Abstract
The RING domain-containing protein CCNB1IP1 (Cyclin B1 Interacting Protein 1) is a putative ubiquitin E3 ligase that is essential for chiasmata formation, and hence fertility, in mice. Previous studies in cultured cells indicated that CCNB1IP1 targets Cyclin B for degradation, thus playing a role in cell cycle regulation. Mice homozygous for a mutant allele (mei4) of Ccnb1ip1 display no detectable phenotype other than meiotic failure from an absence of chiasmata. CCNB1IP1 is not conserved in key model organisms such as yeast and Drosophila, and there are no features of the protein that implicate clear mechanisms for a role in recombination. To gain insight into CCNB1IP1’s function in meiotic cells, we raised a specific antibody and determined that the protein appears in pachynema. This indicates that CCNB1IP1 is involved with crossover intermediate maturation, rather than early (leptotene) specification of a subset of SPO11-induced double strand breaks towards the crossover pathway. Additionally, a yeast 2-hybrid (Y2H) screen revealed that CCNB1IP1 interacts with SUMO2 and a set of proteins enriched for consensus sumoylation sites. The Y2H studies, combined with scrutiny of CCNB1IP1 domains, implicate this protein as an E3 ligase of the sumoylation cascade. We hypothesize CCNB1IP1 represents a novel meiosis-specific SUMO E3 ligase critical to resolution of recombination intermediates into mature chiasmata.
Keywords: meiosis, sumoylation, mouse, crossing over, recombination, yeast two hybrid
1. Introduction
In previous work, our lab conducted forward genetic mutagenesis screens to identify novel genes required for meiosis in mice [1,2]. One of the alleles induced by the point mutagen ENU (N-ethyl-N-nitrosourea), mei4, presented as a recessive male and female sterile. Histological and cytological analyses revealed abnormal alignment and distribution of chromosomes at metaphase/anaphase at the first meiotic division in spermatocytes and oocytes [3]. Immunocytological analyses revealed no abnormalities in non-crossover (NCO) recombination or chromosome synapsis through early pachynema. However, as the meiocytes entered diplonema, the homologous chromosomes failed to maintain interhomolog associations, suggesting an absence of chiasmata. This suspicion was confirmed by an absence of MLH1 and MLH3 foci on pachytene chromosomes [3]. The mismatch repair proteins are well established markers of chiasmata [4].
Positional cloning revealed that mei4 is a mutant allele of Ccnb1ip1 (also called Hei10), a gene not previously known to have a role in meiosis. Ccnb1ip1 encodes a coiled-coil RING domain-containing protein, whereas Ccnb1ip1mei4 bears a donor splice site mutation resulting in an aberrantly spliced transcript [3]. Studies of CCNB1IP1 in cultured somatic cells implicated a role for this putative ubiquitin E3 ligase in Cyclin B regulation, cell cycle progression, and cell invasion [5,6]. However, the exact function of CCNB1IP1 in meiotic recombination remains is unclear. A model proposed by Ward et al. posited that CCNB1IP1 disrupts association of CDK2 with CCNB3, possibly via ubiquitylation, thus permitting CDK2 to recruit or enable binding of MLH1 and MLH3 (and possibly other proteins) to designated crossover sites [3].
To better understand the role of CCNB1IP1 in recombination, and to gain possible support for the aforementioned model, we conducted a yeast two hybrid (Y2H) screen for interacting proteins in the mouse testis, characterized the temporal appearance of CCNB1IP1 during meiosis, and examined bioinformatically the domain structures of CCNB1IP1. Surprisingly, these studies implicate CCNB1IP1 as a SUMO (Small Ubiquitin-like Modifier) E3 ligase. SUMOylation modulates many behaviors of proteins, including interactions with other proteins, subcellular localization, and stabilization though competition with Ubiquitin for lysine residues [7]. The process of SUMO conjugation to target substrates is analogous to that of the well characterized Ub cascade; involving E1, E2 and E3 type ligases [8]. The role SUMO plays in meiosis remains largely unknown; however, immunolocalization studies in mammals have detected SUMO at sites of double strand breaks (DSBs) and at centromeric and heterochromatic regions, including the XY body of mouse pachytene spermatocytes [9,10,11,12]. Additionally, the singular SUMO E2 ligase, UBC9 (UBE2I in the mouse) localizes along synapsed chromosome cores during pachynema and diplonema [13,14]. The evidence we present in support of CCNB1IP1 as a potential SUMO E3 ligase has the potential to reveal hitherto unknown mechanisms in mammalian meiotic recombination.
