Abstract
The multiplicity of Notch receptors raises the question of the contribution of specific isoforms to T-cell development. Notch3 is expressed in CD4–8– thymocytes and is down-regulated across the CD4–8– to CD4+8+ transition, controlled by pre-T-cell receptor signaling. To determine the effects of Notch3 on thymocyte development, transgenic mice were generated, expressing lck promoter-driven intracellular Notch3. Thymuses of young transgenics showed an increased number of thymocytes, particularly late CD4–8– cells, a failure to down-regulate CD25 in post-CD4–8– subsets and sustained activity of NF-κB. Subsequently, aggressive multicentric T-cell lymphomas developed with high penetrance. Tumors sustained characteristics of immature thymocytes, including expression of CD25, pTα and activated NF-κB via IKKα-dependent degradation of IκBα and enhancement of NF-κB-dependent anti-apoptotic and proliferative pathways. Together, these data identify activated Notch3 as a link between signals leading to NF-κB activation and T-cell tumorigenesis. The phenotypes of pre-malignant thymocytes and of lymphomas indicate a novel and particular role for Notch3 in co-ordinating growth and differentiation of thymocytes, across the pre-T/T cell transition, consistent with the normal expression pattern of Notch3.
Keywords: NF-κB/Notch3/pre-TCR/T-cell malignancy
Introduction
The first steps of T-cell differentiation occur within the thymus, as the proliferation, cell fate specification or death of T-cell progenitors is determined by instructive and permissive signals that are either cell autonomous or arise from interactions with the thymic stroma (Boyd and Hugo, 1991). These signals include those generated by T-cell-specific differentiation products, e.g. the pre-T-cell receptor (pre-TCR) that mediates the survival and proliferation of late CD4–8– double negative (DN) cells (von Boehmer et al., 1999) and mature TCRαβ that regulates further development to the CD8+ or CD4+ single positive (SP) stages (Jameson and Bevan, 1998). T-cell differentiation is also controlled by general biological regulators, including members of the Notch receptor family. Four mammalian Notch homologs (Notch1–4) and specific ligands (Jagged 1 and 2 and Delta 1 and 3) have been identified, together with responsive target genes (HES1, HES5 and Deltex) (Artavanis-Tsakonas et al., 1999).
A description of the Notch signaling pathway (Notch family gene members and their cognate ligands and target genes) has been provided for the thymus (Felli et al., 1999) and a significant role for Notch1 in thymocyte survival (Deftos et al., 1998) and in intrathymic differentiation of T-lymphoid cell lineages described (Robey et al., 1996; Washburn et al., 1997). Nonetheless, the presence of multiple Notch receptors raises the question of whether such different receptors play distinct roles during thymocyte differentiation. Indeed, we observed previously that a significantly higher percentage of DN thymocytes express Notch3 relative to Notch1 and that there was a preferential up-regulation of Notch3 (compared with Notch1) in DN thymocytes prior to their transition to the CD4+8+ double positive (DP) stage following interaction with thymic epithelial cells (Felli et al., 1999). Most provocatively, Notch3 is down-regulated across the DN–DP transition (Felli et al., 1999) that is controlled by the pre-TCR signaling pathway.
To determine the capacity of Notch3 to regulate thymocyte development, transgenic [tg(+)] mice were generated in which expression of the Notch3 intracellular domain (Notch3-IC) was driven by the proximal lck promoter. Thymocyte development initially displayed a sustained expression of several markers of late DN cells at or around pre-TCR selection stage (e.g. expansion of stage II and III DN cells, retention of CD25 expression in post-DN cells and constitutively activated NF-κB). After 6–8 weeks, highly aggressive Notch3-positive T-cell lymphomas dominated the spleen and lymph nodes of multiple tg(+) lines. Like the pre-malignant thymocytes, the lymphomas also retained the expression of CD25, pTα, constitutively active NF-κB and an NF-κB-induced anti-apoptotic function. This phenotype, which is distinct from that displayed by Notch1 tg(+) mice, provides evidence that Notch3 plays a novel potent, non-redundant role in the co-ordination of T-cell differentiation and growth control, across the pre-T/T cell transition.
Results
Generation of Notch3-IC transgenic mice
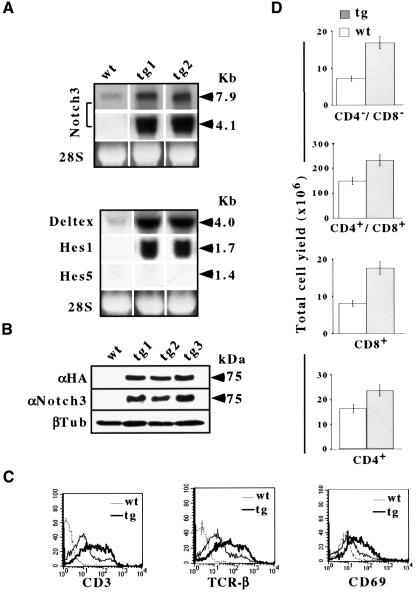
To assess the ability of Notch3 to influence T-cell development, we generated Notch3 tg(+) mice, using the lck proximal promoter to overexpress a hemagglutinin (HA) epitope-tagged Notch3-IC, able to trigger ligand-independent Notch3 intracellular signaling (Lardelli et al., 1996). Fourteen founders were identified. High expression of the Notch3-IC transgene-encoded 4.1 kb mRNA transcript was detected in the thymus of tg(+) mice, together with a significant up-regulation of endogenous 7.9 kb wild-type Notch3 mRNA (Figure 1A), in keeping with the autoregulatory feedback sustained by Notch signaling (Deftos et al., 1998; Apelqvist et al., 1999; Artavanis-Tsakonas et al., 1999). By use of either anti-HA or anti-Notch3 antibodies, the Notch3-IC-HA 75 kDa protein was detected in the thymus of tg(+) but not wild-type mice (Figure 1B). Constitutive activation of Notch signaling in Notch 3 (tg+) mice is suggested by enhanced expression of specific Notch target genes, such as HES-1 and Deltex (Figure 1).
Fig. 1. (A) Northern blot analysis of the endogenous 7.9 kb Notch3 mRNA, transgene 4.1 kb Notch3-IC mRNA and HES-1, HES-5 and Deltex mRNAs in thymocytes of two individual tg(+) mouse lines (tg1 and tg2) and wild-type mice (wt), hybridized with Notch3-IC, HES-1 and HES-5 cDNAs (Felli et al., 1999) and RT–PCR-derived Deltex cDNA probe. (B) Immunoblot analysis of whole-cell lysates from wild-type and individual tg(+) mouse lines, using anti-HA tag (αHA), anti-Notch3 (αNotch3) and anti β-tubulin (β-tub) antibodies. (C) FCA of CD3, TCRβ chain and CD69 in thymocytes from 3-week-old tg(+) (tg) and wild-type (wt) mice. The plots are overlaid and superimposed on the fluorescence obtained with an irrelevant antibody (---). (D) Thymocyte subset total yield per thymus (detected by CD4 versus CD8 two-color FCA) in 3-week-old tg(+) (tg) and wild-type (wt) mice.
