Abstract
Cystalysin is a Cβ–Sγ lyase from the oral pathogen Treponema denticola catabolyzing l-cysteine to produce pyruvate, ammonia and H2S. With its ability to induce cell lysis, cystalysin represents a new class of pyridoxal 5′-phosphate (PLP)-dependent virulence factors. The crystal structure of cystalysin was solved at 1.9 Å resolution and revealed a folding and quaternary arrangement similar to aminotransferases. Based on the active site architecture, a detailed catalytic mechanism is proposed for the catabolism of S-containing amino acid substrates yielding H2S and cysteine persulfide. Since no homologies were observed with known haemolysins the cytotoxicity of cystalysin is attributed to this chemical reaction. Analysis of the cystalysin–l-aminoethoxyvinylglycine (AVG) complex revealed a ‘dead end’ ketimine PLP derivative, resulting in a total loss of enzyme activity. Cystalysin represents an essential factor of adult periodontitis, therefore the structure of the cystalysin–AVG complex may provide the chemical basis for rational drug design.
Keywords: aminoethoxyvinylglycine/cysteine desulfhydrase/haemolysin/periodontitis/protein crystallography
Introduction
Cystalysin is a 46 kDa pyridoxal 5′-phosphate (PLP) protein isolated from Treponema denticola (Chu and Holt, 1994). This organism belongs to the group of oral spirochetes that are located in the human gingival crevice. Especially high concentrations of treponemes have been found at diseased sites in the subgingiva of patients with severe periodontitis. Evidence is increasing that oral treponemes play an important role in disease progression leading to gingival inflammation and bleeding, detachment of connective tissue, formation of lesions and abscesses, and finally to tissue and bone destruction (Chu et al., 1994, 1997).
Since T.denticola appears to be restricted to the ecological niche of periodontal pockets, it had to develop special techniques to cope with an environment that requires complex nutritional needs. In vitro studies revealed the production of a large number of virulence factors by T.denticola, including several proteolytic and cytotoxic enzymes. The equipment with such potential tools for cell adhesion and degrading of host proteins/peptides seems to be a prerequisite for bacterial growth and the progression of periodontal lesions (Chu and Holt, 1994).
Treponema denticola mainly produces one putative haemolysin, which is able to agglutinate and lyse erythrocytes upon incubation. By studying the haemolytic and haemoxidizing properties of the T.denticola strain TD-4, Holt and coworkers could pinpoint both activities to a cytoplasmic 46 kDa protein (Chu et al., 1994). Both the native and the recombinant cystalysin were able to interact with human and sheep red blood cells leading to impressive spikes and protrusions in the erythrocyte membranes and finally to the formation of countless irregular holes (Chu et al., 1994). Additionally, cystalysin causes the oxidation and sulfuration of haemoglobin to methaemoglobin and sulfhaemoglobin (Kurzban et al., 1999), respectively. The cytoplasm leaking from the lysed red blood cells contains a large number of nutrition factors suitable for T.denticola, especially various amino acids and the detached haem from haemoglobin. With the iron from extracellular haem, a rather scarce substance of the periodontal pocket becomes available for T.denticola. The central role of cystalysin in iron acquisition is further supported by the observation that cystalysin synthesis is significantly increased under conditions of iron deprivation (Chu et al., 1994).
Studies of the amino acid sequence from cystalysin (Chu et al., 1995) revealed no relation to previously reported haemolytic proteins but showed significant homologies with the family of PLP-dependent aminotransferases. This suggests that haemolysis as performed by cystalysin is proceeding by a novel, unique mechanism.
PLP could be identified as a cofactor of cystalysin (Chu et al., 1995), forming a Schiff base with the ε-amino group of the active site lysine (Lys238). Due to the nature of the PLP cofactor, the enzymatic activity was tested for a large number of amino acids (Chu et al., 1997; Kurzban et al., 1999). Only compounds with thiol-derived functional groups including cystathionine, reduced glutathione, cystine and cysteine were metabolized by cystalysin. Among these substrates, cysteine was cleaved with highest efficiency (Km = 3.6 mM, kcat = 12 s–1) (Chu et al., 1997). The Cβ–Sγ cleavage reactions catalysed by cystalysin with cysteine, cystathionine and cystine are α,β-elimination reactions, yielding ammonia, pyruvate, H2S, homocysteine and cysteine persulfide, respectively. Accordingly, cystalysin functions as a PLP-dependent cysteine desulfhydrase (Chu et al., 1997), catalyzing reactions similar to cystathionine β-lyase (CBL) (Dwivedi et al., 1982), l-cysteine/cystine C–S lyase from Synechocystis (Leibrecht and Kessler, 1997) and MalY (Zdych et al., 1995).
Interestingly, comparably high levels (>2 mM) of H2S have been reported in periodontal disease pockets. In this concentration, H2S is toxic for most cells, since it is able to cleave disulfide bonds in proteins and to inactivate metalloproteins. It is also known to be a potent inhibitor of oxidative phosphorylation (Beauchamp et al., 1984). Treponema denticola belongs to a limited number of periodontal microbes that can produce and tolerate millimolar levels of H2S (Persson et al., 1990). This ability should give selective advantages to T.denticola in its natural environment. For this reason the production of H2S should be the major function of cystalysin, which can thus be regarded as an important virulence factor (Chu et al., 1997; Kurzban et al., 1999).
To investigate the mechanism by which cystalysin participates in erythrocyte lysis, in haem oxidation and in H2S production, the crystal structure of the protein was determined at high resolution. Here we give a detailed description of the crystal structure of the first haemolytically active PLP protein. Additionally, we have solved the structure of a complex with the inhibitor l-aminoethoxyvinylglycine (AVG), which provides further insight into substrate binding and specificity.
Results and discussion
Overall fold
The structure of cystalysin was solved by molecular replacement at 1.9 Å resolution. As a search model the recently solved structure of the PLP-dependent MalY protein from Escherichia coli was used (Clausen et al., 2000). This enzyme is with 27% sequence identity the most homologous protein with known crystal structure. The electron density contoured at 1.0σ is continuous throughout the structure including the PLP cofactor. Only side chains of the surfacial lysines Lys154, Lys161, Lys163 and Lys184 could not be incorporated in the density. The final model of cystalysin consists of all 394 residues and 328 water molecules per monomer and was refined to a crystallographic R-factor of 20.8% and a free R-factor of 24.7%. The model of the cystalysin–AVG complex has a final R-factor of 19.3% (Rfree 26.0%). The mean error of atom positions as determined from a Luzzatti plot (Luzzatti, 1952) was 0.35 Å. In the Ramachandran plot (Ramachandran and Saisekharan, 1968), 88.0% of the residues were found to be in the most favourable, 11.7% in the favourable, 0.3% in the generously allowed and none in the disallowed region as indicated by the program PROCHECK (Laskowski et al., 1993). During the refinement the model bias was eliminated by 8-fold averaging and simulated annealing. The general correctness of the solution was further attested by the ‘omit’ density that appeared for the PLP cofactor.
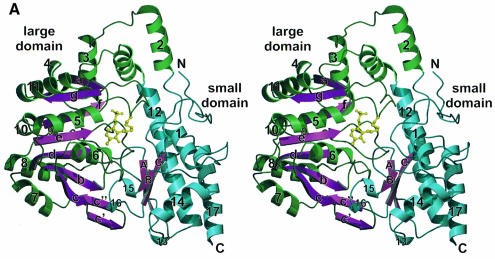
Analysis of the secondary structure of cystalysin with the programs STRIDE (Frischman and Argos, 1995) and DSSP (Kabsch and Sander, 1983) revealed an open α/β-type architecture with 46.2% helical structure, 14.2% β-sheet, 25.4% β-bend and 14.2% unclassified coil structure. Within the helical content there are five 310 helices (4, 9, 13, 15 and 16) three or four residues long. The secondary structural elements are depicted in Figure 1A along with the usual nomenclature.
