Figure 2.
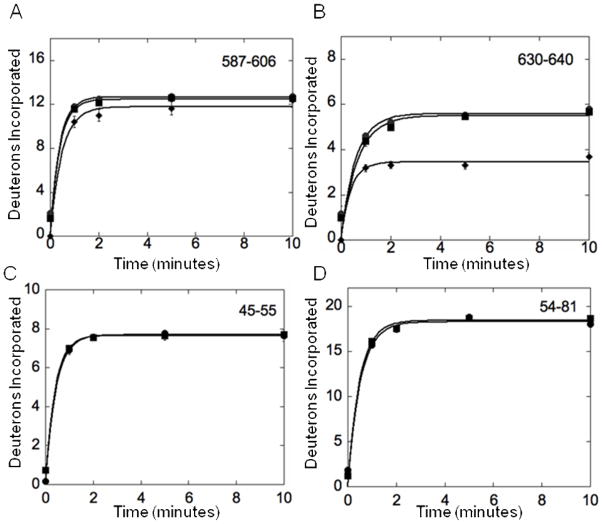
Plots of the amide H/D exchange of Fe65. (A) Deuterons incorporated over 10 minutes for Fe65 1-662 (circles), Fe65 236-662 (squares), and Fe65 236-662 with AICD bound (diamonds). Residues 587-606 of the PID2 domain are depicted and show the same solvent accessibility with or without the N-terminal domain. Fe65 236-662 with AICD bound also showed the same solvent accessibility since this region is not in the Fe65:AICD binding interface. (B) Deuterons incorporated over 10 minutes for Fe65 1-662 (circles), Fe65 236-662 (squares), and Fe65 236-662 with AICD bound (diamonds). Residues 630-640 of the PID2 domain are depicted and show the same solvent accessibility with or without the presence of the N-terminal domain. Fe65 236-662 with AICD bound showed decreased solvent accessibility for this region because it is in the binding interface. (C) Plots of the amide H/D exchange of Fe65 236-662 (circles) and Fe65 236-512 (squares) over 10 minutes. Residues 45-55 of the WW domain are depicted and showed the same solvent accessibility with or without the presence of the PID2 domain. (D) Plots of the amide H/D exchange of Fe65 236-662 (circles) and Fe65 236-512 (squares) over 10 minutes. Residues 54-81 of the WW domain are depicted and showed the same solvent accessibility with or without the presence of the PID2 domain.