Abstract
The effects of bicuculline, a γ-aminobutyric acid (GABA) antagonist, were investigated in 258 immature rats between the third and 22nd postnatal days. Behavioral and electrocorticographic events were correlated. Bicuculline induced both behavioral and electrographic seizures as early as the third postnatal day, an age when the CD50 for bicuculline was lowest, and therefore the sensitivity to it was the greatest. Bicuculline may thus be a suitable convulsant for epilepsy studies involving rats during the first postnatal week.
Keywords: Bicuculline, Seizure, Ontogeny, Rat, γ-Aminobutyric acid, Brain development
INTRODUCTION
Epilepsy in the immature brain presents unique features related to neuronal excitability, cortical connectivity and electrical impulse propagation9,25. The newborn rat brain is less mature than that of most other laboratory animals, and of the fullterm human neonate9. Infant rats thus allow investigation of epileptic phenomena and their relationship to environmental, hormonal or pharmacological agents relatively early in development22,24,25.
Immunocytochemical13 and biochemical4,5 studies suggest that the γ-aminobutyric acid (GABA) neuronal system of the rat may mature relatively early. Thus, pharmacological manipulation of GABA neurotransmission may allow the generation of seizure paradigms useful in the immediately postnatal rat, when many conventional convulsants are not applicable24,25.
Bicuculline, an antagonist of the GABAA receptor, has convulsant properties in both adult and immature animals1,7,17. De Feo7, has reported the convulsant effect of this compound in rats ranging in age from 6 to 30 days, but found poor correlation of behavioral and electrographic events induced by a single bicuculline dose (3 mg/kg). Other reports involve older pups6,15 and lack correlation of behavior to electrocortical changes15.
This study was designed to investigate the usefulness of bicuculline-induced seizures in immature rats beginning on the third postnatal day. The epileptic nature of bicuculline-induced behaviors was corroborated by electrographic changes, and the age-dependent bicuculline dose resulting in epileptic phenomena in 50% of animals (CD50) was determined.
MATERIALS AND METHODS
Animals
26 timed-pregnancy rats were obtained from Zivic-Miller, (Zelionple, PA) on pregnancy days 17–18. Rats were housed under a 12-h light/dark cycle, and fed ad libitum. They were handled daily to prevent cannibalism14. Delivery times were monitored and were accurate to within 12 h. The day of birth was considered day zero. The pups were kept with the mothers, and litters were culled to 12 pups. Pups were mixed, such that study groups contained pups from several litters. 24–81 pups were tested at each age; separate animals were used for electroclinical correlations and for determination of CD50. Each pup was subjected to bicuculline only once.
Surgery and recording
In order to ascertain the epileptic nature of bicuculline-induced behaviors during the first postnatal week, rats not participating in the CD50 experiments were implanted with cortical electrodes and subjected to bicuculline. At least 2 pups from separate litters were implanted 24–48 h prior to recording. Rats were recorded only once, on day 3, 5, 7 or 10. Four tungsten electrodes were implanted under halothane anesthesia, using an infant-rat stereotaxic apparatus, as previously described22. Two frontal and two posteroparietal electrodes were used. The technique allows for electrocorticogram (ECoG) recording from freely moving animals, via long, flexible wires. Recordings were carried out in heated, shielded plexiglass chambers, using a Grass 78E Polygraph. Pups were continuously observed throughout the recording.
Electrocorticograms were obtained for 30 min before, as well as after, bicuculline administration. Since anesthesia may affect seizure threshold, rats implanted with electrodes did not participate in the determination of CD50. The lowest bicuculline dose causing seizures in all rats in each age-group was administered to the implanted rats, and the resulting electrographic and behavioral seizures were correlated.
Seizure induction
All experiments were conducted between 08.00 and 10.30 h, to minimize diurnal fluctuations in seizure susceptibility19. Rats, 4–6 per group, were placed in heated observation chambers, under video photography and observation. Infant rats were observed for 30 min, so that the repertoire of activities and behavior unrelated to bicuculline could be determined for each age. Bicuculline (Sigma, St. Louis), dissolved in 0.1 N HCL, adjusted to pH 5 with NaOH and diluted in saline, was injected intraperitoneally (i.p.). Injection volume was 1–1.5 μl per g body weight, and solutions were warmed to 37 ºC. Rats were observed and videotaped for a minimum of 30 min after the administration of bicuculline.