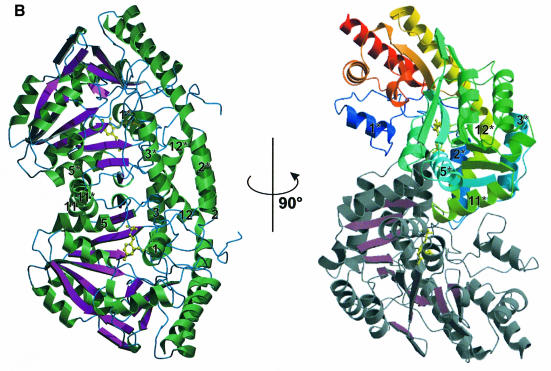
Fig. 1. Overall fold. (A) Structure of the cystalysin monomer showing the large domain with green helices and magenta β-sheets, the small domain with blue helices and the PLP cofactor in yellow. The nomenclature for the secondary structural elements is indicated. (B) Ribbon presentation of the cystalysin dimer emphasizing structural elements essential for dimerization. Helical structures are shown in green, loop structure in blue and β-sheets in magenta. 90° rotation results in the characteristic S-shaped orientation in which the sequential flow is mapped by colour ramp to one monomer (N-terminus, blue; C-terminus, red).
Similarly to most PLP enzyme structures related to l-aspartate aminotransferase, each cystalysin monomer folds into two domains (Figure 1A) and has an approximate size of 67 × 62 × 50 Å. The large domain constitutes residues 48–288 and carries the PLP cofactor covalently bound to Lys238. The small domain is assembled by the two terminal regions of the polypeptide chain, by residues 1–47 from the N-terminal and residues 288–394 from the C-terminal part. The central element of the large domain is a mainly parallel seven-stranded β-sheet that is found in all α-family aminotransferases (Jansonius, 1998). Except for the antiparallel β-strand g all strands run parallel and form a β-sheet with a left-handed twist enveloping helix 5. All cross-overs are right handed. Additionally, strands c′ and c″ following β-sheet c form an antiparallel two-stranded β-sheet at the interface of both domains. Both sheets are wedged between five helices (4, 7, 8, 9 and 10) at the solvent-accessible side, one (6) at the intersubunit and four helices (2, 3, 5 and 11) at the interdomain interfaces. Helices 2 and 12 are mediating the transition to the small domain at the N-terminus and the C-terminus, respectively. The long α-helix 12 (39 residues) with a kink at Cys288 is the most characteristic secondary structural element of the model. The small domain is formed by a three-stranded, antiparallel β-sheet that is protected from solvent by four α-helices (1, 12, 14 and 17) and one 310 helix (13). α-helix 1 (residues 17–25) is contributed by the N-terminal segment, which was found to be well ordered and mainly involved in dimer contacts. Two additional 310 helices (15 and 16) form part of the interdomain interface due to interactions with the loop connecting β-strand b with helix 6 from the large domain. Both domains form a deep cleft in the centre of the cystalysin monomer, at the bottom of which the PLP cofactor is bound to the C-terminal end of the seven-stranded β-sheet.
As deduced from gel filtration experiments, cystalysin occurs as a homodimer of 95 kDa in solution (H.I.Krupka, unpublished data). This quaternary assembly was also observed in the crystalline state. The two monomers are tightly associated through a non-crystallographic 2-fold axis, resulting in an overall arch-shaped appearance (Figure 1B). Figure 1B shows the dimer with one monomer displaying the active site entrance, while the other active site cleft is oriented in the opposite direction. In this orientation the αβα architecture of the dimer is clearly visible, with the two main β-sheets assembled like a winding ladder in the centre of the molecule. Rotating the molecule about 90° demonstrates the S-shaped dimer typical for this family of PLP enzymes (Figure 1B).
Although the dimer displays a rather open structure, the contact surface between the monomers is extensive: ∼11.2% (1906 Å) of the total solvent-accessible area of a monomer is buried upon dimerization. Only considering contacts shorter than 3.8 Å, a total of 16 hydrogen bonds and two salt bridges participate in subunit interactions (Jones and Thornton, 1996). Dimer formation is mediated mainly by two α-helix bundles per monomer (2/3, 11/12 and 2*/3*, 11*/12*). Each helix bundle is connected by a loop that is reaching into the neighbouring subunit, the loop between helix 2 and 3 even protruding into the adjacent active site. Consequently, residue Tyr64, situated within this loop, is even directly involved in binding of the PLP cofactor (see below) of the other subunit. Further dimer stabilization is achieved by the N-terminal coil structure and by helices 1 and 5, which interact with the loop between helices 2* and 3* and the C-terminal part of helix 11*, respectively. Additionally, large hydrophobic patches are formed between helices 2 and 2*, which are positioned in almost perfect antiparallel orientation. The large contact surface with numerous interactions between monomers supports the notion that the physiologically active state of the cystalysin enzyme is the dimer.
Comparison with related proteins
For three-dimensional structural comparisons the DALI algorithm (Holm and Sander, 1993) was employed. The three highest scoring structures of the Protein Data Bank (PDB) database are aspartate aminotransferase from Thermus thermophilus (Nakai et al., 1999) (tAAT, z-score 41.0), tyrosine aminotransferase from Trypanosoma cruzi (Blankenfeldt et al., 1999) (z-score 38.4) and 8-amino-7-oxononate synthase from E.coli (Alexeev et al., 1998) (z-score 23.2). tAAT and MalY from E.coli (which was not taken into account by the DALI server, since the coordinates had not yet been deposited) were used for more detailed structural comparison.
PLP-dependent enzymes have been classified into three families (α-, β- and γ-family) with subdivision of the α-family into subgroups I–IV (Mehta et al., 1993; Käck et al., 1999). According to Okamoto et al. (1996), subgroup I was further subdivided into subgroups Ia and Ib. 8-amino-7-oxononate synthase and tyrosine aminotransferase belong to the α-family, with the latter being assigned to subgroup I. The aspartate aminotransferases (AATs) belong to subgroup Ia [e.g. E.coli, yeast, chicken and pig AAT (pAAT) (Rhee et al., 1997)] or subgroup Ib [e.g. Bacillus sp. (Sung et al., 1991), T.thermophilus (Nakai et al., 1999) and Rhizobium meliloti AAT (Watson and Rastogi, 1993)], sharing >40% sequence identity within each group but only 16% or less between both subgroups. The Cα alignment of cystalysin with AATs clearly revealed higher similarities with subgroup Ib than with subgroup Ia: with tAAT from T.thermophilus, 357 Cα aligned (r.m.s.d. 2.3 Å), compared with pAAT (closed form), 279 aligned Cα (r.m.s.d. 3.3 Å).
