Abstract
Despite CD40’s role in stimulating dendritic cells (DCs) for efficient specific T-cell stimulation, its signal transduction components in DCs are still poorly documented. We show that CD40 receptors on human monocyte-derived DCs associate with sphingolipid- and cholesterol-rich plasma membrane microdomains, termed membrane rafts. Following engagement, CD40 utilizes membrane raft-associated Lyn Src family kinase, and possibly other raft-associated Src family kinases, to initiate tyrosine phosphorylation of intracellular substrates. CD40 engagement also leads to a membrane raft-restricted recruitment of tumor necrosis factor (TNF) receptor-associated factor (TRAF) 3 and, to a lesser extent, TRAF2, to CD40’s cytoplasmic tail. Thus, the membrane raft structure plays an integral role in proximal events of CD40 signaling in DCs. We demonstrate that stimulation of Src family kinase within membrane rafts initiates a pathway implicating ERK activation, which leads to interleukin (IL)-1α/β and IL-1Ra mRNA production and contributes to p38-dependent IL-12 mRNA production. These results provide the first evidence that membrane rafts play a critical role in initiation of CD40 signaling in DCs, and delineate the outcome of CD40-mediated pathways on cytokine production.
Keywords: CD40 signaling/dendritic cells/membrane rafts/Src kinases/TRAF
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) specialized in antigen capture, migration to secondary lymphoid organs and T-cell priming (Banchereau and Steinman, 1998). They are considered to be the most powerful APCs because only DCs are able to induce primary immune responses, thus permitting establishment of immunological memory (Banchereau and Steinman, 1998). Antigen presentation by DCs activates specific helper T cells to express CD40 ligand (CD40L) (Roy et al., 1993), which, in turn, activates CD40+ DCs. CD40 is a member of the tumor necrosis factor (TNF) receptor family (Foy et al., 1996) that conditions DCs for efficient specific T-cell stimulation. Indeed, stimulation of DCs through CD40 strongly enhances their capacity to induce proliferation and cytokine production by T cells (Cella et al., 1996). Furthermore, it has been shown that, in vivo, CD40 triggering by helper T cells enables DCs to activate cytotoxic T cells (Bennett et al., 1998; Ridge et al., 1998; Schoenberger et al., 1998). Signaling through CD40 leads to up-regulation of major histocompatibility complex (MHC) I and II molecules, of accessory molecules such as CD80/CD86 and of cytokines such as interleukin (IL)-12 and IL-1α/β, which all participate in T-cell stimulation (Caux et al., 1994; Cella et al., 1996; Koch et al., 1996). In addition, CD40 engagement provides survival signals to DCs that become resistant to Fas ligand expressed by activated T cells (Bjorck et al., 1997; Koppi et al., 1997). Thus, CD40–CD40L interaction is essential for optimal antigen presentation by DCs.
Multimerization of CD40 molecules following engagement with CD40L or antibodies appears to be a critical initiating step for CD40-mediated signaling (Kehry, 1996). Although the cytoplasmic domain of CD40 lacks sequences indicative of any catalytic activity, it can associate with members 2, 3, 5 and 6 of the TNF receptor-associated factors (TRAFs) (Hanissian and Geha, 1997; Arch et al., 1998; Revy et al., 1999). TRAFs are recruited to the CD40 cytoplasmic tail following receptor engagement (Kuhne et al., 1997; Jalukar et al., 2000), a step that is enhanced by CD40 oligomerization (Pullen et al., 1999). They are adaptors that can mediate the activation of nuclear factor-κB (NF-κB) and the mitogen-activated protein kinase (MAPK) family (Arch et al., 1998). The CD40 cytoplasmic tail also associates constitutively with the Janus family kinase (Jak) 3, which becomes activated following CD40 engagment, and leads to activation of signal transducers and activators of transcription (STATs) (Hanissian and Geha, 1997; Revy et al., 1999). In addition, numerous studies support the idea that early events in CD40 signaling involve stimulation of protein tyrosine kinases. This was best studied in B-cell lines, with observed stimulation of the tyrosine kinases Lyn, Fyn and Syk (Faris et al., 1994; Ren et al., 1994). There has, however, been no biochemical demonstration of direct or indirect association of these tyrosine kinases with CD40. In DCs, the proximal molecular components of CD40 signal transduction remain poorly documented. Downstream of CD40 engagement in these cells is the stimulation of protein tyrosine phosphorylation (pTyr) events (Servet-Delprat et al., 2000), the activation of NF-κB (Garceau et al., 2000) and the activation of the three structurally related MAPKs: the extracellular-regulated kinases (ERKs), the c-jun N-terminal kinases (JNKs) and the p38 MAPK (Aicher et al., 1999). Aicher and collaborators further demonstrated that CD40-induced IL-12 synthesis in DCs was dependent on p38 MAPK activation (Aicher et al., 1999).
The plasma membranes of many cell types contain microdomains commonly referred to as lipid or membrane rafts (for review see Simons and Ikonen, 1997; Brown and London, 1998a). They are enriched in sphingolipids and cholesterol that would preferentially self-associate to constitute a ‘liquid-ordered’ environment (Brown and London, 1998b) that resists solubilization in non-inonic detergent (Schroeder et al., 1998). Such non-solubilized membranes can be isolated from low density fractions after flotation in a sucrose gradient (Brown and Rose, 1992). Membrane rafts incorporate specific proteins, among which are many glycophosphatidylinositol (GPI)-anchored proteins and Src family kinases. Lipidation with saturated acyl chains of many of the raft-associated protein would participate in their preferential membrane raft localization (Melkonian et al., 1999). Strong evidence for membrane raft-dependent scaffolding of signaling complexes has come from studies on immunoreceptor signaling including T- and B-cell antigen receptors and Fcε receptor I (Cheng et al., 1999; Ilangumaran et al., 2000).
Here we show that very proximal to CD40 engagement in human monocytes-derived DCs are the activation of the Src-related tyrosine kinase Lyn and the recruitment of TRAF2 and 3 to the CD40 cytoplasmic tail. We demonstrate that these proximal events require a compartmentation of CD40 in membrane rafts, indicating that these membrane structures provide a platform for the initiation of CD40 signaling. In addition, we delineate the outcome of CD40-mediated Lyn activation, and possibly other raft-located Src family kinases, on cytokine expression. Thus, this study provides important insights into CD40 signaling in dendritic cells, and reinforces the critical role of membrane rafts in immunoreceptor signaling.
Results
CD40 engagement leads to Lyn and Syk kinase phosphorylation in human DCs
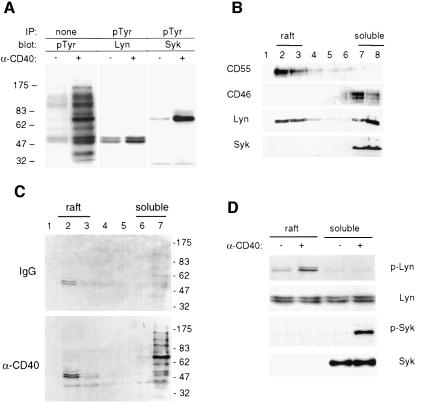
We previously reported that treatment of DCs with anti-CD40 monoclonal antibody (mAb) triggered the tyrosine phosphorylation of several proteins ranging between 30 and 175 kDa (Servet-Delprat et al., 2000; Figure 1A). The apparent molecular weight of the phosphorylated substrates suggested that some of these proteins may be the Src family kinase Lyn (53/56 kDa) and the Syk kinase (72 kDa). Anti-pTyr immunoprecipitates of the same cell lysates were thus immunoblotted with antisera directed against Lyn or Syk (Figure 1A), and confirmed that these two kinases were tyrosine phosphorylated.