Coding of seizures
Epileptic events were defined as behaviors that were: (1) significantly different from any observed during the 30 min observation period, and (2) observed after bicuculline and associated with sharp or rhythmic electrocortical discharge in implanted animals of the same age. Seizures were described rather than graded, and verified independently by a second observer who reviewed the videotapes.
Determination of CD50
CD50, the convulsive dose in 50% of the animals, was determined according to a modification of Swinyard’s staircase method23. Briefly, initial bicuculline doses for each group (4–6 rats) were determined by preliminary experiments. An initial ineffective dose was doubled, that causing 100% response was halved. Mean doses between ineffective and maximal doses were subsequently administered. A bicuculline dose causing convulsions in 50% of the rats was repeated at least once. The series of dose-response data for each age group was analyzed using the SAS program (Scientific Inst., Cary, NC). Since the distribution of convulsive doses could not be presumed to be normal, median convulsive dose (CD50) and 95% fiducial limits were determined26. Values falling outside the 95% fiducial intervals were considered significant at the 0.05 level26.
RESULTS
Ontogeny of the spectrum of bicuculline-induced epileptic phenomena
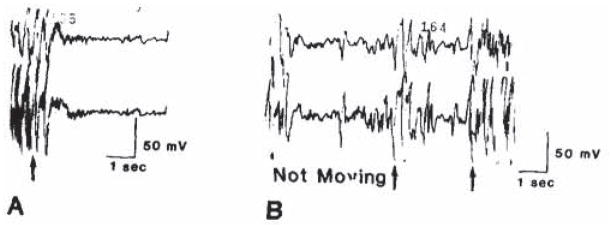
Bicuculline effects were evident within 1–5 min. Rat pups on the third postnatal day were either quiescent, moving purposefully, or displaying random, brief myoclonic jerks. Bicuculline resulted in hyperkinesis in the form of repetitive forepaw treading and tremulousness. Tonic extension of a single extremity, tail, or less frequently, 2 extremities on the same or opposite sides was also observed. ECoG correlates of a tonic event are seen in Fig. 1, and consist of relatively high voltage spikes, quite distinct from the very low voltage, random, poly-rhythmic background at this age. Motion artifacts. clearly differing in contour and velocity from cortical activity, were noted both before and after bicuculline administration.
Fig. 1.
Effect of bicuculline on the electrocorticogram (ECoG) of a 3 day old rat. A: ECoG prior to bicuculline administration. B: ECoG following bicuculline, 2 mg/kg. i.p., The arrows indicate motion artifacts.
On the 5th postnatal day, seizure spectrum was similar to that described above (Fig. 2E). Violent myoclonic jerks, and generalized tonic extension of neck and all extremities, with persistent rhythmic ECoG spikes could be seen with larger bicuculline doses (twice CD50). Fig. 2 shows the progression of ECoG phenomena after bicuculline administration at this age: Abnormal activity was present within 35 s, with no overt movements; hyperkinesis followed, and finally, a general tonic seizure was accompanied by rhythmic, higher voltage spikes (Fig. 2A–D).
Fig. 2.
