Abstract
Mural trophectoderm cells of the mouse embryo possess a phagocytic potential as early as 3.5 days post coitum (d.p.c.). This first differentiated function shows a graded variation along the embryonic–abembryonic axis, from a maximal activity in the non-dividing cells of the abembryonic pole to a complete lack of activity in the replicating polar trophectoderm overlying the inner cell mass (ICM). This pattern can be explained by a negative control exerted by the ICM. Addition of FGF4, a factor secreted by ICM cells, strongly inhibited phagocytosis while inducing resumption of DNA synthesis in mural trophectoderm cells, revealing a reversible, FGF4-dependent differentiation state. Under conditions in which a small cluster of mural trophectoderm cells (<10) had internalized large particles, these otherwise morphologically normal embryos could not implant in the uterus, indicating that cells at the abembryonic pole have a critical role in initiating the implantation process. At post-implantation stages (6.5–8.5 d.p.c.), the ectoplacental cone and secondary giant cells derived from the polar trophectoderm also contained active phagocytes, but at that stage, differentiation was not reversed by FGF4.
Keywords: blastocyst/FGF4/uterus implantation
Introduction
Shortly before implanting in the uterine wall, the mouse blastocyst at 3.5–4.5 days post coitum (d.p.c.) comprises two distinct cell lineages. The inner cell mass (ICM) will eventually generate all embryonic tissues. The eccentric position of the ICM in the blastocyst defines the first developmental axis, the embryonic–abembryonic axis (Gardner et al., 1992). The trophectoderm constitutes a specialized epithelial layer that encloses both the ICM and the blastocoel cavity. Its differentiation represents the first major lineage decision to take place in the developing mouse embryo. The blastocyst trophectoderm consists of two discrete groups of cells that are individualized by both physical location and a number of cellular and molecular properties (reviewed by Hogan et al., 1994; Gardner, 1999). The region in contact with the ICM, designated polar trophectoderm, will generate a portion of the structures connecting the embryo to the maternal tissues, namely the ectoplacental cone (EPC) and extra-embryonic ectoderm, shortly after implantation, and later much of the fetal part of the placenta. The mural trophectoderm, surrounding the blastocoel to the limit of the ICM region, is considered to be the embryo’s first fully differentiated cell type, as defined by the arrest of proliferation and later ability to generate primary trophoblast giant cells, a process that involves endoreduplication of the genome. Secondary giant cells will subsequently be produced by precursors originating in the EPC region.
There is much evidence that normal development and implantation of the blastocyst are dependent on ongoing interactions between the ICM and trophoblast regions of the embryo. Embryological studies have indicated that proper growth and expansion of the trophoblast are dependent on continued contact with cells of the ICM region (Ansell and Snow, 1975), suggesting that the ICM is the producing factor(s) critical for trophectoderm proliferation and differentiation. The identity of one such factor has been revealed by recent studies on the role of the fibroblast growth factor FGF4 and its cognate receptor FGFR2. FGF4 was shown to be produced by ICM [and embryonic stem (ES) cells] while FGFR2 is expressed in trophectoderm, consistent with a paracrine interaction of ligand and receptor (Niswander and Martin, 1992; Orr-Urtreger et al., 1993; Rappolee et al., 1994, 1998; Chai et al., 1998; Fraidenraich et al., 1998; Haffner-Krausz et al., 1999). Null mutations of both the Fgf4 and Fgfr2 genes resulted in similar phenotypes, namely death of homozygous mutant embryos around the time of implantation (Feldman et al., 1995; Arman et al., 1998). FGF4 has also been shown to be critical in the maintenance of the proliferation of trophectoderm and extra-embryonic ectoderm-derived cells and cell lines in vitro (Nichols et al., 1998; Tanaka et al., 1998).
Two distinct mechanisms of internalization of macromolecules and particles by the mouse embryo have previously been recognized, namely pinocytosis at the pre-implantation stage, and phagocytosis after implantation in the uterine wall. Soluble proteins and small particles (0.1–0.2 µm) enter trophectoderm cells of the free blastocyst by pinocytosis (Pemble and Kaye, 1986; Dyce et al., 1987; Dunglison and Kaye, 1995). Intake of larger particles (≥1 µm in diameter) by phagocytosis, a distinct, actin-dependent process, has been considered as a property characteristic of the post-implantation trophoblast cells, especially the giant cells (Albieri and Bevilacqua, 1996 and references therein). We inquired whether the progenitors of the trophoblast cells (mural trophectoderm cells) would already be programmed to exert this highly differentiated activity as early as 3.5 d.p.c. Using as an assay the ability of the cells to internalize large (1–3 µm) latex particles, phagocytosis could be demonstrated at this early stage of mouse development. Furthermore, phagocytosis exhibited a graded pattern along the embryonic–abembryonic axis. This result led us to inquire whether this very early functional differentiation might be regulated by signals from the ICM, in which case FGF4 would be an obvious candidate.
