Abstract
Adolescent developments in limbic structures and the endogenous cannabinoid system suggest that teenagers may be more vulnerable to the negative consequences of marijuana use. This study examined the relationships between amygdala volume and internalizing symptoms in teenaged chronic marijuana users. Participants were 35 marijuana users and 47 controls ages 16–19 years. Exclusions included psychiatric (e.g., mood and anxiety) or neurologic disorders. Substance use, internalizing (anxiety/depression) symptoms and brain scans were collected after 28 days of monitored abstinence. Reliable raters manually traced amygdala and intracranial volumes on high-resolution magnetic resonance images. Female marijuana users had larger right amygdala volumes and more internalizing symptoms than female controls, after covarying head size, alcohol, nicotine and other substance use (p<0.05), while male users had similar volumes as male controls. For female controls and males, worse mood/anxiety was linked to smaller right amygdala volume (p<0.05), whereas more internalizing problems was associated with bigger right amygdala in female marijuana users. Gender interactions may reflect marijuana-related interruptions to sex-specific neuromaturational processes and staging. Subtle amygdala development abnormalities may underlie particular vulnerabilities to sub-diagnostic depression and anxiety in teenage female marijuana users.
Keywords: Adolescence, Anxiety, Depression, Development, Gender, Marijuana, Structural MRI
1. Introduction
With more than 20% of high school seniors reporting recent use [1], marijuana is the most frequently used illicit substance among adolescents. Despite its prevalence, the impact of marijuana use on adolescent brain development is not fully known. Important neuromaturational processes such as synaptic refinement and myelination occur during adolescent years and continue into young adulthood [2]. In light of ongoing structural brain changes as well as concurrent developments in the endogenous cannabinoid system [3, 4], the potential for neurobehavioral consequences of teenage marijuana use is of great interest.
Teenage marijuana use is linked to developing psychopathology, especially in syndromes of emotion dysregulation like mood and anxiety disorders [5]. Furthermore, the effects of marijuana may mimic symptoms of depression such as anhedonia, psychomotor retardation and concentration difficulties [6, 7]. It is probable that these pharmaco-behavioral relationships may be explained by alterations in emotional neurocircuitry that is still developing during teen years; however, the underlying neural link between adolescent marijuana use and internalizing symptoms is not yet known.
Human and animal studies have indicated that limbic brain regions may be particularly vulnerable to chronic marijuana use [8, 9]. Autoradiographic studies have shown high cannabinoid receptor (CB) densities in the amygdala [10, 11], which play a role in affective processes [12]. Specifically, animal research suggests that cannabinoid signaling alters amygdala functioning by facilitating long-term depression of GABAergic interneurons within the amygdala’s basolateral nucleus [13, 14]. Furthermore, rodent models report peak CB binding in adolescent pups [15], suggesting that teens may be more susceptible to disruptions in cannabinoid signaling. For example, mice lacking the expression of cannabinoid receptors (CB1) exhibit more depressive- and anxiety-like behaviors [16, 17] as do genetically intact rodents after administering a CB1 antagonist or following chronic exposure to cannabinoid agonists [18, 19]. Therefore, exogenous cannabinoid exposure during adolescence may alter CB rich brain structures, like the amygdala, resulting in increased risk for mood dysregulation and related psychopathology [20, 21].
Functional magnetic resonance imaging (fMRI) studies in humans have also documented the role of the cannabinoid system in amygdala functioning. Hariri et al. [22] found that enhanced endocannabinoid signaling was associated with decreased threat-related amygdala reactivity. Other studies evidence reduced amygdala reactivity during affective processing tasks with increased marijuana use over time as well as after a period of abstinence [23, 24]. However, the authors of these studies reported on experimental conditions (e.g., angry faces, threat stimuli) contrasted to neutral control trials, so it is possible that less task-related response resulted from greater baseline amygdala activity. Indeed, acute cannabinoid administration to non-using adults is linked to attenuated amygdala reactivity during intoxication but exaggerated amygdala activation following those acute effects [25].
Chronic marijuana use may lead to structural changes in the amygdala through neurotoxic influence [26]. To date, there is only one volumetric study of adult marijuana users, which demonstrated smaller amygdala volumes compared to controls [27]. Amygdala asymmetry is often observed in healthy adults with typically larger right versus left (R>L) volumes [28], although some studies have found minimal to no asymmetry [29, 30]. Asymmetry specific to amygdala volumes has not been confirmed in animal models [31] and it has not been examined in developing animals. Typical amygdala asymmetry is theorized to distinguish functional differences in emotional processing across both hemispheres [32, 33] and abnormal asymmetry (including exaggerated R>L, L>R, and symmetry) has been associated with multiple pathological conditions including Alzheimer’s disease, schizophrenia, depression, obsessive-compulsive disorder and epilepsy [34–38]. In consideration of ongoing brain developments, results from adult samples may not extend to adolescents. Human adolescent studies document aberrant structural morphometry among brain areas that are associated with the cognitive sequelae of marijuana [39]. Irregularities are observed in the prefrontal cortex, hippocampus and cerebellar vermis as well as white matter organization in teenage marijuana users compared to non-using peers, and these abnormalities were associated with poorer neurobehavioral outcomes [40–44].