Aberrant pre-T-cell development in Notch3-IC transgenic mice
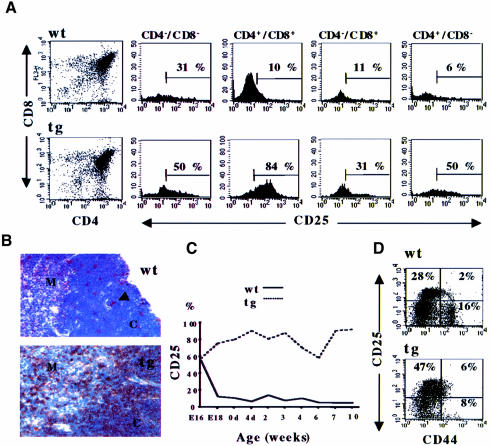
The thymocyte phenotype of wild-type and tg(+) animals was analyzed at different ages, from 16 d.p.c. Until 4 weeks of age, there was a reproducible increase in the thymocyte number in tg(+) mice (3.0 ± 0.4 × 108/thymus compared with 1.8 ± 0.3 × 108/thymus in wild-type 3-week-old mice). Absolute numbers of cells of each subset (DN, DP and SP) were increased (Figure 1D). Although there was no obvious impairment of thymocyte subset distribution with respect to CD4+ and/or CD8+ cells (Figure 2A), most tg(+) thymocytes, including DN thymocytes, stained brightly for CD3 and TCRβ (Figure 1C). A higher percentage of tg(+) thymocytes also stained brightly for CD69, mainly observed in the DP subset (Figure 1C). Thus, overexpression of Notch3-IC disrupts thymocyte differentiation. Further evidence of this was provided by the fact that all tg(+) thymocyte subsets included cells expressing high levels of CD25, broadly distributed in cortex and medulla of thymus sections (Figure 2A and B). CD25+ cells commonly accounted for 60–80%. This phenotype might be interpreted as a failure to down-regulate CD25 expression that occurs ordinarily in DP and SP subsets after 17–18 d.p.c. (Figure 2C) (Jenkinson et al., 1987).
Fig. 2. Overexpression of CD25/IL-2Rα in thymocyte subsets of 3-week-old Notch3 tg(+) (tg) mice compared with wild-type (wt). (A) Three-color analysis of CD25 versus CD4 versus CD8 expression in CD4–8–, CD4+8+, CD4–8+ and CD4+8– thymocyte subsets. Numbers indicate the percentages of CD25+ thymocytes. Left panels indicate the thymocyte subset distribution. (B) Immunohistochemical staining of CD25+ thymocytes in cryostat acetone-fixed sections from frozen thymic tissue, pre-incubated with rabbit serum, and then incubated sequentially with rat anti-mouse monoclonal antibody against CD25 and biotinylated rabbit anti-rat IgGs and streptavidin–HPR (PharMingen, San Diego, CA). CD25+ wt cells are scattered in the cortex (arrowhead), while most of the cortical (C) and medullary (M) tg(+) thymocytes are CD25+. Hematoxilin counterstain. Magnification: 250×. (C) Percentage of CD25+ thymocytes from wild-type (wt) and tg(+) (tg) mice at different ages (16 and 18 d.p.c., E16 and E18; 0 and 4 post-natal days, 0d and 4d; and indicated weeks). (D) Two-color analysis of CD44 versus CD25 expression in FACS-sorted DN thymocytes from wild-type and tg(+) mice. Numbers in the panels indicate the percentage of CD44+25–, CD44+25+ and CD44–25+ cells, respectively.
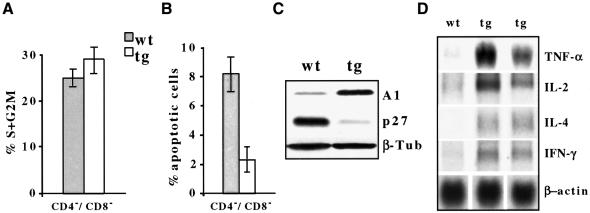
To examine whether the increased number of thymocytes was attributable to changes in the earliest subsets, we examined DN thymocytes, segregated into four subsets (I–IV) based on the discrete expression of CD44 and CD25 (Godfrey et al., 1993). Both CD25+ DN subsets (II and III) were over-represented in tg(+) mice (Figure 2D), the latter consistent with a failure to down-regulate CD25. Ordinarily, the pre-TCR-regulated transition of subset III (CD44–/CD25+) cells into subset IV (CD44–/CD25–) cells is a critical point in thymocyte differentiation, as thymocytes are rescued from apoptosis and induced to expand clonally (Hoffman et al., 1996). DN cells from tg(+) versus non-tg(+) littermates showed little increase in the proportion of cells in S + G2–M phases of the cell cycle (29.0 versus 23.8%, respectively). A noticeably smaller percentage of tg(+) DN cells were apoptotic compared with wild-type mice (2.4 versus 8.1%, respectively) (Figure 3A and B). Tg(+) thymocytes also showed decreased expression of the cyclin-dependent kinase inhibitor p27 and increased levels of the anti-apoptotic Bfl-1/A1 protein (Figure 3), as well as increased mRNA levels of interleukin-2 (IL-2), interferon-γ (IFN-γ), IL-4 and tumor necrosis factor-α (TNF-α) (Figure 3D). Thus, overexpression of Notch3-IC disrupts growth regulation in the early thymocyte subsets.
Fig. 3. Cell cycle (A), apoptosis (B), Bfl-1/A1 and p27 (C) and cytokine mRNA (D) expression analysis in thymocytes of 3-week-old wild-type (wt) and tg(+) (tg) mice. FACS-sorted CD4–8– cells were used for (A) and (B). Bfl-1/A1 and p27 were immunoblotted by using antibodies against p27, Bfl-1/A1 and β-tubulin, to monitor sample loading. (D) IL-2, IFN-γ, IL-4 and TNF-α mRNAs were revealed by northern blot analysis, using murine IL-2, IFN-γ, IL-4 and TNF-α cDNAs as probes.
Increased NF-κB activity in transgenic thymocytes
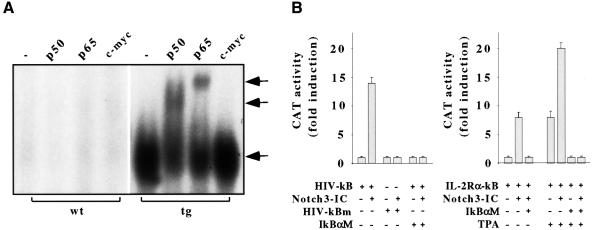
Two transient peaks of NF-κB activity occur in the thymus, one in CD8+ SP cells and one as cells move through the late DN stages (Zùniga-Pflucker et al., 1993; Bakker et al., 1999). We and others observed that the latter subset-specific NF-κB activity provides relief from apoptosis as late DN thymocytes proliferate in response to signals from the pre-TCR (Bakker et al., 1999; S.Ghosh and A.C.Hayday, unpublished). Given the expansion of late DN thymocytes, the reduced levels of apoptosis and the sustained expression of a number of previously described NF-κB target genes, including CD25, IL-2, IFN-γ, IL-4, TNF-α and Bfl-1/A1, in tg(+) mice (Ferreira et al., 1999; Pahl, 1999), we examined NF-κB activity. Compared with the non-tg(+) mice, nuclear extracts from thymocytes of 2- to 4-week-old Notch3 tg(+) mice displayed constitutive high levels of specific p50–p65 NF-κB–DNA complexes (Figure 4A).
Fig. 4. (A) Constitutive activation of the NF-κB complex in Notch3 tg(+) thymocytes. EMSA of NF-κB complexes in nuclear extracts from freshly isolated thymocytes of 3-week-old tg(+) (tg) or wild-type (wt) mice, incubated in the absence (–) (lower arrow) or presence of antibodies against either p50 or p65 or an unrelated antibody against c-myc. Antibody-mediated supershifted complexes (upper arrows) are indicated. (B) Notch3-IC enhances transcriptional activation from κB elements of HIV LTR and IL-2Rα promoter. M31T cells were transfected with 2 µg of HIV-κB-CAT, HIV-κBm-CAT or IL-2Rα-κB-CAT in the absence or presence of 4 µg of CMV-Notch3-IC or pCMX-IκBαM and assayed for CAT activity after 48 h. Phorbol ester (TPA) treatment (30 ng/ml, for 24 h) is indicated. Fold induction of CAT activity relative to basal CAT levels observed with reporter plasmid alone (assigned the value of 1) is indicated. CAT assays are normalized to co-transfected β-galactosidase expression vector.
