Abstract
Developing effective techniques for the cryopreservation of human adipose-derived adult stem cells (ASCs) could increase the usefulness of these cells in tissue engineering and regenerative medicine. To this end, we investigated the post-freeze/thaw viability and apoptotic behavior of Passage 1 (P1) adult stem cells (ASCs) in 11 different media: (i) the traditional media containing Dulbecco’s modified Eagle’s medium (DMEM) with 80% fetal calf serum (FCS) and 10% dimethyl sulfoxide (DMSO), (ii) DMEM with 80% human serum (HS) and 10% DMSO, (iii) DMEM with 1% methyl cellulose (MC) and 10% of either HS or FCS or DMSO, and (iv) DMEM with 0%, 2%, 4%, 6%, 8%, or 10% DMSO. Approximately 1 mL (106 cells/mL) of P1 ASCs were frozen overnight in a −80°C freezer and stored in liquid nitrogen for 2 weeks before being rapidly thawed in a 37°C water bath (1–2 min of agitation), resuspended in culture media, and seeded in separate wells of a 6-well plate for a 24-h incubation period at 37°C. After 24 h, the thawed samples were analyzed by bright-field microscopy and flow cytometry. The results suggest that the absence of DMSO (and the presence of MC) significantly increases the fraction of apoptotic and/or necrotic ASCs. However, the percentage of viable cells obtained with 2% DMSO and DMEM was comparable with that obtained in freezing media with 10% DMSO and 80% serum (HS or FCS), that is, ∼84% ± 5% and ∼84% ± 8%, respectively. Adipogenic and osteogenic differentiation behavior of the frozen thawed cells was also assessed using histochemical staining. Our results suggest that post-thaw ASC viability, adipogenic and osteogenic differentiability can be maintained even when they are frozen in the absence of serum but with a minimal concentration of 2% DMSO in DMEM.
Introduction
At present clinically relevant survival rates for adipose-derived stromal/stem cells (ASCs) are primarily achieved through the use of cryopreservation solution containing fetal bovine serum (FBS) plus 10% dimethyl sulfoxide (DMSO) or growth medium containing serum plus 10% DMSO [1–6]. Although DMSO is regarded as relatively nontoxic, the clinical use of frozen/thawed cells treated with DMSO can cause many adverse effects and toxic reactions [7–14]. It has also been reported that DMSO is not only cytotoxic but it also induces differentiation of stem cells to cardiac or neuronal like cells when added to the cell culture medium [15,16]. It is, therefore, imperative to reduce the toxicity by the removal of DMSO prior to clinical use. However, the total removal of DMSO from the frozen/thawed cells is costly and time consuming and invariably results in cell loss and clumping [7,8]. Therefore, it is necessary to develop cryopreservation protocols either with lower concentrations of DMSO or with nontoxic alternatives to DMSO.
A major constituent of the media used for the cryopreservation of ASCs is serum derived from animals. Serum is routinely added to the cryopreservation media as a source of nutrients, and other undefined factors, in spite of scientific and technical disadvantages to its inclusion, its high cost, and the increasing availability of serum-free alternatives [17–20]. Obviously, for in vivo clinical use, the elimination of animal serum proteins and all possible sources of infectious diseases such as hepatitis, human immunodeficiency virus (HIV), and bovine spongiform encephalopathy (BSE) is a prerequisite [21–23]. Therefore, it is necessary to replace serum as a supplement in freezing media used for cryopreserving ASCs. In contrast to animal serum, the use of an autologous serum for supplementation eliminates the risk of infectious diseases completely. A drawback, however, is that the production of autologous serum is a costly process and requires a preoperative blood donation by the patient. As a replacement for animal serum and to overcome the disadvantages of autologous serum, it is also possible to use human serum albumin in cryopreservation of ASCs. However, the risk of transmission of infectious diseases is not completely eliminated and the production of human serum albumin involves a major capital outlay [23]. Therefore, efforts need to be made to reduce, and preferably remove, serum (animal and human) in freezing (cryopreservation) media.
Methylcellulose (MC) is a high-molecular-weight polymer that has been used previously as a supplement in a serum-free culture medium and in cryopreservation of serum-free cultured cells [24–31]. Some investigators who have added MC to cell culture media have suggested that MC has its main role as a protective agent since it has minimal nutritional value for mammalian cells [24,28]. Thomas and Johnson [29] suggested that besides its protective function, MC also has a stimulatory effect on cells in suspended culture. Kuchler et al. [30] successfully eliminated the use of animal serum in their fibroblast cell-line cultures by the addition of MC and they further suggested that the function of the serum proteins and the methylcellulose in cell culture is similar. Merten et al. [25] claimed that for Vero and BHK-21 cells, no differences could be found between the cells frozen in the standard freezing medium (containing 10% fetal calf serum) and those frozen in serum-free media (containing 0.1% MC), with respect to growth and viability. Ohno et al. [31] showed that the presence of methylcellulose was a necessity in a serum-free media for increasing the viability of HeLa cells after thawing. Hence, we hypothesized that the presence of MC may be a necessary supplement during cryopreservation of ASCs in serum-free conditions.
In an effort to address the challenges posed by the presence of high concentrations of DMSO and potentially dangerous animal serum during current cryopreservation protocols for ASCs, the current study examined the apoptotic and necrotic response of passage 1 (P1) ASCs when the cells are frozen/thawed in the absence of serum and in the presence of several concentrations of DMSO as the sole cryoprotective agent (CPA). Further, we investigated the potential benefits of MC as a supplement in a serum-free freezing media in the presence and absence of DMSO.
Materials and Methods
Materials
All materials were obtained from Sigma-Aldrich (St. Louis, MO, www.sigmaaldrich.com) or Fisher Scientific (Pittsburgh, PA, www.fisherscientific.com) unless otherwise stated.
Isolation, collection, and culture of ASCs
All human protocols were reviewed and approved by the Pennington Biomedical Research Centre Institutional Review Board and specimens were obtained anonymously from subjects with written informed consent. Subcutaneous adipose tissue liposuction aspirates were obtained from 3 female Caucasian patients with the following demographics: mean age of 37.8 ± 9.1 years, mean body mass index (BMI) of 24.0 ± 2. Tissue samples (100–200 mL) were washed 3–4 times in phosphate-buffered saline (PBS) pre-warmed to 37°C, suspended in PBS supplemented with 1% bovine serum albumin and 0.1% collagenase (Type I; Worthington Biochemicals, Lakewood, NJ), and digested with gentle rocking for 45–60 min at 37°C. The digests were centrifuged for 5 min at 1,200 rpm (300g) at room temperature, resuspended, and the centrifugation step repeated. The supernatant was aspirated and the pellet resuspended in stromal medium (DMEM high glucose, 10% FBS, 100 units penicillin/mL, 100 μg streptomycin/mL, and 25 μg amphotericin/mL). The cell suspension was plated at a density equivalent to 0.125 mL of liposuction tissue per square centimeter of surface area, using a 35-mL volume of stromal medium per T225 flask. Cells were cultured for 48 h in a 5% CO2, humidified, 37°C incubator. At which time, the adherent cells were rinsed once with pre-warmed PBS and the cells fed with fresh stromal medium. The cells were fed with fresh stromal medium every 2–3 days until they reached approximately 75%–80% confluence. The medium was then aspirated; the cells were rinsed with pre-warmed PBS, and harvested by digestion with 0.05% trypsin solution (5–8 mL per T225 flask) for 3–5 min at 37°C. The cells were suspended in stromal medium, centrifuged for 5 min at 1,200 rpm (300g), the pellet resuspended in a volume of 10 mL of stromal medium, and the viable cell count determined by trypan blue exclusion. These cells were identified as Passage 0 (P0). The remaining cells were seeded in T225 flasks at a density of 5 × 103 cells/cm2. The cells were maintained in culture and passaged as described earlier to obtain the Passage 1 (P1) ASCs used in this study. A portion of the cells was resuspended in the various media at a concentration of 1.0 × 106 cells/mL of cryopreservation medium [3–6,32].
