Abstract
Objective:
This study aimed to compare the glucose-lowering effect and glycemic variability of insulin glargine with those of insulin detemir.
Material and methods:
This was an open-label, single-center, randomized, two-way crossover study in patients with diabetes on basal-bolus insulin therapy, with neutral protamine Hagedorn (NPH) insulin as basal insulin. Patients switched from NPH insulin to a course either of insulin glargine followed by insulin detemir, or insulin detemir followed by insulin glargine, continuing the same dose of the prior bolus of insulin. To evaluate the glucose-lowering effect, daily glycemic profiles were recorded for 72 hours by continuous glucose monitoring (CGM) in an outpatient setting. The mean amplitude of glycemic excursions, standard deviation (SD), and the mean of daily difference (MODD) were used to assess intraday and day-to-day glycemic variability.
Results:
Eleven patients were enrolled and nine completed the study. Mean blood glucose calculated from CGM values was significantly lower with insulin glargine compared with insulin detemir (9.6 ± 2.4 mmol/L versus 10.4 ± 2.8 mmol/L, P = 0.038). The SD was significantly lower with insulin glargine versus insulin detemir (2.5 ± 0.9 mmol/L vs 3.5 ± 1.6 mmol/L, P = 0.011). The MODD value was significantly lower with insulin glargine than with insulin detemir (2.2 ± 1.1 mmol/L vs 3.6 ± 1.7 mmol/L, P = 0.011). There was no significant difference between the two insulin analogs in terms of hypoglycemia.
Conclusion:
This study suggests that insulin glargine leads to more effective and more stable glycemic control than the same dose of insulin detemir.
Keywords: continuous glucose monitoring, insulin detemir, insulin glargine
Introduction
The Diabetes Control and Complications Trial1 and the Kumamoto Study2 have shown that intensive insulin therapy and the resulting improvements in glycemic control reduce the incidence and delay the progression of microvascular complications. However, neutral protamine Hagedorn (NPH) insulin, which was used as basal insulin in these studies, has several limitations. Of note, its duration of action is only 8–12 hours, with a peak in action occurring within 4–6 hours after subcutaneous administration, thus increasing the risk of hypoglycemia.3–7 Indeed, some patients complain of hypoglycemia before dawn, necessitating a reduction in the NPH insulin dose at bedtime. However, reducing the NPH insulin dose at bedtime increases the blood glucose level in the morning. Additionally, NPH insulin is a suspension, which must be thoroughly resuspended before injection, and inadequate resuspension results in a very large day-to-day glycemic variability of action.8,9 Therefore, to achieve tight glycemic control without increasing the risk of hypoglycemia, insulin preparations with a long duration of action and low day-to-day glycemic variability in terms of glucose-lowering action are needed.
Insulin glargine4,6,10 and insulin detemir5,7,11,12 are basal insulin analogs of the dissolution type that have flatter profiles and longer duration of action compared with NPH insulin. However, there is controversy over which insulin analog has the longer and more stable action. Therefore, in this study, the aim was to compare the glucose-lowering effect and glycemic stability of insulin glargine with those of insulin detemir using continuous glucose monitoring (CGM).
Material and methods
Patients
Diabetic patients who were prescribed basal-bolus insulin therapy with NPH insulin as basal insulin at bedtime for 1 year or more were enrolled in the study. Patients who injected NPH insulin two or more times per day, with proteinuria >1.0 g/day, serum creatinine >132 μmol/L (men) or 106 μmol/L (women), abnormal aspartate aminotransferase/ alanine aminotransferase elevation (>3 ×the upper limit of normal), myocardial infarction or stroke within 6 months prior to study entry, or HbA1c > 10.0% or <5.8%, were excluded from this study.
All patients received an explanation of the procedures and possible disadvantages of participating in the study and gave written informed consent prior to entry. This study was approved by the Institutional Review Board of Kitasato Institute Hospital and was performed in accordance with the Declaration of Helsinki.