Results
Phagocytic activity of the mural trophectoderm
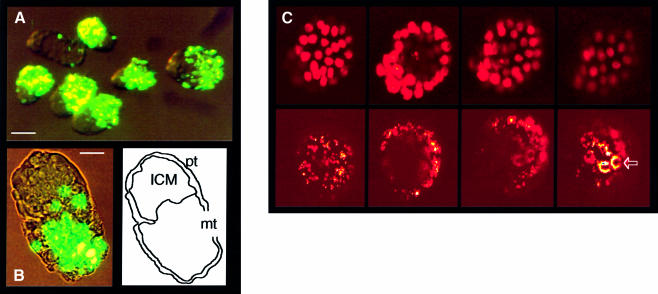
It was previously reported that all trophectoderm cells take up fluorescent latex microparticles (0.2 µm diameter) by endocytosis, a property that was exploited as a marker of this lineage (Dyce et al., 1987). Internalization of larger sized particles (1–3 µm) requires phagocytic potential. Using as an assay the internalization of either colored or fluorescent latex beads, we have observed phagocytic activity in a defined region of the trophectoderm of the pre-implantation mouse embryo. Embryos were collected between 2 and 3.5 d.p.c. and, after removal of the zona pellucida, incubated overnight in M16 medium containing the fluorescent particles. Starting between 2.5 and 3 d.p.c., concomitant with the formation of the blastocoel cavity, internalization was readily shown by fluorescence microscopy in mural trophectoderm cells. Electron microscopic examination of embryo sections after exposure to the beads confirmed their inclusion in cytoplasmic phagocytic vacuoles (not shown). Phagocytosis could not be demonstrated at any of the stages preceding blastocoel formation (zygote to morula; Table I). In the blastocyst, a sharp limit distinguished the labeled phagocytic cells from an inactive region corresponding to the polar trophectoderm in contact with the ICM (Figure 1A and B). A more precise analysis by confocal microscopy analysis of sequential sections through blastocysts exposed to fluorescent particles showed internalized label only in the mural trophectoderm region (Figure 1C). The absence of internalized particles in the polar region was not due to unequal exposure to the particles. The latter were maintained in suspension by Brownian motion and identical results were obtained in experiments in which the plates were gently shaken during the incubation period. When embryos were fixed every third hour after addition of the beads, internalized fluorescent particles were first detected after 6 h of culture (data not shown).
Table I. Phagocytic activity is not detected prior to trophectoderm differentiation.
| Age and developmental stage | No. of experiments | Total No. of embryos | Embryos with internalized beadsa |
|---|---|---|---|
| 1.0–2.0 d.p.c., 4–8 cell morula | 1 | 12 | 0 |
| 2.0–3.0 d.p.c., compacted morula to early blastocyst | 4 | 56 | 0b |
| 3.5–4.5 d.p.c., blastocyst | 3 | 48 | 48 |
aPhagocytosis was monitored by fluorescence microscopy visualization of internalized particles after overnight incubation of embryos collected at the indicated time in the presence of 1–2 µm diameter latex beads. Numbers correspond to embryos with internalized fluorescent material as depicted in Figure 1A (>100 beads per embryo).
bEarly blastocysts occasionally seen with a small number of internalized beads (1–5 per embryo).
Fig. 1. Phagocytic activity of 3.5 d.p.c. mouse embryos. (A) Low magnification fluorescent microscopy photograph of FITC-labeled latex particles (1–2 µm diameter) internalized during overnight incubation in M16 medium of blastocysts collected at 3.5 d.p.c. Bar, 50 µm. (B) Higher magnification (bar, 20 µm) showing internalization in only part of the blastocyst structure and tracing of the limits of the polar trophectoderm (pt), mural trophectoderm (mt) and ICM. (C) Confocal microscopy optical sections of a blastocyst treated as in (A) and (B). Upper row, nuclear labeling (Hoechst 33258); lower row, FITC fluorescence, both in artificial color; 0.95 µm thick optical sections at 2 µm intervals. Shown from left to right are sections at 18, 48, 70 and 80 µm from the slide bottom. The ICM is on the left of the pictures. Internalization occurs only in mural trophectoderm cells, with a cluster of more intensively labeled apical cells (arrow).
A graded pattern of phagocytic activity in the 3.5 d.p.c. blastocyst
A cluster of more brightly fluorescent cells was always observed at the abembryonic pole, opposite to the ICM (see Figure 1C). Variations in the phagocytic activity along the embryonic–abembryonic axis were examined by testing the ability of the cells to internalize particles of increasing sizes (Figure 2). Beads with a broad size distribution between 1 and 2 µm were found internalized in the whole mural trophectoderm region. Cells capable of taking up 2 µm particles homogeneous in size showed a more restricted distribution, which did not extend to the region closest to the ICM. Larger size particles (3 µm) labeled only a small region at the abembryonic pole. These different distributions were clearly correlated with the size of the beads, and not with their surface properties. Identical results were obtained in two series of experiments performed with homogeneous series of beads varying only by their sizes (data not shown; see Materials and methods). In summary, the farther the cells were from the polar–mural border, the more active they were in phagocytosis. Furthermore, we observed that the phagocytic ability of trophectoderm cells is not limited to latex particles, but extends to whole living cells, as shown by the internalization of sperm cells (Figure 2B).
Fig. 2. A graded pattern of phagocytic activity shown by internalization of particles of increasing sizes. (A) Internalization of latex particles of different sizes. (a)–(d) The blastocysts have been exposed to a mixture of 1 µm (yellow stain in c) and 3 µm particles (red stain in d). (a) Hoechst 33258 staining and (b) phase contrast microcopy showing the localization of the ICM and mural trophectoderm (mt); the embryonic–abembryonic axis is indicated by the double-headed arrow. (e and f) Same experiment performed using a mixture of non-fluorescent blue 1 µm beads (e) and red fluorescent 3 µm beads (e and f); the embryo is viewed from the abembryonic pole, with the ICM being partly masked. (B) Phagocytosis of live cells. β-galactosidase-positive epididymal sperm were collected from a male mouse of the ROSA26 transgenic family (Zambrowicz et al., 1997). Live sperm cells were diluted in M16 medium to a concentration of ∼250 000 cells/ml. Blastocysts collected at 3.5 d.p.c. were added, after removal of the zona pellucida, to 2 ml of the suspension of sperm cells. After overnight incubation, they were extensively washed and treated with trypsin as indicated in Materials and methods, and β-galactosidase activity in sperm cells was revealed by XGal staining as described by Hogan et al. (1994).