Preparation of freezing solutions
The CPAs used were: MC (Methocel® MC, viscosity of 3,000–5,500 mPa·s for 2% in water, 20°C) and DMSO (average molecular weight: 78.14). DMSO was used as received while MC was autoclaved at 121°C for 30 min before being added to DMEM. The DMEM-MC solutions (MC concentration of 1%) were prepared by dissolving weighted MC in DMEM at ∼60°C, followed by vortexing for 30 min, and the solutions were then stored overnight at 4°C to obtain a homogeneous preparation. Concentrations above 1% MC were found to be highly viscous and hard to handle and hence were not used in the present study. Table 1 shows the details of the cryopreservation media tested in this study. Before freezing, 1 × 106 P1 ASCs were added to 1 mL of cryopreservation media and were incubated at room temperature for 10 min to allow the CPA to get into the cells and establish the osmotic equilibrium.
Table 1.
The Composition of the Various Freezing Media Tested During Serum-Free Cryopreservation of P1 Adipose-Derived Stromal/Stem Cells (ASCs), in This Study
| Percentage of DMSO and/or MC in DMEM | Percentage of fetal bovine serum (FBS) | Percentage of human serum (HS) |
|---|---|---|
| Control media | ||
| 10% DMSO | 80 | 0 |
| 10% DMSO | 0 | 80 |
| Test media | ||
| 0%, 2%, 4%, 6%, 8%, 10% DMSO | 0 | 0 |
| 1% MC | 0 | 0 |
| 1% MC | 10 | 10 |
| 1% MC + 10% DMSO | 0 | 0 |
Freezing (and thawing) experiments
The P1 ASCs were frozen at −80°C in an ethanol-jacketed closed container overnight. The cells were then subsequently stored in liquid nitrogen for at least 2 weeks until use. Prior to the bright-field microscopy and the flow cytometric analysis, individual cryovials of cells were rapidly thawed in a 37°C water bath (1–2 min of agitation), resuspended in the stromal culture media, and seeded into the separate wells of a 6-well plate for a 24-h incubation period at 37°C.
Cell viability and apoptosis/necrosis assessment
A well-established annexin V apoptosis assay was analyzed by quantitative flow cytometry [33–36]. As a chemically induced apoptotic control, ASCs were incubated for 24 h in fresh medium enriched with etoposide (40 μM), a cytotoxic drug that induces DNA strand breaks by interaction with DNA topoisomerase II. For a necrotic control, ASCs were incubated for 24 h in fresh medium with 5 mM hydrogen peroxide (H2O2). The no treatment control consisted of ASCs treated in fresh medium, free from inducing agents. For each treatment, detached and attached cells were pooled, harvested by trypsinization (0.25% trypsin), washed with 10 mL of culture medium, and resuspended in 100 μL of 1× annexin-binding buffer (included in annexin V-FITC/PI kit). Approximately 100 μL of the cell suspension was mixed with 8 μL of annexin-V-FITC and 8 μL of 100 μg/mL propidium iodide (PI) and incubated in the dark at room temperature for 15 min. Liquid volume was removed by centrifugation and aspiration, and the cells were resuspended by gentle vortexing in 300–500 μL of 1× annexin-binding buffer prior to analysis on the flow cytometer. Apoptotic analyses for ASCs were performed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) utilizing 488-nm laser excitation and fluorescence emission at 530 nm (FL1) and >575 nm (FL3). Forward and side scatter measurements were made using linear amplification, and all fluorescence measurements were made with logarithmic amplification. A total of 20,000 events per sample were acquired using Cell Quest software (BD Biosciences, San Jose, CA).
Apoptosis is characterized by phosphatidylserine (PS) translocation from the inner leaflet to the outer leaflet of the lipid bilayer, while the cell membrane remains intact. Annexin V-positive cells correspond to cells that have experienced PS translocation. PI staining of the cells indicates that the integrity of the cell membrane has been compromised and is used to distinguish living and early apoptotic cells from necrotic cells. The fluorescent dot plots show three cell populations: live (annexin V-FITC-negative and PI-negative; annexin V− and PI−) necrotic (annexin V-FITC-positive and PI-positive; annexin V+ and PI+), and apoptotic (annexin V-FITC-positive and PI-negative; annexin V+ and PI−). Quadrant analysis was performed on the fluorescence dot plot to quantify the percentage of live, necrotic, and apoptotic cell populations. The quadrants positions were determined according to the no treatment control, 40 μM etoposide apoptotic control, and 5 mM H2O2 necrotic control.
Adipogenesis
Confluent cultures of ASCs were induced to adipogenesis by replacing the medium with an adipocyte induction cocktail containing DMEM/F-12 Ham’s with 3% FBS, 33 μM biotin, 17 μM pantothenate, 1 μM bovine insulin, 1 μM dexamethasone, 0.5 mM isobutylmethylxanthine (IBMX), 5 μM rosiglitazone, and 100 U penicillin/100 μg streptomycin/0.25 μg fungizone. After 72 h, the adipocyte induction medium was replaced with adipocyte maintenance media, which contains the same components as the induction medium except IBMX and rosiglitazone. Induced cells were maintained in culture for 9 days, with adipocyte maintenance medium replacement every 3 days. Upon the ninth day, the cultures were washed twice with pre-warmed PBS and fixed in formalin at 4°C. Adipocyte quantification was determined by staining neutral lipids with Oil Red O [32,37,38].
Osteogenesis
Confluent cultures of ASCs were induced to osteogenesis by replacing the medium with an osteogenic induction cocktail containing DMEM/F-12 Ham’s, 10% FBS, 10 mM β-glycerophosphate, 50 μg/mL sodium ascorbate 2-phosphate, and 100 U penicillin/100 μg streptomycin/0.25 μg fungizone. The induced cells were fed fresh osteogenic induction media every 3 days for 3 weeks. The cultures were then washed with 0.9% sodium chloride solution and fixed in 70% ethanol. Osteoblast quantification was determined by Alizarin Red staining for calcium phosphate [32,37,38].
Results
The temperature/time history experienced by the cells in the ethanaol-jacketed container was measured by using a type-T hypodermic needle thermocouples (Omega Technologies, Stamford, CT). Thermocouple voltages were read by a precision temperature data logger (Veriteq Instruments Inc., Richmond, BC, Canada) and transferred to a personal computer for further reduction and data analysis. The various cooling rates experienced by the cells in the ethanol-jacketed container placed in a −80°C freezer when 10% DMSO was used as sole CPA are shown in Figure 1. The plot suggests that the cells were subjected to different cooling rates at different time points within the ethanol-jacketed container. The ice nucleation was observed around −5°C and subsequently, a cooling rate of ∼1.1°C/min was imposed to a temperature of −40°C. The cooling rates experienced by the cells then further drops to 0.3°C/min and 0.1°C/min, before reaching −80°C (Fig. 1).
