design, Setting, and Participants
Retrospective assessment of lung function, stratified by environmental and genetic factors. Data were collected by the US Cystic Fibrosis Twin and Sibling Study with missing data supplemented by the Cystic Fibrosis Foundation Data Registry. All participants were diagnosed with CF, were recruited between October 2000 and October 2006, and were primarily from the United States.
Main Outcome Measures
Disease-specific cross-sectional and longitudinal measures of lung function.
Results
Of 812 participants with data on secondhand smoke in the home, 188 (23.2%) were exposed. Of 780 participants with data on active maternal smoking during gestation, 129 (16.5%) were exposed. Secondhand smoke exposure in the home was associated with significantly lower cross-sectional (9.8 percentile point decrease; P<.001) and longitudinal lung function (6.1 percentile point decrease; P=.007) compared with those not exposed. Regression analysis demonstrated that socioeconomic status did not confound the adverse effect of secondhand smoke exposure on lung function. Interaction between gene variants and secondhand smoke exposure resulted in significant percentile point decreases in lung function, namely in CFTR non-ΔF508 homozygotes (12.8 percentile point decrease; P=.001), TGFβ1-509 TT homozygotes (22.7 percentile point decrease; P=.006), and TGFβ1 codon 10 CC homozygotes (20.3 percentile point decrease; P=.005).
Conclusions
Any exposure to secondhand smoke adversely affects both cross-sectional and longitudinal measures of lung function in individuals with CF. Variations in the gene that causes CF (CFTR) and a CF-modifier gene (TGFβ1) amplify the negative effects of secondhand smoke exposure.
With the availability of the human genome sequence, a new challenge has arisen, namely, determining the role of genetic factors in disease variation and their effects on clinical outcomes. The genes of an organism operate within the context of environmental exposures. Therefore, quantifying the effect of genes on disease variation requires accounting for interactions with key environmental factors. Single gene disorders such as cystic fibrosis (CF) present an opportunity to examine these interactions in a disease of uniform etiology. Cystic fibrosis is caused by mutations in a single gene, the CF transmembrane conductance regulator (CFTR) gene, and affects more than 30 000 individuals in the United States.
Cystic fibrosis is a fatal disorder, although the age of survival varies greatly, ranging from early childhood to later adulthood.1 The major cause of morbidity and mortality in individuals with CF is progressive obstructive lung disease. Variation in CF lung disease is primarily due to genetic and environmental factors operating independently of disease-causing mutations in the CFTR gene.2 The US Cystic Fibrosis Twin and Sibling Study is designed to address the potential gene-environment interactions with pulmonary outcomes. The Twin and Sibling Study collected data on lung phenotypes, genetic factors, and environmental factors.
One key environmental factor for any gene-environment study focusing on lung disease is secondhand smoke exposure. Secondhand smoke exposure both in the home and in utero have been shown to adversely affect pulmonary function in healthy children.3 Despite public health warnings, including a recent US surgeon general’s report stating that there is no risk-free level of secondhand smoke exposure,3 substantial numbers of individuals with CF are exposed to secondhand smoke.4 Unfortunately, published studies have been inconsistent in associating poorer clinical outcomes in patients with CF with secondhand smoke exposure. Four studies have demonstrated a decline in lung function in patients with CF that was associated with secondhand smoke exposure by at least 1 measure,5–8 whereas 3 studies have not.4,9,10 In particular, the best-powered study to date involving 325 individuals with CF found no association between secondhand smoke exposure in the home and lung disease severity.4
The inconsistent results between existing published studies may be due to differences in power to detect the detrimental effects of secondhand smoke exposure. However, it is also possible that environmental and/or genetic modifiers of the effect of secondhand smoke exposure on lung disease differ among these studies. For example, socioeconomic status has been correlated with cigarette smoking yet only 2 studies investigated its effect on the relationship between secondhand smoke exposure and clinical outcomes in patients with CF.5,8 In addition, some studies have demonstrated that exposure to secondhand smoke is associated with poorer nutritional status in patients with CF by at least 1 measure,5,7 whereas others have not.4,8 Finally, 3 studies have explored the effects of secondhand smoke exposure in patients with known CFTR genotypes; in 2 studies only participants carrying 2 copies of the most common CF mutation (ΔF508) were analyzed,4,5 and in the remaining study too few patients were enrolled to examine secondhand smoke interaction with the CFTR genotype.6 There are no published studies examining whether interactions between modifier genes and secondhand smoke exposure contribute to the variation of CF lung disease.
Using data collected by the US Cystic Fibrosis Twin and Sibling Study, we asked the questions: (1) is secondhand smoke exposure associated with worse lung disease and other outcomes in individuals with CF, (2) is there an environment-environment interaction between secondhand smoke exposure and socioeconomic status that correlates with CF lung disease severity, and (3) do gene-environment interactions between the disease-causing gene (CFTR) or the transforming growth factor β1 (TGFβ1) gene influence the effect of secondhand smoke exposure on the pulmonary phenotype? TGFβ1 was selected for study because genotypic variants of TGFβ1 have been shown to modify the severity of CF11–13 and the development and/or severity of other chronic respiratory tract conditions, including asthma14–18 and chronic obstructive pulmonary disease.19–21
METHODS
Participants
Data for this study were collected by the US Cystic Fibrosis Twin and Sibling Study2,22 with pulmonary function, growth parameter, zip code, and ethnicity/race data supplemented through the use of the Cystic Fibrosis Foundation Data Registry (Bethesda, Maryland). Approval and annual review of this study was obtained through the Johns Hopkins University institutional review board with review by local institutional review boards if required by participating institutions. Written informed consent or assent was obtained from all participants and/or guardians. Where possible, Twin and Sibling Study data were used in preference to data from the Cystic Fibrosis Foundation Data Registry.
