Abstract
Post-ictal depression of consciousness occurs after generalized convulsive seizures, and includes analgesia, lasting for hours after electrically- or chemically-induced seizures in animals. The brain sites and mechanisms, mediating post-ictal analgesia, are unclear. The ventrolateral periaqueductal gray (PAG) is an important neuronal network site for mediating analgesia and also in generalized seizures, particularly in genetically epilepsy-prone rats (GEPRs). Endocannabinoids are implicated in mediating analgesia in several brain sites, including the PAG, and generalized seizures result in endocannabinoid release. This study evaluated if post-ictal analgesia occurs in GEPRs, following audiogenic seizures (AGS), and whether this analgesia involves endocannabinoid actions in PAG. Analgesia was evaluated, using thermal stimulation to evoke nociception, measuring changes in paw withdrawal latencies (PWLs) induced by AGS. Endocannabinoid involvement in post-ictal analgesia in GEPRs was evaluated, using focal bilateral microinjection of a cannabinoid (CB1) receptor antagonist (AM251) into PAG. AGS induced a significant increase in PWLs, lasting for ≥120 min. Microinjection of AM251 (100 and 200, but not 50 pmoles/side) into PAG significantly decreased post-ictal analgesia in GEPRs. Endocannabinoids are also known to activate transient receptor potential vanilloid (TRPV1) receptors, but PAG microinjection of a TRPV1 receptor antagonist (capsazepine) did not affect post-ictal analgesia in GEPRs. These results indicate that AGS in GEPRs induce post-ictal analgesia, which is the first observation of this phenomenon in a genetic epilepsy model. These findings suggest an important role of PAG in post-ictal analgesia. The results also suggest that CB1 receptors in PAG are critical for mediating post-ictal analgesia in GEPRs.
Keywords: PAG, Post-ictal analgesia, GEPR, Cannabinoid receptor 1, Endocannabinoids, TRPV1, Pain, Descending modulation
1. Introduction
Several studies have demonstrated that post-ictal analgesia is induced, following electroshock or drug-induced generalized seizures in rats (Coimbra et al., 2001; De Oliveira et al., 2006; Portugal-Santana et al., 2004; Urca et al., 1981). Reduced responsiveness to pain is also known to occur in certain forms of human epilepsy (Guieu et al., 1992). Genetically epilepsy-prone rats (GEPRs) are an inherited model of epilepsy that exhibits generalized tonic-clonic seizures in response to high intensity acoustic stimuli (Jobe and Laird, 1981). Previous studies have also shown that GEPRs exhibit greater susceptibility than normal rats to seizures induced by convulsant drugs, kindling, electroshock and hyperthermia, and GEPRs of the substrain GEPR-9 are also susceptible to severe audiogenic seizures (AGS) (Consroe and Edmonds, 1979; Faingold, 1999; Jobe et al., 1986).
The ventrolateral periaqueductal gray (PAG) is known to be a major nucleus in the network that mediates analgesia (Arvidsson et al., 1995) as well as generalized seizures, particularly in GEPRs (N'Gouemo and Faingold, 1999; Raisinghani and Faingold, 2003; Yang et al., 2003). Electrical stimulation of PAG is known to induce analgesia both in humans and animals (Mayer, 1984; Reynolds, 1969). The PAG plays a key role in the descending modulation of nociception by projecting via the rostral ventromedial medulla to spinal cord dorsal horn neurons (Liebeskind et al., 1973; Moreau and Fields, 1986; Urban and Smith, 1994).
Previous studies have suggested that the PAG is also an important site for endocannabinoid-mediated analgesia (Hohmann et al., 2005; Maione et al., 2006). Endocannabinoids, such as anandamide and 2-arachidonoyl glycerol, are lipid neuromodulators in the brain and activate cannabinoid (CB1 and CB2) receptors, which both modulate nociception. Endocannabinoids are also known to activate transient receptor potential vanilloid (TRPV1) receptors and to exert analgesic effects. Endocannabinoids are released, following seizure induction in rats (Wallace et al., 2001, 2002, 2003). Recent data also indicate that cannabinoid CB1 receptors are expressed in the PAG and may play a role in analgesia (Maione et al., 2006). We evaluated if induction of audiogenic seizures (AGS) in GEPRs would induce post-ictal analgesia. We also examined whether blockade of CB1 receptors or TRPV1 receptors by focal microinjection of a CB1 antagonist or TRPV1 antagonist into the PAG would inhibit any analgesic effects that occurred post-ictally in GEPRs.