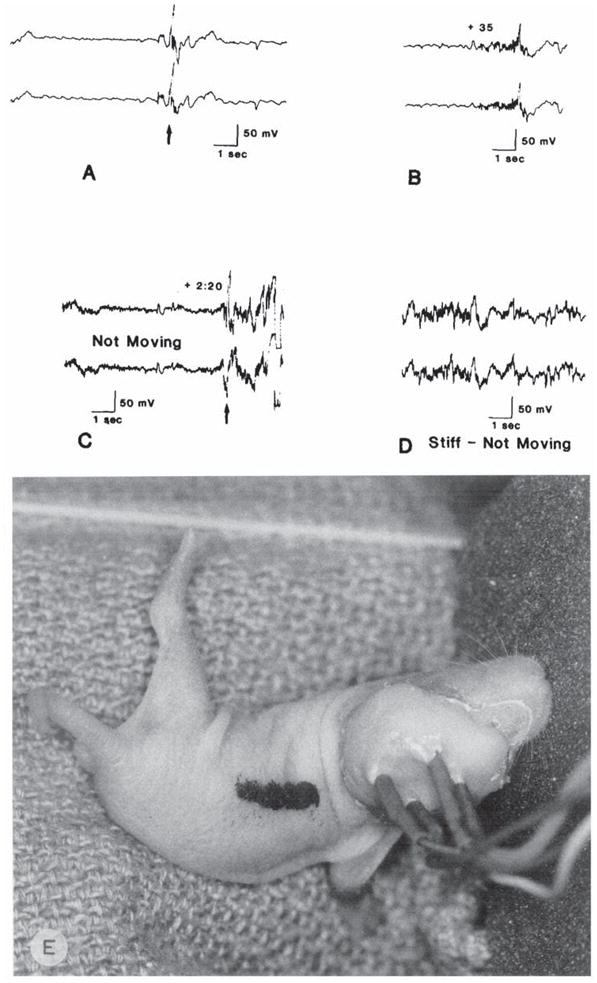
Electrocorticograms of a 5 day old rat: effects of bicuculline (2 mg/kg, i.p.) administration. A: prior to the drug. B: 35 s after bicuculline. C: 150 s after bicuculline. D: 6 min following injection. E: behavioral effect of bicuculline in a different 5 day old rat: tonic extension of a single extremity and tail. Arrows in A and C indicate motion artifacts.
By the end of the first postnatal week (day 7), hyperkinesis as an epileptic phenomenon was less pronounced, and tonic extension commonly involved two or all extremities. Background and post-bicuculline ECoG patterns are seen in Fig. 3.
Fig. 3.

Electrocorticograms of a 7 day old rat prior to (A), and subsequent to (B) bicuculline administration. Arrows indicate motion.
Ten day old infant rats displayed a fairly continuous, low voltage electrocorticographic activity. Bicuculline caused seizures which were mainly tonic, commonly symmetric or generalized, and resulted in loss of the righting reflex. ‘Swimming’ movements also occurred. The ECoG before and after seizure induction is seen in Fig. 4. By the end of the second week, behavioral phenomena conformed to well-described patterns, which were further differentiated by the 22nd postnatal day: clonic forepaw movements were followed in succession by a tonic and a clonic phase, with loss of the righting reflex10.
Fig. 4.
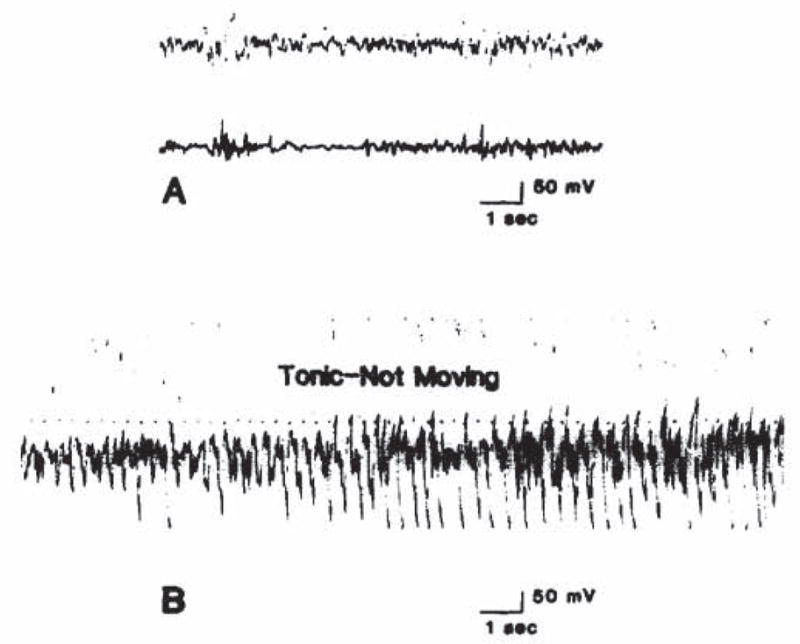
Electrocorticograms of a 10 day old rat injected with 2 mg/kg bicuculline. A: before injection; B: 2.25 min after injection.