The striking difference between the two aminotransferase subgroups is the ability of AATs in subgroup Ia to undergo a large conformational change from the open to the closed form, induced by the binding of substrates or inhibitors (Rhee et al., 1997; Jansonius, 1998). Not surprisingly, typical residues like Gly36, Val37, Gly38, Met326 and the substrate binding Arg292 (Rhee et al., 1997), which enable a transition from the open to the closed conformation of pAAT, are absent in cystalysin. In AATs of subgroup Ib only the N-terminal region (Lys13–Val30) of the small domain moves to close the active site upon substrate binding (Nakai et al., 1999). Remarkably, the construction and location of the N-terminal segment differs strongly between cystalysin and AATs of this subgroup. In tAAT only 11 residues form a short arm, while the N-terminus of cystalysin contains an α-helix (Leu17–Gln25) among coil structures with a total of 48 residues. Furthermore, the high flexibility of the respective N-terminal segments suggests a general role of this segment for active site closure of subgroup Ib AATs and may also be relevant for cystalysin. However, even in the enzyme–inhibitor complex of cystalysin with AVG such a conformational change has not been observed. Overall, the obvious structural similarities with tAAT and MalY (see below) lead to the classification of cystalysin into subgroup Ib.
The structure is more similar to MalY (355 Cα aligned, r.m.s.d. 1.7 Å) than to tAAT in the entire molecule. Differences are mainly restricted to two regions, which are unique for both enzymes and should play an important role for their respective physiological functions. (i) The structural components that contribute to the individual substrate binding pockets vary in length and architecture, generating different morphologies of the catalytic clefts with divergent electrostatic surface topologies. The corresponding active site boundaries can be divided into a proximal part (helix 14 and its C-terminal loop) and a distal part (the N-terminal segment, helix 11* and its C-terminal loop), relative to their location to the PLP cofactor. While in MalY the active site accessibility via the proximal part is more pronounced, the substrate binding pocket of cystalysin is more open in the distal half. The different morphology is paralleled by an inverse active site polarity: while the proximal part of the MalY active site is constructed mainly by hydrophobic residues (Phe25, Leu340, Met351, Tyr354), cystalysin shows a highly acidic surface formed by Gln25, Glu343, Asp355, Glu356 and Tyr358. A similar opposing behaviour is observed in the distal half of the active site crevice, which in cystalysin is built up mainly by hydrophobic residues (Leu17, Leu21, Met271, Phe273), while in MalY this region is dominated by the polar residues Glu23, Arg24, Lys261, Ser267 and Ser268. (ii) MalY contains a binding patch for the MalT protein, which is the central activator of the maltose operon. The most important element of the corresponding protein–protein interaction motif of MalY, the loop comprising residues 212–222, is replaced by α-helix 10 (Glu224–Asp228) in cystalysin, which covers the described hydrophobic groove of the MalY interaction patch (Clausen et al., 2000).
Neither comparison of three-dimensional structures nor amino acid sequences revealed any similarity of the haemolytically active cystalysin with known thiol- activated haemolysins of other species. Most striking is the high content of lysine residues (10.5%) in cystalysin, similar to thiol-activated streptolysins and to α-haemolysin from Staphylococcus aureus. The structure of staphylococcal α-haemolysin from S.aureus is characterized by a mushroom-shaped heptameric pore that contains a lytic transmembrane domain formed by a hydrophobic β-barrel (Song et al., 1996). In cystalysin the total number of 116 charged amino acids (43 lysines, 32 glutamates, 28 aspartates and 13 arginines) out of 394 renders the surface extremely polar and opposes the formation of such a hydrophobic structure element and thus membrane insertion. Also, the creation of irregular holes in erythrocyte membranes upon incubation with cystalysin (Chu et al., 1994) implies that the enzyme should function through a unique mechanism of cell lysis.
Cystalysin is highly soluble, displays remarkable stability over a wide pH and temperature range and is almost inert to protease treatment (Chu et al., 1997). No precursor protein containing an N-terminal signal sequence has been detected as is usually required for extracellular translocation of periplasmic, outer membrane and secreted proteins (Oliver, 1985). Also, the multiple motif LXGGXGXD, previously described for proteins secreted via alternative pathways (Delepelaire and Wandersman, 1990), is absent in cystalysin. Moreover, all PLP enzymes known so far are cytosolic enzymes. These findings suggest that cystalysin is not acting directly as a haemolysin but induces haemolysis and haemoxidation (Chu et al., 1994) by its reaction products (see below).
Active site structure of cystalysin
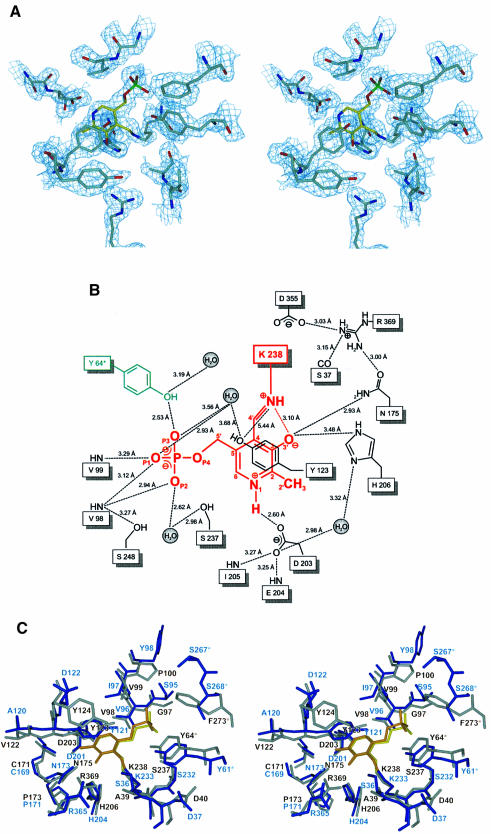
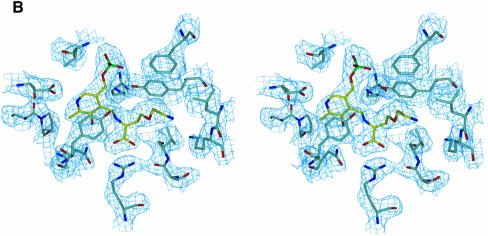
In the centre of each cystalysin monomer the PLP cofactor is bound in a wide catalytic cleft. This crevice is formed by both domains of one subunit and by parts of the large domain from the neighbouring subunit (see below). The boundaries framing the active site cleft are constituted by the C-terminal tails of strands d, e and f, their connecting loops, by the N-terminal ends of helices 5 and 6 and predominantly by helix 1 building up a bordering wall throughout its length. Binding of the cofactor is achieved by Lys238 located at a β-turn connecting β-strands f and g at the bottom of the active site cleft. Continuous electron density confirms the formation of an aldimine bond between the ε-amino group of Lys238 and the aldehyde of the uncomplexed PLP (Figure 2A). Additionally, the cofactor is anchored strongly through its phosphate group, which has a total of six hydrogen bonds with protein residues (Ser237, Val98, Val99 and Tyr64*) and two hydrogen bonds with two water molecules (Figure 2B). Further polar interactions are found between Asp203 and the pyridine N1 as well as between His206 and Asn175 and the deprotonated hydroxyl group O3′. Furthermore, the cofactor undergoes ring-stacking interactions with the phenol ring of Tyr123, which is positioned 3.8 Å above the pyridine ring in parallel orientation. Ring-stacking interactions and stabilization of the positively charged pyridine nitrogen are conserved features in the α-family of PLP enzymes and have been shown to contribute to the electron sink character of the conjugated pyridine system (Yano et al., 1992).