All participants met CF diagnostic criteria23 and were recruited between October 27, 2000, and October 17, 2006, on the basis of having an affected sibling and/or twin. All participants had at least 1 pulmonary function test obtained after age 6 years and prior to any lung transplantation. All participants had information concerning at least 1 form of secondhand smoke exposure. Exclusion criteria include current or past (>1 month) active smoking of tobacco or marijuana. Except for 2 sets of twins, all participants attended CF care centers in the United States.
Smoking exposure, demographic and socioeconomic data, birth parameters, and the presence of nasal polyposis and/or sinus disease and associated surgeries were determined by chart review and/or questionnaires completed by the participant or his/her parent (forms available on request). Lung function measures and postnatal growth parameters were obtained from chart review and/or the Cystic Fibrosis Foundation Data Registry.
Phenotyping
Forced expiratory volume in the first second of expiration (FEV1) was used as the pulmonary phenotype because this measure of lung function correlates well with survival in patients with CF.24 The FEV1 data obtained prior to age 6 years or after lung transplantation were excluded. The FEV1 values in liters were converted into CF-specific percentiles for FEV1.25 Use of CF-specific percentiles, which account for age, height, and sex, allows patients of different ages to be readily compared because the typical longitudinal decline in lung function measures with traditional predictive equations is not seen with CF-specific percentiles.2 The maximum FEV1 CF percentile, a cross-sectional measure of lung function, and the average FEV1 CF percentile, a longitudinal measure, were derived as previously described.2 The estimated FEV1 percentile at 20 years, a longitudinal measure that predicts lung function at age 20 years, was derived as previously described.26
Body mass index (BMI) z scores were calculated using reference equations from the Centers for Disease Control and Prevention.27 The average BMI z score, a longitudinal measure of nutritional status, was derived by averaging BMI z score measurements for participants with a minimum of 4 years of data and a minimum of 4 measurements.
Environmental Factors
Both smoking exposure at home (secondhand smoke at home) and active maternal smoking during pregnancy (secondhand smoke at gestation) were defined as either ever or never having occurred, without reference to location, duration, or severity. Race/ethnicity was designated by the participant. Those participants describing themselves as white or Hispanic were categorized as white; all other participants were categorized as nonwhite.
Parental education and occupation were described as 7- and 10-level categorical variables, respectively.28 Parental occupations were scored separately by 3 reviewers (J.M.C., A.B., K.N.) with discrepancies resolved by consensus. Parental education levels and occupations were converted into family Hollingshead strata, a categorical variable from 1 to 5, in which 5 is the highest socioeconomic stratum.28 Household income was derived using residential zip codes and US Census data from 2000.29
Genetic Factors
Twin zygosity was determined by the AmpFlSTR Profiler kit (Applied Biosystems, Foster City, California). CFTR genotype (GenBank AY399795) was obtained from medical records, by typing 31 common CF alleles, or by sequencing, coding, and flanking regions. The TaqMan Assays-on-Demand (Applied Biosystems) were used to genotype TGFβ1 C-509T (rs1800469, promoter region) and T+869C (rs1982073, codon 10 in exon 1). Reactions were performed in a 10-μL reaction volume with 10 ng of template DNA on an iCycler thermal cycler (Bio-Rad Laboratories, Hercules, California). Fluorescent end points were read using an ABI PRISM 7900HT sequence detection system (Applied Biosystems). Genotypes were called using SDS version 2.1 (Applied Biosystems). Genotype accuracy was assessed by inheritance analysis of participants and their parents using SIB-PAIR version 0.99.9 software.30 C-509T genotypes were verified using sequence-specific oligonucleotide linear arrays (Roche Molecular Systems, Alameda, California), which were hybridized on a BeeBlot 1003 machine (Bee Robotics Ltd, Caernarfon, United Kingdom). The discrepancy rate between the 2 genotyping methods for C-509T was less than 1%. Unclear or erroneous genotypes were repeated by TaqMan or directly sequenced using the BigDye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems).
Data Analysis
Statistical analyses were performed using Intercooled Stata version 8 (StataCorp, College Station, Texas). The robust standard errors and 95% confidence intervals for regressions were examined but did not affect the results. Group means were compared using unpaired t tests and group percentages were compared using χ2 tests. Group medians were compared using Wilcoxon Mann-Whitney tests. Single and multiple linear regression were used to relate lung function measures to individual characteristics. Multiple linear regressions included interaction terms to identify interactions of interest. All regressions estimated standard errors accounting for within-family correlation. All reported regression coefficients are unstandardized. For all tests, P<.05 was deemed statistically significant.
RESULTS
Study Demographics
A total of 132 twins and 698 siblings (830 participants from 450 families) were recruited from CF centers located primarily in the United States. Thirteen participants were excluded from further analysis on the basis of reporting current smoking (4 participants), past smoking (7 participants), or either active or past marijuana use (2 participants). Of the 812 participants with data concerning secondhand smoke exposure in the home, 188 (23.2%) were exposed. Of the 780 participants with data concerning active maternal smoking during gestation, 129 (16.5%) were exposed.
Participants with a history of being exposed to secondhand smoke differed from those not exposed only on the basis of age and zygosity (Table 1). The demographics of the subgroups of participants with longitudinal lung function data were similar to that of the entire study population; differences seen with secondhand smoke exposure included only age and zygosity (data available on request). Participants with a history of any active maternal smoking during their gestation differed from those not exposed only on the basis of age and age at diagnosis (Table 1).
Table 1.