Fig. 1. Lyn tyrosine phosphorylation following CD40 engagement occurs in membrane rafts. (A) Monocyte-derived DCs were stimulated for 10 min with either an irrelevant mouse IgG1 (–) or with anti-CD40 antibody (+), and analyzed as indicated: IP, immunoprecipitation; blot, immunoblotting. Molecular mass markers (kDa) are shown on the left. (B) Membrane rafts of DCs were separated on a bottom-loaded sucrose step gradient. The distribution of CD55, CD46, Lyn and Syk proteins within fractions (fraction 1 represents the top of the gradient) was analyzed by immunoblotting (equal volume loaded) as indicated. (C) Membrane rafts from unstimulated (IgG) or CD40-stimulated (α-CD40) DCs were fractionated as above and pTyr events were detected within each fraction by western blotting. Molecular mass markers (kDa) are shown on the right. (D) Pooled raft or soluble fractions from DCs, stimulated with either IgG control (–) or anti-CD40 antibody (+), were immunoprecipitated with anti-pTyr antibody and eluates were immunoblotted with either anti-Lyn (p-Lyn) or anti-Syk (p-Syk) antibodies. Total pooled samples were also immunoblotted with Lyn and Syk to control their respective distributions.
Lyn kinase phosphorylation, but not Syk phosphorylation, is restricted to membrane raft microdomains
Since dually acylated Src family kinases, including Lyn, have been reported to associate with membrane rafts (Simons and Ikonen, 1997; Brown and London, 1998a), we investigated the membrane raft location of Lyn in human DCs. Raft membranes from DCs were isolated using a flotation assay based on resistance to solubilization by Triton X-100 at 4°C and buoyancy at low-density fractions of a bottom-loaded discontinuous sucrose gradient, (Manie et al., 2000) (Figure 1B). The GPI-anchored CD55, a resident of membrane rafts, was recovered mainly in fractions 2 and 3, which will be referred to as the raft fractions. In contrast, most of the cellular proteins were recovered in fractions 7 and 8, as exemplified by CD46, which will be referred to as the soluble fractions. Approximately 50% of total Lyn kinase co-partitioned with raft fractions, whereas Syk kinase was detected only in the soluble fractions. We next determined the location of CD40-mediated phosphorylated proteins within sucrose gradient fractions of unstimulated or stimulated DCs. Figure 1C shows that upon CD40 stimulation, the majority of phosphorylated proteins were recovered in the soluble fractions (6 and 7). However, a clear increase in phosphorylation of a doublet in the molecular weight range corresponding to that of Lyn (53/56 kDa) was detected in the raft fraction. Both the raft fractions and the soluble fractions were pooled. An aliquot of each pool was then either resuspended directly in gel loading buffer to monitor the quantity of proteins or subjected to anti-pTyr immunoprecipitation. Figure 1D shows that the quantity of Lyn in each pool remained constant whether the cells were stimulated or not (Lyn panel). However, CD40-mediated phosphorylation of Lyn was only observed in the raft compartment of the cells, with barely no detectable phosphorylated Lyn in the soluble fractions, whether the cells were stimulated or not (p-Lyn panel). Conversely, Syk was recovered only in soluble fractions (Syk panel), in which phosphorylated Syk was detected following CD40 stimulation (p-Syk). Thus, these results indicated that CD40-mediated Lyn kinase phosphorylation is restricted to the membrane raft compartment in human DCs.
CD40 associates with membrane rafts
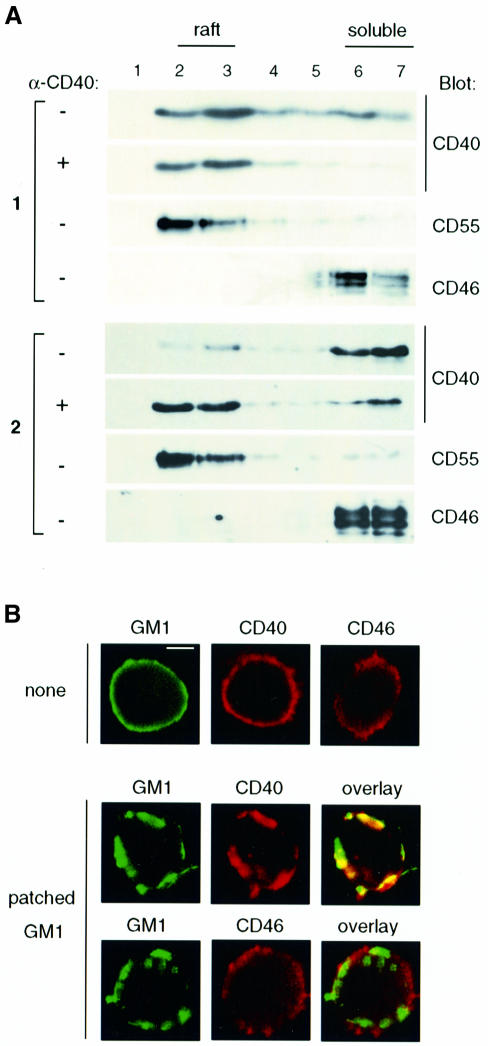
We next investigated whether CD40 is associated with these microdomains. Figure 2A shows two representative experiments from different DC isolates. In the absence of CD40 engagment, a variable proportion, ranging from ∼2 to ∼70% of total CD40, constitutively associated with membrane rafts, even though the two internal controls of raft-associated and non-associated proteins, CD55 and CD46, respectively, were recovered consistently in their expected fractions. However, stimulation of DCs with anti-CD40 antibody consistently increased the proportion of CD40 present in the raft fractions. These results suggest a moderate affinity of CD40 for membrane rafts that is variably sensitive to detergent extraction with regard to DC isolates, but can be stabilized by cross-linking with antibody. This would be similar to what has been reported recently for the T-cell antigen receptor (Janes et al., 1999). To investigate this possibility, we used a non-destructive approach to investigate the localization of CD40 within intact membrane rafts. Membrane rafts were stained with fluorescein isothiocyanate (FITC)-labeled cholera toxin B (CT-B) subunit, which binds to the plasma membrane ganglioside GM1 (Harder et al., 1998; Janes et al., 1999). CD40 and CD46 were stained with phycoerythrin (PE)-labeled specific antibodies. Representative mid-section confocal images of cells (Figure 2B, upper panel) showed punctate staining of GM1 (green), CD40 and CD46 (red), distributed evenly on the plasma membrane. This is consistent with studies that estimated the size of membrane rafts as <100 nm (Friedrichson and Kurzchalia, 1998; Varma and Mayor, 1998), whereas light microscopy has a resolution of ∼200 nm. Membrane raft domains can be manipulated by lateral cross-linking of GM1 that then forms patches (Harder et al., 1998; Janes et al., 1999). This was acheived in human DCs by incubating cells with CT-B followed by a rabbit polyclonal anti-CT-B antibody. Figure 2B, lower panel, shows that GM1 became concentrated in distinct patches within the membrane. Under these conditions, CD40 also showed a substantial membrane redistribution that co-localized (yellow) with GM1 patches. However, CD46 remained evenly distributed and did not co-localize significantly with GM1. The sequestration of CD40 by lateral cross-linking of GM1 strongly suggests that they both reside in the same lipid environment in the plasma membrane and thus supports the notion that CD40 is associated constitutively with membrane rafts. However, this association might be weak and thus sensitive to detergent extraction unless CD40 is cross-linked by an antibody.