Phagocytic activity of trophectoderm cells does not require maintenance of the blastocyst structure
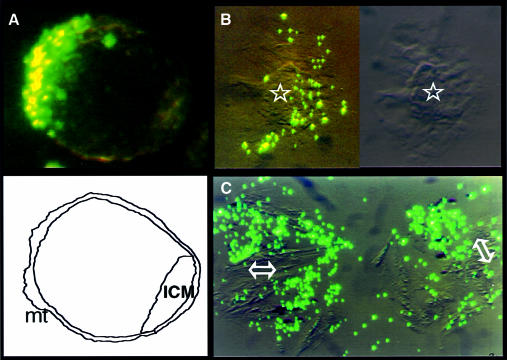
The same analysis was conducted on blastocysts attached to plastic plates in rich medium [Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 15% fetal calf serum]. Before attachment, they displayed the same pattern of uptake of fluorescent particles as the embryos maintained in M16 medium (Figure 3A), and immediately after attachment it was similarly found that only a fraction of the attached trophectoderm cells were capable of phagocytosis (Figure 3B). When the ICM had been removed by micromanipulation, the continued uptake of fluorescent particles still showed active phagocytosis in the resulting trophectoderm preparations (Figure 3C). The phagocytic activity of the mural trophectoderm is therefore not dependent on the presence of ICM, and the phagocytosis profile in the whole embryo suggests that the activity is in fact inhibited by ICM cells. To demonstrate such an inhibitory function, we first employed ES cells as a convenient model.
Fig. 3. Phagocytosis by attached trophectoderm cells in DMEM medium. (A) Blastocysts were maintained in DMEM medium supplemented with 15% fetal calf serum. Pictures were taken after overnight incubation in the presence of the beads, a time at which the blastocysts are still not attached to the plate. (B) Same experiment performed 24 h later, at a time when the blastocysts were attached; the ICM (star) is surrounded by trophectoderm cells. As exemplified in this picture, in 16 out of 16 attached blastocysts analyzed, only a fraction of the trophectoderm cells were taking up particles. (C) Same as in (B), but after removal of the ICM by micromanipulation from the regions indicated by double arrows, active phagocytosis in the attached trophectoderm cells is seen. Identical results were obtained for 10 out of 10 positive preparations.
Inhibition by ES cells of the phagocytic activity of trophectoderm cells
Blastocyst-derived ES cell lines are functionally equivalent to the ICM in their totipotent potential (Hogan et al., 1994 and references therein). Experiments were conducted in cocultures of blastocysts with ES cells in serum-containing DMEM medium, which, in previous experiments, was found to be compatible with the full phagocytic activity of trophectoderm cells (Figure 3). Since in addition, the ES cell culture medium contains exogenous leukemia inhibiting factor (LIF) and feeder cell products, we tested the possible effects of both and found that they did not inhibit phagocytosis (data not shown).
In the experiments summarized in Table II, blastocysts were maintained overnight in suspension in plates containing a preformed layer of ES cells; a second series was maintained in suspension together with trypsinized ES cells, and a third one in medium conditioned by the growth of ES cells. As compared with control cultures in the absence of ES cells, the phagocytic activity of the trophectoderm was in all cases strongly inhibited. The conclusion that inhibitory factor(s) is released by ES cells makes it more likely that the restriction of phagocytic activity to the mural region of the trophectoderm results from a paracrine inhibition by factor(s) released by the ICM.
Table II. Inhibition by ES cell-conditioned medium of the phagocytic activity of blastocyst trophectoderm cells.
| Experimenta | Culture medium | Total No. of embryos | Phagocytic activity (internalized beads per embryo) |
|||
|---|---|---|---|---|---|---|
| 0 | 1–2 | ≤10 | ≥100 | |||
| I | ES growth medium, feeder cells | 32 | 0 | 0 | 0 | 32 |
| II | Same as in I, but in the presence of an attached ES culture | 22 | 14 | 6 | 2 | 0 |
| III | Same medium as in I, but conditioned by prior growth of ES culture | 18 | 10 | 5 | 3 | 0 |
aI, control in ES growth medium with feeder cells and without ES cells. II, ES cells were attached to the plate during incubation of the blastocysts. III, the medium had been conditioned by a prior 24 h incubation with ES cells.
Control by FGF4 of the phagocytic activity of trophectoderm cells
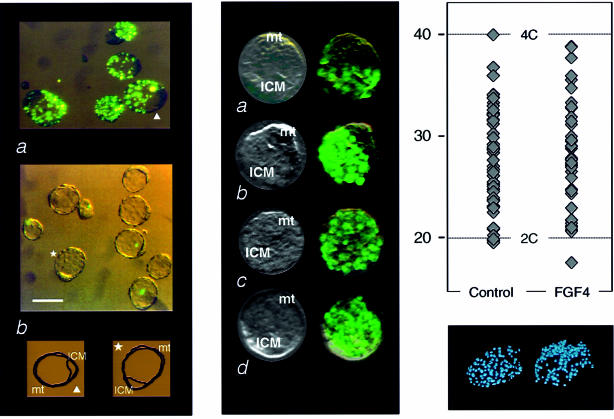
Since recent reports pointed to a role of FGF4 in maintaining the proliferative capacity (undifferentiated state) of trophectoderm cells (Chai et al., 1998; Nichols et al., 1998; Tanaka et al., 1998), we considered the possibility that this factor would be involved in the control of trophectoderm phagocytosis. A simple hypothesis would be that the release of FGF4 by ICM cells would prevent the phagocytic differentiation of polar trophectoderm cells and stimulate cell proliferation. Assays were performed to analyze possible effects of FGF4 on the phagocytic activity of the blastocysts. Embryos were recovered at 3.5 d.p.c. and cultured in parallel in 15% fetal calf serum-supplemented DMEM medium with concentrations ranging from 0.25 to 50 ng/ml recombinant human FGF4, which is known to be active on mouse cells. As shown in Figure 4, embryos maintained overnight in medium containing at least 25 ng/ml FGF4 were strongly inhibited in their ability to take up fluorescent particles (see also Table III), thus supporting the hypothesis that FGF4 secreted by ICM cells prevents the differentiation of trophectoderm cells reflected by their phagocytic potential.
