Abstract
Objective
Antiepileptic drugs (AEDs) may have adverse effects on bone mineral density (BMD) and metabolism. We previously reported biochemical evidence of increased bone turnover in pre-menopausal women with epilepsy on phenytoin monotherapy compared with those on carbamazepine, lamotrigine, and valproate. We therefore hypothesized that rates of bone loss would be higher in young women treated with phenytoin.
Methods
Ninety-three premenopausal women with epilepsy receiving a single AED (carbamazepine, lamotrigine, phenytoin, or valproate) participated. Subjects completed nutritional and physical activity questionnaires. Biochemical indices of bone and mineral metabolism and BMD of the proximal femur and lumbar spine were measured at baseline and 1 year.
Results
Participants reported high calcium intake (>1,000 mg/day) and were physically active. Significant loss (2.6%) was seen at the femoral neck in the phenytoin group. BMD remained stable in the other AED groups. Bone turnover markers and calciotropic hormones were unchanged after 1 year in all groups except for a significant decline in urine N-telopeptide in the phenytoin group. In women receiving phenytoin, lower serum 25-hydroxyvitamin D concentrations were associated with higher parathyroid hormone, bone alkaline phosphatase, and urine N-telopeptide levels, a biochemical pattern consistent with secondary hyperparathyroidism and increased remodeling.
Conclusion
In this study, young women treated with phenytoin had significant femoral neck bone loss over 1 year. In contrast, those treated with carbamazepine, lamotrigine, and valproate did not have detectable adverse effects on bone turnover or bone mineral density. These results raise concerns about the long-term effects of phenytoin monotherapy on bone in young women with epilepsy.
Antiepileptic drug (AED) treatment has adverse effects on bone and mineral metabolism that may ultimately increase the risk of fracture.1 Most studies of AED effects on bone and mineral metabolism are cross-sectional. Of the few longitudinal studies, some revealed bone loss in children and adults as well as changes in indices of bone and mineral metabolism.2–5 None have evaluated the effects of individual AEDs.
We previously reported results of a cross-sectional study of bone mineral density (BMD) and indices of bone and mineral metabolism in premenopausal women with epilepsy taking one of four AEDs in monotherapy.6 We reported significant reductions in serum calcium in women treated with phenytoin, carbamazepine, and valproate compared with women treated with lamotrigine. Similarly, phenytoin was associated with significantly higher markers of bone formation, suggesting increased bone turnover. Serum concentrations of vitamin D metabolites and BMD did not differ among the groups.
Herein, we report our findings in women treated with one of four AEDs in monotherapy followed up for 1 year. Given the biochemical evidence of increased turnover at baseline in women receiving phenytoin, we hypothesized that there would be more bone loss in this group.
METHODS
Subjects
Premenopausal women with epilepsy (n = 147) aged between 18 and 40 years and with normal menstrual cycles participated in the study. Subjects were enrolled at Stanford University (n = 83) or Columbia University (n = 64) between September 1997 and January 2004. Baseline data on the first 93 subjects enrolled were published previously.6 Of the 147 subjects, 54 (37%) discontinued participation. Reasons for discontinuation included lost to follow-up (n = 23), change of AED (n = 19), pregnancy (n = 6), time constraints (n = 4), and moved (n = 2). This report includes 93 subjects with both baseline and 1-year follow-up measures. No a priori power calculations were performed. All were receiving a single AED (carbamazepine, lamotrigine, phenytoin, or valproate) for at least 6 months before enrollment. Because one bone remodeling cycle takes approximately 3 months and two remodeling cycles would have been completed, the effects of prior AED exposure would be unlikely to influence bone turnover markers and rates of bone loss.7 Data on AED dose, duration of AED treatment for studied AED, and total duration of AED treatment were ascertained. Excluded were pregnant and postmenopausal women, those with impaired motor function, diseases that affect the skeleton (primary hyperparathyroidism, Paget disease, multiple myeloma), and those taking glucocorticoids and excessive doses of vitamin D or A.
Study design and analytical methods
Each subject completed validated nutritional and physical activity questionnaires. The nutrition questionnaire is a food frequency questionnaire8 that assesses daily diet, vitamin intake, tobacco, and alcohol. The exercise questionnaire includes questions on specific exercises and exercise frequency.9 In addition, detailed clinical histories were taken, including menarchal age and reproductive history. Height and weight were measured, and body mass index was calculated. These evaluations were completed at baseline and after 1 year of observation.
Fasting morning blood was drawn at baseline and 1 year. Serum measurements included total calcium, 25-hydroxyvitamin D (25-OHD), 1,25-dihydroxyvitamin D (1,25(OH)2D), parathyroid hormone (PTH), markers of bone formation (bone-specific alkaline phosphatase [BSAP] and osteocalcin), and cross-linked N-telopeptide of type I bone collagen (NTx), a marker of bone resorption. Urine was collected for analysis of NTx. Serum and urine were stored at −80°C until batch analysis at Columbia University’s Irving Center for Clinical Research Core Laboratory.