Fig. 2. CD40 associates with membrane rafts. (A) DCs were stimulated with either IgG control (–) or anti-CD40 antibody (+) prior to membrane raft separation, and the protein distribution was assayed by immunoblotting as indicated. Numbers 1 and 2 indicate two independent experiments. (B) Membrane rafts were revealed in DCs by GM1 labeling with FITC-conjugated CT-B. Membrane raft patching was induced with anti-CT-B antibody (patched GM1). CD40 and CD46 (red) were detected on fixed cells with specific PE-conjugated immunostaining. Single confocal sections show fluorescence in FITC (GM1) and PE (CD40 or CD46) channels. Bar: 5 µm.
CD40-mediated pTyr events require both the integrity of membrane rafts and activation of Src family kinases
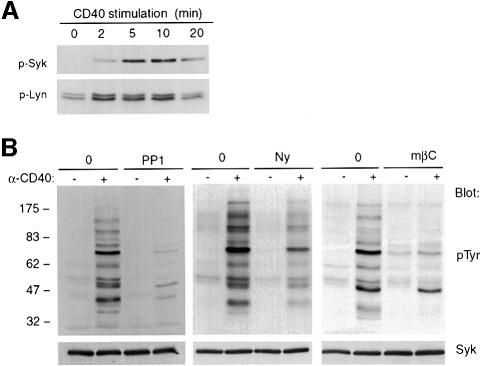
Time-dependent alteration of the CD40-stimulated Lyn and Syk phosphorylation indicated that Lyn was phosphorylated maximally earlier than Syk (Figure 3A). The phosphorylation of Src family kinases is in most cases due to autophosphorylation and can be taken as a measure of the enzymatic activity of the kinase (Thomas and Brugge, 1997), thus suggesting that Lyn kinase activation might be upstream of Syk phosphorylation. In support of this scheme, treatment of cells with the Src family selective tyrosine kinase inhibitor PP1 (Hanke et al., 1996), prior to CD40 stimulation, was found to diminish greatly all inducible pTyr events (Figure 3B), including Lyn and Syk (not shown). These results indicate that activation of Src family kinases, most probably including Lyn, is required for the CD40-mediated pTyr events in human DCs. We next tested whether intact membrane rafts were required for CD40-mediated pTyr events. Two cholesterol-binding agents, the polyene antifungal nystatin and the circular glucose heptamer methyl-β-cyclodextrin, have been reported to disrupt cholesterol-rich membrane domains, leading to inhibition of membrane raft-dependent signaling events (Xavier et al., 1998). Nystatin forms complexes with cholesterol within the membrane, whereas cyclodextrin selectively extracts cholesterol from the membrane. The viability of DCs treated with nystatin or cyclodextrin was comparable with that of control cells (not shown). However, both membrane raft-dispersing drugs significantly reduced the CD40-mediated pTyr events (Figure 3B). Therefore, altogether, these results indicate that initiation of CD40-mediated pTyr signaling requires both the integrity of membrane rafts and activation of Src family kinases.
Fig. 3. CD40-induced pTyr events are initiated in membrane rafts and are dependent on Src family kinase activation. (A) Cellular lysates from DCs stimulated with CD40 for the indicated times were immunoprecipitated with anti-p-Tyr antibody. Eluates were immunoblotted with either anti-Lyn (p-Lyn) or anti-Syk (p-Syk) antibodies. (B) DCs were pre-treated or not with the Src-specific inhibitor PP1, or with the cholesterol-binding agents nystatin (Ny) or methyl-β-cyclodextrin (mβC), before CD40 stimulation. Cellular lysates were analyzed by immunoblotting as indicated. Syk served as a loading control.
Membrane rafts provide a platform for TRAF2 and 3 recruitment to the CD40 cytoplasmic tail
The TRAF family members TRAF2 and TRAF3 have been shown to bind to the CD40 cytoplasmic tail in human B cells following CD40 engagement (Kuhne et al., 1997). We investigated whether a similar recruitment of TRAF2 and 3 would occur in DCs, and whether membrane rafts would be involved. Pooled raft fractions or soluble fractions from unstimulated or stimulated cells were analyzed for their CD40, TRAF2 and 3 content. Figure 4A shows that both TRAF2 and 3 are expressed in DCs and that under resting conditions, they are recovered in the soluble compartment. As a consequence of CD40 stimulation, a significant proportion of total TRAF3 was translocated into the raft compartment, which was mirrored by a decrease in the soluble compartment. However, the reduction in the soluble compartment could only be partially accounted for by recruitment into the raft fractions. Such a loss of TRAFs following CD40 engagement has been reported previously (Kuhne et al., 1997), but reasons for that remain unclear. TRAF2 was also translocated into the raft compartment albeit to a much lower extent when compared with TRAF3, and detection of translocated TRAF2 required a longer revelation step (Figure 4A). We next performed an immunoprecipitation of CD40 to monitor the recruitment of TRAFs to the CD40 cytoplasmic tail. We selected an experiment in which CD40 engagement stabilized only part of CD40 into the raft compartment in the presence of detergent (Figure 4B). Both TRAF2 and TRAF3 could be co-immunoprecipitated with CD40 only from the raft compartment following stimulation. These results indicated that TRAFs are recruited to the CD40 cytoplasmic tail, and that the recruitment correlates with the stabilization of the association of CD40 with membrane rafts. The stabilization process probably involves the coalescence of lipid microdomains with which the receptor is already associated (Harder et al., 1998; Janes et al., 1999), suggesting that TRAF recruitment is, at least in part, dependent on membrane raft reorganization. To substantiate this requirement further, membrane rafts were disrupted with cyclodextrin. Figure 4C shows that disruption of membrane rafts before cell stimulation led to a strong diminution of CD40 in the raft compartment that was mirrored by an increase in the soluble compartment. However, although TRAF3 was co-immunoprecipitated with CD40 when membrane rafts were intact, it was no longer detected in CD40 immunoprecipitates when membrane rafts were disrupted. TRAF2 association with CD40 was barely detectable in this experiment. These results indicate that in human DCs, TRAF3 and, to a lesser extent, TRAF2 are recruited to the CD40 cytoplasmic tail following engagement of the receptor, and that this recruitment requires membrane raft integrity and reorgan ization.