Bicuculline CD50 as a function of age in the first weeks of life in the rat
Fig. 5 is a graphic depiction of the CD50 of bicuculline in rat pups between the third and 22nd postnatal day. As is evident from the graph, bicuculline CD50 is minimal on the third postnatal day, and rises gradually on days 5, 7 and 10. A statistically significant decrease in the bicuculline CD50 occurs at the end of the second week of life, followed by a rise towards adult values.
Fig. 5.
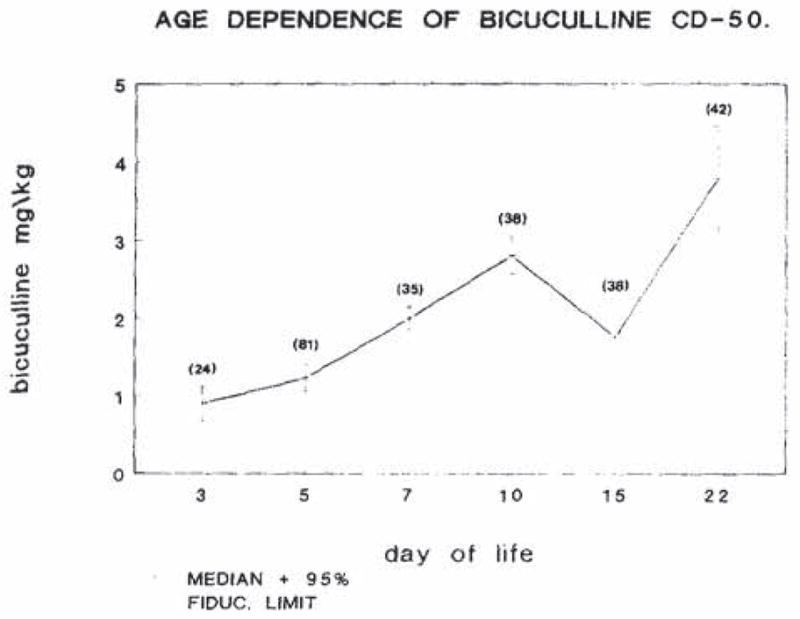
Age dependence of the CD50 for bicuculline in infant rats. CD50 was determined as described in the materials and methods section. The vertical bracketed lines at each point represent 95% fiducial intervals. Numbers in parentheses denote the number of animals used at each age.
DISCUSSION
This study delineates an epilepsy paradigm for infant rats during the first postnatal week. The repertoire of observable seizures at this age is quite limited, and is confined to hyperkinesis and tonic phenomena24,25. Furthermore, some authors have commented on the prolonged latency of bicuculline-induced ECoG changes in young rats, and the poor correlation between observed behavioral events and electrocortical spikes7. Thus, any definition of epileptic events in this age group necessitates both behavioral and ECoG observations. Bicuculline induced seizures in rats younger than 6 days have not been published7,15, and most ontogeny studies have relied on behavioral observations alone15.
The data confirm the presence of recordable ECoG activity in the rat pup as early as the third postnatal day, and the ability to correlate behavioral events to abnormal electrocortical activity22. The data further suggest that the seizure threshold of 3 day old pups to bicuculline may be lower than that at older ages. Whether this is due to better access of bicuculline to the brain, because of the immaturity of the blood-brain barrier to bicuculline at this age is unknown; the penetration of another alkaloid. methadone, into the brain does not seem to vary over the first postnatal weeks in the rat20. In the 7 day old rat, our finding of a CD50 of 2 mg/kg is comparable to the 2.48 mg/kg reported by Liskova et al. for their youngest age group, based on behavioral observation alone15.
The potency of bicuculline in inducing seizures in the immediate postnatal period, in contradistinction to some other convulsants such as pilocarpine3, may be due to differential maturation of the respective neurotransmitter systems involved; the GABA system in the rat has been shown by immunocytochemistry to differentiate prenatally, earlier than other neurotransmitter systems12,13. Total brain concentration of GABA is 50% of adult levels at birth, while adult concentrations are found in the brainstem and cerebellum4,8. GABA uptake is at adult levels at birth20, while the activity of glutamic acid decarboxylase, the GABA synthetic enzyme is only 5% of adult levels4. GABA-receptor activity is present at 25%–33% of adult binding at birth and throughout the first postnatal days, and increases to adult levels by the fourth week4,20. Electrophysiologic studies demonstrated the opening of chloride channels by GABA in cultured astrocytes derived from 0–2 day old rats10. The function of the immediately postnatal GABA system may reflect these different maturational levels of pre- and postsynaptic components.