2. Results
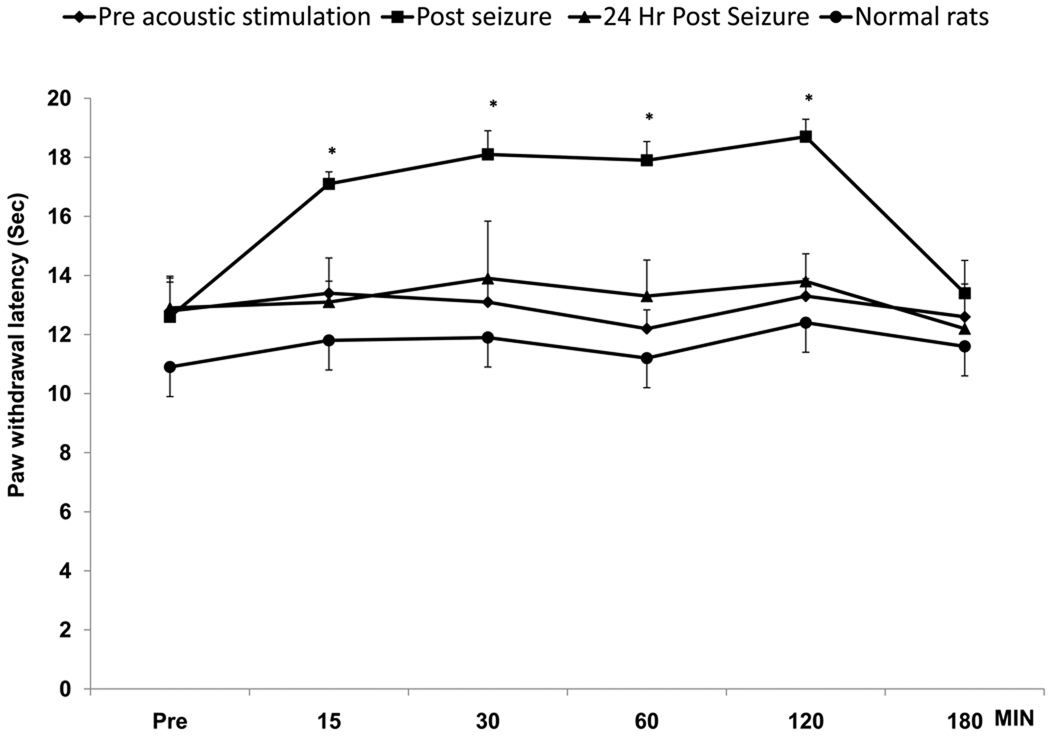
GEPRs showed a significant increase in PWLs after AGS induction as compared to pre-seizure baseline and compared to the PWLs 24 hr later (Fig.1). The increase in PWLs observed post-ictally was significant at 15, 30, 60 and 120 min after AGS (p<0.05, One-way ANOVA). The analgesic effect was no longer present by 180 min in all GEPRs. Audiogenic seizure severity is evaluated using the scale of Jobe (Dailey and Jobe, 1985) wherein, a score of 3 denotes generalized clonus, a score of 5 denotes hind limb tonic flexion, and a score of 9 denotes tonic hind-limb extension. The seizure severity of GEPRs in the present study did not have any significant effect on the incidence or degree of post-ictal increase in PWLs. Thus, GEPRs with seizures ending in generalized clonus (n=6), hind limb tonic flexion (n=6) or tonic hind-limb extension (n=12) all exhibited similar and significant increases in PWLs relative to baseline PWLs following AGS [F (3,23) = 401.2; p<0.05 by one-way ANOVA]. Presentation of the acoustic stimulus did not have any significant effect on PWLs in normal rats. GEPRs demonstrated normal exploratory behavior after induction of seizures, which suggests that the elevated PWLs were not related to motor deficits.
Fig. 1.