Fig. 4. Inhibition by FGF4 of the phagocytic activity and activation of DNA synthesis in mural trophectoderm cells. (A) Inhibition of phagocytosis. (a) Internalization of 1–2 µm fluorescent latex beads by 3.5 d.p.c. blastocysts (same experiment as in Figure 3A); (b) part of the same batch of embryos was maintained overnight in the presence of FGF4 (25 ng/ml; see Materials and methods). Tracing of one embryo in each series, indicated by arrowhead and star, respectively. Bar, 50 µm. (B) Activation of DNA synthesis in mural trophectoderm cells. 3.5 d.p.c. embryos were maintained overnight under the same conditions as in (A), either with (c and d) or without (a and b) the addition of 25 ng/ml FGF4. BrdU was then added to the medium for periods of either 2 (a and c) or 4 h (b and d) and incorporation was monitored by in situ immunodetection (mt, mural trophectoderm). (C) DNA content of blastocyst nuclei. Blastocysts were collected, maintained for 24 h in culture medium with (FGF4) and without (control) addition of 25 ng/ml FGF4. They were fixed and stained with Hoechst 33258 and nuclear DNA content was determined by fluorescence intensity reading performed on digitized images of individual nuclei, as previously described (Rassoulzadegan et al., 1998). Calibration of the diploid DNA content value was deduced from parallel measurements on spleen B lymphocytes (not shown). Top, values recorded for four blastocysts in the control and four in the FGF4 series. Bottom, fluorescence microscopy of one stained embryo in each series.
Table III. Maintenance of the undifferentiated non-phagocytic state requires both FGF4 and serum factor(s) and correlates with the proliferation potential of trophectoderm cells.
| Culture medium | Total No. of embryosa | No. of embryos with |
||
|---|---|---|---|---|
| 0 bead | <10 beads | >100 beads | ||
| DMEM | 12 (3) | 0 | 0 | 12 |
| DMEM + FGF4 | 36 (3) | 0 | 0 | 36 |
| DMEM + 15% fetal calf serum | 20 (3) | 0 | 0 | 20 |
| DMEM + 15% fetal calf serum + FGF4 (25 ng/ml) | 80 (4) | 69 | 11 | 0 |
aNumber of independent experiments in parentheses.
Inhibition by FGF4 of the phagocytic behavior of mural trophectoderm cells correlates with initiation of S phase
The lack of a phagocytic potential would thus appear to correlate with the proliferative state maintained by FGF4 in polar trophectoderm cells (Chai et al., 1998; Nichols et al., 1998; Tanaka et al., 1998). To verify directly this conclusion by in situ assays on whole blastocysts, DNA synthesis was monitored by BrdU incorporation during 2–4 h pulses (see Materials and methods) on embryos that had been kept overnight in 15% fetal calf serum-supplemented DMEM medium with or without addition of FGF4 (25 ng/ml). Results (Figure 4B) showed that the trophectoderm cells of the abembryonic pole, which are the most active phagocytes, and which, as expected, were not in a proliferative state in the normal embryo, resumed DNA synthesis when treated with FGF4. As shown in Figure 4, only a well delimited region of the control embryos, corresponding to the ICM and the polar trophectoderm, showed labeled nuclei, in fact the reverse pattern of phagocytic activity. In the blastocysts maintained in the presence of FGF4, all the trophectoderm nuclei were uniformly stained after BrdU exposure. FGF4 thus appears to convert the mural cells into cells with the properties of polar trophectoderm cells with respect to both DNA replication and phagocytosis.
Since the mural trophectoderm cells will, after implantation, generate the primary giant cells of the trophoblast, a process that involves genomic endoreduplication, it is a possibility that the observed resumption of DNA synthesis upon exposure to FGF4 corresponds to the generation of polytene chromosomes rather than entry into a normal cell cycle. In order to test this hypothesis, we performed measurements of the DNA contents of individual nuclei in blastocysts that have been maintained in the presence of FGF4. Results (Figure 4C) showed that while, as we previously reported (Rassoulzadegan et al., 1998), all the cells of the pre-implantation mouse embryo are still diploid, FGF4 treatment did not result in the appearance of cells with DNA contents greater than the 2–4C range of a cycling diploid cell. The total DNA content of the blastocysts maintained in the presence of FGF4 was significantly increased compared with that of untreated embryos (data not shown). We therefore conclude that FGF4 induces the resumption of cell proliferation in the arrested abembryonic region of the mural trophectoderm.
FGF4 inhibition requires serum factor(s) in culture
In the experiment shown in Figure 4A, the blastocysts had been maintained overnight in serum-supplemented DMEM medium, with or without exposure to the labeled beads. Upon a more prolonged incubation, they would attach to the plastic substrate, spread and proliferate, eventually leading to the establishment of permanent ES and other lines (Hogan et al., 1994; Tanaka et al., 1998). Serum factors are required to maintain the proliferative potential of these embryonic cells, and, in serum-free DMEM, neither attachment nor subsequent cellular growth would be observed. Since FGF4 is clearly involved in the choice between the differentiated and proliferative states, we checked whether the presence of serum was required for inhibition of the phagocytic differentiation. This was indeed the case, as FGF4 addition did not inhibit phagocytosis in serum-free media (DMEM or M16) at a concentration (25 ng/ml) at which it effectively did so in serum-containing medium (Table III). Only irregular and low levels of BrdU labeling were observed after overnight incubation of the blastocysts in serum-free media (not shown).