FIG. 1.
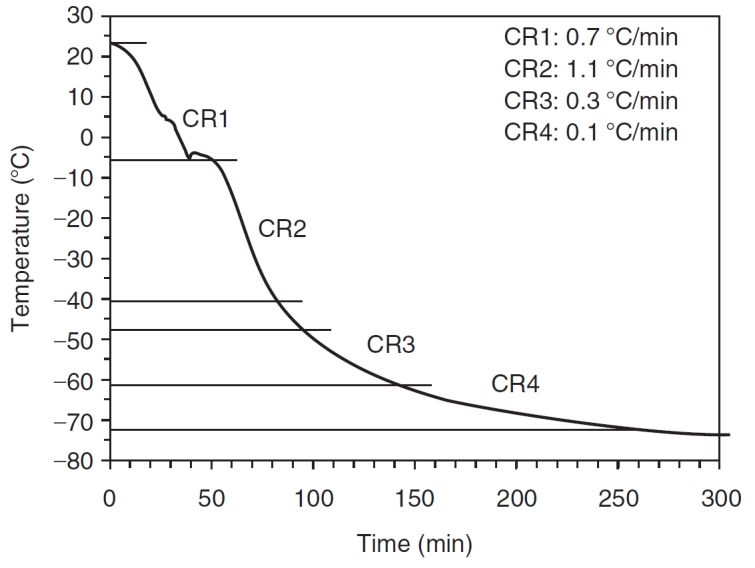
Representative cooling rates experienced by the cells in the ethanol-jacketed closed container placed in the −80°C freezer with 80% FCS and 10% DMSO in DMEM.
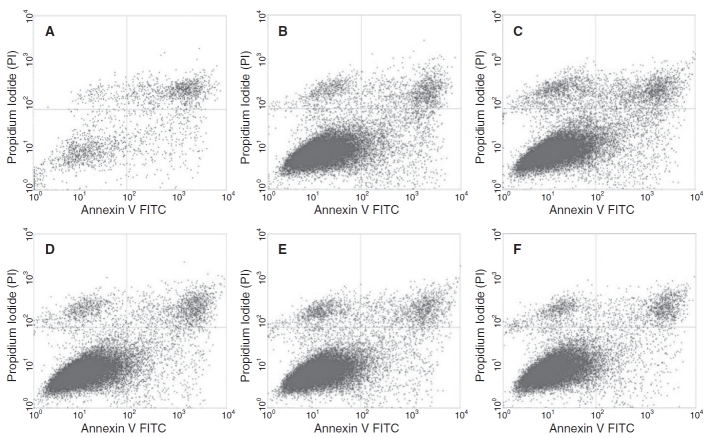
As described earlier, the effect of DMSO with concentrations ranging from 0% to 10% as the sole CPA in DMEM (with no serum components), on the post-freeze/thaw viability, apoptotic and necrotic response of ASCs, was assessed using flow cytometry. Characteristic flow cytometer fluorescence dot plots for ASCs frozen/thawed with different concentrations of DMSO as determined by annexin V staining and PI uptake are shown in Figure 2. The fuorescence dot plots for cells frozen in the presence of 2% and 10% DMSO (Fig. 2B and 2F) show a majority of the cells are in the lower left quadrant (annexin V− and PI−), which corresponds to live cell population. However at 0% DMSO, the number of cells present in the upper right quadrant (annexin V+ and PI+), which corresponds to necrotic cell population, increases. It is also important to note that the number of discrete events collected was ∼20,000 events (or cells) per run but with 0% DMSO this value was reduced to 8,000 to 9,000 events; presumably, because of the significantly larger necrotic cells within the population that are collected as debris during cell processing for flow cytometry. Similar population quadrant analysis was performed on all other experimental treatments to obtain quantitative information on the condition of the cells following the freeze/thaw process for the different media combinations investigated as part of this study.
FIG. 2.
Characteristic flow cytometer fluorescence dot plots showing fluorescence-activated cell sorting (FACS) analysis of P1 adipose-derived stromal/stem cells (ASCs) frozen/thawed in the presence various concentrations (0%, 2%, 4%, 6%, 8%, or 10%) of DMSO in DMEM. A–F represent 0%, 2%, 4%, 6%, 8%, or 10% DMSO in DMEM, respectively. The fluorescent dot plots show 3 cell populations: live (annexin V-FITC-negative and PI-negative; annexin V− and PI−) necrotic (annexin V-FITC-positive and PI-positive; annexin V+ and PI+), and apoptotic (annexin V-FITC-positive and PI-negative; annexin V+ and PI−). The quadrants positions were placed according to the no treatment control, 40 μm etoposide apoptotic control and 5 mM H2O2 necrotic control (see text for further details).
The post-thaw results obtained from the most routinely used cryopreservation media, either 80% fetal calf serum (FCS) with 10% DMSO in DMEM or 80% human serum (HS) with 10% DMSO in DMEM, were used as controls. The post-thaw behavior of cells frozen obtained using this traditional cryopreservation media is shown in Table 2. The viability of P1 ASCs cryopreserved in the control media containing 80% FCS with 10% DMSO in DMEM was ∼84% ± 8% (Table 2). Presumably, this routinely used cryopreservation media produces maximum viability; therefore, this data was used as a control to compare with the data obtained from the various freezing media tested in this study. The control data also suggests that the choice of the serum (human serum, HS or fetal calf serum, FCS) does not significantly alter the P1 ASC survival when frozen/thawed in 10% DMSO and DMEM (Table 2). Additional data from assay experiments (unfrozen cells, apoptotic control treated with etoposide, necrotic cells treated with H2O2) are also shown in Table 2.
Table 2.
The Percentage of Viable, Apoptotic, and Necrotic Passage 1 (P1) Adult Stem Cells (ASC) Obtained Using Fluorescence-Activated Cell Sorting (FACS) Analysis for Cells Frozen in 10% DMSO in DMEM Media With Either 80% Fetal Calf Serum (FCS) or 80% Human Serum (HS)
| % Viable (±SD) | % Apoptotic (±SD) | % Necrotic (±SD) | |
|---|---|---|---|
| Media with serum | |||
| 80% HS with 10% DMSO in DMEM | 82.5 (±8.3) | 5.8 (±3.9) | 8.3 (±3.3) |
| 80% FCS with 10% DMSO in DMEM | 84.1 (±7.7) | 5.7 (±2.8) | 7.5 (±3.7) |
| Assay controls | |||
| Live (untreated, unfrozen control) | 80.2 (±2.2) | 7.3 (±0.4) | 8.6 (±0.8) |
| Apoptotic (etoposide treated) | 46.1 (±5.7) | 38.2 (±8.3) | 10.5 (±4.1) |
| Necrotic (H2O2 treated) | 8.8 (±2.8) | 14.5 (±6.2) | 75.0 (±3.4) |
The percentages of viable, apoptotic, and necrotic P1 adipose-derived stromal/stem cells (ASCs) obtained FACS analysis are also shown for untreated (unfrozen, controls), apoptotic control (treated with 40 μM of etoposide per milliliter of cell culture media), and necrotic control (5 mM of hydrogen peroxide, H2O2).