The predominance of tonic seizures, which are thought to originate in brainstem structures2, during the first postnatal week is compatible with the caudo-rostral maturation of the rodent brain. It is thus not surprising that inhibition of the relatively mature GABAergic system, via bicuculline blockade of GABA receptors, results in largely tonic seizures during the first postnatal week.
The change in bicuculline CD50 as a function of age (Fig. 5) appears to parallel the time course of the CD50s for picrotoxin and pentylenetetrazol. Specifically, after a rise between the fourth and 12th postnatal days24, the CD50 for these drugs decreases significantly around the 15th day, only to increase to adult dose subsequently24. The mechanism of this transient ‘hypersusceptibility’ to bicuculline and other convulsants is unclear; it may be due to the maturation of seizure propagation (e.g., myelinization) prior to that of inhibitory, GABA-specific networks. A likely candidate for such an inhibitory network may be the substantia nigra; Moshe and Albala reported a decrease in seizure susceptibility to flurothyl after intranigral muscimol in adult rats. Such effect was not present in 15 day old animals18.
In summary, consistent induction of electrographic and behavioral seizures by bicuculline in the first postnatal days in the rat is feasible; the paradigm allows the investigation of epileptic phenomena in the very immature brain.
Acknowledgments
The authors are indebted to Linda Schultz for excellent technical assistance. Supported by NINDS Grant NS010307 (T.Z.B.).
References
- 1.Borgstrom L, Chapman AG, Siesjo BK. Glucose consumption in the cerebral cortex of the rat during bicuculline-induced status epilepticus. J Neurochem. 1976;27:971–973. doi: 10.1111/j.1471-4159.1976.tb05165.x. [DOI] [PubMed] [Google Scholar]
- 2.Browning RA, Nelson DK. Variation in threshold and pattern of electroshock-induced seizures in rats depending on site of stimulation. Life Sci. 1985;37:2205–2212. doi: 10.1016/0024-3205(85)90573-9. [DOI] [PubMed] [Google Scholar]
- 3.Cavalheiro EA, Delrio FS, Turski WA, et al. The susceptibility of rats to pilocarpine-induced seizures is age dependent. Dev Brain Res. 1987;37:43–58. doi: 10.1016/0165-3806(87)90227-6. [DOI] [PubMed] [Google Scholar]
- 4.Coyle JT, Enna SJ. Neurochemical aspects of the ontogenesis of GABAergic neurons in the rat brain. Brain Res. 1976;111:119–123. doi: 10.1016/0006-8993(76)91053-2. [DOI] [PubMed] [Google Scholar]
- 5.Coyle JT, Singer H, Beaulieu M, Johnston MV. Development of central neurotransmitter-specified neuronal systems: implications for pediatric neuropsychiatric disorders. Acta Neurol Scand. 1984;69:1–11. doi: 10.1111/j.1600-0404.1984.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 6.Daval JL, Sarfati A. Effects of bicuculline induced seizures on benzodiazepine and adenosine receptors in developing rat brain. Life Sci. 1987;41:1685–1693. doi: 10.1016/0024-3205(87)90595-9. [DOI] [PubMed] [Google Scholar]
- 7.DeFeo M, Mecarelli O, Ricci G. Bicuculline and allylglycine induced epilepsy in developing rats. Exp Neurol. 1985;90:411–421. doi: 10.1016/0014-4886(85)90030-5. [DOI] [PubMed] [Google Scholar]
- 8.Hedner TH, Iversen K, Lundburg P. Central GABA mechanisms during postnatal development in rats. J Neur Trans. 1984;59:105–108. doi: 10.1007/BF01255409. [DOI] [PubMed] [Google Scholar]