Demographics of Participants by Secondhand Smoke Exposurea
| Characteristics | Home
|
Gestation
|
||||
|---|---|---|---|---|---|---|
| Secondhand Smoke Exposure (n = 188) | No Secondhand Smoke Exposure (n = 624) | P Value | Secondhand Smoke Exposure (n = 129) | No Secondhand Smoke Exposure (n = 651) | P Value | |
| Male sex, No. (%) | 109 (58.0) | 329 (52.7) | .21 | 71 (55.0) | 347 (53.3) | .72 |
|
| ||||||
| Age as of January 1, 2007, mean (SD), y | 19.5 (9.6) | 16.8 (7.3) | <.001 | 20.0 (9.9) | 17.0 (7.5) | <.001 |
|
| ||||||
| Age at time of diagnosis, yb | ||||||
| Mean (SD) | 2.3 (5.0) | 2.1 (4.2) | .54 | 3.1 (6.7) | 2.0 (3.8) | .01 |
|
| ||||||
| Median (range) | 0.36 (0–35.53) | 0.38 (0–32.28) | .14 | 0.36 (0–35.53) | 0.40 (0–32.28) | .21 |
|
| ||||||
| Nonwhite race, No./total (%) | 11/188 (5.9) | 31/624 (5.0) | .63 | 5/129 (3.9) | 33/651 (5.1) | .57 |
|
| ||||||
| Monozygous twins, No./total (%) | 33/188 (17.6) | 71/624 (11.4) | .03 | 18/129 (14.0) | 77/651 (11.8) | .50 |
|
| ||||||
| CFTR genotype ΔF508 homozygote, No./total (%) | 82/187 (43.9) | 317/617 (51.4) | .07 | 68/127 (53.5) | 315/647 (48.7) | .32 |
|
| ||||||
| TGFβ1, No./total (%) | ||||||
| −509 genotype TT | 24/174 (13.8) | 60/583 (10.3) | .20 | 13/113 (11.5) | 65/617 (10.5) | .76 |
|
| ||||||
| Codon 10 genotype CC | 27/144 (18.8) | 79/510 (15.5) | .35 | 17/93 (18.3) | 83/541 (15.3) | .47 |
The denominator is provided throughout because some data were not available.
Data were not available for 2 patients.
Environment-Phenotype Effects
Secondhand Smoke Exposure in the Home
Secondhand smoke exposure was associated with decreased lung function for all measures of lung function studied (Table 2), including the cross-sectional measure (maximum FEV1 CF percentile) and both longitudinal measures (average FEV1 CF percentile and estimated FEV1 percentile at 20 years). Because all 3 measures of lung function were adversely affected in the presence of secondhand smoke exposure and were similar in terms of demographics, we used the cross-sectional measure (maximum FEV1 CF percentile) in the analyses that follow to maximize the number of participants available for analyses. The demographic covariate of race/ethnicity may influence the effect of secondhand smoke exposure on lung function because nonwhites had significantly worse lung function than whites. Sex was not a significant demographic covariate. Secondhand smoke exposure did not affect the severity of sinus disease or nasal polyposis in patients with CF.
Table 2.
Clinical Outcomes and Secondhand Smoke Exposurea
| Characteristics | Home
|
Gestation
|
||||
|---|---|---|---|---|---|---|
| Secondhand Smoke Exposure (n = 188) | No Secondhand Smoke Exposure (n = 624) | P Value | Secondhand Smoke Exposure (n = 129) | No Secondhand Smoke Exposure (n = 651) | P Value | |
| Cross-sectional lung function, maximum FEV1 cystic fibrosis percentile, mean (SD) | 0.61 (0.28) | 0.71 (0.25) | <.001 | 0.69 (0.26) | 0.69 (0.26) | .92 |
|
| ||||||
| Longitudinal lung function, mean (SD) | ||||||
| Average FEV1 cystic fibrosis percentile | (n = 149) 0.55 (0.25) | (n = 432) 0.61 (0.23) | .007 | (n = 96) 0.62 (0.23) | (n = 465) 0.60 (0.24) | .51 |
|
| ||||||
| Estimated FEV1 percentile at 20 y | (n = 139) 77.9 (24.7) | (n = 386) 83.8 (24.6) | .02 | (n = 88) 82.6 (21.1) | (n = 417) 82.7 (25.0) | .98 |
|
| ||||||
| Longitudinal nutritional status, average BMI z score, mean (SD) | (n = 159) −0.25 (0.80) | (n = 546) −0.13 (0.80) | .08 | (n = 101) −0.11 (0.83) | (n = 580) −0.16 (0.78) | .58 |
|
| ||||||
| Sinus disease, No./total (%) | 83/180 (46.1) | 267/596 (44.8) | .76 | 58/123 (47.2) | 289/624 (46.3) | .86 |
|
| ||||||
| Sinus surgery, No./total (%) | 47/80 (58.8) | 146/248 (58.9) | .99 | 35/57 (61.4) | 158/268 (59.0) | .73 |
|
| ||||||
| Nasal polyposis, No./total (%) | 71/177 (40.1) | 210/589 (35.7) | .28 | 50/120 (41.7) | 223/615 (36.3) | .26 |
|
| ||||||
| Polyp surgery, No./total (%) | 34/66 (51.5) | 103/188 (54.8) | .65 | 23/46 (50.0) | 113/202 (55.9) | .47 |
|
| ||||||
| Birth length, mean (SD), cm | (n = 93) 49.7 (3.3) | (n = 483) 50.2 (3.4) | .21 | |||
|
| ||||||
| Birth weight, mean (SD), kg | (n = 123) 3.02 (0.56) | (n = 629) 3.15 (0.61) | .03 | |||
|
| ||||||
| Gestation duration, mean (SD), wk | (n = 119) 38.7 (2.5) | (n = 627) 38.3 (2.7) | .16 | |||
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in the first second of expiration.
The denominator is provided throughout because some data were not available.