Figure 3 shows the post-thaw results obtained for P1 ASCs frozen in DMEM with 1% MC supplemented with either no additions or minimal concentrations of serum (10% HS, 10% FCS) or 10% DMSO. The highest percentage of post-thaw cell survival was found for cells frozen with 10% DMSO (79.8% ± 2.8%) and the lowest values were found for cells frozen with 10% HS or 10% FCS alone (∼40.0% ± 8%). It is also interesting to see that the addition of 10% HS or 10% FCS to DMEM with 1% MC did not significantly alter the post-thaw viability results, that is, the cell survival is statistically similar between 1% MC in DMEM (46.8% ± 7.6%) and with 10% HS (or 10% FCS) with 1% MC in DMEM (∼40.0% ± 8%). Intriguingly, the percentage of apoptotic cells essentially remained constant (∼12%–14%) between the four media investigated with 1% MC (see Fig. 3). However, the percentage of necrotic cells with 10% DMSO and 1% MC was significantly lower (4.1% ± 3.1%) from the other 3 treatments investigated with 1% MC (∼35%–40%). Additional experiments conducted with decreasing percentage of MC lead to an even further decrease in cell viability (data not shown). Clearly, the data suggests the following: (i) the use of 1% MC decreased the cell survival significantly from ∼83% to ∼47%, that is, comparing the data with the traditional freezing media from Table 2 and 1% MC with DMEM; (ii) the addition of 10% DMSO significantly improves the cell viability, that is, the post-thaw cell viability with 1% MC in DMEM (∼47%) is significantly lower than that obtained with DMEM containing 1% MC and 10% DMSO (∼80%). Fortunately, the data obtained with 10% DMSO and 1% MC in DMEM is comparable to control data obtained with 80% FCS (or 80% HS) with 10% DMSO in DMEM. Thus, suggesting that the serum (FCS or HS) in the freezing media can be replaced by 1% MC.
FIG. 3.
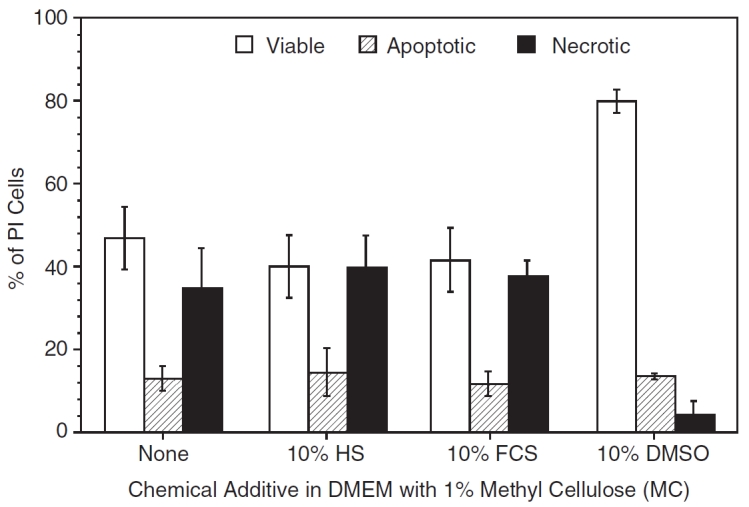
The post-thaw viability (open columns), apoptotic (partially shaded columns), and necrotic (completely shaded columns) response of P1 adipose-derived stromal/stem cells (ASCs) cryopreserved in the presence of 1% methyl cellulose (MC) in DMEM with either (i) no serum or dimethyl sulfoxide (DMSO) or, (ii) 10% human serum (HS) or, (iii) 10% fetal calf serum (FCS) or, (iv) 10% dimethyl sulfoxide (DMSO). The error bars represent the standard deviation in the data. The percentage of cells (viable or apoptotic or necrotic) is shown on the y-axis while the choice of chemical additive (none, 10% HS or 10% FCS or 10% DMSO) in the media (1% MC in DMEM) is shown on the x-axis.
Based on these results, additional experiments were performed without MC and with varying percentages of DMSO in DMEM. Figure 4 shows the post-thaw results obtained for P1 ASCs frozen in DMEM with varying percentage of DMSO (0%, 2%, 4%, 6%, 8%, and 10%). Although the highest percentage of post-thaw survival was achieved with a concentration of 10% DMSO (87.9% ± 2.9%), the post-thaw survival values obtained with DMSO concentrations of 2%, 4%, 6%, and 8% are comparable and were 83.8% ± 5.3%, 80.6% ± 3.1%, 86.0% ± 4.6%, and 85.6% ± 2.8%, respectively. However, freezing P1 ASCs in the absence of DMSO was detrimental to cell survival (the post-thaw cell survival dropped significantly to 25.3% ± 5.7%). Correspondingly, the percentage of apoptotic and necrotic cells are also comparable in DMEM containing 2%–10% DMSO. Specifically, the apoptotic cells ranged from 4.3% ± 2.6% (with 10% DMSO) to 8.2% ± 6.2% (with 2% DMSO) while the necrotic cells ranged from 4.6% ± 2.7 % (with 10% DMSO) to 7.0% ± 5.2% (with 4% DMSO). And finally, it is interesting to see the comparative behavior of MC and DMSO as the CPA of choice. The percentage of cell survival with 1% MC in DMEM was 46.8% ± 7.6% and was significantly lower than the value obtained earlier for cells frozen with 10% DMSO in DMEM (87.9% ± 2.9%) and with 2% DMSO in DMEM (83.8% ± 5.3%). Thus, replacing DMSO with MC as the sole CPA leads to a significant reduction (∼50%) in the post-thaw cell viability of P1 ASCs. Statistically, the only significant differences in the data shown in Figure 4 were found when the cells were frozen with DMSO and without DMSO, that is, even the presence of 2% DMSO was significant and this extremely low concentration was able to cryoprotect P1 ASCs.
FIG. 4.
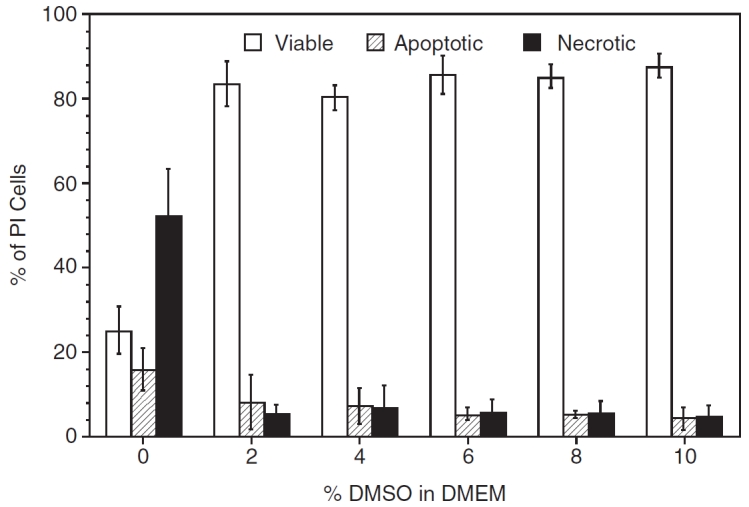
The effect of varying the dimethyl sulfoxide (DMSO) concentration (0%, 2%, 4%, 6%, 8%, or 10%) on the post-thaw viability (open columns), apoptotic (partially shaded columns), and necrotic (completely shaded columns) response of P1 adipose-derived stromal/stem cells (ASCs) cryopreserved in DMEM. The error bars represent the standard deviation in the data. The percentage of cells (viable or apoptotic or necrotic) is shown on the y-axis while the percentage of DMSO in DMEM is shown on the x-axis.