Induction of audiogenic seizures (AGS) in genetically epilepsy-prone rats (GEPRs) resulted in post-ictal analgesia indicated by increased paw withdrawal latencies (PWLs) as compared to baseline PWLs taken prior to acoustic stimulation (Pre) and 24 hr post-seizure. The PWLs to thermal stimulation were measured at 15, 30, 60, 120 and 180 min after AGS. The increases in PWLs were significant at 15, 30, 60 and 120 min. Presentation of the acoustic stimulus did not have any significant effect on PWLs in normal rats (N=6).
* denotes significant differences p<0.05 vs. baseline (Pre) and 24 hr post. (Repeated measure ANOVA test). Error bars represent SEM; N=6 rats.
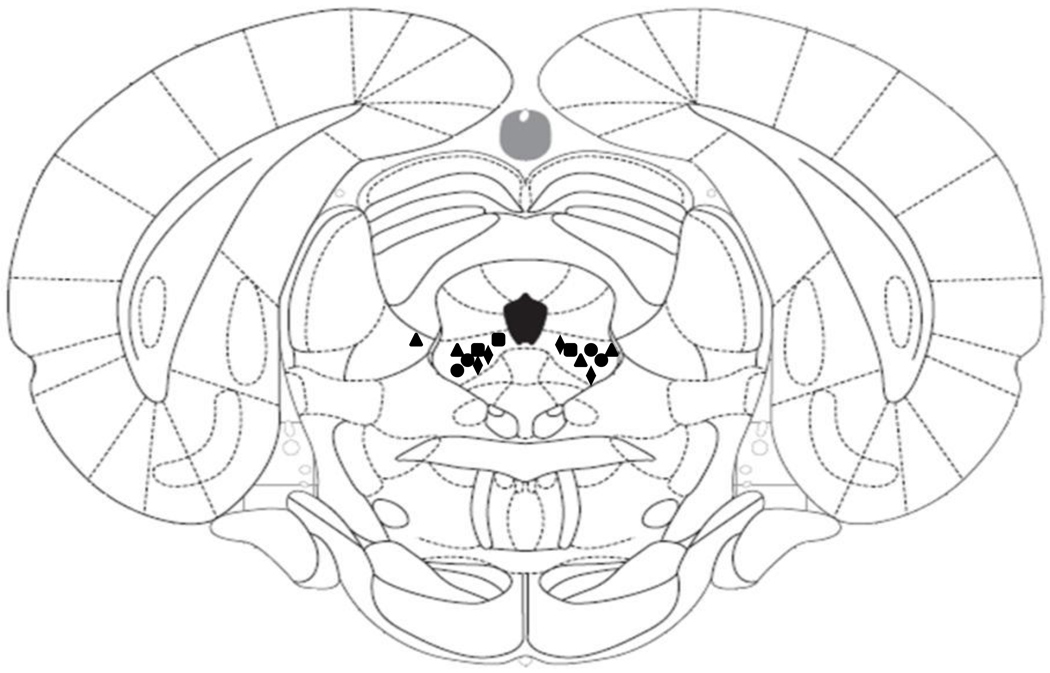
In microinjection studies, histological evaluation of cannula sites indicated that most cannula placements were localized bilaterally to the ventrolateral PAG, as seen in Fig. 2. The sites that were localized outside of the PAG were analyzed separately.
Fig. 2.
Representative ventrolateral periaqueductal gray (PAG) microinjection sites indicate that most cannula placements for microinjection were localized to the PAG, according to the atlas of Paxinos and Watson (2005). The results of near misses were analyzed separately. Squares indicate 200 pmoles/side AM251; circles indicate 100 pmoles/side AM251; triangles indicate 50 pmoles/side AM251; Diamonds indicate capsazepine microinjection sites.