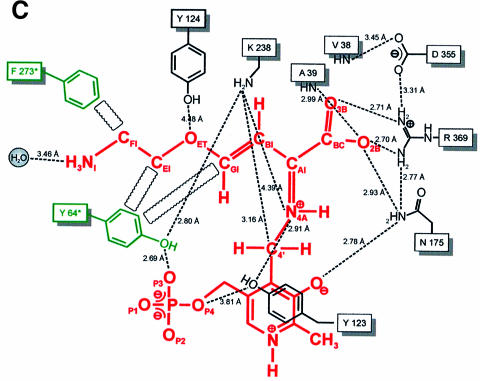
Fig. 2. Active site structure. (A) Final 2Fo – Fc electron density of the PLP cofactor (yellow) and the immediate protein vicinity (colour coding by atom type), contoured at 1.2σ and calculated at 1.9 Å resolution. (B) Schematic presentation of the active site around the PLP cofactor (red). Residues contributing from the neighbouring subunit are coloured green. Hydrogen bonds and interatomic distances (Å) are indicated. (C) Stereo plot showing the superposition of the active sites of cystalysin and MalY from E.coli. Cystalysin residues are coloured grey with the cofactor in yellow, MalY is shown in blue and its PLP in orange.
The superpositions of cystalysin with MalY were performed with the program O (Jones et al., 1991) and revealed similar active site architectures (Figure 2C). Most of the active site residues of MalY are conserved in cystalysin including Asn175, Asp203, Lys238 (Schiff base), Arg369, Tyr64*, Asp40, Val98, Tyr123, Cys171, Pro173, His206 and Ser237. Only in cystalysin, Tyr124 occupies a unique position, reaching towards Tyr123 into the active site and is thus limiting substrate approach to the PLP cofactor above the pyridine ring area. Most probably, Tyr124 is crucial to determine the substrate preference of cystalysin and may act in concert with Phe162, Phe360 and Ile359, which also restrict the active site accessibility in this direction. A noticeable difference comparing the active site structures of various PLP-dependent enzymes is the pyridoxal sandwiching residue. In most aminotransferases a tryptophan or phenylalanine residue is observed in this position, while in cystathionine γ-synthase, CBL, tyrosine aminotransferases and cystalysin a tyrosine is found (Mehta et al., 1989). As reported previously (Clausen et al., 1996), a tyrosine in this position may be of mechanistic importance for substrate activation (see below).
Catalytic mechanism for cystalysin as a Cβ–Sγ lyase
Like MalY and CBL, cystalysin belongs to the group of PLP-dependent Cβ–Sγ lyases that produce ammonium and pyruvate. A common catalytic mechanism for this class of PLP enzymes can be anticipated. Differences are solely restricted to substrate preferences: while MalY prefers cystine over the CBL main substrate cystathionine (Zdych et al., 1995), cystalysin seems to favour cysteine.
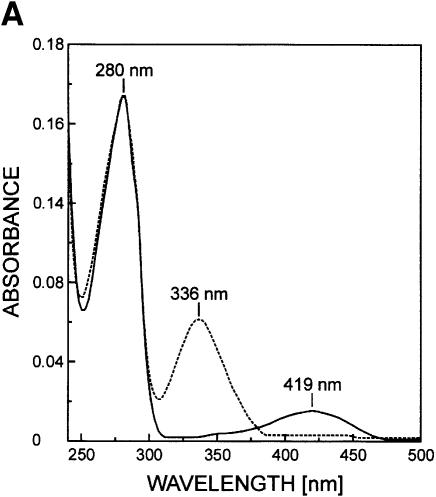
For transaldimination, the amino group of the substrate has to be deprotonated to perform a nucleophilic attack on the C4′ of the internal aldimine. Substrate activation can be achieved in three different ways: (i) an uncharged nitrogen of the internal aldimine can be responsible for substrate deprotonation as commonly encountered with AATs (Jansonius, 1998); (ii) the prevailing pH in solution may be sufficient to shift the chemical equilibrium to the deprotonated substrate amino group (Hayashi and Kagamiyama, 1997); or (iii) deprotonation is mediated by a further active site base, e.g. the phenolate oxygen of the PLP sandwiching tyrosine as proposed for the γ-family of PLP enzymes (Clausen et al., 1996). Since the absorption maximum in the cystalysin spectrum is observed at 419 nm at pH 8.0, the aldimine nitrogen is present in its protonated state, excluding any deprotonating ability. At the pH optimum of cystalysin between 7.8 and 8.0 (Chu et al., 1997) a significant amount of substrate should be present in the deprotonated state to account for the maximum turnover number, since kcat of 12 s–1 of the overall reaction is rather slow (Chu et al., 1997). Additionally, the PLP sandwiching Tyr123 can be expected to exist at least partially as a phenolate at this pH and thus could be further supporting substrate activation in analogy with CBL.
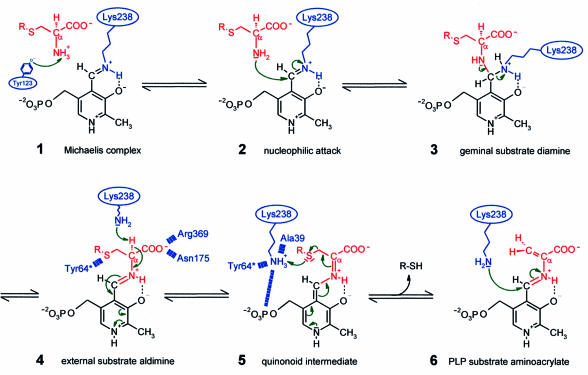
The substrate α-carboxylate should be bound by Arg369, a conserved residue in nearly all PLP-dependent enzymes that is supposed to act as a docking site for carboxylate groups (Mehta et al., 1993) (see below). After transaldimination the released Lys238 abstracts the Cα proton of the substrate with its deprotonated ε-amino group, producing a carbanionic intermediate that is stabilized as the characteristic quinonoid intermediate (Figure 3), which was identified spectroscopically. Charge dissipation into the pyridoxal system is supported by ring-stacking interactions with Tyr123 and N1 charge stabilization by Asp203. For Cβ–Sγ bond cleavage the now positively charged amino group of Lys238 is attracted by the negative charge of the PLP phosphate group and further guided towards Sγ by interaction with Tyr64*, the carbonyl oxygen of Ala39 and one well defined active site water. After reaching an optimal position within hydrogen bonding distance to Sγ, Lys238 protonates the sulfur atom. The resulting thiol is released, which can be H2S (cysteine as a substrate), homocysteine (cystathionine as a substrate) or cysteine persulfide (cystine as a substrate). Finally, reverse transaldimination of the PLP aminoacrylate takes place. Lys238 attacks the C4′ atom, converting the aminoacrylate into an iminopropionate derivative. The reaction end product iminopropionate is released and hydrolysed to pyruvate and ammonia outside the active site.
Fig. 3. Proposed molecular reaction mechanism for cystalysin showing relevant intermediates. The PLP cofactor is coloured black, the substrate and leaving product in red and the enzyme residues in blue.
Altogether, CBL, MalY and cystalysin are catalyzing a C–S lyase reaction with basically the same organization of the active site key residues Tyr64*, Tyr123, Lys238 (CBL: Tyr56*, Tyr111, Lys210; MalY: Tyr61*, Tyr121, Lys233). However, the active site construction of these PLP enzymes results in individual substrate preferences while conservation of a similar reaction mechanism is the consequence of divergent evolution.