A small number of mural trophectoderm cells at the abembryonic pole are required for blastocyst implantation
Experiments were performed to check whether internalization by a small number of cells at the abembryonic pole of the blastocyst of large (3 µm) latex particles, in all likelihood a toxic event, would affect further development. After a limited exposure, <10 cells were estimated to take up these large particles (Figure 2F). When transferred back to the uterus of foster mothers, these embryos were unable to resume their development, and examination of the uterine wall did not reveal implantation sites (Table IV). Under the same conditions, control blastocysts produced a living offspring with the expected efficiency of ∼80%. In spite of the fact that most of the fetal part of the placenta is derived from the polar region of the trophectoderm (EPC, secondary giant cells), i.e. from the region opposite to the cells containing latex particles, this result is indicative of an important role of the latter in the implantation process.
Table IV. Uptake of large size latex particles by a small number of cells at the abembryonic pole is sufficient to prevent implantation.
| Experiment | No. of embryos | Latex particles | No. of foster mothers | Implanted embryos |
|---|---|---|---|---|
| Ia | 20 | none | 2 | 15 |
| 60 | 3 µm beads | 4 | 0 | |
| IIb | 6 | none | 1 | 5 |
| 12 | 3 µm beads | 0 |
aEfficiency of re-implantation of the controls was monitored by counting living births. Implantation of embryos that had been exposed to latex particles was monitored by visual inspection of the uterine wall on the fifth day after transfer.
bAll embryos were re-implanted in the same foster mother, controls in the left uterine horn, embryos exposed to the beads in the right one. Implantation was monitored by visual inspection on the eighth day after transfer.
Phagocytic activity after implantation
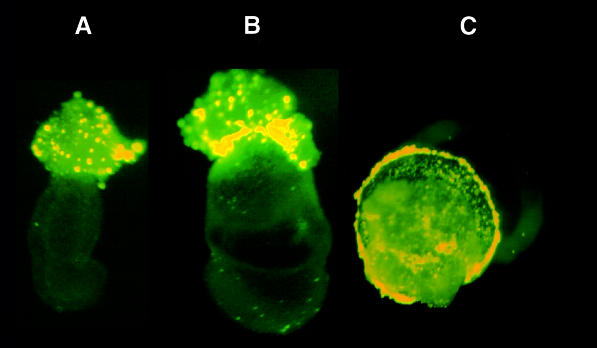
The phagocytic ability of the external cells of the mouse embryo was next evaluated shortly after implantation (6.5–7.5 d.p.c.). Embryos were dissected from the decidua and Reichert’s membrane was removed to allow both culture of the embryos and access of the labeled beads. At 6.5, 7.5 and 8.5 d.p.c., phagocytosis of 1–2 µm fluorescent particles was observed, both with and without serum, almost exclusively in the region of the EPC, a structure that consists of diploid trophoblast cells and secondary giant cells (Figure 5). Maternal decidual cells also possess phagocytic ability (data not shown), but at the stages examined, contamination of the embryo with maternal cells should be minimal (Hogan et al., 1994). Thus, a portion of the post-implantation derivatives of the initially inactive polar trophectoderm, secondary giant cells and/or diploid trophoblast exhibits a phagocytic activity similar to that of the mural trophectoderm in the pre-implantation embryo. Phagocytosis thus appears as an early marker of differentiation in two distinct lineages derived from the polar trophectoderm. As judged by the intensity of the fluorescence of internalized particles, treatment with 25 ng/ml FGF4 had no detectable effect on phagocytosis in extra-embryonic regions of 7.5 and 8.5 d.p.c. embryos (data not shown).
Fig. 5. Phagocytosis by 6.5–8.5 d.p.c. post-implantation whole mouse embryos and extra-embryonic fragments. Whole 6.5 d.p.c. (A) and 7.5 d.p.c. (B) mouse embryos with internalization of fluorescent particles restricted to the EPC. (C) Proximal extra-embryonic fragment of an 8.5 d.p.c. mouse embryo, photographed from its proximal side. Widespread bead internalization is evident, presumably in trophoblast giant cells and/or diploid trophoblast.
Discussion
Phagocytosis assays based on the internalization of latex particles identified a very early functional differentiation starting in the 3.5–4.5 d.p.c. mouse embryo. Among the three lineages individualized at this stage, only one, the mural trophectoderm, showed a phagocytic capacity, while both the polar trophectoderm and the ICM were inactive. Internalization of particles of increasing sizes allowed a semi-quantitative estimate of the activity, a graded pattern was observed in the mural trophectoderm, from a low activity in the part immediately adjacent to the polar region to a maximal activity at the abembryonic pole, both in terms of the number of internalized particles and their maximal size. Phagocytosis had previously been reported after phorbol ester and retinoid activation of cultured post-implantation trophoblast cells (Albieri and Bevilacqua, 1996). We further report a phagocytic potential of cells in the EPC region in intact, freshly explanted 6.5–8.5 d.p.c. embryos, in the absence of specific activating agents. A parallel can be drawn between the requirements for FGF4 in the differentiation of trophectoderm and EPC. Made by the epiblast after implantation, FGF4 prevents the differentiation of trophoblast target cell populations (Nichols et al., 1998; Tanaka et al., 1998). The lack of effect of externally added FGF4 on 7.5 and 8.5 d.p.c. trophoblast phagocytosis in our experiments, clearly contrasting with the inhibition observed in the mural trophectoderm of the blastocyst under the same conditions, suggests that the differentiation state that has been reached by the post-implantation EPC derivatives is now irreversible.