2. Results and Discussion
2.1. Expression of CCNB1IP1 and CCNB1IP1mei4 During Spermatogenesis
CCNB1IP1 is essential for meiotic crossing-over in mice. In S. cerevisiae, although double Holliday junctions characteristic of crossover (CO) recombination appear in early-mid pachynema [15], the partitioning of DSBs to either the NCO or CO pathways is made much earlier, in late leptonema [16]. Like yeast, mammals have genetically distinct NCO and CO pathways [17]. Therefore, CCNB1IP1 may be required either for the specifying a subset of DSBs to the CO fate in leptonema, or subsequent processing of CO recombination intermediates in pachynema.
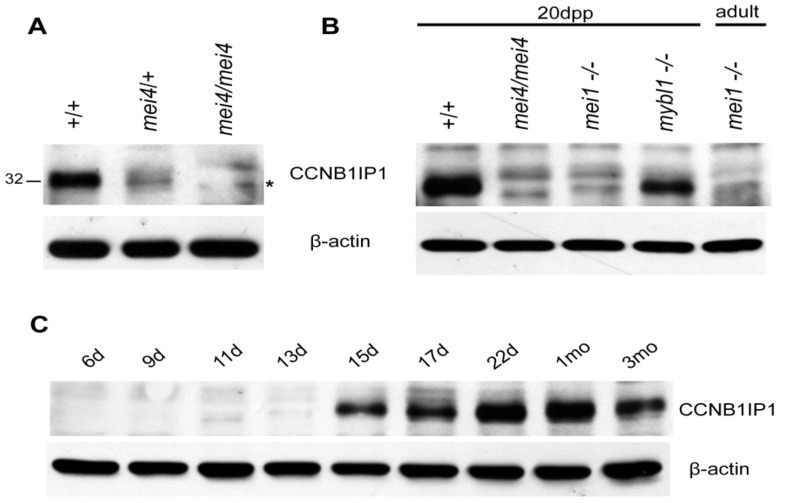
As a first step towards addressing this question, we generated an affinity-purified rabbit polyclonal antibody against N-terminal amino acids 1–245 of CCNB1IP1. The antibody recognized a protein slightly larger than 30 kDa (theoretical MW of CCNB1IP1 = 32 kDa) in Western blots of WT protein from 20 dpp (days post partum) and adult mouse testis. Juvenile Ccnb1ip1mei4/+ extracts had roughly half the amount of the 32 kDa species compared to WT animals. The 32 kDa species was completely lacking in homozygous mutants, consistent with it being CCNB1IP1 (Figure 1a). Notably, both heterozygous and homozygous testis extracts showed an additional, slightly smaller band on the Western blots (Figure 1a,b). This species was not as robust as wild-type CCNB1IP1, and it appeared to be more predominant in the homozygous mutants than in heterozygotes. Considering that the Ccnb1ip1mei4 allele is predicted to encode a protein bearing an internal deletion of 24 amino acids (~2.7 kDa) [3], it is likely that the smaller species in the Western blot is this truncated protein. The mutant CCNB1IP1 allele may retain some function. However, the relatively lower amounts of the smaller species in both hetero- and homozygotes suggests that the CCNB1IP1mei4 protein is less stable, more rapidly cleared, or translated at a lower efficiency than WT CCNB1IP1.
Figure 1.
Western blot analysis of CCNB1IP1 expression in testis. (A) Polyclonal anti-CCNB1IP1 recognizes a ~32 kDa species in 20 dpp testis of WT and heterozygous Ccnb1ip1mei4 animals (size in kDa is shown at left). This band is absent in mutants (third lane), but a lower band of ~30 kDa is evident that not present in WT (asterisk); (B) CCNB1IP1 is greatly decreased or absent from mutant testes that undergo meiotic arrest prior to pachynema (Mei-/-), but not those that progress to approximately diplonema (Mybl1-/-); (C) CCNB1IP1 in testis is first produced between days 13 and 15 dpp, coincident with onset of pachynema.
To further confirm the specificity of the antibody, we performed Western blot analysis of protein from 20 dpp testis extracted from several meiotic mutants (Figure 1b). The 32 kDa product is undetectable in Ccnb1ip1mei4/mei4 animals, but was present in mice homozygous for a mutant Mybl1 allele that causes meiotic arrest at a stage of meiosis similar to that of Ccnb1ip1mei4 spermatocytes (late pachynema/diplonema; [18]). This result indicates that the 32 kDa species is not a cross-reactive product from a class of cells that are missing in Ccnb1ip1mei4/mei4 testes. The product was present at low levels in Mei1/Mei1 20 dpp testis, in which meiosis arrests prior to entry into pachynema due to failed DSB formation and extensive asynapsis [19,20]. This suggests either that Ccnb1ip1mei4 expression is either dependent upon DSB formation (which occurs in leptonema), or it initiates in pachytene spermatocytes.