Mechanisms of haemolysis: role of H2S and sulfane sulfur
As is evident from the presented structure, cystalysin is not a typical pore-forming protein like the classical haemolysins. Obviously, the oral pathogen Treponema denticola has evolved a PLP-dependent enzyme, highly active as an l-cysteine Cβ–Sγ lyase, to generate a toxic substance: H2S. Moxness et al. (1996) proved recently that H2S has the ability to induce cell lysis. The toxic action of H2S is based on two effects, which may occur in combination. (i) Treatment of erythrocytes shows a depletion of phospholipids in the inner membrane leaflet relative to the outer leaflet. This causes an outward bulging of the membrane resulting in spikes and protrusions (Ferrell et al., 1985). The morphological change transforms the normally disc-shaped erythrocytes into balloon-like echinocytes. (ii) H2S is able to increase the permeability of oral mucosal tissue (Ng and Tonzetich, 1984), and seemingly also of erythrocyte membranes. An osmotic intrusion of water molecules is the most likely effect that should finally lead to disintegration of the membrane by gaps and holes, such a scenario would explain the irregularity and variability in size of the membrane lesions observed upon incubation with cystalysin (Chu et al., 1994). Furthermore, the cell adhesion and agglutinating properties of T.denticola could even enhance haemolysis. Generating elevated concentrations of H2S that can diffuse directly through the bacterial to the erythrocyte membrane the bacteria have immediate access to the leaking erythrocyte plasma upon haemolysis. Producing H2S, cystalysin is playing the key role in the mechanism of haemolysis as performed by T.denticola.
Interestingly, also the causative agent of upper respiratory tract disease, Bordetella avium, produces a PLP-dependent β-cystathionase acting as a cytotoxic protein (osteotoxin) (Gentry-Weeks et al., 1993). In osteogenic, osteosarcoma and tracheal cells it causes the formation of balloon-like structures and surfacial blebs that lead finally to plasmolysis and cell death. Similar to cystalysin this β-cystathionase is specific for l-cystathionine, l-cystine and l-cysteine derivatives, showing only weak affinity for l-cysteine (Km = 5 mM). The toxicity of this protein was attributed to the cleavage of l-cystine, for which osteotoxin shows the highest affinity (Km = 77 µM). Thompson and coworkers (Gentry-Weeks et al., 1993) propose a reaction mechanism that could also be valid for cystalysin: sulfane sulfur derivatives containing at least two covalently linked sulfur atoms like persulfide (-SSH), trisulfide (-SSSH), elemental sulfur (S8) and polysulfur (Sn) are supposed to be the chemical basis for the observed biological effects (Toohey, 1989). Sulfane sulfur products like thiocysteine and elementary sulfur are inherently unstable and have been proven to undergo rapid sulfurylation reactions with thiophilic acceptors (Gentry-Weeks et al., 1993). Thereby di- and trisulfides are formed and thiol groups in proteins are oxidized, resulting in an intermolecular transfer of sulfur atoms. The observed effects on enzyme functions can be activatory or inhibitory (Toohey, 1989). At worst, transfer of sulfane sulfur to metabolically or structurally important proteins can lead to cell death. The production of the persulfide thiocysteine and elemental sulfur could be confirmed for osteotoxin and was also observed for cystalysin (H.I.Krupka, unpublished data). Although l-cysteine is the proposed main natural substrate, there is also evidence that cleavage of l-cystine is dramatically enhancing the haemoxidizing and haemolytic activity of cystalysin (Chu et al., 1997). The incorpor ation of sulfur from the cleavage of l-[35S]cystine could be proved for osteotoxin and resulted in labelling of the enzyme itself and other cell proteins (Gentry-Weeks et al., 1993). The effects of reactive sulfane sulfur derivatives like thiocysteine on erythrocytes have been studied by Valentine et al. (1987). Upon incubation, rapid inhibition of the erythrocyte metabolism and vacuole formation was observed, mainly caused by inhibition of many glycolytic enzymes that appeared to be extremely sensitive to sulfane sulfur. Hence, reaction products resulting from cleavage of l-cystine by cystalysin could be an additional important factor in the complex process of haemolysis.
Mechanism of inhibition by l-aminoethoxyvinylglycine
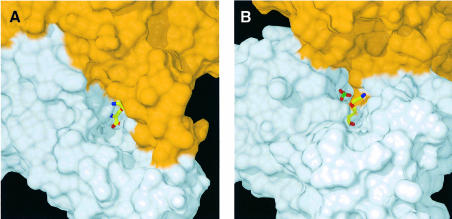
PLP-dependent enzymes catalyzing β-elimination reactions are known to be inhibited by a number of αβ-unsaturated amino acids like the natural toxins rhizobitoxin (Owens et al., 1968) and AVG (Pruess et al., 1974), which act as so-called slow-binding inhibitors (Morrison and Walsh, 1988). Interaction studies of CBL with AVG (Clausen et al., 1997) revealed the time-dependent formation of a long-lived, slowly dissociating enzyme–inhibitor complex upon treatment with AVG. Spectral and fluorescence measurements led to similar observations for cystalysin after incubation with AVG (Figure 4A). Treatment of cystalysin with AVG resulted in a rapid and complete loss of the pyridoxaldimine absorbance of the active enzyme at 419 nm while simultaneously a chromophore at 336 nm was formed. Crystallographic analysis of cystalysin crystals incubated with AVG showed clear and continuous electron density for a covalent PLP–AVG adduct (Figure 4B). The inhibitor is mainly bound by hydrogen bonds to its carboxylate oxygens, which originate from Ala39, Asn175 and Arg369 (Figure 4C). Interestingly, binding of the terminal amino group occurs only over a single hydrogen bond with a well defined water molecule. Hydrophobic interactions are formed with Tyr64* and Phe273*, which are in van der Waals contact with the carbon atoms CFI, CEI and CGI. In contrast to CBL, the amino-tail of the inhibitor is not bound to Tyr123, but is stretched out in the opposite direction towards a large hydrophobic cavity formed by the residues Leu17, Val38, Tyr64* and Phe273*, which is absent in CBL. Comparison of the substrate binding pockets indicates that cystalysin displays a remarkably larger active site cleft (30 × 20 × 15 Å) than CBL (15 × 15 × 10 Å) (Figure 5). The generous construction of the active site cleft implies high accessibility to the PLP cofactor and could grant access even for bulky substrates or inhibitors. Therefore, the design of selective inhibitors that are able to discriminate between the active sites of cystalysin and CBL seems feasible.
Fig. 4. The cystalysin–AVG complex. (A) The spectra of uncomplexed cystalysin and cystalysin after treatment with AVG, recorded at 20°C in 100 mM phosphate buffer. (B) Final 2Fo – Fc electron density of the PLP–AVG complex (yellow) and the immediate protein vicinity (colour coding by atom type), contoured at 1.2σ and calculated at 2.5 Å resolution. (C) Schematic presentation of the PLP–AVG complex (red) depicted with polar and hydrophobic interactions. Interatomic distances are given in Å.
Fig. 5. Active site morphology. Surface plots of the active site cavities of cystalysin (A) and CBL (B) both with the PLP–AVG complex in yellow (colour coding by atom type; P, green). The surface of the subunit, harbouring the complex, is coloured in grey, the neighbouring subunit in orange.
Overall, the PLP cofactor remains fixed in position with only the pyridine ring rotated slightly (<10°), while no major structural changes regarding domain movement have been observed. These findings are contradicting an open/closed mechanism upon substrate binding, since the cystalysin–AVG structure should resemble the physiological enzyme–substrate complex. Due to the similarities in inhibition pattern and spectral changes, AVG should act on cystalysin in a similar manner to that reported for CBL (Clausen et al., 1997). After transaldimination, a proton transfer from Cα to C4′ is mediated by the deprotonated ε-amino group of the released Lys238, instead of protonating the leaving group. The resulting ‘dead end’ ketimine PLP derivative is responsible for the observed 336 nm chromophore and the total loss of enzyme activity. The inability of cystalysin to catalyse a transamination of this ketimine also accounts for the high stability of the final intermediate.