Notch3-dependent NF-κB activation was also demonstrated by the ability of a Notch3-IC expression vector to induce a significant transcriptional enhancement from the κB sites of human immunodeficiency virus long terminal repeat (HIV LTR)-driven CAT reporter gene in a transfected M31T pre-T cell line (Maroder et al., 1996) (Figure 4B). Likewise, Notch3-IC enhanced transcription from the κB sites of the IL-2Rα promoter in either the absence or presence of 12-O-tetradecanoylphorbol- 13-acetate (TPA) (Figure 4B). Furthermore, Notch3-IC-induced effects were abrogated by introducing inactivating mutations in the κB site of the HIV LTR or by accompanying the HIV-κB-CAT or IL-2Rα-κB-CAT transfections with a dominant-negative IκBα mutant (Figure 4B). These data demonstrate that Notch3 promotes NF-κB activation in thymocytes, consistent with improved survival and increased numbers of thymocytes in Notch3 tg(+) mice.
Lymphoproliferative alterations in Notch3-IC transgenic mice
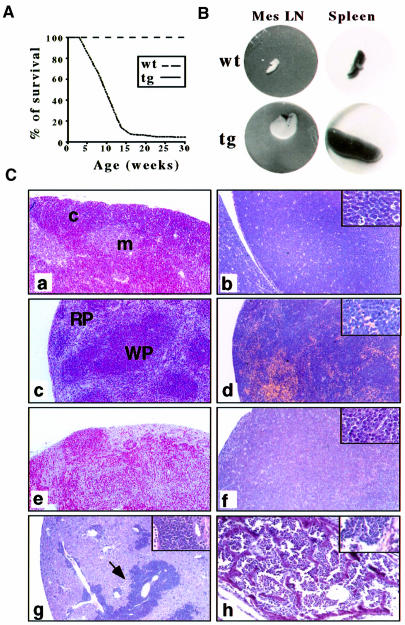
By mutliple criteria, young tg(+) animals appeared normal until ∼7 weeks of age, at which point all 14 founders and progeny developed a rapidly evolving disease, characterized by lethargy, hunched posture and distended abdomen. Eighty percent of tg(+) animals succumbed between 10 and 12 weeks of age, and by 16 weeks 95% of tg(+) mice had died (Figure 5A). By 30 weeks, all mice were sacrificed. Examination of all succumbed and euthanized tg(+) mice revealed, as early as 5–6 weeks and at all ages thereafter, a significant enlargement of the upper media stinum and a 5- to 6-fold increase in the size and weight of the spleen and peripheral (axillary, cervical and abdominal) lymph nodes, compared with control mice (Figure 5B). A hyperplastic thymus was also observed in some tg(+) mice. Histological examination of the thymus, spleen and lymph nodes showed a complete disruption of the normal architecture and a massive infiltration by a monotonous lymphoblastic cell population (Figure 5C). Tumor cell infiltration was also detected in liver, lungs, kidney, bone marrow (Figure 5C and not shown) and peripheral blood (not shown), thus indicating the existence of a leukemic phase of the neoplastic disease.
Fig. 5. (A) Mortality curve of several lines of Notch3 tg(+) mice. Spontaneously dead tg(+) (tg) and wild-type mice (wt) were plotted against their age. Results are indicated as the percentage of surviving animals at each age [n = 100 for Notch3 tg(+) and n = 30 for wild-type mice]. (B) Macro scopic aspect of spleen and mesenteric lymph nodes (MesLN) isolated from wild-type (wt) and tg(+) (tg) mice. (C) Histological analysis of lymphoid and non-lymphoid organs from 12-week-old Notch3 tg(+) mice. Hematoxylin and eosin staining of 4 µm sections of 10% buffered formalin-fixed and paraffin-embedded tissues. Disruption of the architecture of tg(+) thymus (b), spleen (d) and lymph node (f) compared with wild-type organs (a, c and e; WP, white pulp; RP, red pulp; c, cortex; m, medulla) due to the complete substitution of the normal components by a population of lymphoblastic cells. The liver and bone marrow are infiltrated massively by neoplastic lymphoblast-like cells (g, arrow; h and inset). Infiltrating tumor cell (insets of b, d, f and g) show the features of lymphoblastic lymphoma cells: medium to large cells with roundish nuclei, a round central nucleolus and a small rim of cytoplasm. Hematoxylin and eosin staining. Panels: 160×. Insets: 1000×.
Tumorigenic potential of Notch3 transgenic T-cell lymphomas in vivo
All splenic or lymph node lymphomas tested and a cell line [N3-232T, established from the thymocytes of a 6-week-old tg(+) mouse] could be serially transplanted (20 × 106 cells injected subcutaneously) and elicited a lethal infiltration into the lymphoid organs of recipient nude or syngeneic mice, with latency periods of 2 and 3 weeks, respectively. Moreover, N3-232T cells shared phenotypic features of the spontaneously arising lymphomas (see below), e.g. CD3high (>80% of cells), CD25high (30% of cells), pTα expression and activated NF-κB. Interestingly, N3-232T cells also displayed the DN phenotype of immature thymocytes.
Notch3-induced T-lymphoma cells retain pre-T-cell characteristics in spite of progressing towards CD4/CD8 maturation
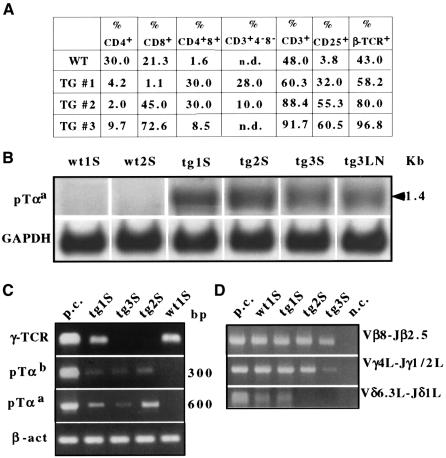
Splenic lymphoma cells from three indepedent Notch3 tg(+) lines displayed variable percentages of CD4 and CD8 expression, ranging from mainly DN to CD8 SP (Figure 6A), suggesting their origin in heterogeneous differentiation stages. Lymphoma cells closely resembled Notch3 tg(+) thymocytes in their expression of CD25, increased in frequency by 10- to 20-fold compared with spleen cells of normal mice (Figure 6A). This evident parallel between the lymphomas and the dysregulated thymocyte development seen in younger tg(+) mice suggested that other early thymocyte markers might also be retained aberrantly in the periphery of the tg(+) mice. Indeed, tg(+) spleen cells showed sustained expression of both pTαa and pTαb (Barber et al., 1998), not normally expressed in mature peripheral T cells (Figure 6B and C).
Fig. 6. (A) Percentages of CD4+, CD8+, CD3+, CD25+ and TCRβ+ chain wild-type splenic T cells and splenic lymphoma cells from representative individual tg(+) animals (Tg#1, #2 and #3) (assayed by FCA). n.d., not detected. (B) Northern blot analysis of the pTαa isoform in splenic T cells of wild type (wt1 and wt2) and in lymphoma cells of Notch3 tg(+) mice (Tg1, Tg2 and Tg3, as indicated in A) (S, spleen; LN, lymph node). (C) Expression of pTαa, pTαb and TCRγ chain genes in splenocytes (S) was assessed by RT–PCR. The positive control (p.c.) is thymocyte mRNA. (D) Rearrangements of TCRβ, δ and γ chains in genomic DNA were detected by PCR amplification of Vβ8–Jβ2.5, Vγ4L–Jγ1/2L and Vδ6.3L–Jδ1L regions. p.c. and n.c., positive and negative controls, respectively.