Design of the study
Patients were randomized using a computer to either Sequence A (NPH insulin was first switched to insulin glargine, then to insulin detemir) or Sequence B (insulin detemir followed by insulin glargine). The patient’s prior NPH insulin was discontinued and replaced with the allocated long-acting insulin analog (insulin glargine or insulin detemir). Patients were asked to continue their other antihyperglycemic medications and to not change their dosage throughout the study. To compare insulin glargine with insulin detemir under the same conditions, the dose of the long-acting insulin analogs was the same as that of NPH, and the doses of bolus insulin (insulin lispro or insulin aspart) were not changed. The study drugs were injected at bedtime. The CGM examination, of 72 hours in duration from 12 pm on day 1 to 12 pm on day 4, was carried out at least 5 days after switching insulin. The study drugs were crossed over on the day when the first CGM examination ended.
The CGM sensor (CGMS® System Gold; Medtronic, Northridge, CA) was applied to the abdominal area by a certified diabetologist. Patients were instructed to measure their capillary blood glucose using finger sticks, at least four times per day (at mealtimes and at bedtime). Glucose meters were calibrated immediately before starting CGM. All patients used the CGM in outpatient settings.
Glycemic control
The outcomes of this study included determining the effectiveness of each type of insulin on glycemic control and glycemic variability. Glycemic control was estimated as the mean blood glucose (MBG), the area under the glucose curve above 7.8 or 10.0 mmol/L (area under the curve [AUC]>7.8,10), and the percentage of time above 7.8 or 10.0mmol/L (t>7.8,10). The AUC was calculated using the trapezoidal method.
Glycemic variability
Intraday glycemic variability was assessed as the standard deviation (SD) and the mean amplitude of glycemic excursions (MAGE). The SD around the mean glucose values is considered the “gold standard” assessment of intraday glycemic variability.13 MAGE, described by Service et al,14 is probably more appropriate for selecting the major glucose swings that are calculated as the arithmetic mean of differences between consecutive peaks and nadirs, provided that the differences are greater than the SD around the mean values.13
Day-to-day glycemic variability was assessed as the mean of daily difference (MODD). MODD, described by Molnar et al,15 is the mean of the absolute difference between glucose values taken on 2 consecutive days at the same time.
Hypoglycemia
Hypoglycemia, which was defined as a sensor value of ≤3.9 mmol/L, was also calculated as a total time at ≤3.9 mmol/L. Severe hypoglycemia was defined as a sensor value of ≤2.8 mmol/L.
Statistical analysis
All values are shown as means with SD. The differences between two insulin analogs were analyzed using the Wilcoxon rank-sum test. A P value of <0.05 was considered statistically significant. SPSS software 14.0J (SPSS Japan Inc, Tokyo, Japan) was used for all statistical analyses.
Results
Patient characteristics
A total of eleven Japanese patients, six with type 1 and five with type 2 diabetes, were enrolled between May 2008 and June 2009. Because of an alert for insulin glargine issued by the European Association for the Study of Diabetes,16 this study was discontinued and the available data was analyzed. Two patients were excluded from analysis because of protocol violation (misuse of NPH). Therefore, nine patients (five in Sequence A and four in Sequence B) completed the study. The demographic and baseline characteristics of these patients are shown in Table 1.
Table 1.
Patient demographics and baseline characteristics
| Type of diabetes (type 1/type 2) | 4/5 |
| Gender (male/female) | 3/6 |
| Age (years) | 64 ± 13 |
| Diabetes duration (years) | 17 ± 11 |
| Duration of insulin therapy (years) | 9 ± 8 |
| Weight (kg) | 57.6 ± 8.0 |
| BMI (kg/m2) | 24.2 ± 4.9 |
| HbA1c (%) | 7.9 ± 1.7 |
| Fasting serum C-peptide (nmmol/L) | 0.29 ± 0.25 |
| Total insulin dose (U/kg/day) | 0.68 ± 0.19 |
| Basal insulin dose (U/kg/day) | 0.24 ± 0.11 |
| Bolus insulin dose (U/kg/day) | 0.44 ± 0.10 |
| Oral glucose-lowering drugs (n) | |
| Metformin | 1 |
| Alpha glucosidase inhibitor | 3 |
Note: Data are mean ± SD.