The polar trophectoderm may be regarded as a proliferation center whose descendants move away in the direction of the abembryonic pole. As they are now subjected to diminished ICM signals, the mural cells will stop proliferation and develop an enhanced phagocytic potential. One signaling system that operates at the peri-implantation stages is the FGF4 factor, secreted by ICM cells, and its receptor FGFR2, present in trophectoderm cells (Niswander and Martin, 1992; Orr-Urtreger et al., 1993; Rappolee et al., 1994, 1998; Chai et al., 1998; Fraidenraich et al., 1998; Haffner-Krausz et al., 1999). Fgfr2–/– mutant blastocysts showed a defect in trophectoderm interactions with the maternal tissue, notably an abnormal random orientation of the implanting embryos within the uterine crypt (Arman et al., 1998). Fgf4–/– homozygous embryos also displayed a peri-implantation lethal phenotype, as they initially attached to the uterine wall and then rapidly degenerated without implanting normally (Feldman et al., 1995). Interfering with FGF receptor signaling through the expression of a dominant negative form of the FGFR2 receptor resulted in inhibition of trophoblast cell proliferation in mosaic blastocysts (Rappolee et al., 1994), and Tanaka et al. (1998) reported that cell lines of trophoblastic origin require FGF4 to maintain their proliferative capacity.
In the 3.5 d.p.c. blastocyst, trophectoderm cells in the abembryonic region appear to enter a reversible differentiated state, exposure to FGF4 inhibiting their phagocytic behavior while inducing nuclear DNA synthesis. FGF4 interaction with the FGFR2 receptor is part of a complex system controlling the alternation between proliferation and differentiation. Other factors are still to be identified, as indicated by the observation that addition of FGF4 was only effective in the presence of serum in the culture medium. The reversibility of the mural trophectoderm fate to that of polar trophectoderm is in agreement with the results of reconstitution experiments in which isolated ICMs were recombined with vesicles of mural trophectoderm to generate viable chimeric blastocysts (Gardner et al., 1973; Papaioannou, 1982; Rossant et al., 1983; reviewed by Cross et al., 1994). The mural trophoblast cells in contact with the transplanted ICM presumably resumed DNA synthesis, and we may assume that FGF4 signaling from ICM cells is involved in this reversible differentiation control.
Our results indicate that resumption of DNA synthesis in cells in the abembryonic region of the trophectoderm corresponds to the induction of proliferation rather than the generation of the polytene chromosomes characteristic of the trophoblast giant cells (Varmuza et al., 1988 and references therein), an event we have shown does not occur at the pre-implantation stage (Rassoulzadegan et al., 1998). Moreover, it was recently reported that cells established in culture from either the trophectoderm of 3.5 d.p.c. blastocysts or the extra-embryonic ectoderm of 6.5 d.p.c. implanted embryos maintained a proliferative capacity only in medium containing FGF4 and differentiated into giant cells after removal of the factor (Tanaka et al., 1998). Therefore, it seems clear that FGF4 is involved in the balance between a differentiated state, which before implantation remains reversible, and a proliferative state.
The physiological function of phagocytosis in the early embryo is a matter of speculation. Several possibilities can be considered. As in the case of macrophages, phagocytosis could be part of an antimicrobial defense system. Other functions may include scavenging of dead cells and debris, possibly residual sperm cells (see Figure 2), but more likely trophectoderm cells engaged in programmed cell death (El-Shershaby and Hinchcliffe, 1975; Coucouvanis and Martin, 1995; Rassoulzadegan et al., 1998). Another likely function is a role in the invasion by the young parasite of the uterine wall. It is noteworthy in this respect that the two regions directly participating in implantation are the embryonic and the abembryonic poles (Hogan et al., 1994). The polar region at the embryonic pole is where the EPC, and later the placenta will develop. It represents one main site of invasion of the uterine wall and its post-implantation derivatives (presumably secondary giant cells) not unexpectedly possess a high phagocytic potential. In the free blastocyst, the most active phagocytes are, however, the mural cells at the abembryonic pole. We observed that implantation was drastically hampered after ingestion of large latex particles by a small number of the abembryonic mural cells, estimated to be <10, in all likelihood interfering with their normal function. This result is reminiscent of previous morphological descriptions of the initial stages of implantation, showing the embryo first attached by its abembryonic pole to the antimesometrial uterine wall (Rugh, 1990). One may speculate that the implantation defects of the homozygous null mutants of FGF4 and FGFR2 could involve this specialized population of abembryonic mural cells.
In other highly phagocytic cell lineages such as the macrophage, phagocytosis involves specific receptors and selective activation of downstream signaling pathways (Kwiatkowska and Sobota, 1999). Whether similar processes are a feature of trophectoderm phagocytosis is also of interest, especially if they are connected to the biology of embryo implantation.
As an early differentiation event in mouse development, phagocytosis is likely to require the activation of specific genes, and it will be of interest in this respect to identify target genes modulated by FGF4 signaling in the early embryo. There would be several genetic and molecular possibilities available to screen gene expression differentially in embryo, primary or established trophoblast cell cultures by gene-trap or cDNA library methodology (Rothstein et al., 1993), using phagocytosis as a marker for trophectoderm differentiation and FGF4 as a lineage modulator.
Materials and methods
Mouse strains
Embryos were generated by crossing C57BL/6×DBA/2 F1 hybrids (B6D2). The ROSA26 strain of transgenic mice [C57BL/6J-TgR(ROSA26)26Sor] was obtained from The Jackson Laboratory (reference number JR2192).
Culture of pre-implantation embryos
Embryos were collected between 2.5 and 3.5 d.p.c. from the oviduct and uterus of fertilized B6D2 mice, and incubated either in M16 medium or in ES cell culture medium after removal of the zona pellucida (Hogan et al., 1994). Experiments involving exposure to human recombinant FGF4 (Sigma F2278) were performed as described (Tanaka et al., 1998).