Active Maternal Smoking
Active maternal smoking during pregnancy was associated with a significantly lower birth weight but did not affect birth length or gestation duration (Table 2). Previous studies have shown that the mean birth weight of infants with CF is statistically lower than that of the general population31–33; thus, maternal cigarette smoking causes a further reduction in birth weight. Active maternal smoking during gestation was not found to affect any of the 3 measures of lung function.
Environment-Environment Interactions
Other environmental factors closely correlated with secondhand smoke exposure may account for or contribute to its negative effect on lung function in patients with CF. There was a significant association between both forms of secondhand smoke exposure and lower socioeconomic status for every measure of socioeconomic status used, which is well documented.3 However, lower socioeconomic status status was associated with more severe lung disease for only 3 of 7 socioeconomic status variables tested: paternal education level (P=.005), Hollingshead strata (P=.02), and periods without health insurance (P= .006). Lower socioeconomic status was not associated with more severe lung disease for the socioeconomic status variables of maternal education level and occupation, paternal occupation, and median income. To weigh the contribution of socioeconomic status factors to lung function in the presence or absence of secondhand smoke exposure, we performed multiple regression analysis. While secondhand smoke exposure was consistently associated with worse lung function, Hollingshead strata were not correlated with lung disease severity (Table 3). In similar regression analyses (data available on request), insurance status and paternal education were not associated with worse lung function, while secondhand smoke exposure remained significant. Thus, socioeconomic status does not confound the relationship between secondhand smoke exposure and reduced lung function in patients with CF.
Table 3.
Effect of Secondhand Smoke Exposure on Lung Functiona
| Single Variableb |
Multiple Variablec |
|||
|---|---|---|---|---|
| β | P Value | β | P Value | |
| Smoking in the home (no secondhand smoke = 0, secondhand smoke = 1) | −0.086 | .003 | −0.067 | .02 |
|
| ||||
| Age | −0.002 | .11 | −0.004 | .03 |
|
| ||||
| Age at diagnosis | 0.006 | .01 | 0.008 | <.001 |
|
| ||||
| Socioeconomic status (lowest = 1, highest = 5) | 0.019 | .08 | 0.014 | .18 |
|
| ||||
| Race (0 = white, 1 = nonwhite) | −0.006 | .30 | −0.006 | .36 |
|
| ||||
| Sex (male = 1, female = 2) | −0.007 | .73 | −0.011 | .59 |
A total of 749 individuals had all data available and were included in all the regressions in this table.
Linear regressions with maximum forced expiratory volume in the first second of expiration (FEV1) cystic fibrosis percentile as the dependent variable with the single independent variable as listed.
Multiple regression with maximum FEV1 cystic fibrosis percentile as the dependent variable and the independent variables as listed.
Gene-Environment Interactions
To assess the effect of variation in the gene that causes CF (CFTR), participants, who by definition have at least 2 deleterious CFTR mutations, were divided into 2 groups: those with 2 copies of the most common mutation (ΔF508 homozygotes) and those with 1 or no copies of that mutation (non-ΔF508 homozygotes). Linear regression revealed that ΔF508 homozygotes did not have a significant reduction in lung function when exposed to secondhand smoke (Table 4). In contrast, individuals carrying other combinations of CF mutations (non-ΔF508 homozygotes) had a reduction of 13 percentile points. Thus, the nature of the mutations in CFTR determines the magnitude of the effect of secondhand smoke exposure on lung function in patients with CF.
Table 4.
Magnitude of Secondhand Smoke Effect by Genotype by Linear Regression of Maximum FEV1 Cystic Fibrosis Percentile
| Genotype Groupa | No. of Individuals | Linear Regression Coefficientb | P Value |
|---|---|---|---|
| CFTR | |||
| ΔF508 homozygote | 399 | −0.064 | .11 |
|
| |||
| 0 or 1 ΔF508 alleles | 405 | −0.128 | .001 |
|
| |||
| TGFβ1 −509 | |||
| CC | 369 | −0.067 | .08 |
|
| |||
| CT | 304 | −0.086 | .05 |
|
| |||
| TT | 84 | −0.227 | .006 |
|
| |||
| TGFβ1 codon 10 | |||
| CC | 106 | −0.203 | .005 |
|
| |||
| CT | 304 | −0.049 | .22 |
|
| |||
| TT | 244 | −0.064 | .16 |
Of the 812 individuals with data on maximum forced expiratory volume in the first second of expiration (FEV1) cystic fibrosis percentile and secondhand smoke exposure at home, only 804, 757, and 654 individuals had CFTR, TGFβ1 −509, and TGFβ1 codon 10 genotypes available, respectively.
This column contains single linear regressions with maximum FEV1 cystic fibrosis percentile as the dependent variable and secondhand smoke exposure in the home as the independent variable.
The TGFβ1 gene has been shown to be a genetic modifier of lung function in patients with CF.12,13 In this study, participants exposed to secondhand smoke bearing 2 copies of the T allele (TT homozygote) at the −509 promoter site of TGFβ1 were found to have a reduction in lung function of more than 22 percentile points compared with TT homozygotes not exposed to secondhand smoke (Table 4). Similarly, participants exposed to secondhand smoke and bearing 2 copies of the C allele (CC homozygote) at codon 10 on TGFβ1 were found to have a reduction in lung function of more than 20 percentile points compared with those not exposed to secondhand smoke. Participants with these high-risk TGFβ1 genotypes (TT homozygotes at −509 and CC homozygotes at codon 10) comprised 11.2% of 762 participants genotyped for TGFβ1 −509 and 16.2% of the 659 participants genotyped for TGFβ1 codon 10. The distributions of these variants are consistent with other white populations.12,13,34
Other genotypes of TGFβ1 at the −509 promoter and codon 10 sites were not found to significantly alter the effect of secondhand smoke exposure on lung function (Table 4). Although the T allele at −509 and the C allele on codon 10 frequently travel together,14,15,17 the number of participants exposed to secondhand smoke in our study carrying TGFβ1 genes with T at −509 and C at codon 10 is too few to permit detailed haplotype analysis to determine whether the T allele at −509, the C allele at codon 10, or the combination of both is responsible for increasing the deleterious effect of secondhand smoke exposure. Removing monozygous twins from both the CFTR and TGFβ1 analyses to reduce the bias of having multiple patients with the same genotypes neither changed the significance of the results nor substantially altered the magnitude of the coefficients.