Abbreviations: BMI, body mass index; Hb A1c, hemoglobin A1c.
Glycemic control
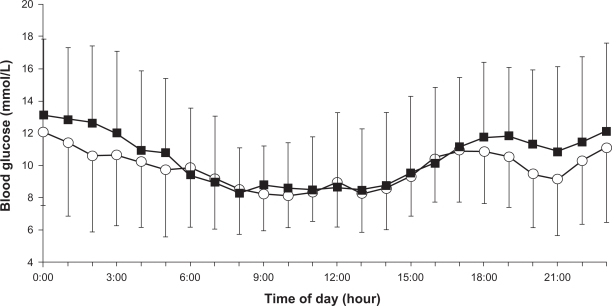
MBG, as calculated from CGM values, was significantly lower with insulin glargine than with insulin detemir (9.6 ± 2.4 mmol/L versus 10.4 ± 2.8 mmol/L, P = 0.038) (type 1: 11.0 ± 2.5 mmol/L versus 12.2 ± 2.7 mmol/L; type 2: 8.4 ± 1.8 mmol/L versus 9.0 ± 2.2 mmol/L). Figure 1 shows the mean daily profiles of day 2 and day 3. This difference between the two insulin analogs was particularly evident at nighttime. In addition, AUC>10 and AUC>7.8 were significantly lower with insulin glargine versus insulin detemir (Table 2).
Figure 1.
24-hour glucose profiles of day 2 and day 3. Each point represents the mean± standard deviation of nine patients treated with insulin glargine (○) or detemir (▪).
Table 2.
Comparison of glargine CGM data with detemir CGM data
| Glargine | Detemir | P value | |
|---|---|---|---|
| Glycemic control | |||
| MBG (mmol/L) | 9.6 ± 2.4 | 10.4 ± 2.8 | 0.038 |
| Type 1 | 11.0 ± 2.5 | 12.2 ± 2.7 | ns |
| Type 2 | 8.4 ± 1.8 | 9.0 ± 2.2 | ns |
| AUC>10 (mmol/L/day) | 1.2 ± 1.5 | 2.1 ± 2.0 | 0.011 |
| Type 1 | 2.1 ± 1.7 | 3.5 ± 1.9 | 0.068 |
| Type 2 | 0.5 ± 0.9 | 1.0 ± 1.3 | 0.080 |
| AUC>7.8 (mmol/L/day) | 2.4 ± 2.1 | 3.3 ± 2.5 | 0.008 |
| Type 1 | 3.6 ± 2.2 | 5.0 ± 2.3 | 0.068 |
| Type 2 | 1.4 ± 1.5 | 2.0 ± 1.7 | 0.043 |
| t>10 (%) | 37.6 ± 31.4 | 44.3 ± 28.6 | 0.051 |
| Type 1 | 57.2 ± 26.3 | 61.7 ± 24.4 | ns |
| Type 2 | 21.9 ± 27.6 | 30.5 ± 25.5 | 0.080 |
| t>7.8 (%) | 61.9 ± 24.1 | 63.8 ± 21.1 | ns |
| Type 1 | 75.1 ± 19.2 | 72.3 ± 19.1 | ns |
| Type 2 | 51.4 ± 23.9 | 57.1 ± 22.0 | ns |
| Hypoglycemic | |||
| Total hypoglycemic (t<3.9) time (min) | |||
| Overtime | 64 ± 81 | 97 ± 177 | ns |
| Type 1 | 94 ± 89 | 65 ± 60 | ns |
| Type 2 | 40 ± 74 | 122 ± 241 | ns |
| Nighttime | 42 ± 74 | 19 ± 48 | ns |
| Type 1 | 83 ± 99 | 43 ± 69 | ns |
| Type 2 | 10 ± 22 | 0 ± 0 | ns |
| Severe hypoglycemic (t<2.8) time (min) | |||
| Overtime | 18 ± 49 | 6 ± 11 | ns |
| Type 1 | 38 ± 75 | 11 ± 16 | ns |
| Type 2 | 3 ± 4 | 2 ± 4 | ns |
| Nighttime | 17 ± 50 | 16 ± 48 | ns |
| Type 1 | 38 ± 75 | 36 ± 73 | ns |
| Type 2 | 0 ± 0 | 0 ± 0 | ns |
Note: Data are mean ± SD.