Since pTα is expressed before the onset of TCR gene rearrangement (von Boehmer et al., 1999), we assessed the gene rearrangement status of the lymphoma cells. All splenic lymphomas carried rearranged TCR β chain genes, as defined by the presence of Vβ8–Jβ2.5 rearrangement in genomic DNA (Figure 6D). While all tumors carried γ chain rearrangements, γ chain mRNA expression could be detected only in some of them (Figure 6C), a fact that together with the deletion of genomic Vδ6.3L–Jδ1L DNA (Figure 6D) indicated that most lymphomas had continued to progress along a pathway of further αβ+ T-cell differentiation (MacDonald and Wilson, 1998), despite retaining pTα expression. Consistent with this, extinction of γ expression and deletion of δ chain gene DNA were more apparent in CD8+ or mixed CD8+/DP tumors, compared with mixed DN/DP tumors (Figure 6A). Sequence analysis of Vβ8–Jβ2.5 and Vγ4L–Jγ1/2L rearrangements revealed a mixture of largely in-frame joinings that together indicated an oligoclonal origin of the tumors (not shown).
Constitutive activation of NF-κB in malignant T cells from Notch3 transgenic mice
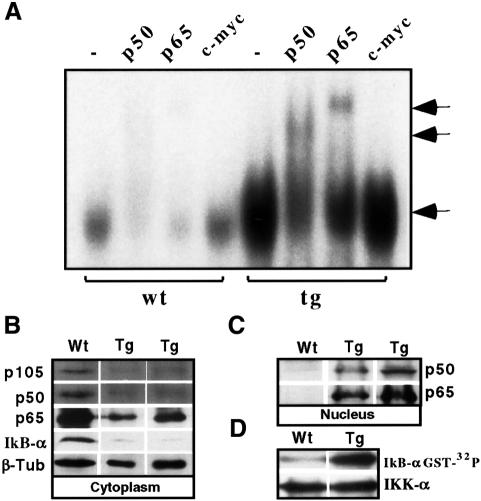
The phenotypic similarities between the lymphoma cells and the thymocytes of younger mice raised the issue of NF-κB activity in the lymphomas. As for Notch3 tg(+) thymocytes, lymphoma cells from the spleens and lymph nodes of 10-week-old Notch3 tg(+) mice showed high constitutive NF-κB activity, by comparison with that in splenic or lymph node T cells of wild-type animals (Figure 7A). Electrophoretic mobility shift assays (EMSAs) of nuclear extracts of lymphoma cells revealed heterodimers, identified as p65 and p50 by a supershifting of the complex by both anti-p50 and anti-p65 antibodies, but not by an unrelated antibody (Figure 7A). Conversely, no p52 was detected in the NF-κB–DNA complex (not shown).
Fig. 7. Constitutive activation of the NF-κB complex in Notch3 tg(+) splenic T-lymphoma cells. (A) EMSA of NF-κB complexes in nuclear extracts from splenic T cells of 10-week-old tg(+) (tg) or wild-type (wt) mice, incubated in the absence (–) (lower arrow) or presence of antibodies against either p50, p65 or c-myc. Antibody-mediated supershifted complexes (upper arrows) are indicated. (B and C) Constitutive translocation of p65/p50 into the nucleus is associated with degradation of IκBα in T-lymphoma cells of Notch3 tg(+) mice. Immunoblots of nuclear and cytoplasmic cell extracts from splenic T cells isolated from two individual lines of 10-week-old tg(+) mice (tg) and wild-type animals (wt) were analyzed using antibodies against p50/105, p65 or IκBα. (D) Notch3 tg(+) T-lymphoma cells express constitutively activated IKKα activity. In vitro kinase assays, using IκBα–GST as an exogenous substrate (upper panel), were performed on IKKα immunoprecipitates (lower panel) from cell lysates of splenic T-lymphoma cells of 10-week-old tg(+) mice (tg) or wild-type splenic T cells (wt).
Nuclear NF-κB subunits p65 and p50 are released from cytoplasmic complexes with IκBα and β proteins following IKK-mediated phosphorylation, ubiquitylation and proteolytic degradation (Israel, 1995). Consistent with the activation of NF-κB in Notch3 tg(+) lymphoma cells, western blot analysis revealed increased levels of both p65 and p50 in the nucleus together with decreased cytoplasmic levels of p65 and p105/50 (Figure 7B and C) that could be attributed to impaired cytoplasmic sequestering by IκB proteins, since there were constitutively lower levels of IκBα in T-lymphoma cells (Figure 7B). IκBα degradation could in turn be correlated with high levels of IKKα kinase activity in Notch3 tg(+) T-lymphoma cells compared with T cells from wild-type mice (Figure 7D). Thus, Notch3-IC-dependent lymphoma cells have height ened constitutive NF-κB nuclear activity that correlates with IKKα kinase activity and reduced levels of the inhibitor IκB.
Activation of anti-apoptotic pathways in T-lymphoma cells of Notch3 transgenic mice
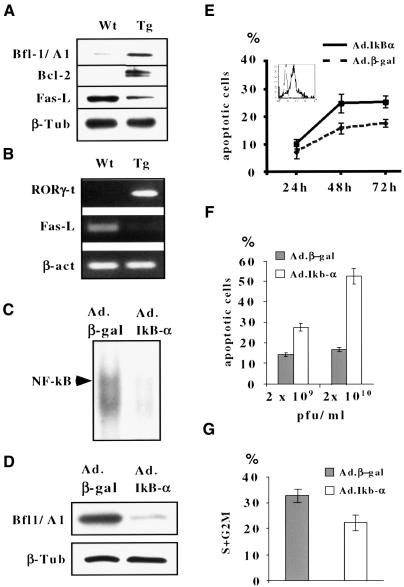
NF-κB has been suggested to rescue DN thymocytes from apoptosis (Bakker et al., 1999; S.Ghosh and A.C.Hayday, unpublished), and likewise to promote oncogenesis via activation of anti-apoptotic programs (Rayet and Gélinas, 1999). Consistent with the prospect that Notch3-induced activation of NF-κB promoted T-cell lymphoma survival, the NF-κB transcriptional, anti-apoptotic target Bfl-1/A1, previously shown to be expressed preferentially in lymphoid tissues (Zong et al., 1999), was found to be expressed at significantly enhanced levels in spleen or lymph node lymphoma cells isolated from Notch3 tg(+) mice (Figure 8A). Moreover, in contrast to pre-malignant thymocytes of Notch3 tg(+) mice, primary lymphoma explants also expressed strikingly higher levels of the anti-apoptotic protein, Bcl-2 (Figure 8A). Possibly this reflects a secondary event associated with the development of frank T-cell malignancy.
Fig. 8. Activation of anti-apoptotic pathways in Notch3-induced T-lymphoma cells in tg(+) mice. (A and B) Splenic T-lymphoma cells overexpress Bfl-1/A1, Bcl2 and RORγ t and down-regulate Fas-L expression. Whole-cell lysates or RNA were isolated from splenic T-lymphoma cells of 10-week-old Notch3 tg(+) mice (tg) or from wild-type splenic T cells (wt). Immunoblot analyses were performed using antibodies against murine Bfl-1/A1, Bcl2 or Fas-L (A). RORγ t and Fas-L mRNAs were evaluated by RT–PCR (B). (C–G) Inactivation of NF-κB by IκBα overexpression decreases spontaneous survival and proliferation of Notch3-induced lymphoma T cells. N3-232T cells were infected with either Ad-IκBα or Ad-β-gal as a control. EMSA of NF-κB DNA-binding activity (C), immunoblot of Bfl-1/A1 levels (D) and FCA of the percentage of apoptotic cells (E and F) or cells in S + G2–M cell cycle phases (G) were assessed 24–72 (E) or 48 h (C, D, F and G) after the infection with 2 × 109 (D, E, F and G) or 2 × 1010 p.f.u./ml (C and F) recombinant adenoviruses. The inset of (E) shows β-gal fluorescence as a measure of adenoviral infection efficiency.