The virulence potential of T.denticola is strongly dependent on the catabolism of thiol substrates to reactive hydrogen sulfide and sulfane sulfur. Thus, inhibition of cystalysin should result in complete loss of haemolytic activity (Kurzban et al., 1999). In vivo, cystalysin inhibition is likely to cause significant shortage of nutrients, especially in terms of haem as the proposed major iron source of the bacteria, resulting in limited growth and probable cell death. Secondly, missing selective advantages created by elevated H2S concentrations in the periodontal ecological niche may stop T.denticola growth. With increasing resistance of bacteria to common antibiotics cystalysin appears to be an interesting novel target for the development of specific inhibitors against the oral pathogen T.denticola. The complex between cystalysin and AVG is a first step for rational drug design of therapeutic agents as powerful weapons to fight periodontitis.
Materials and methods
Enzyme purification and crystallization
Cystalysin was expressed from T.denticola in E.coli as described previously (Chu and Holt, 1994). Briefly, E.coli DH5α transformed with plasmid pLC67 was grown in 2 l Erlenmeyer flasks with 1 l of Luria broth at 37°C. Induction was performed at an OD600 of 0.8 with 1 mM isopropyl-β-d-thiogalactopyranoside for up to 4 h. Cells were harvested by centrifugation, resuspended with 10 ml of buffer A [1× phosphate-buffered saline (PBS) pH 7.2] and stored at –70°C until further processing.
The course of purification was monitored by the cystalysin cystathionase activity (Chu et al., 1997) and by SDS–PAGE. Protein concentration was determined spectroscopically at 280 nm using the extinction coefficient of 63 440 M–1cm–1 and the molecular weight of the cystalysin monomer of 46 258 Da. After cell lysis by ultrasonic treatment and removal of cell debris by centrifugation, the enzyme was basically purified by a four-step procedure. Initially, the soluble cell fraction was submitted to an (NH4)2SO4 fractionation. Solid (NH4)2SO4 was added at 4°C to 60% saturation. The mixture was stirred for 20 min and the precipitate removed by centrifugation at 10 000 r.p.m. for 30 min. (NH4)2SO4 was added to the supernatant to a final concentration of 90%. The precipitated pellet was collected by centrifugation (10 000 r.p.m., 30 min) and dissolved in 12 ml of buffer B (25 mM Tris–HCl pH 7.5, 1 mM EDTA). The protein solution was loaded on a Sephacryl S-300 (Pharmacia; 300 ml) gel filtration column and equilibrated with buffer B. Eluted fractions were pooled, adjusted to 1.0 M (NH4)2SO4 and applied to a Phenyl Sepharose HP (Pharmacia; 80 ml) column and equilibrated with buffer B containing 1.0 M (NH4)2SO4. After washing, elution was performed with buffer B in a linear decreasing salt gradient. Cystalysin-containing fractions were pooled and concentrated with centripreps (Amicon) to 40 mg/ml. The concentrated protein solution was further purified by preparative gel electrophoresis by the method of Reuter and Wehrmeyer (1988). The acrylamide concentration in the gel was 5%. Separation was achieved in buffer C (100 mM Tris–boric acid pH 7.9) using a constant current of 56 mA, which was applied for 3.5 h at 8°C. The protein was eluted with buffer D (10 mM Tris–HCl pH 7.5), concentrated with centricons (Amicon) to 10 mg/ml and stored at –70°C. From 6 l of culture, 60 mg of homogeneous protein were obtained, which appeared to be 99% pure as deduced from SDS–PAGE.
Crystallization was carried out at 20°C using the sitting drop vapour diffusion method. After 2 days crystals were obtained from 10 mg/ml protein solution and 15% (w/v) PEG 4000, 0.2 M magnesium acetate and 100 mM MES–NaOH pH 6.5 as reservoir solution. The rhombic crystal plates of 0.6 × 0.4 × 0.1 µm3 dimensions were bright yellow, indicative of the protonated internal aldimine. They belonged to the monoclinic space group P21 with unit cell constants of a = 89.5, b = 108.4, c = 179.1 Å and β = 90.2°.
Data collection, structure solution and refinement
X-ray data were collected at the wiggler beamline BW6 at DORIS (DESY, Hamburg, Germany) on a CCD-detector (MAR Research) with synchrotron radiation at 1.07 Å. A cryostream (Oxford Cryosystems, Oxford, UK) was used and the crystal was frozen at a temperature of 100 K with 0.1 M MES–NaOH pH 6.5, 0.2 M magnesium acetate, 15% (w/v) PEG 4000 and 20% PEG 400 as cryo-protectant. One crystal was sufficient to collect 450 images in a 90° φ-scan with an exposure time of 30 s per frame. Reflection data of native cystalysin were processed to a maximum resolution of 1.9 Å. Data of the protein–inhibitor complex were collected at an MAR image plate detector equipped with a rotating CuKα anode operated at 120 mA and 50 kV. The dataset of the inhibitor complex was obtained by soaking a native cystalysin crystal for 30 min in the crystallization solution containing 20 mM AVG. Subsequently, the crystal was frozen at a temperature of 100 K (Oxford Cryosystems, Oxford, UK) and cryo-measured in 0.3° oscillation steps.
The diffraction data were processed with the DENZO program package (Otwinowski and Minor, 1996); further reduction and truncation of structure factors were performed with the CCP4 program suite (CCP4, 1994). Collection statistics for both datasets are given in Table I. The structure of the PLP protein MalY from E.coli (Clausen et al., 2000) was chosen as a model to solve the structure with Patterson search techniques using the AMoRe program (Navaza, 1994).
Table I. Statistics for data collection and refinement.
| Cystalysin | Cystalysin–AVG complex | |
|---|---|---|
| Space group | P21 | P21 |
| Cell constants | a = 89.5, b = 108.4, c = 176.1 Å, β = 90.1° | a = 89.4, b = 107.9, c = 176.2 Å, β = 90.2° |
| Resolution range (Å) | 25–1.9 | 20–2.5 |
| Reflections observed | 382 434 | 149 605 |
| Unique reflections | 254 956 | 106 861 |
| Rmergea overall (%) | 7.8 | 4.6 |
| Rmergea outermost shell (%) | 25.5 (1.97–1.90 Å) | 20.4 (2.59–2.50 Å) |
| Completeness overall (%) | 96.5 | 92.4 |
| Completeness outermost shell (%) | 87.9 (1.97–1.90 Å) | 87.0 (2.59–2.50 Å) |
| I/σ overall | 8.6 | 28.9 |
| Reflections used for refinement | 254 953 | 106 861 |
| R-factorb (%) | 20.8 | 19.3 |
| Rfree valuec (%) | 24.7 | 26.0 |
| Active atoms during refinement | ||
| protein atoms | 25 664 | 25 664 |
| cofactor atoms | 120 | 120 |
| inhibitor atoms | – | 88 |
| solvent molecules | 2627 | 1300 |
| r.m.s. deviations | ||
| bond lengths (Å) | 0.006 | 0.007 |
| bond angles (°) | 1.4 | 1.4 |
| dihedral angles (°) | 22.5 | 22.6 |
| improper angles (°) | 0.87 | 0.85 |
| Temperature factors (Å2) | ||
| all atoms | 16.9 | 30.4 |
| protein main chain atoms | 16.0 | 29.9 |
| protein side chain atoms | 17.8 | 30.9 |
| cofactor (and inhibitor) atoms | 20.6 | 39.8 |
| solvent molecules | 39.3 | 43.0 |
aRmerge = Σ|I – <I>|/ΣI
bR = Σ||Fobs| – |Fcalc||/Σ|Fobs|
cRfree was calculated with 5% of the observed reflections that were omitted from the refinement and R-value calculation.