To pinpoint the onset of CCNB1IP1 production, we took advantage of the coordinated first wave of spermatogenesis after birth. Leptotene, pachytene, late pachytene and diplotene cells appear en masse approximately 10, 14, 18, and >18 dpp, respectively [21,22]. As shown in Figure 1c, CCNB1IP1 appears between 13 and 15 dpp, spanning early-mid pachynema. CCNB1IP1 then persists throughout adulthood, although the data does not indicate if it is present in postmeiotic spermatids. These data indicate that CCNB1IP1 is not involved in partitioning DSBs to the CO pathway. Rather, expression after entry into pachynema suggests a requirement for processing CO recombination intermediates.
2.2. Identification of CCNB1IP1 Interacting Proteins
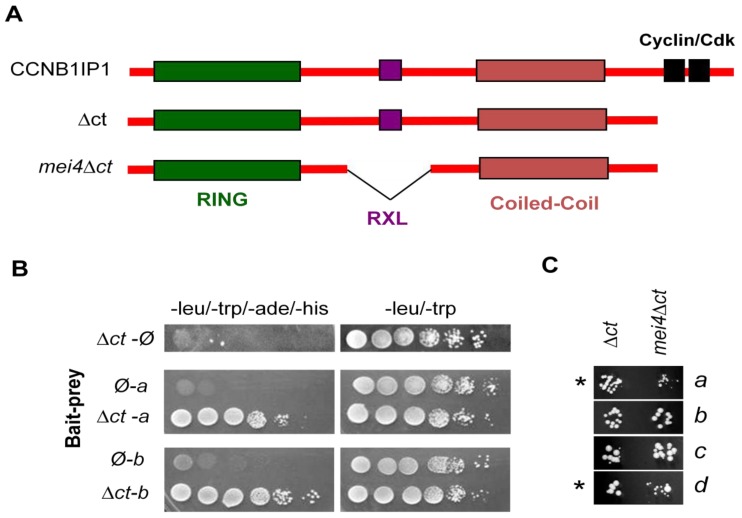
CCNB1IP1 is a coiled-coil RING domain-containing protein shown to have E3 Ubiquitin ligase activity [23]. The RING domain is characteristic of the E3-ligase family of proteins. To identify potential ubiquitylation targets of CCNB1IP1 and other interacting proteins that might illuminate the molecular mechanism by which this protein participates in crossing over, we conducted a yeast two-hybrid (Y2H) screen of a testis library (Figure 2). Full length CCNB1IP1 was found to be auto-activating under the selective growth conditions of the screen. Progressive C-terminal truncations narrowed the autoactivating region to that containing 2 putative Cyclin/Cdk target motifs [5], so these were deleted from the bait vector. Thirty-five interactors were isolated and validated (Table 1; see Methods). None of the CCNB1IP1 interacting genes are known to be essential for meiosis, although Ggn (Gametogenetin) has been implicated to have functions beginning in late pachytene spermatocytes [24]. Additionally, the NCBI GEO Profiles database reveals that several of the genes are transcriptionally up-regulated in the testis, with postnatal testis expression increasing in age and peaking during pachytene of meiosis I (accessions GDS3142, GDS605, GDS401). Interestingly, Hook1 is expressed primarily postmeiotically and is required for proper formation of the sperm head [25], suggesting a possible role for CCNB1IP1 in processes other than recombination.
Figure 2.
Yeast two-hybrid screen for CCNB1IP1 interacting proteins in the testis. (A) Structure of CCNB1IP1 and known motifs/domains. A C-terminal truncated construct was used as bait (∆ct). This lacks two potential phosphorylation sites of cyclin/cdk kinases. A bait construct with the deletion contained in the Ccnb1ip1mei4 allele (mei4∆ct) is shown at the bottom; (B) Confirmation of prey clones under maximal selection stringency (ade and his) with leu and trp selection for presence of bait and prey plasmids; (C) Growth of yeast containing 4 different prey and either the ∆ct bait or the mei4∆ct bait. A subset of CCNB1IP1 interactors (asterisks) were found to reproducibly display weaker interaction with mei4∆ct than ∆ct, as assessed by vigor of individual colony growth. Ø = empty vector; a = EP400; b = OAZ3; c = 5730469M10Rik; d = 4930455F23Rik.
Table 1.