A novel isoform of an orphan receptor, RORγ t, has recently been reported to be expressed specifically in immature (DN and DP) thymocytes, to be up-regulated by signaling through the pre-TCR (Villey et al., 1999) and to be able to protect hybridomas from TCR/CD3-mediated apoptosis, by negatively regulating Fas ligand (FasL) expression (He et al., 1998). Because of the sustained expression in the peripheral lymphomas of early thymocyte markers, we assayed for RORγ t expression. Although the high expression of RORγ t in thymocytes could not be enhanced further during the pre-malignant phase in Notch3 (tg+) mice (not shown), high levels of RORγt were retained in tumors, compared with negligible levels in splenic T cells of wild-type mice (Figure 8B). Consistent with this, FasL expression was barely detectable in tumor tissues at either the RNA or protein level (Figure 8A and B).
Inhibition of NF-κB by IκBα prevents the survival of Notch3-induced T-lymphoma cells
To test the functional significance of Notch3-induced NF-κB activity in the lymphomas, the N3-232T cell line was infected with a recombinant IκBα-expressing adenoviral vector, Ad-IκBα. Adenoviral infection was monitored by infecting N3-232T cells with Ad-βgal carrying, instead of IκBα, the Escherichia coli lacZ gene (Figure 8E, inset). The capacity of Ad-IκBα infection to inhibit NF-κB activity was demonstrated by decreased nuclear levels of NF-κB complexes binding κB target sequences and reduced Bfl-1/A1 protein expression in Ad-IκBα-infected N3-232T cells compared with Ad-βgal-infected cells (Figure 8C and D). Significantly, the basal apoptotic rate in Ad-IκBα-infected N3-232T was increased in a dose-dependent manner compared with cells infected with the Ad-βgal (Figure 8E and F). We also observed a small decrease in the percentage of cells in S + G2–M phase in Ad-IκBα-infected cells (Figure 8G). Together, these data provide evidence that the activation of NF-κB, seen in the thymocytes of young Notch3 tg(+) mice and sustained in the emerging lymphomas, has a functional and pathological capacity to enhance survival and expansion of the lymphoma cells.
Discussion
In this study, we demonstrate that the expression of a constitutively active form of Notch3 in a large number of independent founder tg(+) mice leads to specific alterations in thymocyte development that are sustained in the outgrowth of aggressive T-cell lymphoblastic lymphomas by 6 weeks of age. The specific altered thymocyte phenotype, particularly the persistence of CD25 and pTα expression and activation of NF-κB, and their sustained expression in tumors, suggests that late DN T cells are the initial target of Notch3-induced dysregulation, and that such early dysregulation may constitute the platform for subsequent lymphomagenesis, albeit in parallel with further differentiation.
The phenotype of Notch3 tg(+) mice is different from the occasional incidence of CD8+, low CD3-expressing thymomas developed in older mice expressing constitutively active Notch1 from the same proximal lck promoter (Robey et al., 1996). Nevertheless, the potent lymphomagenic activity of tg(+) Notch3 is consistent with the oncogenic activity of the human rearranged homolog of Notch1 (TAN1) in some T-cell lymphomas (Ellisen et al., 1991), and with T-cell neoplasms induced by bone marrow cells transduced with TAN1 recombinant retrovirus (Pear et al., 1996). The tumorigenic activity of transplanted TAN1-expressing bone marrow cells, compared with overexpression of Notch1 in thymocytes, suggests that the optimal target of the oncogenic activity of Notch1 may be an early pre-thymic progenitor, according to the described role of Notch1 in T-cell development prior to the entry of bone marrow cells into the T lineage (Pui et al., 1999; Radtke et al., 1999).
In contrast to the low incidence of malignancy, the most conspicuous phenotype of Notch1 tg(+) mice is characterized by the production of CD8+ cells that is favored ∼10-fold over the production of CD4+ cells (Robey et al., 1996). Although there was also an apparent bias in lymphoma phenotype toward CD8 versus CD4 thymocytes in Notch3 tg(+) mice, the overall distribution of CD4 versus CD8 thymocyte subsets was unaffected before lymphoma development. Instead, by the measure of a number of parameters, the Notch3-induced dysregulation in thymocyte development seemed specifically to affect late DN cells: thus, there was sustained expression of CD25 and pTα, activation of NF-κB and a significant reduction in apoptosis. This is consistent with the dynamic expression pattern of Notch3 as late DN cells mature, notably its up-regulation before DN to DP commitment and its down-regulation across DN to DP transition (Felli et al., 1999). Preventing such a Notch3 down-regulation by forced transgenesis results in impairment of pre-T-cell development with expansion of the late DN compartment, in parallel with further albeit variable maturation to DP and SP cells. Therefore, we propose that mis-expression of Notch3 dysregulates the co-ordination of growth and differentiation, reproducibly leading to T-cell malignancy. Dysregulated growth of DN cells is also consistent with the Notch3-induced up-regulation of HES1, since this basic helix–loop–helix protein has been described to play a critical role in expansion of early T-cell precursors at the late DN stage (Tomita et al., 1999). Notch3 regulation appears, therefore, to be critical for late DN cells that are subject to intricate regulation of cell survival, expansion and lineage commitment (Dudley et al., 1994).
The differences in phenotype of mice in which Notch1 and Notch3, respectively, are dysregulated further support the emerging evidence that different Notch receptors may have distinct functions. A difference in intracellular signaling of Notch3 versus Notch1 has been described recently, as Notch3, in contrast to Notch1, is an in vitro poor-to-negligible activator of the HES1 and HES5 promoters, respectively, and acts as a repressor of the Notch1-mediated HES activation (Beatus et al., 1999). In support of this notion, Notch3-IC acts as a repressor of Notch1-induced HES activation and exocrine fate during pancreas development (Apelqvist et al., 1999). Cell-specific differences might account for the failure of Notch3 to antagonize HES1 activation, in our model, as the involvement of a common co-activator has been suggested to be responsible for repression of Notch1 activity (Beatus et al., 1999). Indeed, the significant up-regulation of HES1 we observed in Notch3 (tg+) thymocytes, in addition to the low agonistic activity on the HES1 promoter (Beatus et al., 1999), might be attributable to NF-κB-binding sites in the HES1 regulatory region (unpublished data), as we observed activation of NF-κB by Notch3. A further distinction of Notch3 versus Notch1 function may occur on Bcl-2 regulation, which is enhanced in Notch1 (tg+) thymocytes (Deftos et al., 1998) and unaffected in Notch3 (tg+) pre-malignant thymocytes.
The phenotype of T-cell dysregulation in Notch3 tg(+) mice is novel. Although there are some similarities to mice mutant in CD3ζ (Crompton et al., 1994), the persistent expression of pTαa and pTαb in the periphery has not been described previously. This phenotype was most evident in tumors that retained the highest numbers of DN T cells, further suggesting that the aberrant thymic export and loss of growth control in such cells contributes to the reproducible development of tumors, albeit with variable differentiated phenotypes. The rapid development and oligoclonal, in-frame TCR rearrangements of the transplantable tumors indicate that the overexpression of Notch3 coupled with expression of an antigen receptor is a highly potent growth stimulus. This has important ramifications for the widely considered role of Notch in cancer (Artavanis-Tsakonas et al., 1999).
The capacity that we demonstrate of activated Notch3 to lead to constitutive NF-κB activity may explain this potency. The T-cell oncogenic potential of Rel/NF-κB is well established (Rayet and Gélinas, 1999). Transgenic mice expressing v-Rel in T cells develop aggressive multicentric T-cell leukemia/lymphomas (Carrasco et al., 1996), resembling those observed in Notch3 tg(+) mice. Furthermore, NF-κB has been shown to be involved in human T-cell leukemia virus 1 (HTLV-1)-induced lymphomagenesis. Indeed, HTLV-1 Tax induces the degradation of IκBα, thereby activating NF-κB (Geleziunas et al., 1998), which in turn activates survival and autocrine growth stimulatory pathways, e.g. IL-2Rα and IL-2 (Mori et al., 1999; Rayet and Gélinas, 1999). By the criteria of IKKα-mediated phosphorylation and degradation of IκBα and activation of autocrine growth stimulatory pathways (IL-2Rα and IL-2), the Notch3-induced T-cell malignancies described here strikingly resemble HTLV-1-associated T-cell leukemia, and might prove to be a useful model for such. The involvement of activated NF-κB in Notch3-induced T-cell malignancy is supported further by the activation in T cells of Notch3 tg(+) mice of pro-survival pathways that we showed (by use of inhibitors) to be NF-κB dependent.