- 9.Knowles WD. Aspects of epileptogenesis: maturation of neuronal circuits. Cleveland Clin J Med. 1989;56 (suppl):221–225. doi: 10.3949/ccjm.56.s1.221. [DOI] [PubMed] [Google Scholar]
- 10.Kettenmann H, Backus KH, Schachner M. γ-aminobutyric acid opens Cl− channels in cultured astrocytes. Brain Res. 1987;404:1–9. doi: 10.1016/0006-8993(87)91349-7. [DOI] [PubMed] [Google Scholar]
- 11.Kubova H, Mares M. Time course of the anticonvulsant action of clonazepam during ontogenesis in the rat. Arch Int Pharmacodyn. 1989;298:15–24. [PubMed] [Google Scholar]
- 12.Lauder JM. Roles for neurotransmitters in neurogenesis. In: Meisami E, Timiras PS, editors. Handbood of Human Growth and Developmental Biology. I. CRC; Boca Raton: 1988. pp. 53–66. [Google Scholar]
- 13.Lauder JM, Han VK, Henderson P, et al. Prenatal ontogeny of the GABAergic system in the rat brain: an immunocytochemical study. Neuroscience. 1986;19:465–493. doi: 10.1016/0306-4522(86)90275-7. [DOI] [PubMed] [Google Scholar]
- 14.Libbin RM, Person P. Neonatal rat surgery: avoiding maternal cannibalism. Science. 1979;206:66. doi: 10.1126/science.482926. [DOI] [PubMed] [Google Scholar]
- 15.Liskova K, Mares P. Bicuculline induced seizures during ontogenesis in the rat. Physiol Bohemoslov. 1984;33:272. [Google Scholar]
- 16.Massotti M, Alleva FR, Balazs T, Guidotti A. GABA and benzodiazepine receptors in the offspring of dams receiving diazepam: ontogenic studies. Neuropharmacology. 1980;19:951–956. doi: 10.1016/0028-3908(80)90004-0. [DOI] [PubMed] [Google Scholar]
- 17.Meldrum BS. Epilepsy and gamma-aminobutyric acid mediated inhibition. Int Rev Neurobiol. 1975;17:1–36. doi: 10.1016/s0074-7742(08)60205-6. [DOI] [PubMed] [Google Scholar]
- 18.Moshe SL, Albala BJ. Nigral muscimol infusions facilitate the development of seizures in immature rats. Dev Brain Res. 1984;13:305–308. doi: 10.1016/0165-3806(84)90165-2. [DOI] [PubMed] [Google Scholar]
- 19.Oliverio A, Castellano C, Puglisi S, Renzi P. Diurnal variations in electroconvulsive shock induced seizures: involvement of endogenous opioids. Neurosci Lett. 1985;57:237–240. doi: 10.1016/0304-3940(85)90497-5. [DOI] [PubMed] [Google Scholar]
- 20.Peters MA. Development of a ‘blood-brain-barrier’ to methadone in the newborn rat. J Pharmacol Exp Ther. 1975;192:513–520. [PubMed] [Google Scholar]
- 21.Redburn DA, Broome D, Enna SJ. Development of rat brain uptake and calcium-dependent release of GABA. Brain Research. 1978;152:511–519. doi: 10.1016/0006-8993(78)91106-x. [DOI] [PubMed] [Google Scholar]
- 22.Snead OC, III, Stephens HI. Ontogeny of cortical and subcortical electroencephalographic events in unrestrained neonatal and infant rats. Exp Neurol. 1983;82:249–269. doi: 10.1016/0014-4886(83)90400-4. [DOI] [PubMed] [Google Scholar]
- 23.Swinyard EA. Electrically induced seizures. In: Purpura DP, Penry JK, et al., editors. Experimental Models of Epilepsy. Raven Press; New York: 1972. pp. 433–458. [Google Scholar]
- 24.Vernadakis A, Woodbury DM. The developing animal as a model. Epilepsia. 1969;10:163–178. doi: 10.1111/j.1528-1157.1969.tb03841.x. [DOI] [PubMed] [Google Scholar]
- 25.Woodbury DM. Significance of animal models of epilepsy for evaluation of antiepileptic drug therapy in children. In: Morselli PL, Pippinger CE, Penry JK, editors. Antiepileptic Drug Therapy in Pediatrics. Raven Press; New York: 1983. pp. 349–362. [Google Scholar]
- 26.Zar JH. Biostatistical Analysis. 3. Prentice-Hall; Englewood Cliffs: 1984. [Google Scholar]