Proteins Identified in Two Hybrid Screen.
| Kinetically Normal with mei4Δct | Kinetically Defective with mei4Δct |
|---|---|
| SUMO2 | 4930455F23RIK (3) |
| AKAP9 | YPEL2 |
| SPINK10 | 1700006A11RIK |
| 1700019N19RIK (2) | EP400 |
| POLR2B | POMP (4) |
| ENAH | MSL1 |
| H3F3B (3) | DDC8 (2) |
| 5730469M10RIK | HOOK1 |
| PHF12 | GGN (4) |
| MRRF | FHL5 |
| OCIAD1 (2) | 4930503B20RIK (2) |
| B9D1 | 1700021F07RIK (3) |
| MORN2 | SPATA3 (3) |
| ATOH8 | |
| SRGN | |
| PENK1 | |
| BRP44 (3) | |
| INSL3 (2) | |
| EMX1 | |
| GSG1 (3) | |
| OAZ3 (3) | |
| MIIP |
Note: Proteins with predicted SUMOylation sites are underlined. All clones for the corresponding proteins were isolated with CCNB1IP1Δct as bait. If multiple, independent clones were obtained for a prey protein, the number is given in parentheses. One of each prey was individually tested for interaction with the MEI4 deletion version. They were subdivided into two kinetic interaction classes as indicated by the two columns.
In light of the Western blot data indicating that Ccnb1ip1mei4/mei4 testes produced a deleted version of CCNB1IP1, we hypothesized that the mutant protein might have defective interactions with some subset of the Y2H binding partners, thus potentially explaining the recombination phenotype. We therefore tested the full set of 35 CCNB1IP1 interactors against the mutant allele as bait (signified as mei4∆ct). The Ccnb1ip1mei4 deletion did not ablate interaction with any of the prey clones, however it did reproducibly lead to a “kinetically” weaker interaction as assessed by colony size under stringent growth selection conditions (Figure 2c). If this is reflective of the in vivo situation in mice, it is possible that the weaker interactions, coupled with the decreased level of mutant protein, contributes to the phenotype.
Conspicuous amongst the Y2H interactors was SUMO2. This prompted us to consider a potential role for CCNB1IP1 in the SUMOylation cascade. Inspection of the other CCNB1IP1 interacting proteins revealed one notable common motif: Ψ-K-X-E/D (where Ψ is a hydrophobic amino acid; Table 1). This Ψ-K-X-E/D motif is enriched in targets of SUMOylation, and lysine (K) is the residue targeted for SUMO modification in those proteins [26,27]. Notably, of the 13 proteins with kinetic defects in interaction affinity for mei4∆ct, 7 (54%) carry the SUMOylation motif with 5/7 possessing the most common form Y/L-K-X-E. The predicted SUMO-proteome has been estimated to be 38% of all similarly analyzed peptides [28].
2.3. Motif Analysis of CCNB1IP1 Implicates It Is a SUMO E3 Ligase
PIAS4 and other SUMO E3 ligases have been found to interact with SUMO in Y2H assays [29,30,31]. Given that CCNB1IP1 interacts with SUMO2 and other proteins containing consensus SUMOylation sites, we hypothesize that CCNB1IP1 has SUMO E3 ligase function in addition to its reported E3 Ub ligase activity. SUMO E3 ligases often contain a C3H2C3 type RING domain believed to confer interaction specificity to the singular known E2 ligase, UBC9 [32,33]. Alignment of CCNB1IP1 from mouse and other species as well as known SUMO E3 ligases (ZIP3, SIZ1, SIZ2) reveals the presence and conservation of a C3H2C3 type RING domain. Additionally, non-covalent SUMO interacting motifs (SIMs) have been identified within most SUMO E3 ligases [33,34,35]. SIMs are characterized by Ψ-X-Ψ-Ψ where Ψ is V/I or another large hydrophobic residue [34,36]. CCNB1IP1 indeed has such a sequence conserved across mammals (Figure 3b; non-human mammals not shown).
Figure 3.
SUMO E3 ligase-like domain conservation in CCNB1IP1. (A) Alignments of C3H2C3-type RING domains in four CCNB1IP1 orthologs and other known SUMO E3 ligases; (B) CCNB1IP1 contains a canonical SIM motif (blue) just upstream of the region deleted in the Ccnb1ip1mei4 allele.
This putative SIM is just 15 amino acids N-terminal to the deletion in the Ccnb1ip1mei4 allele (Figure 3b). Recently, studies of the Pc2 SUMO E3 ligase showed that pairs of charged and hydrophobic amino acids adjacent to the consensus SIM facilitate E3 function [28]. These residues have been proposed to facilitate interactions with SUMO-conjugated UBC9 [36]. The deletion immediately C-terminal to the putative CCNB1IP1 SIM, if it contains similar facilitating residues, may impair interaction with UBC9-SUMO in vivo.