Gender may represent a differential risk factor for acquiring psychopathology. There is evidence which suggests female adolescent substance users experience negative consequences of drug use earlier than male peers and are more likely to suffer from an internalizing disorder whereas male substance abusers have more externalizing behaviors [45]. The neurocognitive bases for gender-related risk factors for marijuana use and drug use consequences remain relatively unclear. Adolescent girls and boys also differ in the timing of neuromaturational sequences, hormone exposure and neuronal organization [46, 47], thus, male and female teens may be susceptible to gender-specific brain changes resulting from chronic marijuana exposure. In addition, female teens exhibit elevated CB desensitization relative to same-aged males as well as adult men and women [48], suggesting that the endocannabinoid may have a stronger regulatory role in girls. Therefore, marijuana-related brain changes may differ by gender through interactions with adolescent neuromaturational processes.
The current study represents a cross-sectional comparison of amygdala morphometry between adolescent marijuana users and non-drug using controls. Given previous findings of bigger prefrontal cortex and cerebellar volumes in adolescent marijuana users in a different sample of teens [42, 43], we hypothesized that marijuana users will have larger amygdala volumes than will non-drug using controls. As a secondary aim, we examined whether gender moderates the effects of marijuana on amygdala volume. Follow-up analyses assessed the hypothesis that brain-behavior relationships between amygdala morphology and internalizing symptoms [49] differ between marijuana users relative to control teens.
2. Material and Methods
2.1 Participants
Participants were 35 chronic marijuana users (23% female) and 47 demographically similar non-using peers (23% female) recruited from local schools [50]. Teens were eligible if they were ages 16–18 at the time of study enrollment, right-handed and had parent/guardian consent. Each teen and one guardian completed informed assent and consent in accordance with the UCSD Human Research Protections Program. Exclusionary criteria included histories of psychotropic medication use, Axis I psychiatric disorder (other than cannabis abuse or dependence), neurologic problems, prenatal substance exposure, and birth complications. To optimize detection of marijuana-related effects, participants were excluded if criteria were met for abuse or dependence of substances other than marijuana, and for other illicit drug use exceeding 30 lifetime episodes. MRI contraindications (irremovable metal, pregnancy, and claustrophobia) were also exclusionary.
2.2. Measures
2.2.1. Demography, Background, and Mental Health
A semi-structured clinical interview collected eligibility and demographic information from each youth and one primary caregiver, separately, covering socioeconomic status [Hollinghead Index; 51], household income and familial history of substance use disorders [SUD; 52]. Parent interviews assessed the teen’s prenatal and medical histories. The Computerized Diagnostic Interview Schedule for Children [C-DISC; 53] was administered by trained research assistants to youth and parent for screening DSM-IV Axis-I disorders [54].
2.2.2. Verbal Ability
Verbal abilities were estimated using the Wide Range Achievement Test-3 Reading [55] and the Wechsler Abbreviated Scale of Intelligence Vocabulary [56] subtests.
2.2.3. Substance Use
The Customary Drinking and Drug Use Record (CDDR) assessed lifetime and past 3-month marijuana, alcohol, nicotine, and other drug use, withdrawal symptoms, DSM-IV abuse and dependence criteria [54], and substance-related life problems [57]. Fagerström Test of Nicotine Dependence assessed nicotine dependence [58].
2.2.4. Internalizing Symptoms
Measures of internalizing symptoms, collected at the MRI session, were the Beck Depression Inventory [BDI; 59], Hamilton Depression and Anxiety Rating Scales [60, 61], and Spielberger State-Trait Anxiety Inventory [STAI; 62] state anxiety scale (STAI-S). Given that any mood or anxiety disorder diagnosis was exclusionary for both marijuana using and control teens, measures of depression and anxiety in this sample were in the “normal” range. Therefore, standardized z-scores based on the overall sample were computed for the BDI, HAM-D and HAM-A, and age-normalized STAI-S T-scores were transformed to z-scores. In the interest of data reduction, a composite scale was computed as the average z-score from these four measures (Cronbach’s alpha = 0.81). This approach is further supported by pediatric literature, which describes less distinction between diagnostic classes of anxiety and depressive disorders in children and adolescents [63, 64], suggesting that a unified construct of internalization better accounts for these symptoms in young samples.