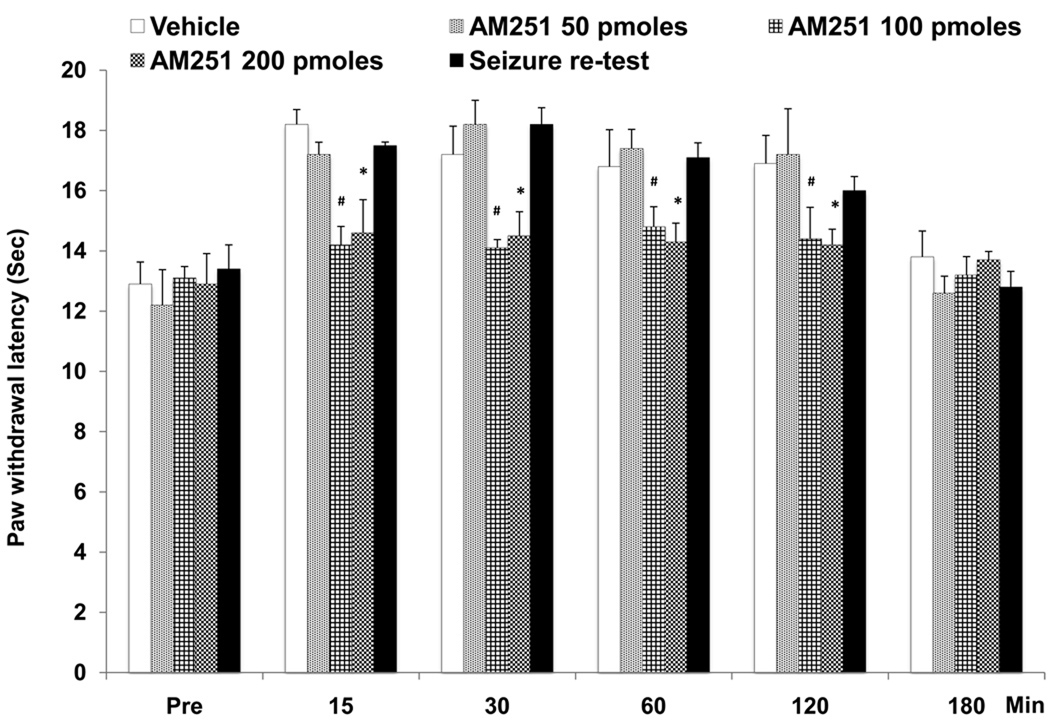
PWLs were recorded before and 7 days after cannula implantation surgery in all rats, and no significant differences were observed. The PWLs were not significantly different between the various animal groups prior to administration of drug or vehicle. Focal bilateral microinjection of the CB1 receptor antagonist, AM251 (100 pmoles and 200 pmoles/side) into the PAG significantly decreased post-ictal analgesia. The decrease in analgesia was observed at 15, 30, 60 and 120 min after AGS (Fig. 3) as compared to vehicle microinjection [ F (1,5) = 524.53; p<0.05 by repeated ANOVA], time versus effect [F (4, 20) = 5.806; p<0.05] and treatment versus time interaction [F (1,5) = 4.58; p<0.05] after microinjection. The dose of 50 pmoles/side of AM251 did not produce a significant change in PWLs. Post-ictal analgesia was no longer present 180 min after microinjection. Bilateral focal microinjection of vehicle into the PAG produced no significant change in the increased PWLs observed post-ictally (Fig.3). The few (N=4) microinjection sites that were localized outside of the PAG were found to induce minimal effects on PWLs and were not statistically significant.
Fig. 3.
Microinjection of cannabinoid (CB1) receptor antagonist, AM251 (200 and 100 pmoles/side) into PAG significantly decreased post-ictal analgesia as measured by changes in PWLs in GEPRs at 15, 30, 60 and 120 min as compared to vehicle. (Note: Baseline PWLs to thermal stimulation were studied 15 min prior to seizure (Pre).The lower dose of AM251 (50 pmoles/side) was ineffective. Seizure retest after 24 hrs was done on all GEPRs (N=24) and showed that increases in PWLs could be induced again.
* denotes significant difference at p<0.05; # denotes significant difference at p<0.01; (Repeated measure ANOVA test). Error bars represent SEM; N= 6 rats per treatment.
All the GEPRs were again subjected to AGS 24 hr after microinjection of the CB1 antagonist and these animals again showed significantly increased PWLs. Thus, there were no residual effects of the PAG microinjections on the ability to induce post-ictal analgesia.
Since endocannabinoids are also known to activate TRPV1 receptors (Maione et al., 2006), we also examined the role of these receptors in the PAG in AGS-induced post-ictal analgesia by administering a TRV1 antagonist. Intra-PAG microinjection of the TRPV1 receptor antagonist, capsazepine (1, 5 and 10 nmoles in DMSO) or the vehicle [F (1,5) = 1.081; p>0.05 by repeated ANOVA], time versus effect [F (4, 20) = 0.148; p>0.05] and treatment versus time interaction [F (1,5) = 822.26; p>0.05] did not have any significant effect on post-ictal analgesia observed after AGS induction.