Abbreviations: MBG, mean blood glucose; AUC, area under the curve; CGM, continuous glucose monitoring; ns, not significant.
Glycemic variability
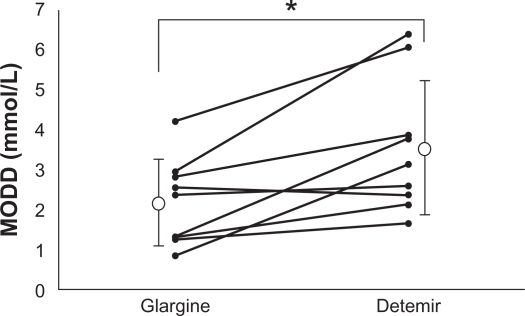
The SD was significantly lower with insulin glargine than with insulin detemir. However, the MAGE value was not significantly different between the two insulin analogs (Table 3). The MODD value was significantly lower with insulin glargine than with insulin detemir (2.2 ± 1.1 mmol/L vs 3.6 ± 1.7 mmol/L, P = 0.011; Figure 2) (type 1: 2.7 ± 1.2 mmol/L vs 4.7 ± 1.8 mmol/L; type 2: 1.8 ± 0.9 mmol/L vs 2.7 ± 0.9 mmol/L).
Table 3.
Comparison of the intraday glycemic variability of glargine with that of detemir
| Glargine | Detemir | P value | |
|---|---|---|---|
| SD (mmol/L) | 2.5 ± 0.9 | 3.5 ± 1.6 | 0.011 |
| Type 1 | 3.2 ± 0.8 | 4.6 ± 1.6 | 0.081 |
| Type 2 | 2.0 ± 0.6 | 2.6 ± 1.0 | ns |
| MAGE (mmol/L) | 6.3 ± 2.4 | 7.8 ± 3.6 | 0.086 |
| Type 1 | 7.8 ± 1.7 | 10.1 ± 3.4 | ns |
| Type 2 | 5.1 ± 2.2 | 5.9 ± 2.7 | ns |
| MODD (mmol/L) | 2.2 ± 1.1 | 3.6 ± 1.7 | 0.011 |
| Type 1 | 2.7 ± 1.2 | 4.7 ± 1.8 | ns |
| Type 2 | 1.8 ± 0.9 | 2.7 ± 0.9 | ns |
Note: Data are mean ± SD.
Abbreviations: MAGE, mean amplitude of glycemic excursions; MODD, mean of daily difference; ns, not significant.
Figure 2.
Comparison of the mean of daily difference (MODD) in nine patients treated with insulin glargine or detemir. The MODD value was significantly lower with insulin glargine than with insulin detemir.
Note: *P = 0.011.
Hypoglycemia
There was no difference between the two insulin analogs in terms of the total hypoglycemic time. This lack of a difference remained even after separating the results according to daytime and nighttime values. Severe hypoglycemic time was also similar for both insulin analogs (Table 2).
Discussion
In this study, the glucose-lowering effect and glucose stability of insulin glargine was compared with those of insulin detemir using CGM. Glycemic control parameters, such as MBG, AUC>10, and AUC>7.8, were better with insulin glargine than with insulin detemir. The present study suggests that insulin glargine has a greater glucose-lowering effect than insulin detemir at the same dose. The difference in glucose-lowering effect can be compensated for by increasing the insulin dose but there might be a difference in the cost-effectiveness and hypoglycemic episode. Because the same tendency was found in both type 1 and type 2 diabetes (MBG in type 1: insulin glargine, 11.0 ± 2.5 mmol/L versus insulin detemir, 12.2 ± 2.7 mmol/L; type 2: 8.4 ± 1.8 mmol/L versus 9.0 ± 2.2 mmol/L), to maintain statistical power, the two types of diabetes were not distinguished.