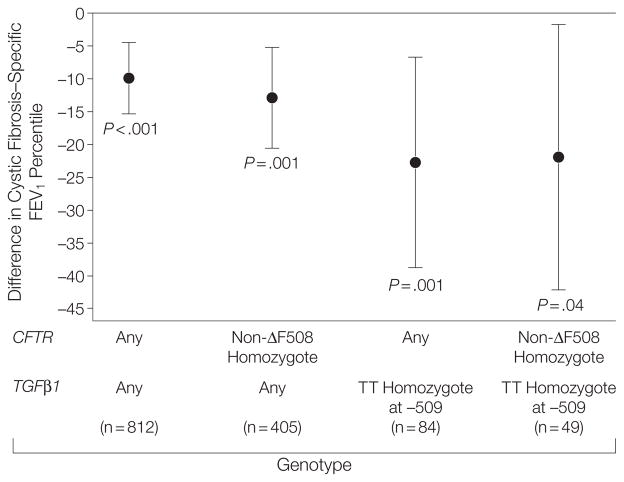
As noted, both the CFTR and TGFβ1 genotypes interacted with secondhand smoke exposure to alter its adverse effect on lung function in patients with CF but each had a different magnitude of effect. The reduction in lung function percentile in patients exposed to secondhand smoke with at-risk TGFβ1 genotypes was almost twice that observed in non-ΔF508 homozygotes (Figure). We next asked whether CFTR and TGFβ1 interact to further amplify the negative effect of secondhand smoke exposure on lung function in patients with CF. However, patients exposed to secondhand smoke who were non-ΔF508 homozygotes and −509 TT homozygotes had similar reductions in lung function as −509 TT homozygotes (Figure). These analyses indicate that both CFTR and TGFβ1 interact with secondhand smoke exposure, but not with a simple additive effect.
Figure.
Difference in Lung Function Among Patients Exposed to Secondhand Smoke Compared With Those Not Exposed by Genotype
Error bars represent the 95% confidence intervals. FEV1 indicates forced expiratory volume in the first second of expiration. The P values refer to t test comparisons between those exposed to secondhand smoke and those who were not exposed.
COMMENT
Our study demonstrates that secondhand smoke exposure is an environmental modifier of both cross-sectional and longitudinal lung function in patients with CF. The effect of secondhand smoke exposure on longitudinal lung function implies that any exposure to secondhand smoke has long-term negative sequelae on lung function in patients with CF. This study had 88% power to detect a 10% difference in lung function; with univariate regression, we detected a 9.8 percentile point decrease in cross-sectional lung function in 812 participants and a 6.1 percentile point decrease in longitudinal lung function in 581 participants. This compares with 58% power for the next best-powered study, which did not observe decreased lung function associated with secondhand smoke exposure.4 Our results also estimate the magnitude of the detrimental effect of secondhand smoke exposure on lung function in patients with CF. The mean reduction in cross-sectional lung function attributed to secondhand smoke exposure roughly translates to a decrease of 8.2% predicted FEV1 (Third National Health and Nutrition Examination Survey) and an 18.6% decrease in patients with a high-risk TGFβ1 −509 genotype using a hypothetical 20-year-old white male (170 cm tall) with median CF lung function as a reference point. Comparing this FEV1 decrease with a reported annual decrease of 2.3% due to CF disease progression (Wang percent predicted)35 implies that for a 17-year-old white male with a TGFβ1 −509 TT genotype with median lung function, the detrimental effect of any secondhand smoke exposure is equal to that expected for an additional 7.3 years of lung function decline; essentially this 17-year-old exposed to secondhand smoke would have the lung function of a 24-year-old male with the same genotype not exposed to secondhand smoke.
In this study, we examined interactions among environmental factors, specifically between secondhand smoke exposure and socioeconomic status. Although we found that socioeconomic status does correlate with secondhand smoke exposure as previously documented,3 multiple regression demonstrated that socioeconomic status is not a significant covariate of the association of secondhand smoke exposure with reduced lung function in patients with CF. To obtain a comprehensive assessment of socioeconomic status, 7 measures were examined in this study. None of these measures significantly altered the detrimental effect of secondhand smoke exposure on lung function in patients with CF. Our results expand the findings of 2 previous CF studies examining the effects of secondhand smoke exposure and socioeconomic status. These studies found that neither socioeconomic status as measured by Hollingshead strata5 nor parental occupation8 affected the association between secondhand smoke exposure and clinical outcomes. Previous studies of socioeconomic status in patients with CF have linked worse lung function with lower income36 and participation in Medicaid,37,38 however none of these studies included secondhand smoke exposure as a factor in the analyses. Our study suggests that secondhand smoke exposure might confound the relationship between socioeconomic status and lung disease in patients with CF and should therefore be considered in any future evaluation of environmental factors on CF disease variation.