2.3 Procedures
Eligibility was determined by confidential interviews with teens and one primary caregiver (who consented to this separate interview). Eligible participants enrolled in a 28-day abstinence period that was monitored with semi-weekly urine toxicology screens (measuring metabolites indicating recent use of cannabis, amphetamines, barbiturates, benzodiazepines, cocaine, codeine, morphine, phencyclidine, and ethanol using cloned enzyme donor immunoassay; Microgenics, Fremont, CA) and breathalyzer (AlcoSensor IV Intoximeter, St. Louis, MO). In the event that biological tests indicated drug use during the abstinence period, participants were offered one opportunity to restart the study. Youths with descending marijuana metabolite levels were scheduled for an MRI session, at which teens were administered mood, anxiety and substance use assessments (day 28). Participants were imaged on a 3-Tesla General Electric scanner (Medical System, Milwaukee, WI). High-resolution anatomical images of the entire brain were collected with a 3D T1-weighted spoiled gradient recalled acquisition (TR = 8 ms, TE = 3 ms, flip angle = 12º, field of view = 240 mm, 176 continuous slices, slice thickness = 1 mm, in-plane resolution = 1 × 1 mm, acquisition time 7:19).
2.4. MRI Data Processing
T1 anatomical images were reconstructed using Analysis of Functional NeuroImages [AFNI; 65]. Trained research assistants inspected the anatomical images for motion artifact and quality. Brains with structural abnormalities that were confirmed by a neuroradiologist were excluded (n = 1). Manual tracings of the left and right amygdalas and the intracranial vault (ICV) were performed by reliable raters (ICCs>0.86; TM, CBP, JP, PL) blind to participant characteristics using a standard protocol [adapted by TM based on 49, 66]. To establish inter-rater reliability, each tracer labeled the amygdala and intracranial volumes of an independent subset of anatomical images. Tracings were defined on high-resolution grayscale images in AC-PC alignment on contiguous coronal slices. The boundaries were as follows: anterior–first coronal slice in which the temporal stalk merges with the white matter of the insula; dorsal–entorhinal sulcus separating the basal forebrain and temporal lobe; posterior–head of the hippocampus as evident in sagittal view; ventral–horizontal boundary extending from the anterior and ventral hippocampal edge; medial–presence of the subarachnoid space; and lateral–surrounding white matter (see Figure 1). Amygdala volumes were calculated by extracting the number of voxels within the defined region then multiplied by the voxel dimensions. ICVs were created by an automated watershed program that uses a deformable surface-based algorithm to produce a brain-only mask [67], which were then inspected and edited by reliable raters (ICC>0.98), and volumes were computed to account for individual variability in head size. For bivariate analyses, amygdala volumes were assessed as proportions to intracranial volume (R/ICV, L/ICV). Amygdala asymmetry was expressed as the relative interhemispheric volume difference [(R−L)/(R+L)] such that positive values correspond with R>L asymmetry.
Figure 1.
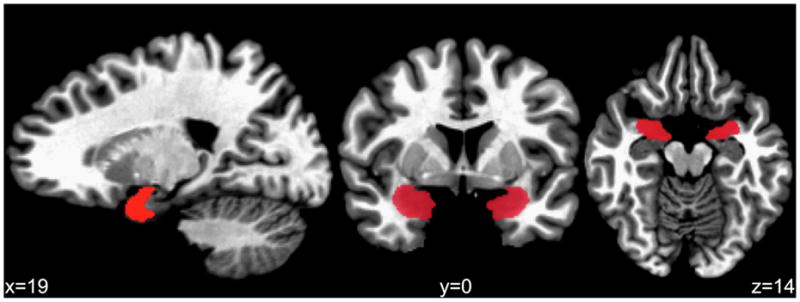
Manually traced amygdala region of interest.
2.5. Data Analysis
Distribution normality of dependent variables was examined using Kolmogorov-Smirnov tests with confirmatory visual inspection. Non-normal variables (i.e., internalizing composite) were logarithmic transformed. Analyses of variance (ANOVAs), Mann-Whitney U and chi-square tests compared groups on important demographic and drug use variables. Pearson’s correlations were computed to compare bivariate relationships between amygdala volumes (R/ICV and L/ICV) and internalizing measures (BDI, STAI-S, HAM-D, HAM-A and mood/anxiety composite). Three series of multiple regressions were run to model asymmetry, right and left amygdala volumes after controlling for ICV, alcohol use (lifetime drinking occasions), nicotine (Fagerström Test of Nicotine Dependence), and other drug use (lifetime episodes of any drug other than alcohol, nicotine or marijuana). Main effects for marijuana use status and gender, and their centered interaction were entered as predictors. As a follow-up, regressions assessed whether gender, group, amygdala volumes, or their centered interactions were related to internalizing symptoms after controlling for ICV. Normal probability plots of standardized residuals for each regression were used to verify analysis assumptions and to detect possible outliers. Data analyses were performed using SPSS (SPSS for Mac, Rel. 17.0.1. 2008. Chicago: SPSS Inc.) and statistical decisions were made if p<.05.