Serum calcium concentrations were measured by standard autoanalzyer technique (normal 8.4–10.2 mg/dL). Serum 25-OHD (normal 9 –55 ng/mL) was measured after extraction by radioimmunoassay (DiaSorin, Stillwater, MN; intra-assay coefficient of variation [CV] 10.5%, interassay CV 8.2%). Serum 1,25(OH)2D (normal 25–66 pg/mL) was measured by radioimmunoassay (DiaSorin; intra-assay CV 7.7%, interassay CV 11.1%). PTH (normal 10–65 pg/mL) was measured by an immunoradiometric assay (Nichols Institute Diagnostics, San Clemente, CA; intra-assay CV 3.4%, interassay CV 5.6%).
Serum BSAP (normal range for premenopausal women 11.6–29.6 μg/L) was measured by competitive enzyme immunoassay (Metra Biosystems, San Diego, CA; intra-assay CV 5.8%, interassay CV 5.2%). Osteocalcin (normal range for premenopausal women 2.4–10.0 ng/mL) was measured using an immunoradiometric assay (Immunotopics, Inc., San Clemente, CA; intra-assay CV 3.9%, interassay CV 5.5%). Serum NTx (normal 6.2–19.0 nM bone collagen equivalents [BCE]) was measured by ELISA (Wampole Laboratories, Princeton, NJ; intra-assay CV 4.6%, interassay CV 6.9%. Urine NTx (normal range for premenopausal women 3.0–63.0 nM BCE/nM creatinine) was measured by ELISA (Wampole Laboratories; intra-assay CV 19.0%, interassay CV 5.0%).
Each subject had BMD measured at entry and 1 year on the same densitometer (Hologic 1000 and 4500 densitometers; Hologic, Waltham, MA). Lumbar spine BMD values were expressed as Z scores, which compare subjects’ data with age-, race-, and sex-matched normative data provided by the manufacturer. Proximal femur results were expressed as Z scores calculated from the National Health and Nutrition Examination Survey of adult women in the United States. T scores were not used because women younger than age 25 to 30 years may not have attained peak bone mass.
Statistical analysis
Data were summarized with means, standard deviations, and 95% confidence intervals surrounding the means for continuous measures and frequencies and percents for categorical data. Baseline differences between AED groups were compared by one-way analysis of variance for continuous measures, with Scheffé adjustment for post hoc comparisons in the presence of a significant overall F test, and by the Cochran–Armitage trend test10 for categorical measures after ordinalizing the groups by descending frequency. The within-subject difference in changes from baseline to 1 year was analyzed with one-way analysis of variance (ANOVA) of the difference scores with a single fixed effect of AED group. Within-subject changes in bone turnover makers from baseline to 1 year were also analyzed as difference scores using one-way ANOVA with the addition of the baseline level of the marker entered as a continuous covariate. Separate models were run for each BMD site and each bone turnover marker without adjustment for multiple comparisons. Pearson correlation analyses were performed to assess relationships among calciotropic hormones and markers of bone turnover in individual AED groups. Graphs are displayed to show means and 1 SEM error bars.
RESULTS
Characteristics of the study population
Of the 93 women (aged 18–40 years) who completed the 1-year study, 41 were taking monotherapy with carbamazepine, 23 with lamotrigine, 15 with phenytoin, and 14 with valproate (table 1). Of these women, 66% were white.
Table 1.