The precise roles of NF-κB proteins in T-cell activation and development remain to be fully defined, particularly regarding the pre-TCR selection stage. Data indicate an NF-κB dependence of the development of intrathymic and peripheral CD8+ T cells, of the inducible expression of multiple cytokine and cytokine receptor genes, and of normal T-cell proliferation in response to TCR/CD3-mediated T-cell activation (Boothby et al., 1997; Ferreira et al., 1999). The observation that thymocytes in Notch3 tg(+) mice express high levels of cytokine and cytokine receptor genes (IL-2, IL-4, IFN-γ, TNF-α and IL-2Rα) is consistent with this, as is the bias in phenotype of the tumors toward CD8 versus CD4. The increased expression of the early activation marker CD69 in pre-malignant thymocytes is also consistent with activation signals related to thymocyte differentiation (e.g. intrathymic positive selection) (Jameson and Bevan, 1998). In contrast, CD69 expression was not increased in peripheral T cells or lymphomas (not shown), thus excluding peripheral T-cell activation.
How Notch3 activates NF-κB needs to be elucidated. Although overexpression of Notch3 is able to lead to transactivation of NF-κB target promoters, Notch3 might require co-regulatory molecules and mediate some or all of its effects by up-regulating the pTα/β pre-TCR complex in late DN cells, which may in turn sustain CD3-dependent and IKKα-mediated activation of NF-κB (Pahl, 1999; S.Ghosh and A.C.Hayday, unpublished). Indeed, this report demonstrates that enforced Notch3 activity prevents pTα silencing along T-cell maturation, sustains the expression of targets of pre-TCR signaling (e.g. RORγ t) and up-regulates CD3 expression in both pre-malignant thymocytes and lymphoma cells. This would be predicted to trigger NF-κB-dependent pathways that result in T-cell survival and proliferation. Notch1 has also been reported to control NF-κB by binding to the p50 subunit and displaying both stimulatory and inhibitory activity upon NF-κB-mediated transactivation (Guan et al., 1996). Additionally, Notch1 enhances NF-κB-2 gene expression (Oswald et al., 1998). Thus, the overall regulation of NF-κB by different Notch family members during lymphocyte development might comprise a multifaceted regulatory web. The high penetrance and dramatic nature of the consequences of a failure to extinguish this regulation in Notch3 tg(+) mice emphasize its importance in the regulation of thymocyte and T-cell growth control and in mediating the oncogenic potential of Notch3.
Materials and methods
Generation of Notch3-IC transgenic mice
The Notch3-IC-encoding cDNA (codons 1664–2318) fused at the C-terminus to the HA nonapeptide epitope tag (Lardelli et al., 1996) was ligated into the BamHI site of the transgenic expression vector p1017 (Chaffin et al., 1990), downstream of the thymocyte-specific mouse lck proximal promoter and containing human growth hormone (hGH) sequences and a polyadenylation site. The lck-Notch3-IC-hGH SacII fragment was microinjected into fertilized eggs from BDF1 (DBA2×C57 Bl/6) mice. Fourteen independent transgenic founders were identified and maintained by backcrossing to C57Bl/6.
Flow cytometry (FCA)
Freshly isolated cells from thymuses, spleens and lymph nodes were prepared and stained as previously described (Maroder et al., 1996) and analyzed on FACScan (Becton Dickinson, Mountain View, CA) using Cell Quest software (Becton Dickinson). Forward- and side-scatter gating were used to exclude dead cells from the analysis.
Fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)- or biotin-conjugated monoclonal antibodies (mAbs) were against: CD4 (H-129.19), CD8a (53-6.7), CD3 (ε chain: 145-2C11), CD25 (IL-2Rα chain, 7D4), CD44 (IM7), B220 (RA3-6B2), Thy-1.2 (53-2.1), βTCR (H57-597), γδTCR (GL3) and CD69 (H1.2F3) (PharMingen, San Diego, CA). Biotinylated mAbs were revealed with streptavidin–Tricolor (Caltag, Burlingame, CA). PE- and FITC-conjugated rat and hamster IgG (PharMingen, San Diego, CA) were used as a control for immunofluorescence. Cell sorting, cell cycle and apoptosis analysis were as previously described (Maroder et al., 1996).
Transfections
The CAT reporter plasmids are driven by κB sites from the IL-2Rα promoter (IL-2R-κB-CAT) and HIV LTR (HIV-κB-CAT) or by HIV-mutated κB (HIV-κBm-CAT), inserted upstream of the minimal c-fos promoter (Franzoso et al., 1992, 1993). PCMX-IκBαM contains IκBα cDNA with mutated Ser32, Ser36, Ser283, Ser288, Ser293, Thr291 and Thr296, which prevent IκBα phosphorylation and degradation (Van Antwerp et al., 1996). CMV-Notch3-IC contains Notch3-IC cDNA driven by the cytomegalovirus (CMV) promoter inserted into pcDNA3.1 vector (Invitrogen Co., Carlsbad, CA). Co-transfected β-galactosidase-expressing pCH110 was used to normalize transfection efficiency between groups. DEAE–dextran-mediated transfections into M31T cells and CAT assays were performed as previously described (De Grazia et al., 1994).
Western blot analysis
Cytoplasmic and nuclear fractions and whole-cell extracts (50 µg of proteins) (Alesse et al., 1998) were analyzed by SDS–PAGE, transferred to nitrocellulose membranes and probed with the following antibodies: mouse mAb against Bcl-2 (N-19) and p27 (F-8) (Santa Cruz Biotechnology, Santa Cruz, CA), Fas ligand (33, Transduction Laboratories, Lexington, KY) and β-tubulin (Ab1, Oncogene Research, La Jolla, CA); rabbit polyclonal antibodies against p50 (H-119), p65 (H-286), IκBα (FL), HA (F-7), Notch3 (M-20) and goat polyclonal antibody against Bfl1/A1 (T-18) from Santa Cruz Biotech. Bound antibodies were detected with horseradish peroxidase (HRP)-conjugated secondary antibodies and enhanced chemiluminescence (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK).
IKKα immunocomplex kinase assay
Cell lysates in KLB [kinase lysis buffer; 50 mM Tris pH 7.5, 250 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100, 0.5% NP-40, 10% glycerol, 0.5 mM dithiothreitol (DTT), 20 mM β-glycerophosphate, 10 mM NaF, 1 mM sodium vanadate and 0.5 mM phenylmethylsulfonyl fluoride (PMSF) on ice for 30 min] were immunoprecipitated with anti-IKKα (M-280) and protein A/G plus agarose (Santa Cruz Biotech) for 2 h at 4°C, washed with 400 mM NaCl/2 M urea KLB, then with 20 mM HEPES pH 7.5, 10 mM MgCl2, 20 mM β-glycerophosphate, 10 mM NaF, 1 mM NaH2PO4, 2 mM DTT, and then incubated with 5 µCi of [γ-32P]ATP and 5 µg of recombinant GST–IκBα (1–317) (Santa Cruz Biotech) at 30°C for 30 min. The samples were analyzed by SDS–PAGE, transferred to nitrocellulose membranes and subsequently probed with anti-IKKα antibody.
Electrophoretic mobility shift assay (EMSA)
A [32P]dATP-labeled double-stranded oligonucleotide spanning the κB site of murine IL-2Rα enhancer was incubated on ice for 20 min with 5 µg of nuclear extract, 2 µg of poly(dIdC) in 75 mM KCl, 10 mM Tris pH 7.5, 1 nM DTT and 20% glycerol and electrophoresed in a non-denaturing 4% polyacrylamide gel. Antibodies against p50 (sc-114-G), p52 (sc-298X), p65 (sc-109X) and c-myc (sc-42X) (Santa Cruz Biotech) were incubated for 20 min on ice before the binding reaction.