3. Experimental Section
3.1. Recombinant Expression of CCNB1IP1 and Anti-CCNB1IP1 Production
cDNA corresponding to amino acids 1–245 of CCNB1IP1 was subcloned into expression plasmid pQE-30 (Qiagen), so as to add a 6X HIS tag. Bacterially expressed peptide was solubilized using standard procedures with the addition of 5 M Urea. The HIS-tagged peptide was purified on a HisPur cobalt resin column according to manufacturer’s protocols (Pierce). The peptide purification was verified via SDS-PAGE and concentrated on a Vivaspin 15R column (SartoriusStedim). The purified CCNB1IP1 was used as immunogen for polyclonal antibody production in rabbit, followed by affinity purification over immobilized CCNB1IP1 as per manufacturer’s procedures (GenScript). Specificity of the IgG was assessed by dot-blot down to 250 pg of recombinant CCNB1IP1.
3.2. SDS-PAGE and Western Blotting
SDS-PAGE and Western blotting were performed by standard procedures. Tissues from mice were dounce homogenized in cold lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris, pH 8.0) supplemented with protease inhibitors (complete®, Roche) followed by sonication at 1 s intervals for 30 s. Lysates were boiled for 5 min followed by clearing via centrifugation. Protein concentrations of cleared lysates were measured by the BCA protein assay (Thermo). SDS-PAGE gels were loaded with 25 μg of total protein lysates per lane. Loading controls were performed with anti-β-actin (Sigma A1978) following SDS/2-MeOH stripping of the PVDF membrane. CCNB1IP1 signal was detected with 1:250 dilution of anti-CCNB1IP1 incubated 3 h at 4 °C followed by an hour incubation using anti-rabbit HRP-conjugated antibody. Detection of signals was performed with Pierce® ECL chemiluminescence substrate.
3.3. Yeast Two-Hybrid Screen for CCNB1IP1 Interactors
Full length CCNB1IP1 was found to be auto-activating under the Y2H conditions used. Following analysis of various truncations for loss of auto-activation under screen conditions, cDNA of a C-terminal truncation of mouse Ccnb1ip1 corresponding to amino acids 1–245 (encoding what we call CCNB1IP1∆ct) was expressed as a “bait” fusion protein with the GAL4 DNA-binding domain. This was constructed in plasmid pGBK. A mouse testis cDNA library in pACT2 (Clontech) was used as “prey” for a protein-protein interaction screen with CCNB1IP1∆ct. Interactions with CCNB1IP1∆ct were selected by colony growth in the absence of histidine on plates supplemented with 7.5 mM 3-AT. Strongly growing colonies were confirmed under more stringent interaction selection on plates lacking histidine and adenine. pACT2 cDNA clones were isolated and analyzed by DNA sequencing. The CCNB1IP1∆ct interactor clones were then directly tested for interaction affinity with the same C-terminal truncation from Ccnb1ip1mei4 cDNA (called mei4∆ct) under the more stringent conditions (Figure 2a). Those clones showing decreased affinity of interaction with mei4∆ct were confirmed in three independent experiments.
4. Conclusions
CCNB1IP1 is required for crossing-over, but there is no known mechanistic linkage to recombination. We conducted the Y2H screen in the hope of identifying proteins of known function that might provide such mechanistic linkage. Although none of the identified interacting proteins have reported roles in crossing-over, our analyses of the aggregate Y2H data suggest a function for CCNB1IP1 as an E3 ligase in the SUMO modification pathway, in addition to the ubiquitin ligase role previously reported in somatic cells [23]. To summarize, we found that CCNB1IP1 interacts with both SUMO2 and proteins containing the consensus SUMOylation motif, properties consistent with computational studies of SUMO E3 ligases [28,31,37]. Furthermore, we observed that CCNB1IP1 contains a C3H2C3 type RING domain, conserved in proven SUMO E3 ligases, that constitutes the interaction surface with the SUMO E2 conjugating enzyme, UBC9. Finally, CCNB1IP1 contains a consensus SIM domain found in the majority of characterized E3s. This sequence has been proposed to function in aiding E3 interaction with SUMO-conjugated UBC9 (not unconjugated UBC9) [38,39]. Whether the putative SIM in CCNB1IP1 actually functions in this manner awaits experimental validation.
There is increasing evidence indicating multiple roles for SUMO modification in regulating DNA repair and meiosis. The S. cerevisiae SUMO E3 ligase Zip3 ensures that SC formation is dependent on recombination initiation, and it interacts with a number of recombination proteins including Mre11, Rad51, Msh4 and Msh5 [33,40,41]. The Zip3 ortholog in C. elegans is required for meiotic crossover formation and is localized to sites of crossing-over in late Prophase I [42]. Variants in the human ortholog RNF212 have been associated with influencing genome-wide meiotic recombination rates [43,44]. Furthermore, the synaptonemal complex component protein Zip1 and axial-element protein Red1 have been demonstrated to bind SUMO-conjugated proteins, the latter of which promotes interhomolog exchange [33,45]. Finally, the SUMO pathway is involved in regulating ubiquitylation in DNA damage responses in mammalian cells [9,46].