3. Discussion
The current results indicate that AGS induction in the GEPRs leads to post-ictal analgesia. The analgesic response observed after seizures was not affected by seizure severity. Thus, the GEPRs that exhibited seizures ending in generalized clonus, complete tonic extension or tonic hind limb extension, which are differing degrees of seizure severity according to the established AGS severity scale (Jobe et al., 1986), all showed a similar degree and duration of post-ictal analgesia. Although, all the GEPRs were derived from the severe seizure strain, and all exhibited tonic extension when tested at 10 weeks of age, AGS in some GEPRs become less severe in older animals, as seen in the present study.
The duration of post-ictal analgesia in the present study was greater than 120 min but less than 180 min after generalized convulsive seizures. The duration of analgesic effects observed in this study are consistent with the previous studies that observed post-ictal analgesia after tonic-clonic convulsions induced by convulsant drugs or electroshock. (Coimbra et al., 2001; De Oliveria et al., 2006; Freitas et al., 2008, 2009; Portugal-Santana et al., 2004). To our knowledge the present findings are the first report of post-ictal analgesia in a genetic epilepsy model and may have relevance to epileptic patients, since long-lasting pain threshold elevations are seen in certain forms of human epilepsy (Guieu et al., 1992).
Previous studies on post-ictal analgesia used the tail-flick test as a nociceptive assay, which measures the latency of the avoidance response to a thermal stimulus in rodents (Coimbra et al., 2001; De Oliveria et al., 2006; Freitas et al., 2008). In the current study, we used the PWL test in which radiant heat is applied, and the latency for the rat to withdraw and lick its paw, allows evaluation of both the sensory (paw raising) and emotional (paw licking) aspects of pain. We would expect similar results using both tests.
The involvement of the PAG in post-ictal analgesia in the present study is consistent with previous studies, which observed that electrical stimulation of PAG results in analgesia in humans and in rats (Mayer, 1984; Reynolds, 1969). The present study also showed that the CB1 receptor antagonist, AM251, blocked post-ictal analgesia, suggesting that activation of endocannabinoid receptors in PAG plays a major role in post-ictal analgesia after AGS in GEPRs. Endocannabinoids are also known to activate TRPV1 receptors. Previous studies have shown that PAG neurons express TRPV1 receptors, but microinjection of the TRPV1 receptor antagonist, capsazepine, into PAG did not affect post-ictal analgesia in the present study.
Endocannabinoid mechanisms are strongly implicated in endogenous antinociceptive mechanisms (Hohmann et al., 2005; Suplita et al., 2005). Release of endocannabinoids has previously been observed following induction of seizures by convulsant drugs (Wallace et al., 2003). The onset of analgesia observed in the present study is consistent with the time course of endocannabinoid release after seizure induction observed in the previous studies (Wallace et al., 2003).
Earlier studies suggested that CB1 receptors in the PAG modulate local inhibitory networks to regulate nociception (Vaughan et al., 2000). Several studies suggest that CB1 receptor-mediated analgesia may involve disinhibition of PAG antinociceptive output neurons by inhibition of inhibitory interneurons in PAG (Meng et al., 1998; Pan et al., 1990; Vaughan et al., 1999).
The current study indicates that AGS in a genetic model of epilepsy, GEPRs, induces post-ictal analgesia and implicates the PAG in the analgesic response observed post-ictally, since the response can be blocked by microinjection into this structure. The blockade of post-ictal analgesia by microinjection of the CB1 antagonist into the PAG suggests that seizures result in elevation of the endocannabinoid levels in the PAG, activating CB1 receptors, which may, in part, mediate the post-ictal analgesia observed in GEPRs. Further work is needed to provide additional experimental evaluation of these findings.
4. Materials and methods
4.1 Animals
Twenty-four GEPRs (250–450 g, 6 males and 18 females) from the severe seizure strain (GEPR-9s) were screened for consistent AGS susceptibility at three weekly intervals according to established screening procedures (Dailey and Jobe, 1985). GEPRs used in the study were bred in the animal care facility of Southern Illinois University School of Medicine. Male Sprague Dawley (SD) rats (250–350 g; Harlan Laboratories) were also utilized in this study. The experimental protocol used in this study includes measures to lessen pain and discomfort and also to use the minimum number of animals and was approved by the Southern Illinois University School of Medicine Laboratory Animal Care and Use Committee.