Characteristics of study participants at baseline
| CBZ, n = 41 | LTG, n = 23 | PHT, n = 15 | VPA, n = 14 | ANOVA p* | |
|---|---|---|---|---|---|
| Background | |||||
| Age, y | 34 ± 5 | 30 ± 6 | 33 ± 5 | 30 ± 7 | 0.03 |
| (33–36) | (28–33) | (30–36) | (27–34) | ||
| White, n (%) | 31 (76) | 14 (61) | 7 (47) | 10 (67) | 0.07† |
| Height, cm | 163 ± 9 | 162 ± 8 | 167 ± 5 | 163 ± 6 | 0.23 |
| (160–166) | (158–165) | (164–170) | (159–166) | ||
| Weight, kg | 66 ± 18 | 73 ± 17 | 70 ± 23 | 66 ± 17 | 0.48 |
| (60–72) | (66–81) | (57–83) | (56–75) | ||
| BMI, kg/m2 | 24.6 ± 5.1 | 27.6 ± 5.9 | 25.2 ± 8.3 | 24.9 ± 6.0 | 0.33 |
| (23.0–26.2) | (24.9–30.4) | (20.6–29.8) | (21.5–28.2) | ||
| Age at menarche | 13 ± 1 | 12 ± 1 | 13 ± 2 | 12 ± 3 | 0.24 |
| (12–13) | (12–13) | (12–14) | (10–13) | ||
| Ever pregnant, n (%) | 13 (32) | 8 (35) | 5 (33) | 3 (20) | 0.35† |
| No. of pregnancies | 1.5 ± 0.9 | 1.7 ± 0.9 | 2.4 ± 0.9 | 1.3 ± 0.6 | 0.26 |
| (1.0–2.1) | (1.0–2.5) | (1.3–3.5) | (−0.1–2.8) | ||
| Diet | |||||
| Daily calcium, mg/day | 1,097 ± 690 | 1,285 ± 579 | 1,095 ± 673 | 958 ± 706 | 0.57 |
| (868–1,328) | (997–1,573) | (667–1,522) | (568–1,349) | ||
| Calcium supplements, n (%) | 21 (54) | 9 (38) | 5 (34) | 6 (40) | 0.18 |
| Daily vitamin D, IU/d | 232 ± 261 | 220 ± 212 | 256 ± 190 | 308 ± 686 | 0.90 |
| (145–319) | (118–322) | (135–377) | (−78–688) | ||
| Vitamin D supplements, n (%) | 23 (59) | 12 (50) | 5 (33) | 9 (60) | 0.97 |
| Exercise | |||||
| Any physical activity, n (%) | 20 (46) | 12 (52) | 9 (60) | 8 (53) | 0.45† |
| Hours/week among exercisers | 4 ± 3 | 7 ± 6 | 8 ± 9 | 4 ± 4 | 0.29 |
| (3–6) | (3–11) | (1–14) | (1–7) | ||
| Antiepileptic drug exposure | |||||
| Medication dose, n (range)‡, mg/d | 39 | 23 | 13 | 14 | |
| 684 ± 384 | 383 ± 263 | 384 ± 54 | 858 ± 556 | ||
| (200–1,800) | (100–1,250) | (260–400) | (250–2,250) | ||
| Total years | 9 ± 10 | 3 ± 3 | 9 ± 8 | 4 ± 4 | 0.04 |
| (5–12) | (1–4) | (4–14) | (1–6) | ||
| Specific drug years | 9 ± 10 | 2 ± 3 | 8 ± 8 | 4 ± 4 | 0.06 |
| (5–12) | (0.7–4) | (3–14) | (1.3–7) | ||
Data are expressed as mean ± SD and 95% confidence interval.
p value of one-way analysis of variance (ANOVA) F test of main effect of antiepileptic drug group with 3,89 degrees of freedom, except hours/week among exercisers with 3,45 degrees of freedom.
Cochran–Armitage trend test of antiepileptic drug group ordinalized in descending frequency.
n = number of subjects with dosage data. The range of doses, not the confidence interval, is provided.
CBZ = carbamazepine; LTG = lamotrigine; PHT = phenytoin; VPA = valproate; BMI = body mass index.
The average age was 32 ± 6 years. Subjects in the lamotrigine and valproate groups were approximately 3 years younger on average than those in the carbamazepine and phenytoin groups (p = 0.03). However, this age difference is unlikely to be clinically relevant, because there is little change in gonadal hormone production or bone mass at this age.11–13 The women had long-term epilepsy, as evidenced by an average total duration of AED therapy of 9 ± 11 years. Although women taking phenytoin and carbamazepine tended to have had longer total exposure to AEDs in general and longer exposure to the study-specific AED, there were no significant differences in between group comparisons for either total duration of AED treatment or duration of time on the individual AED. The mean dose was therapeutic for all AEDs studied. Dietary intake of calcium and vitamin D, amount of exercise, age at menarche, percent ever pregnant, and number of pregnancies among those who had been pregnant did not differ among groups.
Bone mineral density
As we reported previously,6 baseline BMD was similar among the AED groups at the lumbar spine, femoral neck, and total hip (table 2). After 1 year of AED treatment, significant loss was seen only in the group that received phenytoin (−0.023 ± 0.030 g/cm2 or 2.6%) and only at the femoral neck (figure 1).
Table 2.