These results, together with the growing understanding of SUMOylation in higher order eukaryotes, are beginning to shed light on the role for SUMO in DNA damage responses and recombination in meiosis. The defect of Ccnb1ip1mei4 in meiotic crossing over and its putative role as a SUMO E3 ligase offer us a novel element in our understanding of the mechanisms regulating crossover formation. The result of the Y2H screen, which identified a number of putative SUMOylation target proteins with no known roles in meiosis, suggest that further study of CCNB1IP1 will reveal novel mechanisms of meiotic recombination in mammals.
Acknowledgements
This work was supported by National Institutes of Health grants R01 GM45415 to JCS, and T32 HD052471 (training slot to E.R.S.).
References
- 1.Munroe R.J., Bergstrom R.A., Zheng Q.Y., Libby B., Smith R., John S.W.M., Schimenti K.J., Browning V.L., Schimenti J.C. Mouse mutants from chemically mutagenized embryonic stem cells. Nat. Genet. 2000;24:318–321. doi: 10.1038/73563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward J.O., Reinholdt L.G., Hartford S.A., Wilson L.A., Munroe R.J., Schimenti K.J., Libby B.J., O’Brien M., Pendola J.K., Eppig J., Schimenti J.C. Toward the genetics of mammalian reproduction: induction and mapping of gametogenesis mutants in mice. Biol. Reprod. 2003;69:1615–1625. doi: 10.1095/biolreprod.103.019877. [DOI] [PubMed] [Google Scholar]
- 3.Ward J.O., Reinholdt L.G., Motley W.W., Niswander L.M., Deacon D.C., Griffin L.B., Langlais K.K., Backus V.L., Schimenti K.J., O’Brien M.J., Eppig J.J., Schimenti J.C. Mutation in mouse Hei10, an e3 ubiquitin ligase, disrupts meiotic crossing over. PLoS Genet. 2007;3:e139. doi: 10.1371/journal.pgen.0030139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcon E., Moens P. MLH1p and MLH3p localize to precociously induced chiasmata of okadaic-acid-treated mouse spermatocytes. Genetics. 2003;165:2283–2287. doi: 10.1093/genetics/165.4.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gronholm M., Muranen T., Toby G.G., Utermark T., Hanemann C.O., Golemis E.A., Carpen O. A functional association between merlin and HEI10, a cell cycle regulator. Oncogene. 2006;25:4389–4398. doi: 10.1038/sj.onc.1209475. [DOI] [PubMed] [Google Scholar]
- 6.Singh M.K., Nicolas E., Gherraby W., Dadke D., Lessin S., Golemis E.A. HEI10 negatively regulates cell invasion by inhibiting cyclin B/Cdk1 and other promotility proteins. Oncogene. 2007;26:4825–4832. doi: 10.1038/sj.onc.1210282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geiss-Friedlander R., Melchior F. Concepts in sumoylation: A decade on. Nat. Rev. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 8.Ulrich H.D. The SUMO system: An overview. Methods Mol. Biol. 2009;497:3–16. doi: 10.1007/978-1-59745-566-4_1. [DOI] [PubMed] [Google Scholar]
- 9.Galanty Y., Belotserkovskaya R., Coates J., Polo S., Miller K.M., Jackson S.P. Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature. 2009;462:935–939. doi: 10.1038/nature08657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers R.S., Inselman A., Handel M.A., Matunis M.J. SUMO modified proteins localize to the XY body of pachytene spermatocytes. Chromosoma. 2004;113:233–243. doi: 10.1007/s00412-004-0311-7. [DOI] [PubMed] [Google Scholar]
- 11.Shrivastava V., Pekar M., Grosser E., Im J., Vigodner M. SUMO proteins are involved in the stress response during spermatogenesis and are localized to DNA double-strand breaks in germ cells. Reproduction. 2010;139:999–1010. doi: 10.1530/REP-09-0492. [DOI] [PubMed] [Google Scholar]
- 12.Vigodner M., Morris P.L. Testicular expression of small ubiquitin-related modifier-1 (SUMO-1) supports multiple roles in spermatogenesis: Silencing of sex chromosomes in spermatocytes, spermatid microtubule nucleation, and nuclear reshaping. Dev. Biol. 2005;282:480–492. doi: 10.1016/j.ydbio.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 13.Shrivastava V., Pekar M., Grosser E., Im J., Vigodner M. SUMO proteins are involved in the stress response during spermatogenesis and are localized to DNA double-strand breaks in germ cells. Reproduction. 2010;139:999–1010. doi: 10.1530/REP-09-0492. [DOI] [PubMed] [Google Scholar]
- 14.La Salle S., Sun F., Zhang X.D., Matunis M.J., Handel M.A. Developmental control of sumoylation pathway proteins in mouse male germ cells. Dev. Biol. 2008;321:227–237. doi: 10.1016/j.ydbio.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter N., Kleckner N. The single-end invasion: an asymmetric intermediate at the double-strand break to double-holliday junction transition of meiotic recombination. Cell. 2001;106:59–70. doi: 10.1016/S0092-8674(01)00430-5. [DOI] [PubMed] [Google Scholar]
- 16.Borner G.V., Kleckner N., Hunter N. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell. 2004;117:29–45. doi: 10.1016/S0092-8674(04)00292-2. [DOI] [PubMed] [Google Scholar]
- 17.Guillon H., Baudat F., Grey C., Liskay R.M., de Massy B. Crossover and noncrossover pathways in mouse meiosis. Mol. Cell. 2005;20:563–573. doi: 10.1016/j.molcel.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Bannister L., Schimenti J. (Cornell University, Ithaca, NY 14850, USA). Personal communication. 2010.