RNA analysis
Total RNA was isolated from thymocytes and splenocytes and processed further for northern blot analysis and RT–PCR (Felli et al., 1999). Sample loading was monitored by GAPDH or β-actin expression.
PCR was performed at an annealing temperature of 58°C using the following primers: β-actin, 5′-TCCCTGTATGCCTCTGGTGCTACCA C-3′ and 5′-CAGGATCTTCATGAGGTAGTCTGTCAG-3′; FasL, 5′-T CTCTGGAGCAGTCAGCGTCAGAG-3′ and 5′-GGTTCCCTGTTAA ATGGGCCACAC-3′; RORγ t, 5′-ATGGACAGGGCCCCACAGAGA C-3′ and 5′-CAAGTTCAGGACGCCTGGTTTCCTC-3′; preTαb, 5′ (5′-CTACCATCAGGGGAATCT-3′); preTαa, 5′ (5′-CTACCATCAG GCATCGCT-3′); preTαa,b, 3′ (5′-CTATGTCCAAATTCTGTGGGT G-3′); and γTCR, 5′-ACCGTTAACAACTACAGCAGACCATGAA-G-3′ and 5′-TCCTTCTGCAAATACCTTGTG-3′.
Analysis of TCR rearrangements
Genomic DNA was used as substrate of PCR using the following primers: Vβ8, 5′-TGGCAGCACTGAGAAAGGAGATAT-3′; Jβ2.5, 5′-GAGCC GAGTGCCTGGCCCAAAGTA-3′; Vγ4L, 5′-ACCGTTAACAACTAC AGCAGACCATGAAG-3′; Jγ1/2L, 5′-TCCTTCTGCAAATACCTTGT G-3′; Vδ6.3L, 5′-GTCACATGCCTCCTCACAGC-3′; and Jδ1L, 5′-AG TCACTTGGGTTCCTTGTCCAAAGACGAGTTTGTCGTT-3′. Ampli cons were cloned using the TA cloning kit (Invitrogen Co.), sequenced by an ABI Prism 377 DNA sequencer (Perkin-Elmer, Foster City, CA) and analyzed using Gene Works (IntelliGenetics Inc., Mountain View, CA) for multiple sequence comparison.
Adenoviral infection
N3-232T cells [107/ml RPMI medium, 10–5 M 2-mercaptoethanol, 10 mM HEPES pH 7, 2% fetal calf serum (FCS)] were infected with recombinant adenoviruses (2 × 109–2 × 1010 p.f.u./ml) Ad-IκBα carrying IκBα fused to a nuclear localization sequence, which was previously reported to inhibit the NF-κB activity (Wrighton et al., 1996), or with control Ad-βgal (Muhlhauser et al., 1995). After 1 h at 37°C, the viruses were washed off and cells were cultured in 10% FCS growth medium for 1–3 days. Detection of β-gal by FCA was performed on cells pre-incubated at 37°C for 5 min [107/ml in 0.1 ml of phosphate-buffered saline (PBS), 2% FCS], then loaded with pre-warmed 2 mM fluorescein di-β-d-galactopyranoside (Sigma, St Louis, MO), stopped by 300 µM chloroquine in ice-cold PBS and analyzed by FCA after 1 h incubation on ice.
Acknowledgments
Acknowledgements
We thank Drs M.C.Capogrossi, R.de Martin, I.Verma, G.Franzoso and F.Guillemot for providing Ad-βgal and Ad-IκBα viruses, expression vector plasmids and HES1 and HES5 cDNAs, Dr L.Pozzi for assistance in generation of transgenic mice, B.Milana, P.Birarelli and Dr S.Morrone for FCA and cell sorting, and M.Zani for DNA sequencing. This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), the Consiglio Nazionale delle Ricerche (CNR), Biotechnology Project, the Ministero dell’Università e della Ricerca Scientifica e Tecnologica, the Telethon Foundation (B40 Project), the Wellcome Trust (UK) and the National Institutes of Health (USA).
References
- Alesse E., Zazzeroni,F., Angelucci,A., Giannini,G., Di Marcotullio,L. and Gulino,A. (1998) The growth arrest and downregulation of c-myc transcription induced by ceramide are related events dependent on p21 induction, Rb underphosphorylation and E2F sequestering. Cell Death Differ., 5, 381–389. [DOI] [PubMed] [Google Scholar]
- Apelqvist A., Li,H., Sommer,L., Beatus,P., Anderson,D.J., Honjio,T., Hrabe de Angelis,M., Lendahl,U. and Edlund,H. (1999) Notch signalling controls pancreatic cell differentiation. Nature, 400, 877–881. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Rand,M.D. and Lake,R. (1999) Notch signaling: cell fate control and signal integration in development. Science, 284, 770–776. [DOI] [PubMed] [Google Scholar]
- Bakker T.R., Renno,T. and Jongeneel,C.V. (1999) Impaired fetal thymocyte development after efficient adenovirus-mediated inhibition of NF-κB activation. J. Immunol., 162, 3456–3462. [PubMed] [Google Scholar]
- Barber D.F., Passoni,L., Wen,L., Geng,L. and Hayday,A.C. (1998) The expression in vivo of a second isoform of pTα: implications for the mechanism of pTα action. J. Immunol., 161, 11–16. [PubMed] [Google Scholar]
- Beatus P., Lundkvist,J., Oberg,C. and Lendahl,U. (1999) The Notch3 intracellular domain represses Notch1-mediated activation through Hairy/Enhancer of split (HES) promoters. Development, 126, 3925–3935. [DOI] [PubMed] [Google Scholar]
- Boothby M.R., Mora,A.L., Scherer,D.C., Brockman,J.A. and Ballard,D.W. (1997) Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-κB. J. Exp. Med., 185, 1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R.L. and Hugo,P. (1991) Towards an integrated view of thymopoiesis. Immunol. Today, 12, 71–79. [DOI] [PubMed] [Google Scholar]
- Carrasco D., Rizzo,C.A., Dorfman,K. and Bravo,R. (1996) The v-rel oncogene promotes malignant T-cell leukemia/lymphoma in transgenic mice. EMBO J., 15, 3640–3650. [PMC free article] [PubMed] [Google Scholar]
- Chaffin K.E., Beals,C.R., Wilkie,T.M., Forbush,K.A., Simon,M.I. and Perlmutter,R.M. (1990) Dissection of thymocyte signaling pathways by in vivo expression of pertussis toxin ADP-ribosyltransferase. EMBO J., 9, 3821–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton T., Moore,M., MacDonald,H.R. and Malissen,B. (1994) Double-negative thymocyte subsets in CD3ζ chain-deficient mice: absence of HSA+CD44–CD25– cells. Eur. J. Immunol., 24, 1903–1907. [DOI] [PubMed] [Google Scholar]
- De Grazia U. et al. (1994) Positive and negative regulation of the composite octamer motif of the interleukin 2 enhancer by AP-1, Oct-2 and retinoic acid receptor. J. Exp. Med., 180, 1485–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deftos M.L., He,Y.-W., Ojala,E.W. and Bevan,M.J. (1998) Correlating Notch signaling with thymocyte maturation. Immunity, 9, 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley E.C., Petrie,H.T., Shah,L.M., Owen,M.J. and Hayday,A.C. (1994) T cell receptor β chain gene rearrangement and selection during thymocyte development in adult mice. Immunity, 1, 83–93. [DOI] [PubMed] [Google Scholar]
- Ellisen L.W., Bird,J., West,D.C., Soreng,A.L., Reynolds,T.C., Smith,S.D. and Sklar,J. (1991) TAN-1, the human homolog of the Drosophila Notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell, 66, 649–661. [DOI] [PubMed] [Google Scholar]
- Felli M.P. et al. (1999) Expression pattern of Notch1, 2 and 3 and Jagged1 and 2 in lymphoid and stromal thymus components: distinct ligand–receptor interactions in intrathymic T cell development. Int. Immunol., 11, 1017–1025. [DOI] [PubMed] [Google Scholar]
- Ferreira V., Sidenius,N., Tarantino,N., Hubert,P., Chatenoud,L., Blasi,F. and Korner,M. (1999) In vivo inhibition of NF-κB in T-lineage cells leads to a dramatic decrease in proliferation and cytokine production and to increased cell apoptosis in response to mitogenic stimuli, but not to abnormal thymopoiesis. J. Immunol., 162, 6442–6450. [PubMed] [Google Scholar]