Bone mineral density at baseline and after 1 year of antiepileptic drug treatment
| CBZ, n = 41 | LTG, n = 23 | PHT, n = 15 | VPA, n = 14 | ANOVA p* | |
|---|---|---|---|---|---|
| Lumbar spine | |||||
| Baseline, g/cm2† | 1.016 ± 0.12 | 1.068 ± 0.11 | 1.073 ± 0.16 | 0.998 ± 0.15 | 0.21 |
| 1 Year, g/cm2† | 1.021 ± 0.12 | 1.066 ± 0.09 | 1.077 ± 0.17 | 0.999 ± 0.16 | 0.25 |
| Baseline Z score† | −0.20 ± 1.11 | 0.20 ± 0.84 | 0.19 ± 1.41 | −0.35 ± 1.34 | |
| 1-Year Z score† | −0.14 ± 1.09 | 0.26 ± 0.82 | 0.32 ± 1.53 | −0.33 ± 1.43 | |
| Femoral neck | |||||
| Baseline, g/cm2 | 0.792 ± 0.10 | 0.835 ± 0.11 | 0.871 ± 0.18 | 0.838 ± 0.18 | 0.21 |
| 1 Year, g/cm2 | 0.794 ± 0.10 | 0.828 ± 0.11 | 0.849 ± 0.16 | 0.843 ± 0.18 | 0.41 |
| Baseline Z score | −0.51 ± 0.96 | −0.17 ± 0.95 | 0.22 ± 1.46 | −0.28 ± 1.60 | |
| 1-Year Z score | −0.36 ± 0.89 | −0.25 ± 1.08 | 0.07 ± 1.31 | −0.12 ± 1.60 | |
| Total hip | |||||
| Baseline, g/cm2 | 0.889 ± 0.12 | 0.922 ± 0.11 | 0.962 ± 0.15 | 0.926 ± 0.18 | 0.32 |
| 1 Year, g/cm2 | 0.892 ± 0.11 | 0.920 ± 0.11 | 0.961 ± 0.15 | 0.931 ± 0.19 | 0.39 |
| Baseline Z score | −0.47 ± 0.97 | −0.22 ± 0.89 | 0.14 ± 1.13 | −0.23 ± 1.51 | |
| 1-Year Z score | −0.47 ± 0.93 | −0.25 ± 0.97 | 0.15 ± 1.12 | −0.13 ± 1.60 | |
p value of one-way analysis of variance (ANOVA) F test of main effect of antiepileptic drug group with 3,89 degrees of freedom for within-subject difference scores of 12-month change in bone mineral density.
Mean ± standard deviation.
CBZ = carbamazepine; LTG = lamotrigine; PHT = phenytoin; VPA = valproate.
Figure 1.
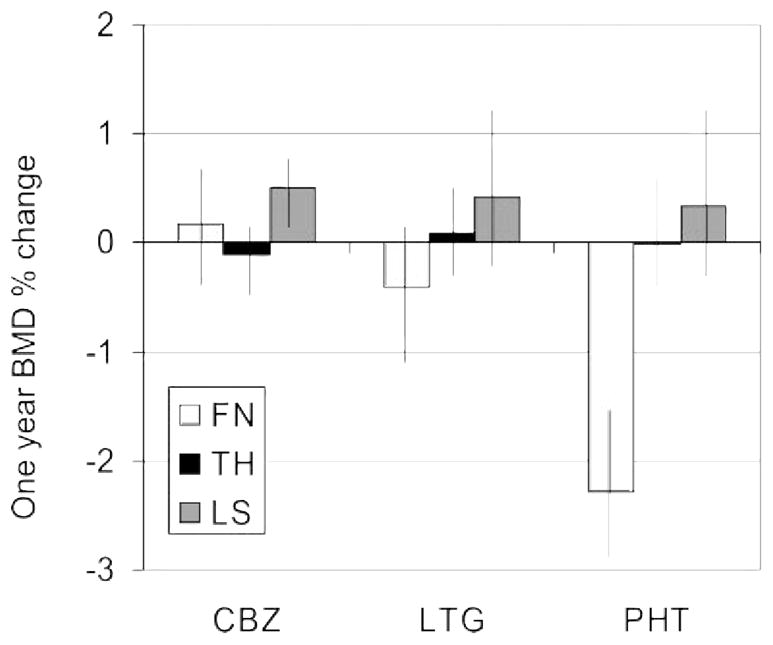
Group mean percent change in femoral neck bone mineral density
The group mean percent change at 1 year with bars showing ± one standard error are presented. The sites studied are femoral neck (FN) total hip (TH), and lumbar spine (LS). In the phenytoin (PHT) group, the percent loss in femoral neck bone mineral density (BMD) at 1 year was significantly greater than in the carbamazepine (CBZ) group (p < 0.03) or the valproate (VPA) group (p < 0.02). LTG = lamotrigine.
Because women taking phenytoin and carbamazepine tended to have had longer total exposure both to AEDs and to the study-specific AED, we used multiple regression to explore associations between duration of prior AED exposure and the second-order interaction of AED type and exposure duration. We did not detect a significant influence of duration of AED use on 1-year change in BMD at any site. However, there was a trend (p = 0.09) for longer AED duration, regardless of specific AED, to be associated with lower 1-year rates of bone loss at the total hip.