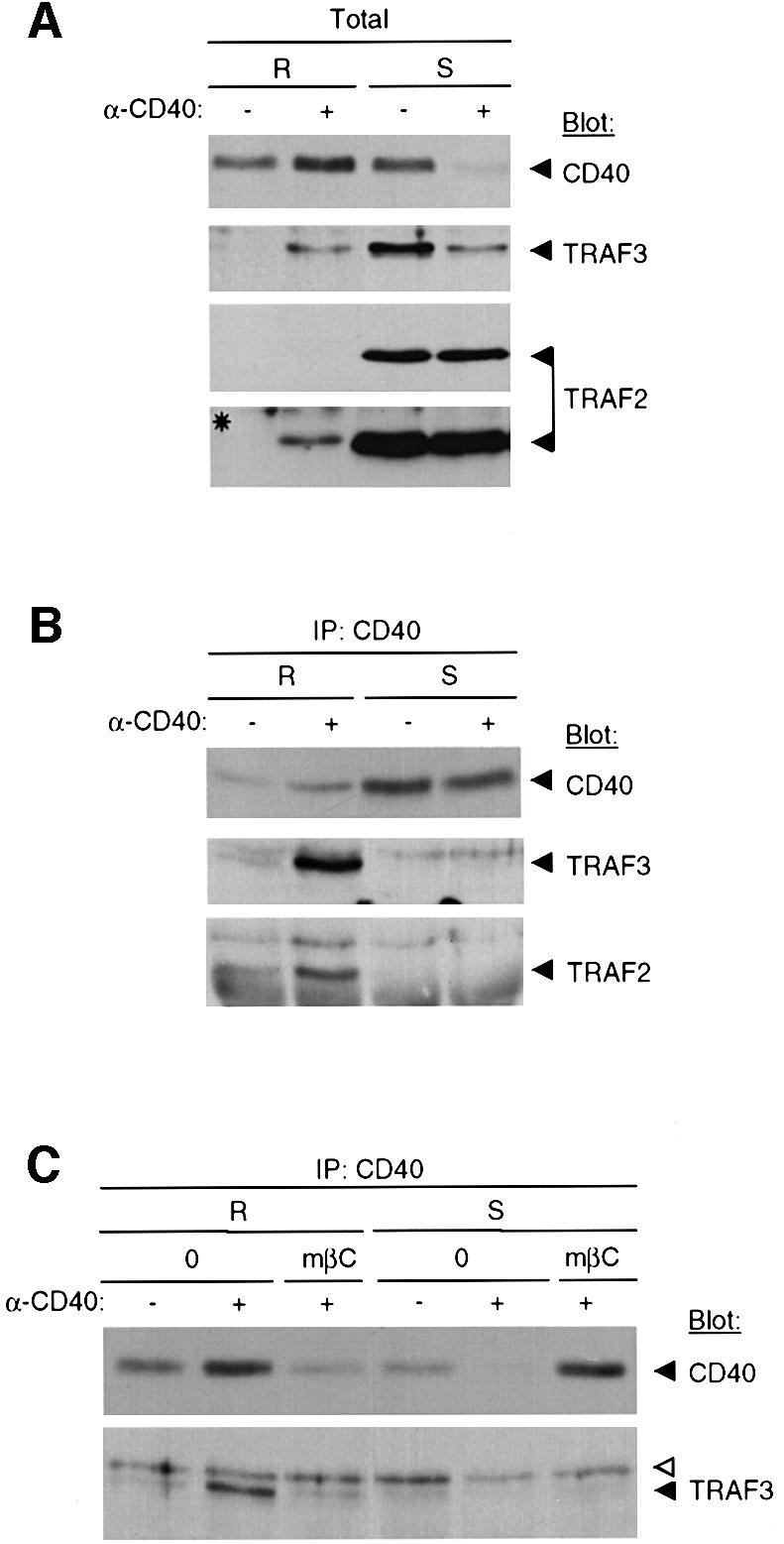
Fig. 4. Membrane rafts provide a platform for TRAF2 and 3 recruitment to the CD40 cytoplasmic tail. (A) Total pooled raft fractions (R) or soluble fractions (S) from IgG-treated (–) or CD40-stimulated (+) DCs were analyzed for CD40, TRAF3 and TRAF2 content by immunoblotting with the corresponding antibodies as indicated. The asterisk indicates a longer exposure of the TRAF2 immunoblot. (B) CD40 was immunoprecipitated (IP) from raft or soluble fractions and eluates were immunoblotted with either anti-CD40, anti-TRAF2 or anti-TRAF3 antibodies as indicated. (C) DCs were pre-treated or not with methyl-β-cyclodextrin (mβC), before CD40 stimulation. Then CD40 was immunoprecipitated (IP) from raft or soluble fractions and eluates were immunoblotted with either CD40 or anti-TRAF3 antibodies as indicated. The open arrow indicates a non-specific band observed with the anti-TRAF3 antibody.
CD40-mediated IL-1α, IL-1β and IL-1Ra production are downstream of a Src family kinase/MEK/ERK pathway, which minimally contributes to p38-dependent IL-12 production
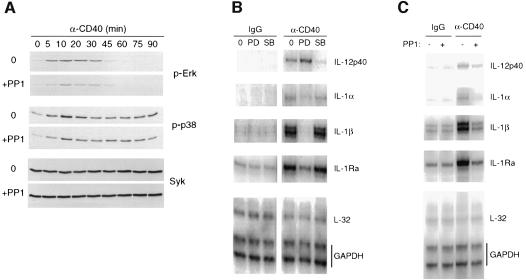
CD40 engagement activates MAPKs in DCs, and p38 MAPK activation regulates IL-12 production (Aicher et al., 1999). Since the Src family selective tyrosine kinase inhibitor PP1 was found to diminish greatly all CD40-mediated pTyr events (Figure 3B), we used PP1 to define the outcome of CD40-mediated pTyr events on p38 MAPK and ERK activation. The active form of enzymes, i.e. Thr180/Tyr182-phosphorylated p38α and Thr202/Tyr204-phosphorylated ERK1/2, were monitored using specific anti-phosphoprotein antibodies. Figure 5A shows that CD40-mediated maximal ERK2 (p42) phosphorylation peaked at 10 min and returned to the unstimulated level at 45 min. ERK1 (p44) could also be stimulated, although not consistently and to a much lower extent than ERK2 (not shown). PP1 efficiently diminished CD40-induced ERK2 phosphorylation over the time course, indicating that ERK phosphorylation is downstream of CD40-mediated pTyr events. p38 MAPK phosphorylation also peaked at 10 min, but then declined to ∼50% of maximal phosphorylation at 30 min (as indicated by quantification of the autoradiograms), which lasted throughout the 90 min of stimulation. PP1 reduced the early phase of p38 phosphorylation, but it only modestly inhibited the sustained plateau of phosphorylation. Therefore, unlike the situation for ERK, a large part of p38 phosphorylation is independent of CD40-mediated pTyr events.
Fig. 5. Effects of CD40-induced pTyr events on cytokine mRNA expression in DCs. (A) DCs were stimulated with anti-CD40 antibody for the indicated times in the presence or abscence of the Src-specific inhibitor PP1. Total cell lysates were analyzed by immunoblotting for the phos phorylated active form of ERK (p-ERK) or p38 MAPK (p-p38) enzymes. Syk served as a loading control. (B and C) Cells were pre-treated or not with MEK1/2 or p38 MAPK inhibitors, PD98059 or SB203580, respectively (B), or Src-specific inhibitor PP1 (C) before stimulation with either an irrelevant mouse IgG1 or with anti-CD40 antibody as indicated. After 14 h of culture, RNAs were extracted and used for RNase protection analysis. L32 and GAPDH correspond to internal control probes.
To define the role of ERK and p38 MAPK activation on CD40-induced IL-1α/β and IL-1Ra mRNAs, we use inhibitors of ERK and p38 activation. SB203580 is a highly specific inhibitor of p38 kinase activity with no effect on ERK (Cuenda et al., 1995), and PD98059 is a highly selective inhibitor of the upstream activator of ERK, MEK1/2 (Alessi et al., 1995). Figure 5B shows that inhibition of p38 activation resulted in almost complete prevention of the CD40-induced increase in IL-12 mRNA, but did not affect IL-1α/β and IL-1Ra mRNA levels. Conversely, inhibition of ERK activation strongly impaired the increase in IL-1α/β and IL-1Ra mRNAs, with no inhibition but rather a slight stimulation of the IL-12 mRNA level. Thus, these results indicate that in CD40-stimulated DCs, p38 activation is upstream of IL-12 regulation, in agreement with the results of Aicher et al. (1999), whereas ERK activation is upstream of IL-1α/β and IL-1Ra regulation.
Consistent with the results above, it was found that PP1 strongly diminished CD40-induced mRNAs for IL-1α/β and IL-1Ra and partially inhibited mRNA for IL-12 (∼50% inhibition) (Figure 5C). Together, these results delineate the outcome of CD40-mediated Src family kinase activation on cytokine expression.