In the partial model of dimeric MalY, the PLP cofactor was omitted and all non-identical and non-related residues were changed to alanine. The ‘cross’-rotation function yielded four solutions with a peak height of 9.5σ above the mean, clearly superior to the next solutions (7.2σ). From the translation function calculated for the resolution range 10.0–4.5 Å, the position of the first dimer was obtained (correlation coefficient 18.2%, R-factor 52.6%), and subsequently, after fixing this position, the position of three other dimers yielding a final correlation coefficient of 35.5% and an R-factor of 47.9%. Inspection of the packing revealed no steric clashes. Furthermore, this solution is in good agreement with the value for the Matthews coefficient of 2.31 Å3/Da, corresponding to a solvent content of 47%.
Initial phases for the protein–inhibitor complex were obtained by molecular replacement with the refined coordinates of native cystalysin. The data were processed likewise in the following: the initial model was refined by energy restrained rigid-body and positional refinement as well as by simulated annealing using CNS (Brünger et al., 1998) and the target parameters of Engh and Huber (1991). To improve the original 2Fo – Fc electron density map, the program AVE (Jones, 1992) was applied for 8-fold real-space averaging. The non-crystallographic symmetry (NCS) operators were extracted with LSQMAN (Kleywegt, 1996). The resulting electron density map was of excellent quality and allowed the incorporation of the complete molecular structure of cystalysin. Model building was done with the program O (Jones et al., 1991) implemented on an ESV-30 graphics station (Evans and Sutherland, Salt Lake City, UT) and on Silicon Graphics Indigo and Octane workstations. Crystallographic refinement during model building was carried out applying weak NCS restraints. After the first refinement cycle the R-factor calculated at 1.90 Å resolution dropped from 44.4% (Rfree 45.6%) to 28.4% (Rfree 31.7%). In later stages, positional and B-factor refinement were carried out omitting NCS restraints. After the addition of solvent molecules the refinement converged at a crystallographic R-factor of 20.8% (Rfree 24.7%).
Accession numbers
The atomic coordinates and structure factor amplitudes of the native and the complexed cystalysin structure have been deposited in the PDB with entry codes 1C7N and 1C7O, respectively.
Acknowledgments
Acknowledgements
The authors wish to acknowledge Wolfgang Reuter for technical assistance with native gel electrophoresis and Gleb P.Bourenkov and Hans Bartunik for excellent support at the BW6 beamline.
References
- Alexeev D., Alexeeva,M., Baxter,R.L., Campopiano,D., Webster,S.P. and Sawyer,L. (1998) The crystal structure of 8-amino-7-oxononoate synthase: a bacterial PLP-dependent Acyl-CoA-condensing enzyme. J. Mol. Biol., 284, 401–419. [DOI] [PubMed] [Google Scholar]
- Beauchamp R.O., Bus,J.S., Popp,J.A., Boreiko,C.J. and Andjelkovich,D.A. (1984) A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol., 13, 25–97. [DOI] [PubMed] [Google Scholar]
- Blankenfeldt W., Nowicki,C., Hunter,G.R., Montemartini-Kalisz,M., Kalisz,H.M. and Hecht,H.J. (1999) Crystal structure of Trypanosoma cruzi tyrosine aminotransferase: substrate specificity is influenced by cofactor binding mode. Protein Sci., 8, 2406–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünger A.T. et al. (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. [DOI] [PubMed] [Google Scholar]
- Chu L. and Holt,S.C. (1994) Purification and characterization of a 45 kDa hemolysin from Treponema denticola ATCC 35404. Microb. Pathog., 16, 197–212. [DOI] [PubMed] [Google Scholar]
- Chu L., Kennell,W. and Holt,S.C. (1994) Characterization of hemolysis and hemoxidation activities of Treponema denticola. Microb. Pathog., 16, 183–195. [DOI] [PubMed] [Google Scholar]
- Chu L., Burgum,A., Kolodrubetz,D. and Holt,S.C. (1995) The 46-kilodalton-hemolysin gene from Treponema denticola encodes a novel hemolysin homologous to aminotransferases. Infect. Immun., 63, 4448–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L., Ebersole,J.L., Kurzban,G.P. and Holt,S.C. (1997) Cystalysin, a 46-kilodalton cysteine desulfhydrase Treponema denticola, with hemolytic and hemoxidative activities. Infect. Immun., 65, 3231–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Huber,R., Laber,B., Pohlenz,H.D. and Messerschmidt,A. (1996) Crystal structure of the pyridoxal 5′-phosphate dependent cystathionine β-lyase from Escherichia coli at 1.83 Å. J. Mol. Biol., 262, 202–224. [DOI] [PubMed] [Google Scholar]
- Clausen T., Huber,R., Messerschmidt,A., Pohlenz,H.D. and Laber,B. (1997) Slow-binding inhibition of Escherichia coli cystathionine β-lyase by l-aminoethoxyvinylglycine: a kinetic and X-ray study. Biochemistry, 36, 12633–12643. [DOI] [PubMed] [Google Scholar]
- Clausen T. et al. (2000) X-ray structure of MalY from Escherichia coli: a pyridoxal 5′-phosphate-dependent enzyme acting as a modulator in mal gene expression. EMBO J., 19, 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Computational Project Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D, 50, 760–763. [DOI] [PubMed] [Google Scholar]
- Delepelaire P. and Wandersman,C. (1990) Protein secretion in Gram-negative bacteria. The extracellular metalloprotease B from Erwinia chrysanthemi contains a C-terminal secretion signal analogous to that of Escherichia coli α hemolysin. J. Biol. Chem., 265, 17118–17125. [PubMed] [Google Scholar]
- Dwivedi C.M., Ragin,R.C. and Uren,J.R. (1982) Cloning, purification and characterization of β-cystathionase from E.coli. Biochemistry, 21, 3064–3069. [DOI] [PubMed] [Google Scholar]
- Engh R.A. and Huber,R. (1991) Accurate bond and angle parameters for X-ray protein-structure refinement. Acta Crystallogr. A, 47, 392–400. [Google Scholar]
- Ferrell J.E. Jr, Lee,K.J. and Huestis,W.H. (1985) Membrane bilayer balance and erythrocyte shape: a quantitative assessment. Biochemistry, 24, 2849–2857. [DOI] [PubMed] [Google Scholar]
- Frischman D. and Argos,P. (1995) Knowledge-based secondary structure assignment. Proteins, 23, 566–579. [DOI] [PubMed] [Google Scholar]
- Gentry-Weeks C.R., Keith,J.M. and Thompson,J. (1993) Toxicity of Bordetella avium β-cystathionase toward MC3T3-E1 osteogenic cells. J. Biol. Chem., 268, 7298–7314. [PubMed] [Google Scholar]
- Hayashi H. and Kagamiyama,H. (1997) Transient-state kinetics of the reaction of aspartate aminotransferase with aspartate at low pH reveals dual routes in the enzyme–substrate association process. Biochemistry, 36, 13558–13569. [DOI] [PubMed] [Google Scholar]
- Holm L. and Sander,C. (1993) Protein structure comparison by alignment of distance matrices. J. Mol. Biol., 233, 123–138. [DOI] [PubMed] [Google Scholar]