However, the hypoglycemic time was comparable for both of the two insulin analogs. These findings suggest that, if higher doses of insulin detemir are administered to achieve equivalent glycemic control to insulin glargine, the hypoglycemic time might increase.
Furthermore, the intraday and day-to-day glycemic variability of insulin glargine was compared with those of insulin detemir. The results of CGM revealed that SD and MODD were lower with insulin glargine than with insulin detemir. These findings suggest that insulin glargine might have better glycemic variability compared with insulin detemir. It is expected that the risk of hypoglycemia is lower with insulin glargine than with insulin detemir, if the aim of treatment is near-normal glycemia.
Several previous studies have compared insulin glargine with insulin detemir.17–29 Three studies using insulin-clamp tests yielded very conflicting results.17–19 Heise et al reported that the same doses (0.4 U/kg) of insulin glargine and insulin detemir are very similar in terms of the mean shape of their pharmacodynamic profiles and duration of action in patients with type 1 diabetes.17 They also reported that the day-to-day glycemic variability is lower with insulin detemir. Klein et al reported a similar duration of action and lower day-to-day glycemic variability with insulin detemir versus insulin glargine in patients with type 2 diabetes (0.8–1.6 U/kg).18 By contrast, Porcellati et al reported that 0.35 U/kg of insulin detemir has similar effects to the same dose of insulin glargine during the first 12 hours, and that the metabolic effects of insulin detemir are lower at 12–24 hours.19
Six clinical studies have compared insulin glargine with insulin detemir. In five studies, in which all patients or more than half of the patients were injected with insulin detemir twice daily, glycemic control was similar to that of once-daily insulin glargine.20–24 The other study, in which the majority (87.4%) of patients were injected with insulin detemir once a day, suggested inferiority of glycemic control for insulin detemir versus once-daily insulin glargine.25
These clinical studies clearly showed that, at similar daily doses, insulin detemir has a shorter action time and weaker action than insulin glargine. These findings are consistent with the results of the present study and with the results of a clamp study reported by Porcellati et al.19
Tone et al compared the day-to-day glycemic variability of insulin glargine with that of insulin detemir.26 However, CGM was not used and the study evaluated only fasting plasma glucose; therefore, the study was inconclusive. However, King et al and Wiesli et al have reported comparisons of insulin glargine with insulin detemir using CGM. Although these studies concluded that once-daily insulin detemir provided 24-hour glycemic control similar to that of insulin glargine in patients with type 2 diabetes, did glycemic variability was not reported.27–29 To the best of the present authors’ knowledge, the present study is the first to show that insulin glargine has better glycemic variability than insulin detemir based on CGM.
There are several limitations to this study. First, because of the alert for insulin glargine, it was deemed inappropriate to continue enrolling patients to receive insulin glargine. Therefore, the number of subjects enrolled was too small to reach a definitive conclusion and patients with type 1 and type 2 diabetes were combined. However, as shown in Figure 2, most of the patients had lower MODD values with insulin glargine than with insulin detemir. In fact, our finding of lower MODD was consistent after stratification for the type of diabetes (insulin glargine versus insulin detemir, type 1: 2.7 ± 1.2 mmol/L versus 4.7 ± 1.8 mmol/L; type 2: 1.8 ± 0.9 mmol/L versus 2.7 ± 0.9 mmol/L). Therefore, the authors’ believe this conclusion may be robust. Second, the doses of basal insulin used in the present study were different from those used in the earlier clinical studies. The average basal insulin dose was 0.24 U/kg/day in this study (type 1: 0.22 U/kg/day; type 2: 0.26 U/kg/day), which was much lower than in earlier clinical studies (insulin glargine: 0.3321–0.7525 U/kg/day; insulin detemir: 0.4021–0.8223 U/kg/day). However, these studies were performed in European countries or in the USA. The doses of basal insulin used in our study are similar to those used in the Japanese national phase III program for insulin glargine for Japanese patients with type 1 diabetes (mean: 0.21 U/kg/day)30 and insulin detemir for Japanese patients with type 1 or type 2 diabetes (mean: 0.27 U/kg/day).31 Therefore, the insulin dose in the present study appears to be appropriate for Japanese patients.