Biochemical indices of bone and mineral metabolism and bone turnover
There were no significant between-groups differences in baseline or 1-year serum concentrations of 25-OHD, 1,25(OH)2D, PTH, or markers of bone formation (table 3). In women on phenytoin, urine NTx, a marker of bone resorption, was significantly lower at 1 year than at baseline. Serum calcium was significantly higher in subjects receiving lamotrigine than in those receiving carbamazepine, phenytoin, and valproate (p = 0.04) at baseline and 1 year. Other bone turnover markers and calciotropic hormones were unchanged after 1 year on the same AED.
Table 3.
Within-group change in biochemical indices of bone mineral metabolism
| Antiepileptic drug group
|
||||||||
|---|---|---|---|---|---|---|---|---|
| CBZ
|
LTG
|
PHT
|
VPA
|
|||||
| Baseline | 1 Year | Baseline | 1 Year | Baseline | 1 Year | Baseline | 1 Year | |
| Serum Ca | 9.5 ± 0.5 | 9.6 ± 0.5 | 10.0 ± 0.4 | 10.0 ± 0.0.4 | 9.6 ± 0.4 | 9.6 ± 0.3 | 9.4 ± 0.9 | 9.6 ± 0.4 |
|
| ||||||||
| 25-OHD | 25.3 ± 6.8 | 23.4 ± 7.2 | 23.8 ± 6.5 | 25.2 ± 7.0 | 23.3 ± 11.5 | 23.8 ± 10.7 | 25.9 ± 13.1 | 27.7 ± 15.7 |
|
| ||||||||
| 1,25(OH)2D | 37.8 ± 14.1 | 36.0 ± 11.6 | 35.9 ± 14.9 | 41.4 ± 13.5 | 33.4 ± 12.2 | 32.5 ± 9.6 | 32.2 ± 14.6 | 36.3 ± 19.4 |
|
| ||||||||
| PTH | 25.4 ± 9.6 | 29.8 ± 18.5 | 28.2 ± 14.9 | 24.4 ± 13.2 | 26.3 ± 13.0 | 32.0 ± 17.2 | 22.7 ± 12.4 | 22.0 ± 12.3 |
|
| ||||||||
| BSAP | 23.0 ± 6.5 | 23.1 ± 7.3 | 19.6 ± 6.5 | 20.8 ± 6.8 | 22.6 ± 5.9 | 26.0 ± 5.7 | 18.6 ± 6.2 | 18.8 ± 7.9 |
|
| ||||||||
| Osteocalcin | 8.2 ± 3.3 | 9.0 ± 3.9 | 9.2 ± 3.3 | 9.4 ± 3.3 | 8.3 ± 4.9 | 8.7 ± 4.2 | 10.1 ± 4.6 | 9.0 ± 3.2 |
|
| ||||||||
| Serum NTx | 12.2 ± 2.2 | 12.5 ± 3.3 | 12.4 ± 2.8 | 13.8 ± 4.2 | 12.9 ± 2.3 | 14.0 ± 1.8 | 12.5 ± 3.0 | 13.2 ± 2.0 |
|
| ||||||||
| Urine NTx | 42.4 ± 24.5 | 43.4 ± 27.9 | 35.0 ± 18.2 | 35.3 ± 17.8 | 55.9 ± 44.1 | 45.4 ± 25.1* | 50.4 ± 31.2 | 43.8 ± 24.4 |
Data are expressed as mean ± SD.
Within-drug group change from baseline p value < 0.05.
CBZ = carbamazepine; LTG = lamotrigine; PHT = phenytoin; VPA = valproate; 25-OHD = 25-hydroxyvitamin D; 1,25(OH)2D = 1,25-dihydroxyvitamin D; PTH = parathyroid hormone; BSAP = bone-specific alkaline phosphatase; NTx = cross-linked N-telopeptide of type I bone collagen.
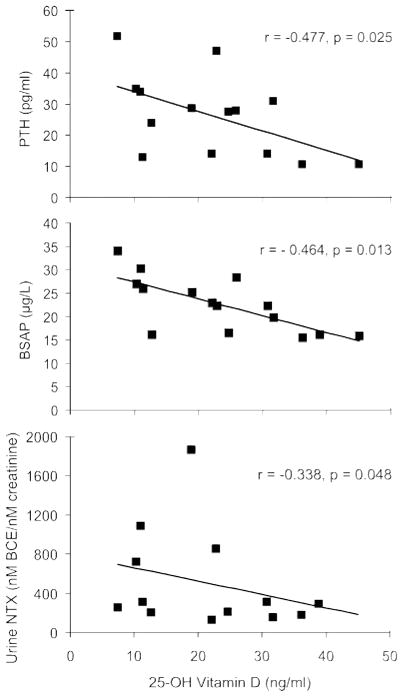
To assess relationships between the calciotropic hormones and markers of bone turnover, Pearson correlation coefficients were performed. No significant relationships were detected in the carbamazepine, valproate, and lamotrigine groups. In contrast, lower 25-OHD concentrations in the women receiving phenytoin were significantly associated with higher serum PTH, BSAP, and urine NTx (figure 2), a biochemical pattern suggestive of secondary hyperparathyroidism with increased bone turnover.