In examining gene-environment interactions, we found that the disease-causing gene CFTR modifies the association between secondhand smoke and worse lung function in patients with CF. Specifically, participants who carry at least 1 non-ΔF508 CFTR mutation were found to have a greater difference in lung function in the presence of secondhand smoke exposure than ΔF508 homozygotes. The ΔF508 mutation leads to degradation of the CFTR protein and complete loss of function, whereas some non-ΔF508 mutations permit residual function.39 Patients with CF who have mutations other than ΔF508 may have some functioning CFTR protein that is susceptible to the effects of secondhand smoke exposure. The recent demonstration that active smoking decreases CFTR expression at the gene and protein levels in participants without CF supports this hypothesis.40 The observed effect of the CFTR genotype may explain why the best-powered study to date on lung function in patients with CF and secondhand smoke exposure did not detect the effect of secondhand smoke exposure on lung function in their subgroup of ΔF508 homozygotes.4 Our findings suggest that future studies of gene-environment interactions in CF lung disease should not be solely confined to ΔF508 homozygotes because non-ΔF508 homozygotes may be better suited to detect the negative effects of modifiers of lung function.
In addition to interactions with the CFTR mutations, we found that the adverse effects of secondhand smoke exposure on lung function were amplified by specific variants of a CF modifier gene, TGFβ1.12,13 A case-control study involving 1306 participants found that the TT genotype at TGFβ1 position −509 was associated with more severe lung disease.13 Our results demonstrated that secondhand smoke exposure was associated with a 22.7 percentile point decrease in cross-sectional lung function in patients with the TT genotype exposed to secondhand smoke compared with nonexposed TT homozygotes. Because that prior study13 did not include secondhand smoke exposure as an environmental modifier, it is unclear whether the interaction between secondhand smoke exposure and TGFβ1 accounted for most of the deleterious effect observed with the genetic modifier alone.
Previous CF studies of TGFβ1 codon 10 variants have demonstrated differing results including no association with lung disease,11 a more rapid deterioration of lung function in TT variants compared with CC variants in ΔF508 homozygotes,12 and that the CC genotype was associated with more severe lung disease.13 Our results demonstrated that secondhand smoke exposure was associated with a 20.3 percentile point decrease in cross-sectional lung function in patients with the CC genotype exposed to secondhand smoke compared with nonexposed CC homozygotes. Similarly, because none of the prior studies included secondhand smoke exposure as an environmental modifier, it is possible that the differing results regarding the effect of this TGFβ1 variant could be attributed to differences in secondhand smoke exposure. Future studies of gene-environment interactions in CF lung disease should consider inclusion of secondhand smoke exposure as an environmental factor.
Although we have associated exposure to secondhand smoke in the home with worse lung function in patients with CF, one caveat to this finding is our definition of secondhand smoke exposure. For this study, we used an ever/never dichotomy in documenting secondhand smoke exposure. Although this definition of secondhand smoke exposure disregards dose-exposure effects, it does reflect current understanding of the effects of secondhand smoke exposure (ie, there is no level of risk-free exposure, thus any exposure to secondhand smoke carries risk).3 However, documentation using this definition may be subject to recall bias and underreporting. Two studies have observed that urine and salivary cotinine levels accurately reflect CF households’ reported smoking8,9; however one study has demonstrated underreporting of smoking in CF households using urine cotinine levels.41 Regardless of potential recall bias or underreporting, the population of our family-based study reports a lower prevalence of secondhand smoke exposure (23% in the home) than has been seen in the general CF population (30%).4
We speculate that families with 2 or more children with lung disease enrolled in our study may be more reluctant to smoke as opposed to families with only 1 child with CF. Additionally, mothers may be less likely to smoke during subsequent pregnancies if they already have 1 child with CF. To generalize to larger populations, family-based studies also must contend with the issue of individuals who share a similar environment for much of childhood and high rates of gene sharing. Our results do not substantially change when monozygous twins are removed from genetic analyses and we used cluster analysis by family to minimize other sharing effects. Participants with CF with a history of being exposed to prenatal and/or postnatal secondhand smoke were older than those with no history of exposure. Age is linked with cohort effects because younger cohorts have access to more therapies than did older cohorts at the same chronological age. To address this issue, we used CF-specific FEV1 percentiles, which are age-corrected and were derived from a cohort of patients with CF comparable with those in this study. Measured by longitudinal lung function (average FEV1 CF percentile), participants in the Twin and Sibling Study do have milder lung function (mean [SD], 0.60 [0.24]) than the general CF population (0.50). This may reflect the recruitment criteria of having a twin or sibling with CF because families with milder disease are more likely to have both members with CF surviving. Finally, a major analytic limitation of studies of multiple genetic or environmental factors is the number of patients required for adequate power to detect the interaction. While we were able to detect interactions between secondhand smoke exposure and CFTR or TGFβ1 with reasonable confidence, interactions between these 3 factors or additional factors will require more study participants. Hence, enrollment of patients in the Cystic Fibrosis Twin and Sibling Study is continuing.
The findings of this study may be applicable to other chronic respiratory tract diseases. TGFβ1 has been found to modify the development and severity of asthma.14–16 Although 2 studies did not demonstrate a gene-environment interaction between secondhand smoke exposure in the home and TGFβ1 in the development of asthma,17,18 detection of interactions in common chronic lung diseases such as asthma may be hampered by etiologic heterogeneity. It may be easier to detect subtle interactions affecting disease severity using a continuous variable, and patients with CF often perform pulmonary function tests on a quarterly basis yielding the continuous variable outcome of FEV1. Thus, CF may be a good model for uncovering gene-environment interactions that are detrimental to lung function. This study also raises the specter that healthy children bearing certain genetic variants (eg, TGFβ1 −509 and codon 10 high-risk genotypes) may be at much higher risk for worse outcomes as a result of secondhand smoke exposure. Demonstration that genetically defined subsets of patients with CF exposed to secondhand smoke in the home have a substantial lifetime reduction in lung function provides potent justification for eradication of cigarette smoke exposure for all individuals with this life-limiting disorder.