- 19.Libby B.J., de La Fuente R., O’Brien M.J., Wigglesworth K., Cobb J., Inselman A., Eaker S., Handel M.A., Eppig J.J., Schimenti J.C. The Mouse Meiotic Mutation mei1 Disrupts Chromosome Synapsis with Sexually Dimorphic Consequences for Meiotic Progression. Dev. Biol. 2002;242:174–187. doi: 10.1006/dbio.2001.0535. [DOI] [PubMed] [Google Scholar]
- 20.Libby B.J., Reinholdt L.G., Schimenti J.C. Positional cloning and characterization of Mei1, a vertebrate-specific gene required for normal meiotic chromosome synapsis in mice. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15706–15711. doi: 10.1073/pnas.2432067100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nebel B.R., Amarose A.P., Hacket E.M. Calendar of gametogenic development in the prepuberal male mouse. Science. 1961;134:832–833. doi: 10.1126/science.134.3482.832. [DOI] [PubMed] [Google Scholar]
- 22.Bellve A., Cavicchia J., Millette C., O’Brien D., Bhatnagar Y., Dym M. Spermatogenic cells of the prepubertal mouse. Isolation and morphological characterization. J. Cell. Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toby G.G., Gherraby W., Coleman T.R., Golemis E.A. A novel RING finger protein, human enhancer of invasion 10, alters mitotic progression through regulation of cyclin B levels. Mol. Cell Biol. 2003;23:2109–2122. doi: 10.1128/MCB.23.6.2109-2122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu B., Bishop C.E. Mouse GGN1 and GGN3, two germ cell-specific proteins from the single gene Ggn, interact with mouse POG and play a role in spermatogenesis. J. Biol. Chem. 2003;278:16289–16296. doi: 10.1074/jbc.M211023200. [DOI] [PubMed] [Google Scholar]
- 25.Mendoza-Lujambio I., Burfeind P., Dixkens C., Meinhardt A., Hoyer-Fender S., Engel W., Neesen J. The Hook1 gene is non-functional in the abnormal spermatozoon head shape (azh) mutant mouse. Hum. Mol. Genet. 2002;11:1647–1658. doi: 10.1093/hmg/11.14.1647. [DOI] [PubMed] [Google Scholar]
- 26.Melchior F., Schergaut M., Pichler A. SUMO: ligases, isopeptidases and nuclear pores. Trends Biochem. Sci. 2003;28:612–618. doi: 10.1016/j.tibs.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Denison C., Rudner A.D., Gerber S.A., Bakalarski C.E., Moazed D., Gygi S.P. A proteomic strategy for gaining insights into protein sumoylation in yeast. Mol. Cell. Proteomics. 2005;4:246–254. doi: 10.1074/mcp.M400154-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Zhou F., Xue Y., Lu H., Chen G., Yao X. A genome-wide analysis of sumoylation-related biological processes and functions in human nucleus. FEBS Lett. 2005;579:3369–3375. doi: 10.1016/j.febslet.2005.04.076. [DOI] [PubMed] [Google Scholar]
- 29.Weger S., Hammer E., Engstler M. The DNA topoisomerase I binding protein topors as a novel cellular target for SUMO-1 modification: characterization of domains necessary for subcellular localization and sumolation. Exp. Cell Res. 2003;290:13–27. doi: 10.1016/S0014-4827(03)00292-1. [DOI] [PubMed] [Google Scholar]
- 30.Kahyo T., Nishida T., Yasuda H. Involvement of PIAS1 in the sumoylation of tumor suppressor p53. Mol. Cell. 2001;8:713–718. doi: 10.1016/S1097-2765(01)00349-5. [DOI] [PubMed] [Google Scholar]
- 31.Goehler H., Lalowski M., Stelzl U., Waelter S., Stroedicke M., Worm U., Droege A., Lindenberg K.S., Knoblich M., Haenig C., et al. A protein interaction network links GIT1, an enhancer of huntingtin aggregation, to Huntington’s disease. Mol. Cell. 2004;15:853–865. doi: 10.1016/j.molcel.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Johnson E.S., Gupta A.A. An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell. 2001;106:735–744. doi: 10.1016/s0092-8674(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 33.Cheng C.H., Lo Y.H., Liang S.S., Ti S.C., Lin F.M., Yeh C.H., Huang H.Y., Wang T.F. SUMO modifications control assembly of synaptonemal complex and polycomplex in meiosis of Saccharomyces cerevisiae. Genes Dev. 2006;20:2067–2081. doi: 10.1101/gad.1430406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song J., Durrin L.K., Wilkinson T.A., Krontiris T.G., Chen Y. Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc. Natl. Acad. Sci. U. S. A. 2004;101:14373–14378. doi: 10.1073/pnas.0403498101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merrill J.C., Melhuish T.A., Kagey M.H., Yang S.H., Sharrocks A.D., Wotton D. A role for non-covalent SUMO interaction motifs in Pc2/CBX4 E3 activity. PLoS ONE. 2010;5:e8794. doi: 10.1371/journal.pone.0008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song J., Zhang Z., Hu W., Chen Y. Small ubiquitin-like modifier (SUMO) recognition of a SUMO binding motif: a reversal of the bound orientation. J. Biol. Chem. 2005;280:40122–40129. doi: 10.1074/jbc.M507059200. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi Y., Toh-e A., Kikuchi Y. A novel factor required for the SUMO1/Smt3 conjugation of yeast septins. Gene. 2001;275:223–231. doi: 10.1016/s0378-1119(01)00662-x. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J., Zhu S., Guzzo C.M., Ellis N.A., Sung K.S., Choi C.Y., Matunis M.J. Small ubiquitin-related modifier (SUMO) binding determines substrate recognition and paralog-selective SUMO modification. J. Biol. Chem. 2008;283:29405–29415. doi: 10.1074/jbc.M803632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin D.Y., Huang Y.S., Jeng J.C., Kuo H.Y., Chang C.C., Chao T.T., Ho C.C., Chen Y.C., Lin T.P., Fang H.I., Hung C.C., Suen C.S., Hwang M.J., Chang K.S., Maul G.G., Shih H.M. Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Mol. Cell. 2006;24:341–354. doi: 10.1016/j.molcel.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 40.Macqueen A.J., Roeder G.S. Fpr3 and Zip3 ensure that initiation of meiotic recombination precedes chromosome synapsis in budding yeast. Curr. Biol. 2009;19:1519–1526. doi: 10.1016/j.cub.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal S., Roeder G.S. Zip3 provides a link between recombination enzymes and synaptonemal complex proteins. Cell. 2000;102:245–255. doi: 10.1016/S0092-8674(00)00029-5. [DOI] [PubMed] [Google Scholar]
- 42.Bhalla N., Wynne D.J., Jantsch V., Dernburg A.F. ZHP-3 acts at crossovers to couple meiotic recombination with synaptonemal complex disassembly and bivalent formation in C. elegans. PLoS Genet. 2008;4:e1000235. doi: 10.1371/journal.pgen.1000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chowdhury R., Bois P.R., Feingold E., Sherman S.L., Cheung V.G. Genetic analysis of variation in human meiotic recombination. PLoS Genet. 2009;5:e1000648. doi: 10.1371/journal.pgen.1000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kong A., Thorleifsson G., Stefansson H., Masson G., Helgason A., Gudbjartsson D.F., Jonsdottir G.M., Gudjonsson S.A., Sverrisson S., Thorlacius T., Jonasdottir A., Hardarson G.A., Palsson S.T., Frigge M.L., Gulcher J.R., Thorsteinsdottir U., Stefansson K. Sequence variants in the RNF212 gene associate with genome-wide recombination rate. Science. 2008;319:1398–1401. doi: 10.1126/science.1152422. [DOI] [PubMed] [Google Scholar]
- 45.Lin F.M., Lai Y.J., Shen H.J., Cheng Y.H., Wang T.F. Yeast axial-element protein, Red1, binds SUMO chains to promote meiotic interhomologue recombination and chromosome synapsis. EMBO J. 2010;29:586–596. doi: 10.1038/emboj.2009.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris J.R., Boutell C., Keppler M., Densham R., Weekes D., Alamshah A., Butler L., Galanty Y., Pangon L., Kiuchi T., Ng T., Solomon E. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature. 2009;462:886–890. doi: 10.1038/nature08593. [DOI] [PubMed] [Google Scholar]