Discussion
Although CD40 receptor plays a dominant role in enhancing the functions of DCs (Banchereau and Steinman, 1998), the question of how signaling through CD40 is achieved in these cells remains poorly documented. This study provides evidence that CD40 molecules associate with membrane rafts in human monocyte-derived DCs, and that these microdomains provide a platform for proximal events of CD40 signaling, i.e TRAF recruitment to the CD40 cytoplasmic tail and the activation of Lyn Src family kinase. In addition, we provide evidence that CD40-mediated Src family kinase activation initiates a pathway, implicating ERK activation, which leads to IL-1α/β and IL-1Ra mRNA production, and minimally contributes to the p38-dependent IL-12 mRNA production.
The quantity of CD40 constitutively associated with membrane rafts (∼40% on average from multiple experiments) is variable from one DC isolate to another, as exemplified in Figure 2A. The reasons for this are not clear but may reflect differences in the maturation and/or activation status of the isolate. However, this association is increased consistently following CD40 engagement. This behavior suggests that, unlike the GPI-linked protein CD55, CD40 has only a moderate affinity for membrane rafts in vivo that can be partly disrupted by non-ionic detergent, but is stabilized following antibody cross-linking. Indeed, the sequestration of CD40 by lateral cross-linking of GM1 strongly suggests that CD40 is constitutively associated with membrane rafts. Thus, the biochemical means of membrane raft isolation used in this study underestimates the amount of raft-associated CD40 on intact cells. Following cross-linking of CD40 with an antibody, the weak interactions existing between CD40 and membrane rafts might be strengthened and lead to stabilization of this association in the presence of detergent. In support of this is a recent report from Janes et al. (1999) that demonstrates that the T cell antigen receptor is associated with lipid raft patches on intact cells; however, this association is not stable to Triton X-100 extraction unless the TCR is cross-linked by an antibody. The stabilization process might simply involve the coalescence of the same lipid environment with which the receptor is already associated (Harder et al., 1998; Janes et al., 1999) and/or others processes triggered by the engagement of CD40. The question as to how CD40 associates with membrane rafts is at present unclear. It is notable that monocyte-derived human DCs expressed no detectable caveolin-1 and -2 by western blotting (not shown), indicating that caveolae do not contribute to the membrane rafts isolated here. Interestingly, a similar association of CD40 with membrane rafts was also observed in the human B-cell line BJAB (not shown), indicating that membrane raft association of CD40 is not restricted to human monocyte-derived DCs.
This study indicates that membrane rafts provide a cellular location allowing CD40 signaling in DCs. Several pieces of evidence strongly suggest that CD40-initiated pTyr events in DCs occur within membrane raft microdomains and require Src family kinase activation, including at least Lyn: (i) perturbation of the structural integrity of membrane rafts by the cholesterol-binding agents nystatin or methyl-β-cyclodextrin profoundly affects CD40-mediated pTyr events; (ii) the increase in phosphorylated Lyn following CD40 engagement is located exclusively in the membrane raft fractions; and (iii) the temporal sequence of Lyn phosphorylation suggested that Lyn kinase activation is upstream of Syk phosphorylation and, indeed, the Src family kinase inhibitor PP1 inhibits all CD40-mediated pTyr events. Due to the limitations of using detergent insolubility as the only criterion to demonstrate the association of a protein with membrane rafts, it remains to be determined whether Syk is, or is not, recruited to membrane rafts for phosphorylation. In addition, CD40 engagement in DCs leads to TRAF3, and to a lesser extent TRAF2, recruitment to the CD40 cytoplasmic tail, and this recruitment requires membrane raft integrity and is likely to be dependent on membrane raft reorganization. High-affinity interaction of TRAFs with the CD40 cytoplasmic tail has been reported to require both TRAF trimerization and CD40 multimerization (Pullen et al., 1998). This supports the idea that avidity-driven recruitment of TRAFs to the cytoplasmic tail of the receptor would be required to initiate intracellular signaling cascades. In this respect, coalescence of membrane rafts following CD40 engagement could provide a membrane compartmentation stabilizing CD40 clustering and thereby favoring TRAF recruitment.
The cytoplasmic domain of CD40 does not posses recognizable homologies to kinases or other sequences that would link this molecule to a canonical transduction cascade, therefore leaving unexplained the mechanism of Lyn activation in the above scheme. This might possibly be achieved through ligand-induced aggregation of membrane rafts as proposed for GPI-linked protein signaling (Horejsi et al., 1999) or T-cell antigen-mediated signaling (Janes et al., 1999). Although CD40 can associate constitutively with membrane rafts in human DCs, initiation of the signaling events investigated in this study requires an antibody-mediated cross-linking of the receptor. Thus, cross-linking of raft-associated CD40 might lead to coalescence of membrane rafts, and such aggregation would facilitate the transactivation of raft-associated Lyn at their cytoplasmic side (Horejsi et al., 1999), thereby initiating the intracellular cascades. Another scenario, although not exclusive of the former, may implicate CD40-associated proteins. We investigated TRAF2 and TRAF3 recruitment here, but other candidates for CD40 signaling proteins also include TRAF5 and 6, as well as Jak3 and p23 (Morio et al., 1995; Hanissian and Geha, 1997; Arch et al., 1998). TRAFs are adaptors thought to mediate the activation of serine/threonine kinases. However, a recent report suggested that c-Src can be regulated through an association with TRAF6 in a signaling complex initiated by TNF-related activation-induced cytokine (TRANCE) receptor (Wong et al., 1999). Interestingly, in this study, TRAF3, but not TRAF2, was also shown to associate with c-Src, thus providing a putative link to CD40-mediated Lyn activation in membrane rafts of DCs.
The use of the Src family kinase inhibitor PP1 indicates that downstream of CD40-mediated Lyn activation, and possibly other Src family kinases, is the phosphorylation of Syk and the activation of ERK. The use of PP1 and of the specific MEK1/2 inhibitor PD98059 allowed us to delineate a Src-family kinase/MEK/ERK pathway leading to IL-1α, IL-1β and IL-1Ra mRNA expression in CD40-stimulated DCs. PD98059 does not inhibit, but rather slightly potentiates, the CD40-mediated increase in IL-12 mRNA. This potentiation might reflect a negative regulatory role for ERK toward IL-12 production, as described in murine macrophages (Feng et al., 1999; Hacker et al., 1999). In contrast, CD40-induced IL-12 mRNA expression was abrogated by the p38 MAPK-specific inhibitor SB203580, in agreement with results described by Aicher et al. (1999), but had no significant effects on IL-1α/β or IL-1Ra mRNA production. Thus, in the CD40-induced cytokine pattern in DCs, ERK activation leads to IL-1α/β and IL-1Ra mRNA expression, whereas p38 MAPK activation leads to IL-12 production. PP1 diminishs the early phase of p38 MAPK phosphorylation and reduces IL-12 mRNA production by 50%, indicating that the Src family kinase-initiated pathway might be partly involved in p38 MAPK activation. In support of this, Lyn and Syk have been shown to activate p38 MAPK following BCR engagement (Jiang et al., 1998); thus they might participate in p38 MAPK activation in DCs. However, a large part of CD40-mediated p38 MAPK phosphorylation is not inhibited by PP1 and probably results from a TRAF-mediated pathway. Indeed, it has been shown that TRAF3 signaling mediates p38 MAPK activation following CD40 ligation on human B cells (Grammer et al., 1998).
In conclusion, Figure 6 proposes a model of CD40-mediated signaling in human DCs. Initiation of signaling events requires a membrane raft reorganization triggered by engagement of CD40, which allows Lyn activation and TRAF2 and 3 recruitment. Lyn activation, and possibly that of other Src family kinases, leads to IL-1α, IL-1β and IL-1Ra mRNA expression via a MEK/ERK pathway. p38 MAPK activation, which induces IL-12 mRNA expression, is probably stimulated through a TRAF-initiated pathway, and to some extent in the early phase of CD40 signaling, through a Src family kinase-dependent pathway.