- Jansonius J.N. (1998) Structure, evolution and action of vitamin B6-dependent enzymes. Curr. Opin. Struct. Biol., 8, 759–769. [DOI] [PubMed] [Google Scholar]
- Jones S. and Thornton,J.M. (1996) Principles of protein–protein interactions derived from structural studies. Proc. Natl Acad. Sci. USA, 93, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.A. (1992) a, yaap, asap, @**? A set of averaging programs. In Dodson,E.F., Gover,S. and Wolf,W. (eds), Molecular Replacement (CCP4). SERC Daresbury Laboratory, Warrington, UK, pp. 92–105. [Google Scholar]
- Jones T.A., Zou,J.Y., Cowan,S.W. and Kjeldgaard,M. (1991) Improved methods for building protein models in electron density maps and location of errors in these models. Acta Crystallogr. A, 47, 110–119. [DOI] [PubMed] [Google Scholar]
- Kabsch W. and Sander,C. (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen bonded and geometrical features. Biopolymers, 22, 2577–2637. [DOI] [PubMed] [Google Scholar]
- Käck H., Sandmark,J., Gibson,K., Schneider,G. and Lindqvist,Y. (1999) Crystal structure of diaminopelargonic acid synthase: evolutionary relationships between pyridoxal 5′-phosphate-dependent enzymes. J. Mol. Biol., 291, 857–876. [DOI] [PubMed] [Google Scholar]
- Kleywegt G.T. (1996) Use of non-crystallographic symmetry in protein structure refinement. Acta Crystallogr. D, 52, 842–857. [DOI] [PubMed] [Google Scholar]
- Kurzban G.P., Chu,L., Ebersole,J.L. and Holt,S.C. (1999) Sulfhemoglobin formation in human erythrocytes by cystalysin, an l-cysteine desulfhydrase form Treponema denticola. Oral Microbiol. Immunol., 14, 153–164. [DOI] [PubMed] [Google Scholar]
- Laskowski R.A., MacArthur,M.W., Mass,S.D. and Thornton,J.M. (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr., 26, 283–291. [Google Scholar]
- Leibrecht I. and Kessler,D. (1997) A novel l-cysteine/cystine C–S-lyase directing [2Fe–2S] cluster formation of Synechocystis ferredoxin. J. Biol. Chem., 272, 10442–10447. [DOI] [PubMed] [Google Scholar]
- Luzzatti V. (1952) Traitement statistique des erreurs das la détermination des structures cristallines. Acta Crystallogr. A, 5, 802–810. [Google Scholar]
- Mehta P.K., Hale,T.I. and Christen,P. (1989) Evolutionary relationships among aminotransferases. Eur. J. Biochem., 186, 249–253. [DOI] [PubMed] [Google Scholar]
- Mehta P.K., Hale,T.I. and Christen,P. (1993) Aminotransferases: demonstration of homology and division into evolutionary subgroups. Eur. J. Biochem., 214, 549–561. [DOI] [PubMed] [Google Scholar]
- Morrison J.F. and Walsh,C.T. (1988) The behavior and significance of slow-binding inhibitors. Adv. Enzymol. Relat. Areas Mol. Biol., 61, 201–301. [DOI] [PubMed] [Google Scholar]
- Moxness M.S., Brunauer,L.S. and Huestis,W.H. (1996) Hemoglobin oxidation products extract phospholipids from the membrane of human erythrocytes. Biochemistry, 35, 7181–7187. [DOI] [PubMed] [Google Scholar]
- Nakai T., Okada,K., Akutsu,S., Miyahara,I., Kawaguchi,S., Kato,R., Kuramitsu,S. and Hirotsu,K. (1999) Structure of Thermus thermophilus Hb8 aspartate aminotransferase and its complex with maleate. Biochemistry, 38, 2413–2424. [DOI] [PubMed] [Google Scholar]
- Navaza J. (1994) AMoRe: an automated package for molecular replacement. Acta Crystallogr. A, 50, 157–163. [Google Scholar]
- Ng W. and Tonzetich,J. (1984) Effect of hydrogen sulfide and methyl mercaptan on the permeability of oral mucosa. J. Dent. Res., 63, 994–997. [DOI] [PubMed] [Google Scholar]
- Okamoto A., Kato,R., Masui,R., Yamagishi,A., Oshima,T. and Kuramitsu,S. (1996) An aspartate aminotransferase from an extremely thermophilic bacterium, Thermus thermophilus HB8. J. Biochem., 119, 135–144. [DOI] [PubMed] [Google Scholar]
- Oliver D. (1985) Protein secretion in Escherichia coli. Annu. Rev. Microbiol., 39, 615–648. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z. and Minor,W. (1996) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol., 276, 307–326. [DOI] [PubMed] [Google Scholar]
- Owens L.D., Guggenheim,S. and Hilton,J.L. (1968) Rhizobium synthesized phytotoxin: an inhibitor of β-cystathionase in Salmonella typhimurium. Biochim. Biophys. Acta, 158, 219–225. [DOI] [PubMed] [Google Scholar]
- Persson S., Edlund,M.B., Claesson,R. and Carlsson,J. (1990) The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol. Immunol., 5, 195–201. [DOI] [PubMed] [Google Scholar]
- Pruess D.L., Scannell,J.P., Kellet,M., Ax,H.A., Janecek,J., Williams,T.H., Stempel,A. and Berger,J. (1974) Antimetabolites produced by microorganisms. X. l-2-amino-4-(2-aminoethoxy)-trans-3-butenoic acid. J. Antibiot., 27, 229–233. [DOI] [PubMed] [Google Scholar]
- Ramachandran G.N. and Sasisekharan,V. (1968) Conformation of polypeptides and proteins. Adv. Protein Chem., 23, 283–437. [DOI] [PubMed] [Google Scholar]
- Reuter W. and Wehrmeyer,W. (1988) Core substructure in Mastigocladus laminosus phycobilisomes I. Microheterogeneity in two of three allophycocyanin core complexes. Arch. Microbiol., 150, 534–540. [Google Scholar]
- Rhee S., Silva,M.M., Hyde,C.C., Rogers,P.H., Metzler,C.M., Metzler,D.E. and Arnone,A. (1997) Refinement and comparisons of the crystal structures of pig cytosolic aspartate aminotransferase and its complex with 2-methylaspartate. J. Biol. Chem., 272, 17293–17302. [PubMed] [Google Scholar]
- Song L., Hobaugh,M.R., Shustak,C., Cheley,S., Bayley,H. and Gouaux,J.E. (1996) Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science, 274, 1859–1866. [DOI] [PubMed] [Google Scholar]
- Sung M., Tanizawa,K., Tanaka,H., Kuramitsu,S., Kagamiyama,H., Hirotsu,K., Higuchi,T. and Soda,K. (1991) Thermostable aspartate aminotransferase from a thermophilic Bacillus sp gene. Cloning sequence determination and preliminary X-ray characterization. J. Biol. Chem., 266, 2567–2572. [PubMed] [Google Scholar]
- Toohey J.I. (1989) Sulfane sulfur in biological systems: a possible regulatory role. Biochem. J., 264, 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine W.N., Toohey,J.I., Paglia,D.E., Nakatani,M. and Brockway,R.A. (1987) Modification of erythrocyte enzyme activities by persulfides and methanethiol: possible regulatory role. Proc. Natl Acad. Sci. USA, 84, 1394–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R.J. and Rastogi,V.K. (1993) Cloning and nucleotide sequencing of Rhizobium meliloti aminotransferase genes: an aspartate aminotransferase required for symbiotic nitrogen fixation is atypical. J. Bacteriol., 175, 1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T., Kuramitsu,S., Tanase,S., Morino,Y. and Kagamiyama,H. (1992) Role of Asp222 in the catalytic mechanism of Escherichia coli aspartate aminotransferase. Biochemistry, 25, 5878–5887. [DOI] [PubMed] [Google Scholar]
- Zdych E., Peist,R., Reidl,J. and Boos,W. (1995) MalY of Escherichia coli is an enzyme with the activity of a β-C–S lyase (cystathionase). J. Bacteriol., 177, 5035–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]