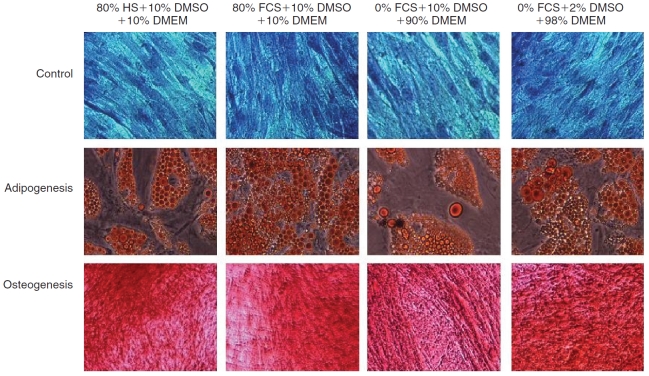
To address whether the presence and absence of serum and DMSO during cryopreservation affects post-thaw adipogenic and osteogenic differentiation in ASCs, we utilized Toluidine Blue, Oil Red O, and Alizarin Red to stain the undifferentiated ASCs, adipocytes, and osteoblasts, respectively (Fig. 5). The ASCs after 14 days of adipogenic induction and 21 days of osteogenic induction displayed morphological features consistent with adipogenesis or osteogenesis comparable to unfrozen cells (data not shown). Representative photomicrographs in Figure 5 show undifferentiated ASCs stained positive with Toluidine Blue (Row 1), adipocytes stained positive with Oil Red O (Row 2), and mineralized (osteoblast) cultures stained positive with Alizarin Red (Row 3). Our experiments showed no significant morphological differences between the cells cryopreserved in the presence of either 80% FCS with 10% DMSO in DMEM (Column 1) or 80% HS with 10% DMSO in DMEM (Column 2), or 0% FCS with 10% DMSO in DMEM (Column 3), or 0% FCS with 2% DMSO in DMEM (Column 4). More importantly, the results indicated that the adipogenic or osteogenic morphology of the cells cryopreserved in the presence of 2% DMSO in DMEM and without any serum (Column 4) is consistent with, and no different from, the control experiments with FCS (Column 1) and with HS (Column 2).
FIG. 5.
Representative phase contrast photomicrographs of P1 adipose-derived stromal/stem cells (ASCs) cultured under untreated (first row; Toludine Blue staining), adipogenic (second row; Oil Red O staining), or osteogenic (third row; Alizarin Red staining) conditions. Adipogenic cultures were stained with Oil Red O 14 days after induction while osteogenic cultures were stained with Alizarin Red after 21 days of culture. Images in column 1 represent cells that were cryopreserved in media containing 80% HS with 10% DMSO in DMEM. Images in column 2 represent cells that were cryopreserved in media containing 80% fetal calf serum (FCS) with 10% DMSO in DMEM. Images in column 3 represent cells that were cryopreserved in media containing 0% FCS with 10% DMSO in DMEM. And finally, images in column 4 represent cells that were cryopreserved in media containing 0% FCS with 2% DMSO in DMEM.
Discussion
Earlier reports suggested that MC can simultaneously act as a CPA and a serum supplement [24–31]. Although, our data suggested that the presence of 1% MC, as the sole CPA, in DMEM significantly increased the viability when compared to its absence (i.e., DMEM with 1% MC and DMEM alone); the overall outcome still yielded a significant loss (∼50%) of viable cell population when compared with controls (i.e., DMEM with 1% MC and DMEM with 80% serum and 10% DMSO). Intriguingly, the use of 1% MC in conjunction with 10% DMSO yielded post-thaw viabilities that are comparable to that of controls. It should be noted, as stated earlier, that concentrations above 1% MC were found to be highly viscous and hard to handle and hence were not used in the present study. This limitation on the percentage of MC in solution was due to the a priori selection of a commercially available high-viscosity grade MC (Methocel® MC, viscosity of 3,000–5,500 mPa·s for 2% in water, 20°C). Future studies with possibly lower or higher viscosity grade MC are clearly warranted and will be performed as time and resources permit.
Recently, there have been several reports suggesting that cells can be successfully cryopreserved with minimal concentrations of DMSO [39–51]. For example, Zhao et al. [46] showed that the use of 5% or 10% DMSO in combination with either 20% or 70% serum produced similar results during cryopreservation of fetal human liver CD34+ cells. Abrahamsen et al. [49] showed that cryopreserving peripheral blood progenitor cells (PBPC) with 5% DMSO instead of 10% DMSO produced improved CD34+ cell viability, while Stiff et al. [50] developed an alternative cryopreservation protocols for hematopoietic stem cells using 5% DMSO in conjunction with 6% hydroxyethyl starch (HES). Furthermore, Liseth et al. [51] concluded that the post-thaw survival for CD34+ was almost identical when frozen with either 4% or 5% DMSO but further lowering the concentration of DMSO to 2% had an adverse effect on post-thaw cell survival.
To the best of our knowledge no study has as yet investigated or reported the effect of DMSO during cryopreservation of ASCs in serum-free conditions. Considering the current status of adipose stem cell tissue engineering, we believe the time is ripe for the development of alternative cryopreservation methods for ASCs that can be directly translated to in vivo clinical trials. Thus, as described earlier, we have performed the current set of experiments on ASCs using media without serum (either FCS or HS) and minimal concentrations of DMSO. The present study indicated no significant differences in ASC cell viability and apoptosis when cells were cryopreserved with DMSO concentration ranging between 2% and 10%. However, a further decrease in DMSO from 2% to 0% was extremely detrimental. This critical finding suggests that there must be a “minimal” threshold concentration of DMSO, between 0% and 2%, that should successfully cryopreserve ASCs without any significant loss in viability. Experiments to determine this “minimal” threshold of DMSO are currently being performed. One possible explanation for this cryoprotective ability of DMSO is its ability to stabilize the cell membrane bilayer gel phase rather than the interdigitated gel phase, even at low concentrations [52–54].
Recent evidence suggests that the freeze/thawing process induces extensive early apoptosis stress activation pathways, which can lead to a time-dependent decline in viability and function at culture temperatures [55–61]. Therefore, consideration must be given to the adoption of methods that simultaneously detect early apoptotic cells along with necrotic cells for a more accurate assessment of post-thaw cell viability for in vivo transplantation applications [58]. For example, Hollister et al. [59] demonstrated that apoptosis is a significant component of cell death in a human prostate cancer cell line (PC3) exposed to cryopreservation temperatures. de Boer et al. [60] observed a significant reduction in the quantity of viable CD34+ cells mainly due to early but irreversible apoptosis. Recently, Sparrow et al. [61] reported that post-thaw umbilical cord blood samples contained significant portion of apoptotic CD34+ cells and lymphocytes when compared to fresh samples. Since, apoptosis is implicated in the delayed onset of cell death and is accompanied by decline in attachment rate and long-time survival, it is important to perform viability assessment at different time points of post-thaw cultured cells. However, assessments at 24 h post-thaw allow for a manifestation of most components of the cell stress cascades and encompass the peak of apoptotic (8–12 h) and necrotic (4–8 h) activities [56–58]. Hence we have performed flow cytometry analysis of apoptosis and necrosis of ASCs, after a 24-h post-thaw incubation, for all the cryopreservation media investigated.