4.2 Cannula implantation
The chronic stereotaxic implantations of guide cannulae were performed bilaterally under ketamine/xylazine anesthesia (85/5 mg/kg, i.p.). Rats were placed in a stereotaxic device, and the incisor bar was set at −3.3 mm in the vertical plane to hold the skull in the flat position. Stainless steel guide cannulae (21 G) were implanted stereotaxically over the PAG (7.6 posterior from bregma; ±3.6 mediolateral from the midline) according to the atlas of Paxinos and Watson (2005) and cemented into place with dental acrylic. Stylets were placed in the guide cannulae to prevent clogging.
4.3 Microinjection technique
At least one week after surgical implantation, each rat received a single bilateral microinjection of the vehicle or drug. The infusion (0.5 µl/side, at 0.25 µl/min for 2 min) was delivered via the infusion cannulae (26 G) inserted to a vertical depth of 5.4 mm into PAG through the guide cannulae and left in place for 30 s. Using these microinjection parameters, the distribution of drugs was limited to a radius of ~0.5 mm around the microinjection site in previous studies (Millan et al., 1986). All microinjections were done 5 min after seizure testing in order to avoid potential interference with seizure susceptibility.
4.4 Acoustic stimulation
AGS were induced with an electrical bell (122 dB SPL, re: 0.0002 dyn/cm2) mounted above a plastic cylinder (40 cm diameter) within a sound-attenuating chamber. The stimulus was presented until the onset of AGS or for a maximum duration of 60 s. The behavioral responses to acoustic stimulation were recorded on videotape, which were subsequently analyzed for the incidence and behavioral pattern of the convulsion. Normal Sprague-Dawley rats (N=6), the strain from which GEPRs were derived, were also subjected to the same acoustic stimulation and served as controls.
4.5 Behavioral analgesic testing
Evaluation of thermal nociception was performed after the rat had been acclimatized for 30 min in the testing apparatus (Ugo Basile, Camerio, Italy), which included a clear plastic box on a glass surface. A calibrated radiant heat source was focused sequentially on both hind paws, and the latency to paw withdrawal was recorded, using a 20-s maximum exposure to avoid tissue injury (Hargreaves et al., 1988). Paw withdrawal latencies (PWLs) were measured three times each for both hind paws. A mean of these three observations was calculated (Hargreaves et al., 1988).
The PWLs to thermal stimulation were measured at 15, 30, 60,120 and 180 min after AGS. In PAG studies, the microinjection began 5 min after cessation of the seizure, when the rat had regained the righting reflex. Baseline PWLs to thermal stimulation were studied 15 min prior to seizure (Pre). The effects of microinjection of a CB1 antagonist, TRPV1, or vehicle on PWLs to thermal stimulation were studied 15 min prior to seizure and at 15 to 180 min after microinjection. Seizure re-test was done 24 hrs later to see if post-ictal analgesia could be induced again after recovery from drug treatment.
4.6 Drugs
The agents used included, CB1 receptor antagonist N-(piperidin-1yl)-5-(4-iodophenyl)-1-(2, 4-dichlorophenyl)-4-methyl-H-pyrazole-3-carboxamide (AM251), (50, 100 and 200 pmoles/side), the TRPV1 antagonist, capsazepine (1,5, and 10 nmoles/side) (Tocris Bioscience, Missouri, USA). The drugs for microinjection were dissolved in vehicle [0.2 % dimethyl sulfoxide (DMSO)] in saline. The solutions were prepared just before use and protected from the light during the experimental sessions. A control group of GEPRs received microinjection of the vehicle only.
4.7 Histology
At the end of the microinjection experiments, rats were sacrificed by administering pentobarbital (100 mg/kg, i.p), and 0.5µl of fast green dye (2%) was microinjected at a rate of 0.25 µl/s. through an infusion cannula, to allow identification of the cannula sites. Brains were fixed by cardiac injection of normal saline followed by formalin. The brains were subsequently removed and stored in formalin. Coronal sections (40 µm) were taken using a freezing microtome, and the tissue was stained with cresyl violet to identify the sites of microinjection.