Phagocytosis assay
Either colored or fluorescent latex beads were added to the embryo culture medium to a final latex concentration of 10–20 µg/ml. At various times thereafter, embryos were washed twice with M2 buffer (Hogan et al., 1994), incubated in 1 ml of M2 containing 0.02% trypsin (Gibco-BRL) for 5 min at 25°C. They were washed twice with M2 buffer. The whole procedure efficiently eliminates particles bound to the cell surface (Nishioka et al., 1994; Grandjean et al., 1997). Experiments presented in this report used the following four types of latex beads (Warrington, Inc., PA), conveniently visualized due to their different colors and/or fluorescent properties: 1.0 µm diameter surfactant-free Royal Carboxyl latex beads (blue colored, non-fluorescent); 2–3 µm diameter surfactant-free fluorescent Red CML latex beads (excitation/emission: 580/605 nm); 1–2 µm diameter fluorescein isothiocyanate (FITC)-labeled paramagnetic latex particles (excitation/emission: 488/518 nm); and 3.015 ± 0.136 µm diameter Fluoresbrite Carboxy NYO microspheres (excitation/emission: 494/518 nm). Identical experiments performed with Carboxyl Latex beads of 0.973, 1–2 and 3 µm, and with surfactant-free Carboxyl Latex beads of 1, 1.1 and 2–3 µm, led to the same conclusions. Internalized particles were visualized by either phase contrast or fluorescence microscopy. Distribution of fluorescent beads within the embryo was determined by confocal microscopy with a Leica CLSM microscope equipped with an argon–krypton ion laser emitting light from 488 and 514 nm and producing an excitation wavelength of 515 and 580 nm and a UV laser (excitation/emission: 352/405 nm). A series of optical sections (32 per embryo, each of ∼0.95 µm thickness) was recorded throughout the z-axis, using an objective of 43× and a zoom of 1.99.
Cell culture
The WW6 ES cell line (Ioffe et al., 1995) was cultured on mitomycin C-treated STO feeder cells in DMEM (Gibco-BRL) containing 15% fetal bovine serum (HyClone) and 1000 µg/ml leukemia inhibitory factor (Gibco-BRL).
In situ determination of DNA synthesizing cells
Blastocysts recovered at 3.5 d.p.c. were incubated for 3–6 h in M16 culture medium in the presence of BrdU at a concentration of 1 µM. The embryos were then washed in M2. Fluorescence determination of the incorporated analog was performed using the ‘In situ Cell Proliferation Kit’ (Boehringer Mannheim, Cat. No. 1810740) following the manufacturer’s instructions.
DNA content of individual cells
Embryos were collected at 3.5 d.p.c., the zona pellucida was removed by treatment with acidic tyrode solution (Hogan et al., 1994), and the embryos were fixed overnight in 4% formaldehyde in M2 medium, then for 5 min in acetic acid:methanol (1:3). They were dried, washed with phosphate-buffered saline (PBS), then stained and mounted in mounting medium with Hoechst 33258 (Vectashield/H-1200). An inverted microscope (Zeiss Axiophot) equipped with a CCD color camera (Hamamatsu) was used for digital image capture. Image analysis was performed using the ‘MacBas v2.2’ program (Kohshin Graphic System, Inc.).
Post-implantation embryos
Embryos were dissected from decidua at 6.5 and 7.5 d.p.c., and Reichert’s membrane removed as described (Hogan et al., 1994). Proximal extra-embryonic fragments of 8.5 d.p.c. embryos were cut in the region of the chorion. Incubation with fluorescent beads was at 37°C for 16 h, either in M16 medium, or in DMEM with 10% fetal calf serum for the analysis of FGF4 effects, followed by washing in PBS and paraformaldehyde fixation as described.
Acknowledgments
Acknowledgements
The expert technical help of E.Couchi, M.Cutajar, Y.Fantei and M.Radjkumar is gratefully acknowledged.
References
- Albieri A. and Bevilacqua,E. (1996) Induction of erythrophagocytic activity in cultured mouse trophoblast cells by phorbol myristate acetate and all-trans-retinal. Placenta, 17, 507–512. [DOI] [PubMed] [Google Scholar]
- Ansell J.D. and Snow,M.H. (1975) The development of trophoblast in vitro from blastocysts containing varying amounts of inner cell mass. J. Embryol. Exp. Morphol., 33, 117–185. [PubMed] [Google Scholar]
- Arman E., Haffner-Krausz,R., Chen,Y., Heath,J.K. and Lonai,P. (1998) Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc. Natl Acad. Sci. USA, 95, 5082–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai N., Patel,Y., Jacobson,K., McMahon,J., McMahon,A. and Rappolee,D.A. (1998) FGF is an essential regulator of the fifth cell division in pre-implantation mouse embryos. Dev. Biol., 198, 105–115. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E. and Martin,G.R. (1995) Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell, 83, 279–287. [DOI] [PubMed] [Google Scholar]
- Cross J.C., Werb,Z. and Fischer,S.J. (1994) Implantation and the placenta: key pieces of the development puzzle. Science, 266, 1508–1518. [DOI] [PubMed] [Google Scholar]
- Dunglison G.F. and Kaye,P.L. (1995) Endocytosis in mouse blastocysts: characterization and quantification of the fluid phase component. Mol. Reprod. Dev., 41, 225–231. [DOI] [PubMed] [Google Scholar]
- Dyce J., George,M., Goodall,H. and Fleming,T.P. (1987) Do trophectoderm and inner cell mass cells in the mouse blastocyst maintain discrete lineages? Development, 100, 685–698. [DOI] [PubMed] [Google Scholar]