Our post-thaw flow cytometry data indicated that there was no significant difference in apoptosis and necrosis in cells preserved in DMSO when compared with that of controls. This was true for all the values of DMSO concentration (2%–10%) that was investigated in the present study; although, the percentage of apoptotic cells increased from ∼4% to ∼8% as the concentration of DMSO was lowered from 10% to 2%. Cryopreservation of the ASCs in the absence of DMSO significantly increased magnitude of apoptotic and necrotic cells by a factor of 2–4× and 10×, respectively. Similarly, the presence of MC in the cryopreservation media led to an increase in the percentage of apoptotic (∼12%–15%) cells when compared with controls (∼6%). The percentage of cells exhibiting apoptosis in the presence of either MC alone or MC and serum was comparable to that obtained with MC and DMSO. However, the percentage of necrotic cells decreased dramatically from ∼38% to ∼4% when DMSO was added to the freezing media with MC. Thus, almost all of the increase in the cell viability obtained by adding DMSO to the media with MC can be correlated to the decrease in necrotic cells. This suggests that DMSO acts a true cryoprotectant and reduces the cell damage during the freeze/thaw process while MC has a minimal effect, at best, in preventing freeze/thaw injury (and apoptosis) of ASCs.
And finally, it is important to consider our results in terms of the broader area of cryobiology and water/ice biophysics; namely, the effect of imposed cooling rates, storage temperature, and thawing rates. As shown in Figure 1, extracellular ice nucleation was observed around −5°C and subsequently, a cooling rate of ∼1.1°C/min was imposed to a temperature of –40°C. The cooling rates experienced by the cells then further drops to 0.3°C/min and 0.1°C/min, before reaching −80°C. Obviously, these cooling rates are slightly modified, ±10%, based on the freezing media (data not shown). However, based on our prior experience with the biophysics of freezing these cells [4–6], these cooling rates are slow enough to avoid damaging intracellular ice formation during freezing while being fast enough to prevent prolonged exposure to concentrated extracellular salt solutions. As described earlier, the cells were stored in liquid nitrogen (approximately −160°C) for 2 weeks, before being rapidly thawed. The temperature of liquid nitrogen is below the glass transition temperature of the freezing solutions used in this study [62–64] and hence ensure the total solidification (and long-term stability) of the frozen sample. The thawing rate was based on an earlier study by Thirumala et al. [5] that investigated the effect of various freezing parameters on the immediate post-thaw integrity of frozen/thawed ASCs and was chosen to be 40°C/min (or rapid thawing). Rapid thawing has also been postulated to prevent the coalescence of large and possibly, damaging intracellular and extracellular ice crystals during the thawing process [65] and hence was utilized in this study as well.
Conclusion
The results of this study demonstrate: (i) the lack of sensitivity in the post-thaw data to the choice of serum, that is, either FCS or HS, in the freezing media; (ii) the ability of MC to act as replacement for serum in the freezing media only when used in conjunction with DMSO and not on its own; and (iii) the cryoprotective ability of DMSO even at the extremely low concentration of 2% in DMEM. Most importantly, the P1 ASCs frozen/thawed solely in the presence of 2% DMSO (ie, in the absence of serum) displayed similar phenotype (morphology and growth/differentiation characteristics) when compared to the cells cryopreserved in 80% serum with 10% DMSO in DMEM.
Acknowledgments
S.T. is supported by an EDA fellowship from LSU. J.M.G. was partially supported by a CNRU Center Grant 1P30 DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” sponsored by NIDDK. The authors thank Dr. Elizabeth Clubb and Dr. James Wade for supplying the liposuction aspirates and their many patients for consenting to participate in this protocol; Marilyn Dietrich of the LSU School of Veterinary Medicine Flow Cytometry Core Facility and Gang Yu, MS, of the Stem Cell Biology Laboratory and CNRU Molecular Mechanism Core for their technical assistance.
Contributor Information
Sreedhar Thirumala, Bioengineering Laboratory, Department of Mechanical Engineering, Louisiana State University, Baton Rouge, Louisiana.; Stem Cell Laboratory, Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, Louisiana.
Jeffrey M. Gimble, Stem Cell Laboratory, Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, Louisiana.
Ram V. Devireddy, Bioengineering Laboratory, Department of Mechanical Engineering, Louisiana State University, Baton Rouge, Louisiana.
Author Disclosure Statement
The authors declare no conflicting commercial interests.
References
- 1.Liu G, Zhou H, Li Y, Li G, Cui L, Liu W, Cao Y. Evaluation of the viability and osteogenic differentiation of cryopreserved human adipose-derived stem cells. Cryobiology. 2008;57:18–24. doi: 10.1016/j.cryobiol.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Gonda K, Shigeura T, Sato T, Matsumoto D, Suga H, Inoue K, Aoi N, Kato H, Sato K, Murase S, Koshima I, Yoshimura K. Preserved proliferative capacity and multipotency of human adipose-derived stem cells after long-term cryopreservation. Plast Reconstr Surg. 2008;121:401–410. doi: 10.1097/01.prs.0000298322.70032.bc. [DOI] [PubMed] [Google Scholar]
- 3.Goh BC, Thirumala S, Kilroy G, Devireddy RV, Gimble JM. Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen. 2007;1:322–324. doi: 10.1002/term.35. [DOI] [PubMed] [Google Scholar]
- 4.Thirumala S, Gimble JM, Devireddy RV. Transport phenomena during freezing of adipose tissue derived adult stem cells. Biotechnol Bioeng. 2005;92:372–383. doi: 10.1002/bit.20615. [DOI] [PubMed] [Google Scholar]
- 5.Thirumala S, Zvonic S, Floyd E, Gimble JM, Devireddy RV. Effect of various freezing parameters on the immediate post-thaw membrane integrity of adipose tissue derived adult stem cells. Biotechnol Prog. 2005;21:1511–1524. doi: 10.1021/bp050007q. [DOI] [PubMed] [Google Scholar]
- 6.Devireddy RV, Thirumala S, Gimble JM. Cellular response of adipose derived passage-4 adult stem cells to freezing stress. J Biomech Eng. 2005;127:1081–1086. doi: 10.1115/1.2073673. [DOI] [PubMed] [Google Scholar]
- 7.Fleming KK, Hubel A. Cryopreservation of hematopoietic and non-hematopoietic stem cells. Transfus Apher Sci. 2006;34:309–315. doi: 10.1016/j.transci.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan SS, Gross SA, Acker JP, Toner M, Carpenter JF, Pyatt DW. Cryopreservation of stem cells using trehalose: evaluation of the method using a human hematopoietic cell line. Stem Cells Dev. 2004;13:295–305. doi: 10.1089/154732804323099226. [DOI] [PubMed] [Google Scholar]