4.8 Statistical analysis
One-way analysis of variance ANOVA and repeated measure was used for statistical analysis of post-ictal analgesia in GEPRs after AGS. This involved comparison of mean ± SEM PWLs of the vehicle-treated group with the different doses of cannabinoid antagonist, AM251 and TRPV1 antagonist, capsazepine, as well as within the group by subjecting the data to repeated measure. To analyze treatment versus time interaction, one-way ANOVAs followed by Duncan test, at each time interval, were performed. P < 0.05 was used to determine statistically significant differences.
Acknowledgements
The authors wish to thank Marcus Randall for technical assistance, Stephen Verhulst, Ph.D. for statistical assistance and Diana Smith and Trish Ellis for manuscript assistance. This work was supported by grants from National Institutes of Health (NS042296 and DK065742) and EAM award from SIUSOM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J.Neurosci. 1995;15(5 Pt 1):3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra NC, Freitas RL, Savoldi M, Castro-Souza C, Segato EN, Kishi R, Weltson A, Resende GC. Opioid neurotransmission in the post-ictal analgesia: involvement of mu(1)-opioid receptor. Brain Res. 2001;903(1–2):216–221. doi: 10.1016/s0006-8993(01)02366-6. [DOI] [PubMed] [Google Scholar]
- Consroe P, Edmonds HL., Jr Genetic animal models of epilepsy. Introduction. Fed.Proc. 1979;38(10):2397–2398. [PubMed] [Google Scholar]
- Dailey JW, Jobe PC. Anticonvulsant drugs and the genetically epilepsy-prone rat. Fed.Proc. 1985;44(10):2640–2644. [PubMed] [Google Scholar]
- De Oliveira RC, de Oliveira R, Ferreira CM, Coimbra NC. Involvement of 5-HT(2) serotonergic receptors of the nucleus raphe magnus and nucleus reticularis gigantocellularis/paragigantocellularis complex neural networks in the antinociceptive phenomenon that follows the post-ictal immobility syndrome. Exp.Neurol. 2006;201(1):144–153. doi: 10.1016/j.expneurol.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Faingold CL. Neuronal networks in the genetically epilepsy-prone rat. Adv.Neurol. 1999;79:311–321. [PubMed] [Google Scholar]
- Freitas RL, Bassi GS, de Oliveira AM, Coimbra NC. Serotonergic neurotransmission in the dorsal raphe nucleus recruits in situ 5-HT(2A/2C) receptors to modulate the post-ictal antinociception. Exp.Neurol. 2008;213(2):410–418. doi: 10.1016/j.expneurol.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Freitas RL, Ferreira CM, Urbina MA, Marino AU, Carvalho AD, Butera G, de Oliveira AM, Coimbra NC. 5-HT1A/1B, 5-HT6, and 5-HT7 serotonergic receptors recruitment in tonic-clonic seizure-induced antinociception: role of dorsal raphe nucleus. Exp.Neurol. 2009;217(1):16–24. doi: 10.1016/j.expneurol.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Guieu R, Mesdjian E, Roger J, Dano P, Pouget J, Serratrice G. Nociceptive threshold in patients with epilepsy. Epilepsy Res. 1992;12(1):57–61. doi: 10.1016/0920-1211(92)90092-8. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435(7045):1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Jobe PC, Laird HE. Neurotransmitter abnormalities as determinants of seizure susceptibility and intensity in the genetic models of epilepsy. Biochem.Pharmacol. 1981;30(23):3137–3144. doi: 10.1016/0006-2952(81)90510-4. [DOI] [PubMed] [Google Scholar]
- Jobe PC, Dailey JW, Reigel CE. Noradrenergic and serotonergic determinants of seizure susceptibility and severity in genetically epilepsy-prone rats. Life Sci. 1986;39(9):775–782. doi: 10.1016/0024-3205(86)90455-8. [DOI] [PubMed] [Google Scholar]
- Liebeskind JC, Guilbaud G, Besson JM, Oliveras JL. Analgesia from electrical stimulation of the periaqueductal gray matter in the cat: behavioral observations and inhibitory effects on spinal cord interneurons. Brain Res. 1973;50(2):441–446. doi: 10.1016/0006-8993(73)90748-8. [DOI] [PubMed] [Google Scholar]