Conclusion
The results of the present study suggest that insulin glargine provides more effective and more stable glycemic control than insulin detemir. Because this study was too small to make a final conclusion, however, large-scale studies are required to confirm these findings.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Diabetes Control and Complications Trial (DCCT) Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 2003;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174–183. doi: 10.1056/NEJMra040832. [DOI] [PubMed] [Google Scholar]
- 4.Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142–2148. doi: 10.2337/diabetes.49.12.2142. [DOI] [PubMed] [Google Scholar]
- 5.Plank J, Bodenlenz M, Sinner F, et al. A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care. 2005;28:1107–1112. doi: 10.2337/diacare.28.5.1107. [DOI] [PubMed] [Google Scholar]
- 6.Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23:644–649. doi: 10.2337/diacare.23.5.644. [DOI] [PubMed] [Google Scholar]
- 7.Fanelli GC, Pampanelli S, Porcellati F, Rossetti P, Brunetti P, Bolli GB. Administration of neutral protamine Hagedorn insulin at bedtime versus dinner in type 1 diabetes mellitus to avoid nocturnal hypoglycemia and improve control. A randomized, controlled trial. Ann Intern Med. 2002;136:504–514. doi: 10.7326/0003-4819-136-7-200204020-00007. [DOI] [PubMed] [Google Scholar]
- 8.Jehle PM, Micheler C, Jehle DR, Breitig D, Boehm BO. Inadequate suspension of neutral protamine Hagedorn (NPH) insulin in pens. Lancet. 1999;354:1604–1607. doi: 10.1016/S0140-6736(98)12459-5. [DOI] [PubMed] [Google Scholar]
- 9.Kølendorf K, Bojsen J, Deckert T. Clinical factors influencing the absorption of 125I-NPH insulin in diabetic patients. Horm Metab Res. 1983;15:274–278. doi: 10.1055/s-2007-1018694. [DOI] [PubMed] [Google Scholar]
- 10.Wang F, Carabino JM, Vergara CM. Insulin glargine: a systematic review of a long-acting insulin analogue. Clin Ther. 2003;25:1541–1577. doi: 10.1016/s0149-2918(03)80156-x. [DOI] [PubMed] [Google Scholar]
- 11.Kurtzhals P, Havelund S, Jonassen I, et al. Albumin binding of insulins acylated with fatty acids: characterization of the ligand-protein interaction and correlation between binding affinity and timing of the insulin effect in vivo. Biochem J. 1995;312:725–731. doi: 10.1042/bj3120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Havelund S, Plum A, Ribel U, et al. The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm Res. 2004;21:1498–1504. doi: 10.1023/b:pham.0000036926.54824.37. [DOI] [PubMed] [Google Scholar]
- 13.Monnier L, Colette C, Owens DR. Glycemic variability: the third component of the dysglycemia in diabetes. Is it important? How to measure it? J Diabetes Sci Technol. 2008;2:1094–1100. doi: 10.1177/193229680800200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycaemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 15.Molnar GD, Taylor WF, Ho MM. Day-to-day variation of continuously monitored glycaemia: a further measure of diabetic instability. Diabetologia. 1972;8:342–348. doi: 10.1007/BF01218495. [DOI] [PubMed] [Google Scholar]
- 16.Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–1744. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614–1620. doi: 10.2337/diabetes.53.6.1614. [DOI] [PubMed] [Google Scholar]
- 18.Klein O, Lynge J, Endahl L, Damholt B, Nosek L, Heise T. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab. 2007;9:290–299. doi: 10.1111/j.1463-1326.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 19.Porcellati F, Rossetti P, Busciantella NR, et al. Comparison of pharmacokinetics and dynamics of the long-acting insulin analogs glargine and detemir at steady state in type 1 diabetes: a double-blind, randomized, crossover study. Diabetes Care. 2007;30:2447–2452. doi: 10.2337/dc07-0002. [DOI] [PubMed] [Google Scholar]