Acknowledgments
Funding/Support: This study is supported by National Heart, Lung, and Blood Institute grant HL68927, Cystic Fibrosis Foundation funding to Drs Collaco and Cutting, and by the Flight Attendant Medical Research Institute.
Role of the Sponsor: The sponsors approved the design and conduct of the study but did not participate in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosures: None reported.
Author Contributions: Drs Collaco and Cutting had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Collaco, Vanscoy, Cutting.
Acquisition of data: Collaco, Vanscoy, Bremer, McDougal, Blackman, Bowers, Naughton, Cutting.
Analysis and interpretation of data: Collaco, Vanscoy, Bremer, McDougal, Blackman, Bowers, Naughton, Jennings, Ellen, Cutting.
Drafting of the manuscript: Collaco, Cutting.
Critical revision of the manuscript for important intellectual content: Collaco, Vanscoy, Bremer, McDougal, Blackman, Bowers, Naughton, Jennings, Ellen, Cutting.
Statistical analysis: Collaco, Vanscoy, Jennings, Ellen, Cutting.
Obtained funding: Collaco, Cutting.
Administrative, technical, or material support: Bremer, McDougal, Blackman, Bowers, Naughton, Jennings, Ellen, Cutting.
Study supervision: Cutting.
Additional Contributions: We thank the North American Cystic Fibrosis Foundation for use of the Cystic Fibrosis Foundation Data Registry; Ase Sewall, BS, Monica Brooks, BS (both of the Cystic Fibrosis Foundation), and the staff at the Data Registry; Nulang Wang, BS, of Johns Hopkins University for CFTR genotyping; Michael Kulich, PhD, of Charles University, Prague, Czech Republic, and Mark Schluchter, PhD, of Case Western Reserve University, Cleveland, Ohio, for providing conversion programs for cystic fibrosis–specific percentiles and Bayes estimates for forced expiratory volume in the first second of expiration, respectively; John McGready, PhD, of Johns Hopkins University and David Cutler, PhD, of Emory University, Atlanta, Georgia, for helpful statistical discussions; Rita McWilliams, PhD, Julie Hoover-Fong, MD, PhD, and Ada Hamosh, MD, MPH, all of Johns Hopkins University, for designing questionnaires; and most importantly the patients with cystic fibrosis and their families, research coordinators, nurses, and physicians who are participating in the Cystic Fibrosis Twin and Sibling Study. Ms Wang and Dr Cutler received funding through grants.
References
- 1.Strausbaugh SD, Davis PB. Cystic fibrosis: a review of epidemiology and pathobiology. Clin Chest Med. 2007;28(2):279–288. doi: 10.1016/j.ccm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Vanscoy LL, Blackman SM, Collaco JM, et al. Heritability of lung disease severity in cystic fibrosis. Am J Respir Crit Care Med. 2007;175(10):1036–1043. doi: 10.1164/rccm.200608-1164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moritsugu KP. The 2006 report of the Surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med. 2007;32 (6):542–543. doi: 10.1016/j.amepre.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Kovesi T, Corey M, Levison H. Passive smoking and lung function in cystic fibrosis. Am Rev Respir Dis. 1993;148(5):1266–1271. doi: 10.1164/ajrccm/148.5.1266. [DOI] [PubMed] [Google Scholar]
- 5.Campbell PW, III, Parker RA, Roberts BT, Krishnamani MR, Phillips JA., III Association of poor clinical status and heavy exposure to tobacco smoke in patients with cystic fibrosis who are homozygous for the F508 deletion. J Pediatr. 1992;120(2 pt 1):261–264. doi: 10.1016/s0022-3476(05)80438-x. [DOI] [PubMed] [Google Scholar]
- 6.Beydon N, Amsallem F, Bellet M, et al. Pulmonary function tests in preschool children with cystic fibrosis. Am J Respir Crit Care Med. 2002;166(8):1099–1104. doi: 10.1164/rccm.200205-421OC. [DOI] [PubMed] [Google Scholar]
- 7.Rubin BK. Exposure of children with cystic fibrosis to environmental tobacco smoke. N Engl J Med. 1990;323(12):782–788. doi: 10.1056/NEJM199009203231203. [DOI] [PubMed] [Google Scholar]
- 8.Smyth A, O’Hea U, Williams G, Smyth R, Heaf D. Passive smoking and impaired lung function in cystic fibrosis. Arch Dis Child. 1994;71(4):353–354. doi: 10.1136/adc.71.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyth A, O’Hea U, Feyerabend C, Lewis S, Smyth R. Trends in passive smoking in cystic fibrosis, 1993–1998. Pediatr Pulmonol. 2001;31(2):133–137. doi: 10.1002/1099-0496(200102)31:2<133::aid-ppul1021>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Gilljam H, Stenlund C, Ericsson-Hollsing A, Strandvik B. Passive smoking in cystic fibrosis. Respir Med. 1990;84(4):289–291. doi: 10.1016/s0954-6111(08)80055-8. [DOI] [PubMed] [Google Scholar]
- 11.Brazova J, Sismova K, Vavrova V, et al. Polymorphisms of TGF-β1 in cystic fibrosis patients. Clin Immunol. 2006;121(3):350–357. doi: 10.1016/j.clim.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Arkwright PD, Laurie S, Super M, et al. TGF-β(1) genotype and accelerated decline in lung function of patients with cystic fibrosis. Thorax. 2000;55(6):459–462. doi: 10.1136/thorax.55.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drumm ML, Konstan MW, Schluchter MD, et al. Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med. 2005;353(14):1443–1453. doi: 10.1056/NEJMoa051469. [DOI] [PubMed] [Google Scholar]