Figure 2.
Correlations between 25-OH vitamin D levels and PTH, BSAP, and NTx
Significant correlations between lower 25-OH vitamin D levels and higher parathyroid hormone (PTH), bone-specific alkaline phosphatase (BSAP), and urine cross-linked N-telopeptide of type I bone collagen (NTx) suggest a pattern consistent with secondary hyperparathyroidism with increased turnover. BCE = bone collagen equivalent.
DISCUSSION
In this longitudinal study of premenopausal women with epilepsy on AED monotherapy, we found that those receiving phenytoin sustained significant femoral neck bone loss after 1 year but did not have bone loss at any other site. In contrast, those treated with carbamazepine, valproate, or lamotrigine did not lose bone mass at any site. Consistent with our previous findings in the cross-sectional study,6 serum calcium concentrations were higher at baseline and also after 1 year of treatment in women taking lamotrigine than in those taking phenytoin, carbamazepine, and valproate. Given the absence of other biochemical differences in the carbamazepine and lamotrigine groups, the clinical significance of the higher serum calcium levels is unclear. There were no significant differences among the groups in markers of bone formation either at baseline or after 1 year. There was a significant decline in urine NTx, a marker of bone resorption in the phenytoin group. The clinical relevance of this decrease is also not clear, particularly in view of the significant femoral neck bone loss that occurred in this group. As we reported previously, serum vitamin D metabolites and PTH concentrations did not differ among the groups. However, within the group of women receiving phenytoin, there was sufficient variation in serum 25-OHD levels to reveal a biochemical pattern consistent with secondary hyperparathyroidism (lower serum, higher serum PTH and markers of bone turnover) in those with lower serum 25-OHD levels.
Measurement of BMD is the most commonly used predictor of fracture risk in postmenopausal women and older men. However, a number of other important risk factors also contribute to increased fracture risk. Of these, the most important are older age, female sex, low body weight, history of fracture after age 50 years, excess alcohol intake, cigarette smoking, family history of fracture, and glucocorticoid treatment.14 In addition, excess bone loss associated with medications such as phenytoin can also contribute to increased future fracture risk. A study of postmenopausal women found significant bone loss at the calcaneus in women with epilepsy treated with phenytoin in comparison with women without epilepsy.15 Similarly, we found significant femoral neck bone loss in young, premenopausal women treated with phenytoin monotherapy. To put this in context, in 614 premenopausal women aged 24 to 44 years, femoral neck BMD declined by 0.3%.16 The 2.6% loss we observed in the women receiving phenytoin is more than eight times greater than that observed in this cohort of young women. If such rates of bone loss were to be sustained over time, premenopausal women receiving long-term phenytoin therapy might enter their menopausal years with lower than normal bone mass, and their vulnerability to postmenopausal fractures might be considerably increased.
AEDs that induce the hepatic CYP450 enzyme system are most commonly associated with a negative impact on bone. Phenytoin is a potent CYP450 enzyme inducer. The significant femoral neck bone loss we observed in women taking phenytoin, together with a biochemical pattern suggesting that vitamin D insufficiency among some women in that group was associated with secondary hyperparathyroidism and increased bone turnover, supports the notion that the adverse effects of this drug on the skeleton may be mediated by this mechanism.
Although both phenytoin and carbamazepine are CYP450 enzyme–inducing AEDs, there was no significant bone loss after 1 year of treatment in the carbamazepine group, nor was there any evidence of secondary hyperparathyroidism or increased bone turnover. In this regard, our results differ from those of other investigators who have reported increased markers of bone turnover in subjects after 1 and 2 years of treatment and decreased BMD in association with this AED.2,3 In contrast, other clinical studies have not found significant changes in BMD or abnormalities in mineral metabolism in patients receiving carbamazepine and thus are consistent with our results.17–19
There were no demographic differences between the phenytoin and carbamazepine groups that could account for our observation, excepting that there were fewer white women in the phenytoin group. However, the racial composition of the phenytoin group was diverse and no racial group predominated. Phenytoin has also been reported to have direct effects on bone resorption20–22 and formation,23,24 may directly impair calcium absorption,24 and may impair the response of osteoblasts to PTH.22,25 Although carbamazepine has also been shown to inhibit bone formation23 and intestinal calcium absorption,24 it is possible that the bone loss we observed may be mediated by one or more direct effects of phenytoin on some aspect of bone and mineral metabolism that are not shared by carbamazepine, rather than by an indirect effect of phenytoin on vitamin D metabolism. This observation requires confirmation and, if confirmed, further exploration.
This study has several important strengths. It is one of very few longitudinal studies evaluating the individual effects of several commonly used AEDs on BMD and markers of bone and mineral metabolism. Other longitudinal studies have evaluated either a single drug or have studied patients taking multiple AEDs. In addition, we controlled for other factors known to affect bone health.