- Franzoso G., Bours,V., Park,S., Tomita-Yamaguchi,M., Kelly,K. and Siebenlist,U. (1992) The candidate oncoprotein Bcl-3 is an antagonist of p50/NFκB-mediated inhibition. Nature, 359, 339–342. [DOI] [PubMed] [Google Scholar]
- Franzoso G., Bours,V., Azarenko,V., Park,S., Tomita-Yamaguchi,M., Kanno,T. and Siebenlist,U. (1993) The oncoprotein Bcl-3 can facilitate NF-κB-mediated transactivation by removing inhibiting p50 homodimers from select κB sites. EMBO J., 12, 3893–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleziunas R. et al. (1998) Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase α (IKKα) and IKKβ cellular kinases. Mol. Cell. Biol., 18, 5157–5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey D.I., Kennedy,J., Suda,T. and Zlotnik,A. (1993) A developmental pathway involving four phenotypically and functionally distinct subsets of CD3–CD4–CD8– triple negative adult mouse thymocytes defined by CD44 and CD25 expression. J. Immunol., 150, 4244–4252. [PubMed] [Google Scholar]
- Guan E., Wang,J., Laborda,J., Norcross,M., Bauerle,P.A. and Hoffman,T. (1996) T cell leukemia-associated human Notch/Translocation-associated Notch homologue has Iκ-B-like activity and physically interacts with nuclear factor-κB proteins in T cells. J. Exp. Med., 183, 2025–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.-W., Deftos,M.L., Ojala,E. and Bevan,M.J. (1998) RORγ t, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity, 9, 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E.S., Passoni,L., Crompton,T., Leu,T.M.J., Schatz,D.G., Koff,A., Owen,M.J. and Hayday,A.C. (1996) Productive T-cell receptor β-chain gene rearrangement: coincident regulation of cell cycle and clonality during development in vivo. Genes Dev., 10, 948–962. [DOI] [PubMed] [Google Scholar]
- Israel A. (1995) A role for phosphorylation and degradation in the control of NF-κB activity. Trends Genet., 11, 203–205. [DOI] [PubMed] [Google Scholar]
- Jameson S. and Bevan,M. (1998) T-cell selection. Curr. Opin. Immunol., 10, 214–219. [DOI] [PubMed] [Google Scholar]
- Jenkinson E.J., Kingstone,R. and Owen,J.J.T. (1987) Importance of IL2 receptors in intrathymic generation of cells expressing T cell receptors. Nature, 329, 160–162. [DOI] [PubMed] [Google Scholar]
- Lardelli M., Williams,R., Mitsiadis,T. and Lendahl,U. (1996) Expression of the Notch3 intracellular domain in mouse central nervous system progenitors cells is lethal and leads to disturbed neural tube development. Mech. Dev., 59, 177–190. [DOI] [PubMed] [Google Scholar]
- MacDonald H.R. and Wilson,A. (1998) The role of T-cell receptor (TCR) in αβ/γδ lineage commitment: clues for intracellular TCR staining. Immunol. Rev., 165, 87–94. [DOI] [PubMed] [Google Scholar]
- Maroder M. et al. (1996) Expression of TrκB neurotrophin receptor during T cell development. Role of brain derived neurotrophic factor in immature thymocyte survival. J. Immunol., 157, 2864–2872. [PubMed] [Google Scholar]
- Mori N., Fujii,M., Ikeda,S., Yamada,Y., Tomonaga,M., Ballard,D.W. and Yamamoto,N. (1999) Constitutive activation of NF-κB in primary adult T-cell leukemia cells. Blood, 93, 2360–2368. [PubMed] [Google Scholar]
- Muhlhauser J. et al. (1995) VEGF165 expressed by a replication-deficient recombinant adenovirus vector induces angiogenesis in vivo. Circ. Res., 77, 1077–1086. [DOI] [PubMed] [Google Scholar]
- Oswald F., Liptay,S., Adler,G. and Schmid,R.M. (1998) NFκB2 is a putative target gene of activated Notch1 via RBP-Jκ. Mol. Cell. Biol., 18, 2077–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl H.L. (1999) Activators and target genes of Rel/NFκB transcription factors. Oncogene, 18, 6853–6866. [DOI] [PubMed] [Google Scholar]
- Pear W.S., Aster,J.C., Scott,M.L., Hasserjian,R.P., Soffer,B., Sklar,J. and Baltimore,D. (1996) Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J. Exp. Med., 183, 2283–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui J.C. et al. (1999) Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity, 11, 299–308. [DOI] [PubMed] [Google Scholar]
- Radtke F., Wilson,A., Stark,G., Bauer,M., van Meerwijk,J., MacDonald,H.R. and Aguet,M. (1999) Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity, 10, 547–558. [DOI] [PubMed] [Google Scholar]
- Rayet B. and Gélinas,C. (1999) Aberrant rel/nf-κb genes and activity in human cancer. Oncogene, 18, 6938–6947. [DOI] [PubMed] [Google Scholar]
- Robey E., Chang,D., Itano,A., Cado,D., Alexander,H., Lans,D., Weinmaster,G. and Salmon,P. (1996) An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell, 87, 483–492. [DOI] [PubMed] [Google Scholar]
- Tomita K., Hattori,M., Nakamura,E., Nakanishi,S., Minato,N. and Kageyama,R. (1999) The bHLH gene Hes1 is essential for expansion of early T cell precursors. Genes Dev., 13, 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Antwerp D.J., Martin,S.J., Kafri,T., Green,D.R. and Verma,I.M. (1996) Suppression of TNFα-induced apoptosis by NF-κB. Science, 274, 787–789. [DOI] [PubMed] [Google Scholar]
- Villey I., de Chasseval,R. and de Villartay,J.-P. (1999) RORγT, a thymus specific isoform of the orphan nuclear receptor RORγ/TOR, is up-regulated by signaling through the pre-T cell receptor and binds to the TEA promoter. Eur. J. Immunol., 29, 4072–4080. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Aifantis,I., Feinberg,J., Lechner,O., Saint-Ruf,C., Walter,U., Buer,J. and Azogui,O. (1999) Pleiotropic changes controlled by the pre-T-cell receptor. Curr. Opin. Immunol., 11, 135–142. [DOI] [PubMed] [Google Scholar]
- Washburn T., Schweighoffer,E., Gridley,T., Chang,D., Fowlkes,B.J., Cado,D. and Robey,E. (1997) Notch activity influences the αβ versus γδ T cell lineage decision. Cell, 88, 833–843. [DOI] [PubMed] [Google Scholar]
- Wrighton C.J., Hofer-Warbinek,R., Moll,T., Eytner,R., Bach,F.H. and de Martin,R. (1996) Inhibition of endothelial cell activation by adenovirus-mediated expression of IκBα, an inhibitor of the transcription factor NF-κB. J. Exp. Med., 183, 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong W.-X., Edelstein,L.C., Chen,C., Bash,J. and Gélinas,C. (1999) The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-κB that blocks TNFα-induced apoptosis. Genes Dev., 13, 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zùniga-Pflucker J.C., Schwartz,H.L. and Lenardo,M.J. (1993) Gene transcription in differentiating immature T cell receptorneg thymocytes resembles antigen-activated mature T cells. J. Exp. Med., 178, 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]