- El-Shershaby A.M. and Hinchcliffe,J.R. (1975) Epithelial autolysis during implantation of the mouse blastocyst: an ultrastructural study. J. Embryol. Exp. Morphol., 33, 1067–1080. [PubMed] [Google Scholar]
- Feldman B., Poueymirou,W., Papaioannou,V.E., DeChiara,T.M. and Goldfarb,M. (1995) Requirement of FGF4 for post-implantation mouse development. Science, 267, 246–249. [DOI] [PubMed] [Google Scholar]
- Fraidenraich D., Lang,R. and Basilico,C. (1998) Distinct regulatory elements govern Fgf4 gene expression in the mouse blastocyst, myotomes and developing limb. Dev. Biol., 204, 197–209. [DOI] [PubMed] [Google Scholar]
- Gardner R.L. (1999) Polarity in early mammalian development. Curr. Opin. Genet. Dev., 9, 417–421. [DOI] [PubMed] [Google Scholar]
- Gardner R.L., Papaioannou,V.E. and Barton,S.C. (1973) Origin of the ectoplacental cone and secondary giant cells in mouse blastocysts reconstituted from isolated trophoblast and inner cell mass. J. Embryol. Exp. Morphol., 30, 561–572. [PubMed] [Google Scholar]
- Gardner R.L., Meredith,M.R. and Altman,D.G. (1992) Is the anterior–posterior axis of the fetus specified before implantation in the mouse? J. Exp. Zool., 264, 437–443. [DOI] [PubMed] [Google Scholar]
- Grandjean V., Sage,J., Ranc,F., Cuzin,F. and Rassoulzadegan,M. (1997) Stage-specific signals in germ line differentiation: control of Sertoli cell phagocytic activity by spermatogenic cells. Dev. Biol., 184, 165–174. [DOI] [PubMed] [Google Scholar]
- Haffner-Krausz R., Gorivodsky,M., Chen,Y. and Lonai,P. (1999) Expression of Fgfr2 in the early mouse embryo indicates its involvement in pre-implantation development. Mech. Dev., 85, 167–172. [DOI] [PubMed] [Google Scholar]
- Hogan B., Costantini,F. and Lacy,L. (1994) Manipulating The Mouse Embryo—A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Ioffe E., Liu,Y., Bhaumik,M., Poirier,F., Factor,S.M. and Stanley,P. (1995) WW6: an embryonic stem cell line with an inert genetic marker that can be traced in chimeras. Proc. Natl Acad. Sci. USA, 92, 7357–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowska K. and Sobota,A. (1999) Signaling pathways in phagocytosis. BioEssays, 21, 422–431. [DOI] [PubMed] [Google Scholar]
- Nichols J., Zevnik,B., Anastassiadis,K., Niwa,H., Klewe-Nebenius,D., Chambers,I., Scholer,H. and Smith,A. (1998) Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell, 95, 379–391. [DOI] [PubMed] [Google Scholar]
- Nishioka K., Wagle,J.R., Rodriguez,T.J., Maeta,M., Kubo,S. and Dessens,S.E. (1994) Studies of human granulocyte phagocytosis stimulation by tuftsin. J. Surg. Res., 56, 94–101. [DOI] [PubMed] [Google Scholar]
- Niswander L. and Martin,G.R. (1992) Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development, 114, 755–768. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A., Bedford,M.T., Burakova,T., Arman,E., Zimmer,Y., Yayon,A., Givol,D. and Lonai,P. (1993) Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR2). Dev. Biol., 158, 475–486. [DOI] [PubMed] [Google Scholar]
- Papaioannou V.E. (1982) Lineage analysis of inner cell mass and trophectoderm using microsurgically reconstituted mouse blastocysts. J. Embryol. Exp. Morphol., 68, 199–209. [PubMed] [Google Scholar]
- Pemble L.B. and Kaye,P.L. (1986) Whole protein uptake by mouse blastocysts. J. Reprod. Fertil., 78, 149–157. [DOI] [PubMed] [Google Scholar]
- Rappolee D.A., Basilico,C., Patel,Y. and Werb,Z. (1994) Expression and function of FGF4 in peri-implantation development in mouse embryos. Development, 120, 2259–2269. [DOI] [PubMed] [Google Scholar]
- Rappolee D.A., Patel,Y. and Jacobson,K. (1998) Expression of fibroblast growth factor receptors in peri-implantation mouse embryos. Mol. Reprod. Dev., 51, 254–264. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Yang,Y. and Cuzin,F. (1998) APLP2, a member of the Alzheimer precursor protein family, is required for correct genomic segregation in dividing mouse cells. EMBO J., 17, 4647–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J., Vijh,M., Siracusa,L.D. and Chapman,V.M. (1983) Identification of embryonic cell lineages in histological sections of M.musculus in-equilibrium M.caroli chimaeras. J. Embryol. Exp. Morphol., 73, 179–191. [PubMed] [Google Scholar]
- Rothstein J.L., Johnson,D., Jessee,J., Skowronski,J., DeLoia,J.A., Solter,D. and Knowles,B.B. (1993) Construction of primary and subtracted cDNA libraries from early embryos. Methods Enzymol., 225, 587–610. [DOI] [PubMed] [Google Scholar]
- Rugh R. (1990) The Mouse, Its Reproduction and Development. Oxford University Press, Oxford, UK. [Google Scholar]
- Tanaka S., Kunath,T., Hadjantonakis,A.K., Nagy,A. and Rossant,J. (1998) Promotion of trophoblast stem cell proliferation by FGF4. Science, 282, 2072–2075. [DOI] [PubMed] [Google Scholar]
- Varmuza S., Pideaux,V., Kothary,R. and Rossant,J. (1988) Polytene chromosomes in mouse trophoblast giant cells. Development, 102, 127–134. [DOI] [PubMed] [Google Scholar]
- Zambrowicz B.P., Imamoto,A., Fiering,S., Herzenberg,L.A., Kerr,W.G. and Soriano,P. (1997) Disruption of overlapping transcripts in the ROSA β geo 26 gene trap strain leads to widespread expression of β-galactosidase in mouse embryos and hematopoietic cells. Proc. Natl Acad. Sci. USA, 94, 3789–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]