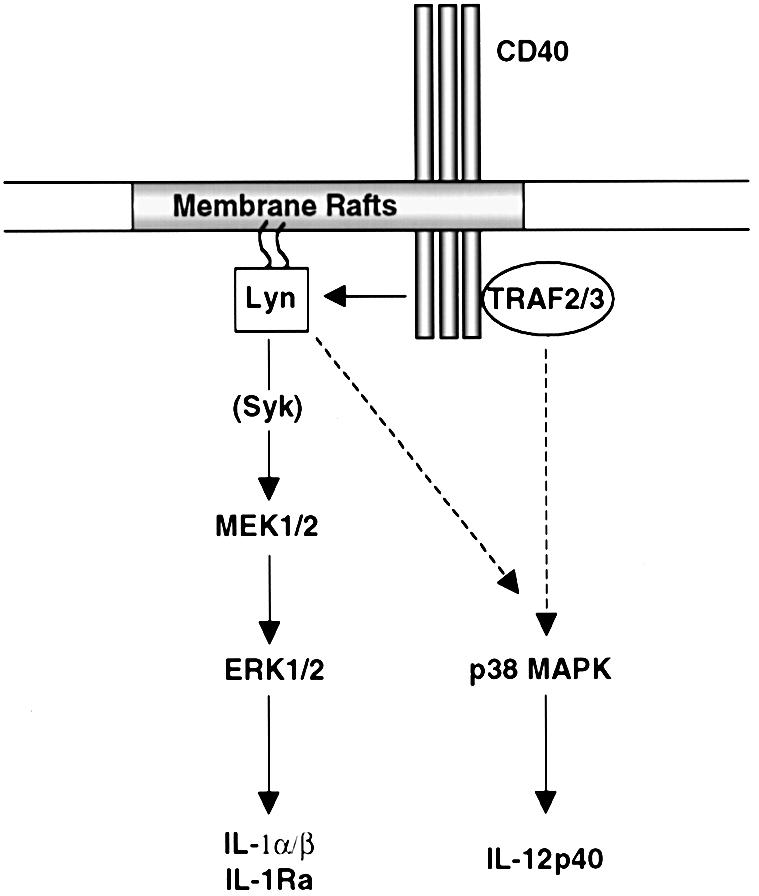
Fig. 6. Model of CD40-mediated signaling in human DCs.
Materials and methods
Cells
Human peripheral blood was obtained from the Etablissement de Transfusion Sanguine (Lyon, France). Mononuclear cells were isolated by density gradient centrifugation using Ficoll/hypaque, then centrifuged through a 50% Percoll gradient (Pharmacia Fine Chemicals, Uppsala, Sweden) for 20 min at 400 g. The light density fraction was recovered and incubated for 10 min at room temperature in 3% human serum–phosphate-buffered saline (PBS). Monocytes were purified by immunomagnetic depletion (Dynal, Oslo, Norway) using a cocktail of mAbs anti-CD19 (4G7 hybridoma, a gift from Dr Ron Levy), CD3 (OKT3; American Type Culture Collection, Rockville, MD) and CD56 (NKH1, Coulter Corp., Miami, FL). The recovered monocytes were >91% pure as shown by flow cytometry with anti-CD14–FITC (Immunotech, Marseille, France). DCs were generated in vitro from purified monocytes, as previously described (Fugier-Vivier et al., 1997). After 6 days of culture in the presence of 50 ng/ml human recombinant granulocye–macrophage colony-stimulating factor (hrGM-CSF) and 500 U/ml hrIL-4 (generously provided by the Schering-Plough Laboratory for Immunological Research, Dardilly, France), >95% of the cells were DCs as assessed by CD1a labeling. Cultures of the DCs were performed in RPMI 1640 (Life Technologies) supplemented with 10 mM HEPES (Life Technologies), 2 mM l-glutamine (Life Technologies), 40 µg/ml gentamicin (Life Technologies) and 10% fetal calf serum (FCS; Boehringer Mannheim, Meylan, France).
Antibodies
Antibodies used in this study include: anti-Lyn, anti-Syk, anti-CD40, anti-CD55 and anti-CD46 antibodies obtained from Santa Cruz Biotechnology (Santa Cruz, CA); mouse monoclonal anti-ERK1/2, anti-phospho-ERK1/2 and anti-P-tyr (4G10) antibodies from Upstate Biotechnology (Lake Placid, NY); rabbit polyclonal anti-p38 MAPK and anti-phospho-p38 MAPK antibodies from New England Biolabs (Beverly, MA); and peroxidase-coupled secondary antibodies from Promega (Madison, WI).
Patching and immunofluorescence confocal microscopy
For all fluorescence confocal microscopy experiments, cells were washed in PBS and incubated in labeling buffer [PBS, 1% bovine serum albumin (BSA), 100 µg/ml human IgG F(c) fragments] for 10 min at 4°C. Cells were incubated with FITC-conjugated CT-B (10 µg/ml in labeling buffer) from Sigma Chemical Co. (St Louis, MO) for 20 min at 4°C. To induce membrane raft patching, cells were then incubated with anti-CT-B antibody (1/250) from Sigma Chemical Co. for 20 min at 37°C. Before antibody staining, cells were fixed with 3% paraformaldehyde in PBS for 30 min, washed extensively and blocked with labeling buffer for 10 min. Membrane CD40 was labeled with PE-conjugated anti-CD40 antibody (10 µg/ml; Immunotech). CD46 was labeled with biotinylated anti-CD46 antibody (20.6 clone, from our laboratory) and revealed with PE-conjugated streptavidin from Caltag Lab (Burlingame, CA). Confocal microscopy was performed with a Zeiss Axioplan 2 and 63× objective lens, using laser excitation at 488 and 543 nm. The widths of FITC and PE emission channels were set such that bleed through across channels was negligible.
CD40 stimulation
DCs were stimulated in serum-free RPMI 1640 (Life Technologies) with 10 µg/ml mouse monoclonal anti-CD40 antibody (mAb89) generously provided by the Schering-Plough Laboratory for Immunological Research or irrelevant mouse IgG1 control antibodies from Sigma Chemical Co. (St Louis, MO) for 10 min or the indicated time at 37°C. p38 MAPK inhibitor SB203580 (5 µM; Calbiochem, San Diego, CA), MEK1/2 inhibitor PD95098 (50 µM; New England Biolabs) or Src family-selective tyrosine kinase inhibitor PP1 (10 µM; Biomol, Plymouth Meeting, PA) was used for 30 min at 37°C before stimulation with anti-CD40. Treatment with methyl-β-cyclodextrin (10 mM; Sigma Chemical Co.) or nystatin (60 µg/ml; Sigma Chemical Co.) was performed in serum-free medium containing 5 mM HEPES for 20 min at 37°C before detergent extraction or anti-CD40 stimulation.
Detergent extraction and flotation assay
For total protein extraction, including membrane raft-associated proteins, lysis was performed in RIPA buffer (150 mM NaCl, 50 mM Tris–HCl pH 8.0, 1% NP-40, 0.5% deoxycholate and 0.1% SDS) containing 5 mM EGTA, 0.2 mM Na2VO4 and a mixture of protease inhibitors (Complete; Boehringer Mannheim) for 15 min at 4°C. Insoluble materials were removed by centrifugation at 10 000 g for 10 min. For membrane raft isolation, cells (5 × 106) were lysed in an equal volume of 2× ice-cold TNE buffer (25 mM Tris–HCl pH 7.5, 150 mM NaCl, 5 mM EGTA, 0.2 mM Na2VO4) containing 0.5% Triton X-100 plus a cocktail of protease inhibitors (Complete; Boehringer Mannheim). The ratio of lysis buffer volume to cell number was kept constant throughout the experiments. After a 30 min incubation on ice, the preparation was made 40% with respect to sucrose. Then 0.8 ml of lysate–sucrose mixture was overlaid sequentially with 2 ml of 30% sucrose and 1 ml of 4% sucrose prepared in TNE, and the mixture was centrifuged at 200 000 g for 14–16 h in an SW50.1 rotor (Beckman). The gradient was fractionated into 0.5 ml fractions from the top of the tube.