- 9.Alessandrino EP, Bernasconi P, Caldera D, Colombo A, Bonfichi M, Malcovati L, Klersy C, Martinelli G, Maiocchi M, Pagnucco G, Varettoni M, Perotti C, Bernasconi C. Adverse events occurring during bone marrow or peripheral blood progenitor cell infusion: analysis of 126 cases. Bone Marrow Transplant. 1999;23:533–537. doi: 10.1038/sj.bmt.1701609. [DOI] [PubMed] [Google Scholar]
- 10.Liseth K, Abrahamsen JF, Bjorsvik S, Grottebo K, Bruserud O. The viability of cryopreserved PBPC depends on the DMSO concentration and the concentration of nucleated cells in the graft. Cytotherapy. 2005;7:328–333. doi: 10.1080/14653240500238251. [DOI] [PubMed] [Google Scholar]
- 11.Benekli M, Anderson B, Wentling D, Bernstein S, Czuczman M, McCarthy P. Severe respiratory depression after dimethylsulphoxide-containing autologous stem cell infusion in a patient with AL amyloidosis. Bone Marrow Transplant. 2000;25:1299–1301. doi: 10.1038/sj.bmt.1702452. [DOI] [PubMed] [Google Scholar]
- 12.Higman MA, Port JD, Beauchamp NJ, Jr, Chen AR. Reversible leukoencephalopathy associated with re-infusion of DMSO preserved stem cells. Bone Marrow Transplant. 2000;26:797–800. doi: 10.1038/sj.bmt.1702589. [DOI] [PubMed] [Google Scholar]
- 13.Windrum P, Morris TC. Severe neurotoxicity because of dimethyl sulphoxide following peripheral blood stem cell transplantation. Bone Marrow Transplant. 2003;31:315. doi: 10.1038/sj.bmt.1703848. [DOI] [PubMed] [Google Scholar]
- 14.Windrum P, Morris TC, Drake MB, Niederwieser D, Ruutu T. Variation in dimethyl sulfoxide use in stem cell transplantation: a survey of EBMT centres. Bone Marrow Transplant. 2005;36:601–603. doi: 10.1038/sj.bmt.1705100. [DOI] [PubMed] [Google Scholar]
- 15.Young DA, Gavrilov S, Pennington CJ, Nuttall RK, Edwards DR, Kitsis RN, Clark IM. Expression of metalloproteinases and inhibitors in the differentiation of P19CL6 cells into cardiac myocytes. Biochem Biophys Res Commun. 2004;322:759–765. doi: 10.1016/j.bbrc.2004.07.178. [DOI] [PubMed] [Google Scholar]
- 16.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 17.Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122:501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- 18.Leist CH, Meyer HP, Fiechter A. Potential and problems of animal cells in suspension culture. J Biotechnol. 1990;15:1–46. doi: 10.1016/0168-1656(90)90049-h. [DOI] [PubMed] [Google Scholar]
- 19.Jochems CE, van der Valk JB, Stafleu FR, Baumans V. The use of fetal bovine serum: ethical or scientific problem? Altern Lab Anim. 2002;30:219–227. doi: 10.1177/026119290203000208. [DOI] [PubMed] [Google Scholar]
- 20.Even MS, Sandusky CB, Barnard ND. Serum-free hybridoma culture: ethical, scientific and safety considerations. Trends Biotechnol. 2006;24:105–108. doi: 10.1016/j.tibtech.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Wessman S, Levings R. Benefits and risks due to animal serum used in cell culture production. Dev Biol Stand. 1999;99:3–8. [PubMed] [Google Scholar]
- 22.van der Valk J, Mellor D, Brands R, Fischer R, Gruber F, Gstraunthaler G, Hellebrekers L, Hyllner J, Jonker FH, Prieto P, Thalen M, Baumans V. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol In Vitro. 2004;18:1–12. doi: 10.1016/j.tiv.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Reuther T, Kettmann C, Scheer M, Kochel M, Iida S, Kübler AC. Cryopreservation of osteoblast-like cells: viability and differentiation with replacement of fetal bovine serum in vitro. Cells Tissues Organs. 2006;183:32–40. doi: 10.1159/000094904. [DOI] [PubMed] [Google Scholar]
- 24.Mizrahi A, Moore GE. Partial substitution of serum in hematopoietic cell line media by synthetic polymers. Appl Microbiol. 1970;19:906–910. doi: 10.1128/am.19.6.906-910.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merten OW, Petres S, Couve E. A simple serum-free freezing medium for serum-free cultured cells. Biologicals. 1995;23:185–189. doi: 10.1006/biol.1995.0030. [DOI] [PubMed] [Google Scholar]
- 26.Bryant JC. Mammalian cells in chemically defined media in suspension cultures. Ann N Y Acad Sci. 1966;139:143–161. doi: 10.1111/j.1749-6632.1966.tb41192.x. [DOI] [PubMed] [Google Scholar]
- 27.Bryant JC. Methylcellulose effect on cell proliferation and glucose utilization in chemically defined medium in large stationary cultures. Biotechnol Bioeng. 1969;11:155–179. doi: 10.1002/bit.260110205. [DOI] [PubMed] [Google Scholar]
- 28.Merchant DJ, Hellman KB, Schneider H, Muirhead EE. Protection of animal cells with methylcellulose. Bact. Proc.: Abstr. 1962:141. [Google Scholar]
- 29.Thomas JA, Johnson MJ. Trace-metal requirements of NCTC clone 929 strain L cells. J Natl Cancer Inst. 1967;39:337–345. [PubMed] [Google Scholar]
- 30.Kuchler RJ, Marlowe ML, Merchant DJ. The mechanism of cell binding and cell-sheet formation in L strain fibroblasts. Exp Cell Res. 1960;20:428–437. doi: 10.1016/0014-4827(60)90171-3. [DOI] [PubMed] [Google Scholar]
- 31.Ohno T, Kurita K, Shin-ichiro A, Eimori N, Ikawa Y. A simple freezing medium for serum-free cultured cells. Cytotechnology. 1988;1:257–260. doi: 10.1007/BF00145029. [DOI] [PubMed] [Google Scholar]
- 32.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 33.van Engeland M, Nieland LJW, Frans CS, Schutte B, Reutelingsperger CPM. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 34.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin-V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 35.Aubry JP, Blaecke A, Lecoanet-Henchoz S, Jeannin P, Herbault N, Caron G, Moine V, Bonnefoy JY. Annexin-V used for measuring apoptosis in the early events of cellular cytotoxicity. Cytometry. 1999;37:197–204. doi: 10.1002/(sici)1097-0320(19991101)37:3<197::aid-cyto6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 36.Thirumala S, Forman JM, Monroe WT, Devireddy RV. Freezing and post-thaw apoptotic behaviour of cells in the presence of palmitoyl nanogold particles. Nanotechnology. 2007;18:195104–119516. [Google Scholar]
- 37.Bunnell BA, Estes BT, Guilak F, Gimble JM. Differentiation of adipose stem cells. Methods Mol Biol. 2008;456:155–171. doi: 10.1007/978-1-59745-245-8_12. [DOI] [PubMed] [Google Scholar]
- 38.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Windrum P, Morris TC, Drake MB, Niederwieser D, Ruutu T. Variation in dimethyl sulfoxide use in stem cell transplantation: a survey of EBMT centres. Bone Marrow Transplant. 2005;36:601–603. doi: 10.1038/sj.bmt.1705100. [DOI] [PubMed] [Google Scholar]