- 14.Mak JC, Leung HC, Ho SP, et al. Analysis of TGF-β(1) gene polymorphisms in Hong Kong Chinese patients with asthma. J Allergy Clin Immunol. 2006;117(1):92–96. doi: 10.1016/j.jaci.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 15.Pulleyn LJ, Newton R, Adcock IM, Barnes PJ. TGFβ1 allele association with asthma severity. Hum Genet. 2001;109(6):623–627. doi: 10.1007/s00439-001-0617-y. [DOI] [PubMed] [Google Scholar]
- 16.Silverman ES, Palmer LJ, Subramaniam V, et al. Transforming growth factor-β1 promoter polymorphism C-509T is associated with asthma. Am J Respir Crit Care Med. 2004;169(2):214–219. doi: 10.1164/rccm.200307-973OC. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Romieu I, Wu H, et al. Genetic polymorphisms in transforming growth factor β-1 (TGFβ1) and childhood asthma and atopy. Hum Genet. 2007;121(5):529–538. doi: 10.1007/s00439-007-0337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salam MT, Gauderman WJ, McConnell R, Lin PC, Gilliland FD. Transforming growth factor-β1 C-509T polymorphism, oxidant stress, and early onset childhood asthma. Am J Respir Crit Care Med. 2007;176(12):1192–1199. doi: 10.1164/rccm.200704-561OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celedón JC, Lange C, Raby BA, et al. The transforming growth factor-β1 (TGFβ1) gene is associated with chronic obstructive pulmonary disease (COPD) Hum Mol Genet. 2004;13(15):1649–1656. doi: 10.1093/hmg/ddh171. [DOI] [PubMed] [Google Scholar]
- 20.Wu L, Chau J, Young RP, et al. Transforming growth factor-β1 genotype and susceptibility to chronic obstructive pulmonary disease. Thorax. 2004;59 (2):126–129. doi: 10.1136/thorax.2003.005769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su ZG, Wen FQ, Feng YL, Xiao M, Wu XL. Transforming growth factor-β1 gene polymorphisms associated with chronic obstructive pulmonary disease in Chinese population. Acta Pharmacol Sin. 2005;26(6):714–720. [PubMed] [Google Scholar]
- 22.Blackman SM, Deering-Brose R, McWilliams R, et al. Relative contribution of genetic and nongenetic modifiers to intestinal obstruction in cystic fibrosis. Gastroenterology. 2006;131(4):1030–1039. doi: 10.1053/j.gastro.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. J Pediatr. 1998;132(4):589–595. doi: 10.1016/s0022-3476(98)70344-0. [DOI] [PubMed] [Google Scholar]
- 24.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr. 1997;131(6):809–814. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 25.Kulich M, Rosenfeld M, Campbell J, et al. Disease-specific reference equations for lung function in patients with cystic fibrosis. Am J Respir Crit Care Med. 2005;172(7):885–891. doi: 10.1164/rccm.200410-1335OC. [DOI] [PubMed] [Google Scholar]
- 26.Schluchter MD, Konstan MW, Davis PB. Jointly modelling the relationship between survival and pulmonary function in cystic fibrosis patients. Stat Med. 2002;21(9):1271–1287. doi: 10.1002/sim.1104. [DOI] [PubMed] [Google Scholar]
- 27.US Centers for Disease Control and Prevention. [Accessed January 6, 2008];Body mass index z score calculation. http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.htm.
- 28.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 29.US Census Bureau. [Accessed January 6, 2008];Census data from 2000. http://factfinder.census.gov/home/saff/main.html?_lang=en.
- 30.Duffy D. Sib-pair: a program for non-parametric linkage/association analysis. Am J Hum Genet. 1997;61(suppl):A197. [Google Scholar]
- 31.Boyer PH. Low birth weight in fibrocystic disease of the pancreas. Pediatrics. 1955;16(6):778–784. [PubMed] [Google Scholar]
- 32.Müller AE, Thamm B, Lietz T, Handrick W, Walter S. Cystic fibrosis: a cause of reduced birth weight? Eur J Pediatr. 1999;158(3):264. doi: 10.1007/s004310051065. [DOI] [PubMed] [Google Scholar]
- 33.Festini F, Taccetti G, Repetto T, et al. Gestational and neonatal characteristics of children with cystic fibrosis: a cohort study. J Pediatr. 2005;147(3):316–320. doi: 10.1016/j.jpeds.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 34.Buranawuti K, Boyle MP, Cheng S, et al. Variants in mannose-binding lectin and tumor necrosis factor α affect survival in cystic fibrosis. J Med Genet. 2007;44(3):209–214. doi: 10.1136/jmg.2006.046318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konstan MW, Morgan WJ, Butler SM, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151(2):134–139. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 36.O’Connor GT, Quinton HB, Kneeland T, et al. Median household income and mortality rate in cystic fibrosis. Pediatrics. 2003;111(4 pt 1):e333–e339. doi: 10.1542/peds.111.4.e333. [DOI] [PubMed] [Google Scholar]
- 37.Schechter MS, Margolis PA. Relationship between socioeconomic status and disease severity in cystic fibrosis. J Pediatr. 1998;132(2):260–264. doi: 10.1016/s0022-3476(98)70442-1. [DOI] [PubMed] [Google Scholar]
- 38.Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331–1337. doi: 10.1164/ajrccm.163.6.9912100. [DOI] [PubMed] [Google Scholar]
- 39.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73(7):1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- 40.Cantin AM, Hanrahan JW, Bilodeau G, et al. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173(10):1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 41.Köhler E, Sollich V, Schuster R, Thal W. Passive smoke exposure in infants and children with respiratory tract diseases. Hum Exp Toxicol. 1999;18(4):212–217. doi: 10.1191/096032799678839932. [DOI] [PubMed] [Google Scholar]