This study also has several important limitations. We did not include a group of women without epilepsy to serve as normal controls. Thus, we may have missed subtle differences in bone and mineral metabolism. Subjects were not randomly assigned to the studied AED but were receiving the drug best able to control their seizure activity. The findings therefore may in part be explained by a selection bias influencing AED treatment for the enrolled subjects. Fifty-four women (37%) withdrew from the study before completion. The baseline outcome variables of these women did not differ when compared with those who completed the study, excepting that those who withdrew were approximately 2.5 years younger. This age difference is unlikely to be clinically important, because bone mass is generally stable at this age. Therefore, we do not believe that the number of women who withdrew influenced our results. However, the reduced and small sample sizes due to the large dropout may have limited our ability to detect small differences which may be clinically meaningful. The subjects had long-term epilepsy, and many were treated with other agents before the investigated AED. We therefore could not control for prior AED exposure. Although the longitudinal study design provides an assessment of effects of current AED treatment, 1 year of observation may not be sufficient to detect minor changes that may accumulate over time. The women studied had well-controlled epilepsy and seemed to be health conscious in that they were on relatively high intakes of calcium and vitamin D and exercised for several hours each week. This may limit generalizability of these results to other groups of women. In addition, our subjects were estrogen replete because they were premenopausal and had regular menses. Adverse effects of AED therapy may be more apparent in postmenopausal women or older men, in whom estrogen levels may be insufficient to counter the effects of these drugs on bone turnover.
Acknowledgments
The authors thank Robert Marcus, MD, for his help in designing the study.
This study was supported by an investigator-initiated research grant from GlaxoSmithKline, and the serologic and urine analysis was supported by NIH RR00645.
GLOSSARY
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- AED
antiepileptic drug
- ANOVA
analysis of variance
- BCE
bone collagen equivalent
- BMD
bone mineral density
- BMI
body mass index
- BSAP
bone-specific alkaline phosphatase
- CBZ
carbamazepine
- FN
femoral neck
- LS
lumbar spine
- LTG
lamotrigine
- NTx
cross-linked N-telopeptide of type I bone collagen
- OHD
25-hydroxyvitamin D
- PHT
phenytoin
- PTH
parathyroid hormone
- TH
total hip
- VPA
valproate
Footnotes
Disclosure: A.M.P.: Research: GlaxoSmithKline; Speakers’ Bureau: Abbott, Novartis, GlaxoSmithKline; Consulting: GlaxoSmithKline. M.J.M.: Employee: Neuropace, Inc.; Speakers’ Bureau: Pfizer, GlaxoSmithKline; Consulting: GlaxoSmithKline. A.R.: The author reports no conflicts of interest. D.J.M.: The author reports no conflicts of interest. E.S.: The author reports no conflicts of interest.
References
- 1.Souverein PC, Webb DJ, Petri H, Weil J, Van Staa TP, Egberts T. Incidence of fractures among epilepsy patients: a population-based retrospective cohort study in the General Practice Research Database. Epilepsia. 2005;46:304–310. doi: 10.1111/j.0013-9580.2005.23804.x. [DOI] [PubMed] [Google Scholar]
- 2.Verrotti A, Greco R, Morgese G, Chiarelli F. Increased turnover in epileptic patients treated with carbamazepine. Epilepsia. 2000;47:353–358. [PubMed] [Google Scholar]
- 3.Verrotti A, Greco R, Latini G, Morgese G, Chiarelli F. Increased bone turnover in prepubertal, pubertal, and postpubertal patients receiving carbamazepine. Epilepsia. 2002;43:1488–1492. doi: 10.1046/j.1528-1157.2002.13002.x. [DOI] [PubMed] [Google Scholar]
- 4.Tekgul H, Dizdarer G, Demir N, Ozturk C, Tutuncuoglu S. Bone mineral status in pediatric outpatients on antiepileptic drug monotherapy. J Child Neurol. 2004;19:26–30. doi: 10.1177/08830738060210050101. [DOI] [PubMed] [Google Scholar]
- 5.Wu S, Legido A, DeLuca F. Effects of valproic acid on longitudinal bone growth. J Child Neurol. 2004;19:26–30. doi: 10.1177/088307380401900105011. [DOI] [PubMed] [Google Scholar]