- 40.Galmes A, Besalduch J, Bargay J, Novo A, Morey M, Guerra JM, Duran MA. Long-term storage at -80 degrees C of hematopoietic progenitor cells with 5-percent dimethyl sulfoxide as the sole cryoprotectant. Transfusion. 1999;39:70–73. doi: 10.1046/j.1537-2995.1999.39199116897.x. [DOI] [PubMed] [Google Scholar]
- 41.Bakken AM, Bruserud O, Abrahamsen JF. No differences in colony formation of peripheral blood stem cells frozen with 5 or 10% dimethyl sulfoxide. J Hematother Stem Cell Res. 2003;12:351–358. doi: 10.1089/152581603322023089. [DOI] [PubMed] [Google Scholar]
- 42.Halle P, Tournilhac O, Knopinska-Posluszny W, Kanold J, Gembara P, Boiret N, Rapatel C, Berger M, Travade P, Angielski S, Bonhomme J, Deméocq F. Uncontrolled-rate freezing and storage at -80 degrees C, with only 3.5-percent DMSO in cryoprotective solution for 109 autologous peripheral blood progenitor cell transplantations. Transfusion. 2001;41:667–673. doi: 10.1046/j.1537-2995.2001.41050667.x. [DOI] [PubMed] [Google Scholar]
- 43.Lakota J, Fuchsberger P. Autologous stem cell transplantation with stem cells preserved in the presence of 4.5 and 2.2% DMSO. Bone Marrow Transplant. 1996;18:262–263. [PubMed] [Google Scholar]
- 44.Curcoy AI, Alcorta I, Estella J, Rives S, Toll T, Tuset E. Cryopreservation of HPCs with high cell concentration in 5-percent DMSO for transplantation to children. Transfusion. 2002;42:962–962. doi: 10.1046/j.1525-1438.2002.00198.x. [DOI] [PubMed] [Google Scholar]
- 45.Liseth K, Abrahamsen JF, Bjorsvik S, Grottebo K, Bruserud O. The viability of cryopreserved PBPC depends on the DMSO concentration and the concentration of nucleated cells in the graft. Cytotherapy. 2005;7:328–333. doi: 10.1080/14653240500238251. [DOI] [PubMed] [Google Scholar]
- 46.Zhao J, Hao HN, Thomas RL, Lyman WD. An efficient method for the cryopreservation of fetal human liver hematopoeitic progenitor cells. Stem Cells. 2001;19:212–218. doi: 10.1634/stemcells.19-3-212. [DOI] [PubMed] [Google Scholar]
- 47.Beaujean F, Bourhis JH, Bayle C, Jouault H, Divine M, Rieux C, Janvier C Le Forestier, Pico JL. Successful cryopreservation of purified autologous CD34+ cells: influence of freezing parameters on cell recovery and engraftment. Bone Marrow Transplant. 1998;22:1091–1096. doi: 10.1038/sj.bmt.1701494. [DOI] [PubMed] [Google Scholar]
- 48.Richter E, Eichler H, Raske D, Leveringhaus A, Zieger W, Kerowgan M, Goldmann SF. 5% Me2SO is sufficient to preserve stem cells derived from cord blood. Bone Marrow Transplant. 1998;22(Suppl 1):S16. [PubMed] [Google Scholar]
- 49.Abrahamsen JF, Bakken AM, Bruserud O. Cryopreserving human peripheral blood progenitor cells with 5-percent rather than 10-percent DMSO results in less apoptosis and necrosis in CD34+ cells. Transfusion. 2002;42:1573–1580. doi: 10.1046/j.1537-2995.2002.00242.x. [DOI] [PubMed] [Google Scholar]
- 50.Stiff PJ, Koester AR, Weidner MK, Dvorak K, Fisher RI. Autologous bone marrow transplantation using unfractionated cells cryopreserved in dimethylsulfoxide and hydroxyethyl starch without controlled-rate freezing. Blood. 1987;70:974–978. [PubMed] [Google Scholar]
- 51.Liseth K, Abrahamsen JF, Bjorsvik S, Grottebo K, Bruserud O. The viability of cryopreserved PBPC depends on the DMSO concentration and the concentration of nucleated cells in the graft. Cytotherapy. 2005;7:328–333. doi: 10.1080/14653240500238251. [DOI] [PubMed] [Google Scholar]
- 52.Kennedy A, Long CJ, Hmel PJ, Reid TJ. The interaction of DMSO with model membranes. II. Direct evidence of DMSO binding to membranes: an NMR study. J Liposome Res. 2003;13:259–267. doi: 10.1081/lpr-120026391. [DOI] [PubMed] [Google Scholar]
- 53.Yamashita Y, Kinoshita K, Yamazaki M. Low concentration of DMSO stabilizes the bilayer gel phase rather than the interdigitated gel phase in dihexadecylphosphatidylcholine membrane. Biochim Biophys Acta. 2000;1467:395–405. doi: 10.1016/s0005-2736(00)00237-6. [DOI] [PubMed] [Google Scholar]
- 54.Moldovan D, Pinisetty D, Devireddy RV. Molecular dynamics simulation of pore growth in lipid bilayer membranes in the presence of edge-active agents. Appl Phys Lett. 2007;91:204104. [Google Scholar]
- 55.Baust JM, Van Buskirk RG, Baust JG. Cell viability improves following inhibition of cryopreservation-induced apoptosis. In Vitro Cell Dev Biol Anim. 2000;36:262–270. doi: 10.1290/1071-2690(2000)036<0262:cvifio>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 56.Baust JM, Van Buskirk RG, Baust JG. Gene activation of the apoptotic caspase cascade following cryogenic storage. Cell Preserv Technol. 2002;1:63–80. [Google Scholar]
- 57.Baust JM, Vogel MJ, Van Buskirk R, Baust JG. A molecular basis of cryopreservation failure and its modulation to improve cell survival. Cell Transplant. 2001;10:561–571. [PubMed] [Google Scholar]
- 58.Mathew AJ, Baust JG, Van Buskirk RG. Improved hypothermic preservation of human renal cells through suppression of both apoptosis and necrosis. Cell Preserv Technol. 2003;1:239–253. [Google Scholar]
- 59.Hollister WR, Mathew AJ, Baust JG, Van Buskirk RG. The effects of freezing on cell viability and mechanisms of cell death in an in vitro human prostate cancer cell line. Mol Urol. 1998;2:13–18. [Google Scholar]
- 60.de Boer F, Drager AM, Pinedo HM, Kessler FL, van der Wall E, Jonkhoff AR, van der Lelie J, Huijgens PC, Ossenkoppele GJ, Schuurhuis GJ. Extensive early apoptosis in frozen–thawed CD34-positive stem cells decreases threshold doses for haematological recovery after autologous peripheral blood progenitor cell transplantation. Bone Marrow Transplant. 2002;29:249–255. doi: 10.1038/sj.bmt.1703357. [DOI] [PubMed] [Google Scholar]
- 61.Sparrow RL, Komodromou H, Tippett E, Georgakopoulos T, Xu W. Apoptotic lymphocytes and CD34+ cells in cryopreserved cord blood detected by the fluorescent vital dye SYTO 16 and correlation with loss of L-selectin (CD62L) expression. Bone Marrow Transplant. 2006;38:61–67. doi: 10.1038/sj.bmt.1705405. [DOI] [PubMed] [Google Scholar]
- 62.Luyet B. On various phase transitions occurring in aqueous solutions at low temperatures. Proc Natl Acad Sci USA. 1965;125:549–569. doi: 10.1111/j.1749-6632.1960.tb49982.x. [DOI] [PubMed] [Google Scholar]
- 63.Franks F. Properties of aqueous solutions a subzero temperatures. In: Franks F, editor. Water a comprehensive treatise: Water And Aqueous Solutions at Subzero Temperatures. Vol. 7. Plenum Press; New York: 1982. pp. 292–309. [Google Scholar]
- 64.Aitkins PW. Physical Chemistry. 6th edn. Oxford University Press; Oxford, UK: 1999. [Google Scholar]
- 65.Rubinsky B. Principles of low temperature cell preservation. Heart Fail Rev. 2003;8:277–284. doi: 10.1023/a:1024734003814. [DOI] [PubMed] [Google Scholar]