Immunoprecipitation and immunoblot analysis.
Cell lysates were pre-cleared by incubation at 4°C with 10 µg/ml irrelevant antibodies pre-absorbed on protein G-coupled Sepharose beads for 2 h. Sucrose fractions from the flotation assay were diluted in TNE containing 1% octyl-glucoside (final sucrose concentration <8%) before pre-clearing. These pre-cleared lysates were immunoprecipitated at 4°C by adding 10 µg/ml specific antibodies for 1 h, followed by 1 h of incubation with protein G-coupled Sepharose beads. For CD40 immunoprecipitation, the pre-clearing step was omitted. Immunoprecipitates were washed five times with TNE containing 1% Triton X-100 and dissolved in SDS sample buffer. Proteins from cell lysates were separated by SDS–PAGE then transferred to Immobilon-P membranes (Millipore, Bedford, MA). Membranes were blocked using 5% non-fat dried milk in TBS-T (20 mM Tris pH 7.6, 130 mM NaCl, 0.1% Tween-20) and incubated for 1 h. Blots were then incubated with specific antibodies, followed by the appropriate horseradish peroxidase (HRP)-coupled secondary antibodies.
RNase protection assays
RNA was extracted from 107 treated DCs, using RNA NOW-TC reagent (Biogentex, Seabrook, TX). The RNase protection was performed using 4 µg of RNA with the RiboQuant multi-probe RNase assay system (PharMingen, San Diego, CA) following the manufacturer’s specification. In brief, RNA was hybridized overnight with the in vitro-translated 32P-labeled probe (hCK-2 kits, PharMingen). Following hybridization, samples were treated with RNase A + T1 and proteinase K, phenol–chloroform extracted and ethanol precipitated. The protected fragments were resolved by electrophoresis on a 5% acrylamide/urea gel and exposed on a Phosphor Screen (Molecular Dynamics Inc., Sunnyvale, CA) for 12 h to quantify the intensity of the bands with ImageQuant software (Molecular Dynamics Inc.). Under the conditions of cell stimulation used here, increased mRNAs for IL-1α, IL-1β and IL-1Ra were already detectable 4 h post-stimulation, whereas increased mRNAs for IL-12 required 14 h to become clearly detectable.
Acknowledgments
Acknowledgements
We thank Dr Dale Christiansen for critically reading the manuscript. This work was supported by institutional grants from CNRS, INSERM and MENRT, and additional support from ARC (SM 9501 and CRC 6108), programme PRFMMIP and Region Rhone-Alpes.
Note added in proof
During revision of this manuscript, we became aware of a report from Hostager et al. (2000) which indicates that during CD40 signaling in mouse B cell lines, CD40 along with TRAF2 and 3 were recruited to membrane rafts. These results are consistent with our findings in human DCs and further strengthen the important role of membrane rafts in CD40-mediated signaling in different cell types.
References
- Aicher A., Shu,G.L., Magaletti,D., Mulvania,T., Pezzutto,A., Craxton,A. and Clark,E.A. (1999) Differential role for p38 mitogen-activated protein kinase in regulating CD40-induced gene expression in dendritic cells and B cells. J. Immunol., 163, 5786–5795. [PubMed] [Google Scholar]
- Alessi D.R., Cuenda,A., Cohen,P., Dudley,D.T. and Saltiel,A.R. (1995) PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem., 270, 27489–27494. [DOI] [PubMed] [Google Scholar]
- Arch R.H., Gedrich,R.W. and Thompson,C.B. (1998) Tumor necrosis factor receptor-associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev., 12, 2821–2830. [DOI] [PubMed] [Google Scholar]
- Banchereau J. and Steinman,R.M. (1998) Dendritic cells and the control of immunity. Nature, 392, 245–252. [DOI] [PubMed] [Google Scholar]
- Bennett S.R., Carbone,F.R., Karamalis,F., Flavell,R.A., Miller,J.F. and Heath,W.R. (1998) Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature, 393, 478–480. [DOI] [PubMed] [Google Scholar]
- Bjorck P., Banchereau,J. and Flores-Romo,L. (1997) CD40 ligation counteracts Fas-induced apoptosis of human dendritic cells. Int. Immunol., 9, 365–372. [DOI] [PubMed] [Google Scholar]
- Brown D.A. and London,E. (1998a) Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol., 14, 111–136. [DOI] [PubMed] [Google Scholar]
- Brown D.A. and London,E. (1998b) Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol., 164, 103–114. [DOI] [PubMed] [Google Scholar]
- Brown D.A. and Rose,J.K. (1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell, 68, 533–544. [DOI] [PubMed] [Google Scholar]
- Caux C., Massacrier,C., Vanbervliet,B., Dubois,B., Van Kooten,C., Durand,I. and Banchereau,J. (1994) Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med., 180, 1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Scheidegger,D., Palmer-Lehmann,K., Lane,P., Lanzavecchia,A. and Alber,G. (1996) Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J. Exp. Med., 184, 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P.C., Dykstra,M.L., Mitchell,R.N. and Pierce,S.K. (1999) A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J. Exp. Med., 190, 1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A., Rouse,J., Doza,Y.N., Meier,R., Cohen,P., Gallagher,T.F., Young,P.R. and Lee,J.C. (1995) SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett., 364, 229–233. [DOI] [PubMed] [Google Scholar]
- Faris M., Gaskin,F., Parsons,J.T. and Fu,S.M. (1994) CD40 signaling pathway: anti-CD40 monoclonal antibody induces rapid dephosphorylation and phosphorylation of tyrosine-phosphorylated proteins including protein tyrosine kinase Lyn, Fyn and Syk and the appearance of a 28-kD tyrosine phosphorylated protein. J. Exp. Med., 179, 1923–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G.J., Goodridge,H.S., Harnett,M.M., Wei,X.Q., Nikolaev,A.V., Higson,A.P. and Liew,F.Y. (1999) Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J. Immunol., 163, 6403–6412. [PubMed] [Google Scholar]
- Foy T.M., Aruffo,A., Bajorath,J., Buhlmann,J.E. and Noelle,R.J. (1996) Immune regulation by CD40 and its ligand GP39. Annu. Rev. Immunol., 14, 591–617. [DOI] [PubMed] [Google Scholar]
- Friedrichson T. and Kurzchalia,T.V. (1998) Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature, 394, 802–805. [DOI] [PubMed] [Google Scholar]
- Fugier-Vivier I., Servet-Delprat,C., Rivailler,P., Rissoan,M.C., Liu,Y.J. and Rabourdin-Combe,C. (1997) Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med., 186, 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garceau N., Kosaka,Y., Masters,S., Hambor,J., Shinkura,R., Honjo,T. and Noelle,R.J. (2000) Lineage-restricted function of nuclear factor κB-inducing kinase (NIK) in transducing signals via CD40. J. Exp. Med., 191, 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammer A.C., Swantek,J.L., McFarland,R.D., Miura,Y., Geppert,T. and Lipsky,P.E. (1998) TNF receptor-associated factor-3 signaling mediates activation of p38 and Jun N-terminal kinase, cytokine secretion and Ig production following ligation of CD40 on human B cells. J. Immunol., 161, 1183–1193. [PubMed] [Google Scholar]