- 20.Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomized, 52 week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naïve people with type 2 diabetes. Diabetologia. 2008;51:408–416. doi: 10.1007/s00125-007-0911-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heller S, Koenen C, Bode B. Comparison of insulin detemir and insulin glargine in a basal-bolus regimen, with insulin aspart as the mealtime insulin, in patients with type 1 diabetes: a 52-week, multinational, randomized, open-label, parallel-group, treat-to-target noninferiority trial. Clin Ther. 2009;31:2086–2097. doi: 10.1016/j.clinthera.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Pieber TR, Treichel HC, Hompesch B, et al. Comparison of insulin detemir and insulin glargine in subjects with Type 1 diabetes using intensive insulin therapy. Diabet Med. 2007;24:635–642. doi: 10.1111/j.1464-5491.2007.02113.x. [DOI] [PubMed] [Google Scholar]
- 23.Hollander P, Cooper J, Bregnhøj J, Pedersen CB. A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with type 2 diabetes. Clin Ther. 2008;30:1976–1987. doi: 10.1016/j.clinthera.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Swinnen SG, Dain MP, Aronson R, et al. A 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin detemir twice-daily in patients with type 2 diabetes inadequately controlled on oral glucose-lowering drugs. Diabetes Care. 2010;33:1176–1178. doi: 10.2337/dc09-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raskin P, Gylvin T, Weng W, Chaykin L. Comparison of insulin detemir and insulin glargine using a basal-bolus regimen in a randomized, controlled clinical study in patients with type 2 diabetes. Diabetes Metab Res Rev. 2009;25:542–548. doi: 10.1002/dmrr.989. [DOI] [PubMed] [Google Scholar]
- 26.Tone A, Iseda I, Higuchi C, et al. Comparison of insulin detemir and insulin glargine on glycemic variability in patients with type 1 and type 2 diabetes. Exp Clin Endocrinol Diabetes. 2010;118:320–324. doi: 10.1055/s-0029-1243230. [DOI] [PubMed] [Google Scholar]
- 27.King AB. Once-daily insulin detemir is comparable to once-daily insulin glargine in providing glycaemic control over 24 h in patients with type 2 diabetes: a double-blind, randomized, crossover study. Diabetes Obes Metab. 2009;11:69–71. doi: 10.1111/j.1463-1326.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- 28.King AB. No higher dose requirements with insulin detemir than glargine in type 2 diabetes: a crossover, double-blind, and randomized study using continuous glucose monitoring. J Diabetes Sci Technol. 2010;4:151–154. doi: 10.1177/193229681000400119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiesli P, Krayenbuhl P, Uthoff H, Seifert B, Schmid C. Omitting breakfast and lunch after injection of different long-acting insulin preparations at bedtime: a prospective study in patients with type 2 diabetes. Diabetologia. 2009;52:1816–1819. doi: 10.1007/s00125-009-1439-z. [DOI] [PubMed] [Google Scholar]
- 30.Kawamori R, Iwamoto Y, Kadowaki T, Iwasaki M. Comparison of efficacy between insulin glargine and NPH human insulin in type 1 diabetes patients undergoing intensive insulin treatment – phase II/III clinical studies in Japan [in Japanese] Rinsho Iyaku. 2003;19:423–440. [Google Scholar]
- 31.Kobayashi M, Iwamoto Y, Kaku K, Kawamori R, Tajima N. 48-week randomized multicenter open-label parallel group phase 3 trial to compare insulin detemir and NPH insulin efficacy and safety in subjects with insulin requiring diabetes mellitus in a basal-bolus regimen [in Japanese] J Japan Diab Soc. 2007;50:649–663. [Google Scholar]