- Maione S, Bisogno T, de N V, Palazzo E, Cristino L, Valenti M, Petrosino S, Guglielmotti V, Rossi F, Di M. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J.Pharmacol.Exp.Ther. 2006;316(3):969–982. doi: 10.1124/jpet.105.093286. [DOI] [PubMed] [Google Scholar]
- Mayer DJ. Analgesia produced by electrical stimulation of the brain. Prog.Neuropsychopharmacol.Biol.Psychiatry. 1984;8(4–6):557–564. doi: 10.1016/0278-5846(84)90015-0. [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395(6700):381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- Millan MH, Patel S, Mello LM, Meldrum BS. Focal injection of 2-amino-7-phosphonoheptanoic acid into prepiriform cortex protects against pilocarpine-induced limbic seizures in rats. Neurosci.Lett. 1986;70(1):69–74. doi: 10.1016/0304-3940(86)90439-8. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Fields HL. Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res. 1986;397(1):37–46. doi: 10.1016/0006-8993(86)91367-3. [DOI] [PubMed] [Google Scholar]
- N'Gouemo P, Faingold CL. The periaqueductal grey is a critical site in the neuronal network for audiogenic seizures: modulation by GABA(A), NMDA and opioid receptors. Epilepsy Res. 1999;35(1):39–46. doi: 10.1016/s0920-1211(98)00128-4. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT, Osborne PB. Opioid actions on single nucleus raphe magnus neurons from rat and guinea-pig in vitro. J.Physiol. 1990;427:519–532. doi: 10.1113/jphysiol.1990.sp018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. fifth ed. San Diego: Academic Press; 2005. [Google Scholar]
- Portugal-Santana P, Doretto MC, Tatsuo MA, Duarte ID. Involvement of prolactin, vasopressin and opioids in post-ictal antinociception induced by electroshock in rats. Brain Res. 2004;1003(1–2):1–8. doi: 10.1016/j.brainres.2003.09.083. [DOI] [PubMed] [Google Scholar]
- Raisinghani M, Faingold CL. Identification of the requisite brain sites in the neuronal network subserving generalized clonic audiogenic seizures. Brain Res. 2003;967(1–2):113–122. doi: 10.1016/s0006-8993(02)04232-4. [DOI] [PubMed] [Google Scholar]
- Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164(878):444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Suplita RL, Farthing JN, Gutierrez T, Hohmann AG. Inhibition of fatty-acid amide hydrolase enhances cannabinoid stress-induced analgesia: sites of action in the dorsolateral periaqueductal gray and rostral ventromedial medulla. Neuropharmacology. 2005;49(8):1201–1209. doi: 10.1016/j.neuropharm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Urban MO, Smith DJ. Localization of the antinociceptive and antianalgesic effects of neurotensin within the rostral ventromedial medulla. Neurosci.Lett. 1994;174(1):21–25. doi: 10.1016/0304-3940(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Urca G, Yitzhaky J, Frenk H. Different opioid systems may participate in post-electro-convulsive shock (ECS) analgesia and catalepsy. Brain Res. 1981;219(2):385–396. doi: 10.1016/0006-8993(81)90301-2. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br.J.Pharmacol. 1999;127(4):935–940. doi: 10.1038/sj.bjp.0702636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Connor M, Bagley EE, Christie MJ. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol.Pharmacol. 2000;57(2):288–295. [PubMed] [Google Scholar]
- Wallace MJ, Wiley JL, Martin BR, DeLorenzo RJ. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur.J.Pharmacol. 2001;428(1):51–57. doi: 10.1016/s0014-2999(01)01243-2. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Martin BR, DeLorenzo RJ. Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur.J.Pharmacol. 2002;452(3):295–301. doi: 10.1016/s0014-2999(02)02331-2. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J.Pharmacol.Exp.Ther. 2003;307(1):129–137. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- Yang L, Long C, Randall ME, Faingold CL. Neurons in the periaqueductal gray are critically involved in the neuronal network for audiogenic seizures during ethanol withdrawal. Neuropharmacology. 2003;44(2):275–281. doi: 10.1016/s0028-3908(02)00367-2. [DOI] [PubMed] [Google Scholar]