- Hacker H., Mischak,H., Hacker,G., Eser,S., Prenzel,N., Ullrich,A. and Wagner,H. (1999) Cell type-specific activation of mitogen-activated protein kinases by CpG-DNA controls interleukin-12 release from antigen-presenting cells. EMBO J., 18, 6973–6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanissian S.H. and Geha,R.S. (1997) Jak3 is associated with CD40 and is critical for CD40 induction of gene expression in B cells. Immunity, 6, 379–387. [DOI] [PubMed] [Google Scholar]
- Hanke J.H., Gardner,J.P., Dow,R.L., Changelian,P.S., Brissette,W.H., Weringer,E.J., Pollok,B.A. and Connelly,P.A. (1996) Discovery of a novel, potent and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem., 271, 695–701. [DOI] [PubMed] [Google Scholar]
- Harder T., Scheiffele,P., Verkade,P. and Simons,K. (1998) Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol., 141, 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horejsi V., Drbal,K., Cebecauer,M., Cerny,J., Brdicka,T., Angelisova,P. and Stockinger,H. (1999) GPI-microdomains: a role in signalling via immunoreceptors. Immunol. Today, 20, 356–361. [DOI] [PubMed] [Google Scholar]
- Hostager B.S., Catlett,I.M. and Bishop,G.A. (2000) Recruitment of CD40 and tumor necrosis factor receptor-associated factors 2 and 3 to membrane microdomains during CD40 signaling. J. Biol. Chem., 275, 15392–15398. [DOI] [PubMed] [Google Scholar]
- Ilangumaran S., He,H.T. and Hoessli,D.C. (2000) Microdomains in lymphocyte signalling: beyond GPI-anchored proteins. Immunol. Today, 21, 2–7. [DOI] [PubMed] [Google Scholar]
- Jalukar S.V., Hostager,B.S. and Bishop,G.A. (2000) Characterization of the roles of TNF receptor-associated factor 6 in CD40-mediated B lymphocyte effector functions. J. Immunol., 164, 623–630. [DOI] [PubMed] [Google Scholar]
- Janes P.W., Ley,S.C. and Magee,A.I. (1999) Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol., 147, 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A., Craxton,A., Kurosaki,T. and Clark,E.A. (1998) Different protein tyrosine kinases are required for B cell antigen receptor-mediated activation of extracellular signal-regulated kinase, c-Jun NH2-terminal kinase 1 and p38 mitogen-activated protein kinase. J. Exp. Med., 188, 1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehry M.R. (1996) CD40-mediated signaling in B cells. Balancing cell survival, growth and death. J. Immunol., 156, 2345–2348. [PubMed] [Google Scholar]
- Koch F., Stanzl,U., Jennewein,P., Janke,K., Heufler,C., Kampgen,E., Romani,N. and Schuler,G. (1996) High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med., 184, 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppi T.A., Tough-Bement,T., Lewinsohn,D.M., Lynch,D.H. and Alderson,M.R. (1997) CD40 ligand inhibits Fas/CD95-mediated apoptosis of human blood-derived dendritic cells. Eur. J. Immunol., 27, 3161–3165. [DOI] [PubMed] [Google Scholar]
- Kuhne M.R., Robbins,M., Hambor,J.E., Mackey,M.F., Kosaka,Y., Nishimura,T., Gigley,J.P., Noelle,R.J. and Calderhead,D.M. (1997) Assembly and regulation of the CD40 receptor complex in human B cells. J. Exp. Med., 186, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manie S.N., Debreyne,S., Vincent,S. and Gerlier,D. (2000) Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J. Virol., 74, 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkonian K.A., Ostermeyer,A.G., Chen,J.Z., Roth,M.G. and Brown,D.A. (1999) Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem., 274, 3910–3917. [DOI] [PubMed] [Google Scholar]
- Morio T., Hanissian,S. and Geha,R.S. (1995) Characterization of a 23-kDa protein associated with CD40. Proc. Natl Acad. Sci. USA, 92, 11633–11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen S.S., Miller,H.G., Everdeen,D.S., Dang,T.T.A., Crute,J.J. and Kehry,M.R. (1998) CD40-tumor necrosis factor receptor-associated factor (TRAF) interactions: regulation of CD40 signaling through multiple TRAF binding sites and TRAF hetero-oligomerization. Biochemistry, 37, 11836–11845. [DOI] [PubMed] [Google Scholar]
- Pullen S.S., Labadia,M.E., Ingraham,R.H., McWhirter,S.M., Everdeen,D.S., Alber,T., Crute,J.J. and Kehry,M.R. (1999) High-affinity interactions of tumor necrosis factor receptor-associated factors (TRAFs) and CD40 require TRAF trimerization and CD40 multimerization. Biochemistry, 38, 10168–10177. [DOI] [PubMed] [Google Scholar]
- Ren C.L., Morio,T., Fu,S.M. and Geha,R.S. (1994) Signal transduction via CD40 involves activation of lyn kinase and phosphatidylinositol-3-kinase and phosphorylation of phospholipase Cγ 2. J. Exp. Med., 179, 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revy P., Hivroz,C., Andreu,G., Graber,P., Martinache,C., Fischer,A. and Durandy,A. (1999) Activation of the Janus kinase 3–STAT5a pathway after CD40 triggering of human monocytes but not of resting B cells. J. Immunol., 163, 787–793. [PubMed] [Google Scholar]
- Ridge J.P., Di Rosa,F. and Matzinger,P. (1998) A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature, 393, 474–478. [DOI] [PubMed] [Google Scholar]
- Roy M., Waldschmidt,T., Aruffo,A., Ledbetter,J.A. and Noelle,R.J. (1993) The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J. Immunol., 151, 2497–2510. [PubMed] [Google Scholar]
- Schoenberger S.P., Toes,R.E., van der Voort,E.I., Offringa,R. and Melief,C.J. (1998) T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature, 393, 480–483. [DOI] [PubMed] [Google Scholar]
- Schroeder R.J., Ahmed,S.N., Zhu,Y., London,E. and Brown,D.A. (1998) Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J. Biol. Chem., 273, 1150–1157. [DOI] [PubMed] [Google Scholar]
- Servet-Delprat C., Vidalain,P.O., Bausinger,H., Manie,S., Le Deist,F., Azocar,O., Hanau,D., Fischer,A. and Rabourdin-Combe,C. (2000) Measles virus induces abnormal differentiation of CD40 ligand-activated human dendritic cells. J. Immunol., 164, 1753–1760. [DOI] [PubMed] [Google Scholar]
- Simons K. and Ikonen,E. (1997) Functional rafts in cell membranes. Nature, 387, 569–572. [DOI] [PubMed] [Google Scholar]
- Thomas S.M. and Brugge,J.S. (1997) Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol., 13, 513–609. [DOI] [PubMed] [Google Scholar]
- Varma R. and Mayor,S. (1998) GPI-anchored proteins are organized in submicron domains at the cell surface. Nature, 394, 798–801. [DOI] [PubMed] [Google Scholar]
- Wong B.R., Besser,D., Kim,N., Arron,J.R., Vologodskaia,M., Hanafusa,H. and Choi,Y. (1999) TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol. Cell, 4, 1041–1049. [DOI] [PubMed] [Google Scholar]
- Xavier R., Brennan,T., Li,Q., McCormack,C. and Seed,B. (1998) Membrane compartmentation is required for efficient T cell activation. Immunity, 8, 723–732. [DOI] [PubMed] [Google Scholar]