- 6.Pack AM, Morrell MJ, Marcus R. Bone mass and turnover in women with epilepsy on antiepileptic drug monotherapy. Ann Neurol. 2005;57:252–257. doi: 10.1002/ana.20378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delmas PD, Eastell R, Garnero P, et al. The use of biochemical markers of bone turnover in osteoporosis. Osteoporosis Int. 2000;11 (suppl 6):S2–S17. doi: 10.1007/s001980070002. [DOI] [PubMed] [Google Scholar]
- 8.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 9.Katzman DK, Bachrach LK, Carter DR, Marcus R. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab. 1991;73:1332–1339. doi: 10.1210/jcem-73-6-1332. [DOI] [PubMed] [Google Scholar]
- 10.Margolin BH. Test for trend in proportions. In: Johnson NL, editor. Encyclopedia of Statistical Sciences. New York: John Wiley & Sons; 1988. pp. 334–336. [Google Scholar]
- 11.Bainbridge KE, Sowers M, Lin X, Harlow SD. Natural history of bone loss over 6 years among premenopausal and early postmenopausal women. Am J Epidemiol. 2002;156:410–417. doi: 10.1093/aje/kwf049. [DOI] [PubMed] [Google Scholar]
- 12.Sowers MR, Finkelstein JS, Ettinger B, et al. The association of endogenous hormone concentrations and bone mineral density measures in pre- and perimenopausal women of four ethnic groups: SWAN. Osteoporos Int. 2003;14:44–52. doi: 10.1007/s00198-002-1307-x. [DOI] [PubMed] [Google Scholar]
- 13.Sowers MR, Greendale GA, Bondarenko I, et al. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int. 2003;14:191–197. doi: 10.1007/s00198-002-1329-4. [DOI] [PubMed] [Google Scholar]
- 14.Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 15.Ensrud KE, Walczak TS, Blackwell T, Ensrud ER, Bowman PJ, Stone KL. Antiepileptic drug use increases rates of bone loss in older women: a prospective study. Neurology. 2004;62:2051–2057. doi: 10.1212/01.wnl.0000125185.74276.d2. [DOI] [PubMed] [Google Scholar]
- 16.Sowers M, Crutchfield M, Bandekar R, et al. Bone mineral density and its change in pre- and perimenopausal white women: the Michigan Bone Health Study. J Miner Res. 1998;13:1134–1140. doi: 10.1359/jbmr.1998.13.7.1134. [DOI] [PubMed] [Google Scholar]
- 17.Tjellesen J, Nilas L, Christiansen C. Does carbamazepine cause disturbances in calcium metabolism in epileptic patients. Acta Neurol Scand. 1983;68:13–19. doi: 10.1111/j.1600-0404.1983.tb04809.x. [DOI] [PubMed] [Google Scholar]
- 18.Valimaki MJ, Tiihonen M, Laitinen K, et al. Bone mineral density measured by dual-energy x-ray absorptiometry and novel markers of bone formation and resorption in patients on antiepileptic drugs. J Bone Miner Res. 1994;9:631–637. doi: 10.1002/jbmr.5650090507. [DOI] [PubMed] [Google Scholar]
- 19.Bramswig S, Zitterman A, Berthold HK. Carbamazepine does not alter biochemical parameters of bone turnover in healthy male adults. Calcif Tissue Int. 2003;73:356–360. doi: 10.1007/s00223-002-0018-9. [DOI] [PubMed] [Google Scholar]
- 20.Nakade O, Baylink DJ, Lau KH. Phenytoin at micromolar concentrations is an osteogenic agent for human-mandible-derived bone cells in vitro. J Dent Res. 1995;74:331–337. doi: 10.1177/00220345950740010801. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi A, Onodera K, Shinoda H, et al. Phenytoin and its metabolite, 5-(4-hydroxyphenyl)-5-phenylhydantoin, show bone resorption in cultured neonatal mouse calvaria. Jpn J Pharmacol. 2000;82:82–84. doi: 10.1254/jjp.82.82. [DOI] [PubMed] [Google Scholar]
- 22.Hahn TJ, Scharp CR, Richardson CA, et al. Interaction of diphenylhydantoin (phenytoin) and phenobarbital with hormonal mediation of fetal rat bone resorption in vitro. J Clin Invest. 1978;62:406–414. doi: 10.1172/JCI109142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldkamp J, Becker A, Witte OW, et al. Long-term anticonvulsant therapy leads to low bone mineral density: evidence for direct drug effects of phenytoin and carbamazepine on human osteoblast-like cells. Exp Clin Endocrinol Diabetes. 2000;108:37–43. doi: 10.1055/s-0032-1329213. [DOI] [PubMed] [Google Scholar]
- 24.von Burstel Smith M, Crofoot K, Rodriguez-Proteau R, et al. Effects of phenytoin and carbamazepine on calcium transport in Caco-2 cells. Toxicol In Vitro. 2007;21:855–862. doi: 10.1016/j.tiv.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Auszmann JM, Vernillo AT, Fine AS, et al. The effect of phenytoin on parathyroid hormone simulated cAMP activity in cultured murine osteoblasts. Life Sci. 1990;46:351–357. doi: 10.1016/0024-3205(90)90014-i. [DOI] [PubMed] [Google Scholar]