3. Results
3.1. Sample Characteristics
ANOVAs, Mann-Whitney U, and chi-square tests examined whether background characteristics differed between group status and gender (see Table 1). Marijuana users (n = 35) and controls (n = 47) were statistically similar in gender and ethnic distribution, age, socioeconomic status, familial SUD, and verbal abilities, and these characteristics were similar across genders. Marijuana users reported 446 lifetime marijuana episodes (range 180 to 1,800 occasions), had been smoking marijuana for about 3 years prior to participation, and had used approximately 12 occasions in the past month with around 10 hits per episode. Male (81%) and female (50%) marijuana users did not differ in terms of the proportion of individuals who met criteria for cannabis abuse or dependence (p>0.05). Marijuana users reported more lifetime (p<0.01) and past 3-month alcohol drinking occasions (p<0.01). On average, marijuana users smoked cigarettes 6 days per month, with two users reporting daily use (10 and 20 cigarettes per day); no participant met criteria for nicotine dependence (Fagerström range = 0–3 of a maximum possible = 10). Lifetime uses of other drugs were more (p<0.01) in users (range = 0–27) than in controls (range = 0–13). ICVs were larger for boys than girls (p<0.01), but did not differ by group.
Table 1.
Demographic, substance use, and morphometry measures by marijuana use group and gender
| Female Controls (n = 11) | Male Controls (n = 36) | Female Users (n = 8) | Male Users (n = 27) | |
|---|---|---|---|---|
| Ethnicity (% Caucasian) | 36% | 40% | 25% | 48% |
| Family history of SUD (% positive) | 9% | 17% | 57% | 26% |
| Cannabis abuse/dependence (% positive) | 0% | 0% | 50% | 81% |
|
|
||||
| M ± SD | M ± SD | M ± SD | M ± SD | |
|
| ||||
| Age | 17.85 ± 0.73 | 17.65 ± 0.90 | 18.15 ± 0.86 | 17.92 ± 0.91 |
| Hollingshead Index | 26.73 ± 15.56 | 33.22 ± 16.92 | 30.25 ± 13.35 | 25.37 ± 14.25 |
| Vocabulary T-score | 58.82 ± 9.91 | 59.89 ± 8.76 | 58.63 ± 8.35 | 57.70 ± 8.98 |
| Reading standard score | 109.64 ± 5.63 | 110.06 ± 7.49 | 106.38 ± 9.09 | 106.74 ± 8.55 |
| Internalizing composite z-scorea | −0.43 ± 0.58 | −0.47± 0.69 | 0.51 ± 1.25 | −0.33 ± 0.50 |
| Lifetime marijuana use episodesa | 0.36 ± 0.81 | 1.58 ± 2.56 | 397.00 ± 243.03 | 460.07 ± 391.36 |
| Lifetime alcohol use episodesa | 26.60 ± 61.24 | 21.03 ± 27.53 | 239.38 ± 211.15 | 185.70 ± 138.93 |
| Lifetime other drugs use episodesa | 0.18 ± 0.60 | 0.47 ± 2.20 | 8.13 ± 6.10 | 4.48 ± 7.11 |
| Fagerström Test for Nicotine Dependencea | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.30 ± 0.78 |
| Intracranial volume (cc)b | 1,512.61 ± 160.00 | 1,616.23 ± 131.51 | 1,472.83 ± 82.22 | 1,617.80 ± 123.75 |
| Right amygdala (cc) b | 2.03 ± 0.19 | 2.38 ± 0.33 | 2.20 ± 0.29 | 2.30 ± 0.33 |
| Left amygdala (cc) b | 1.84 ± 0.23 | 2.04 ± 0.32 | 1.92 ± 0.20 | 2.06 ± 0.30 |
Marijuana users > Controls, p<0.05;
Males > Females, p<0.05
3.2. Amygdala Morphometry by Marijuana Status and Gender
Multiple regression analyses determined whether right or left amygdala volumes were associated with marijuana use status, gender, or their interaction after controlling for ICV, alcohol, nicotine and other drug use. In the absence of main effects for group and gender, significant interactions between group and gender were observed in modeling right amygdala volume (R2Δ = 0.05, β = −0.22, p = 0.03). Plots of amygdala volumes show that female marijuana users had significantly larger volumes than female controls, while male users and controls had similar volumes (see Figure 2). Neither asymmetry nor left amygdala volume were predicted by marijuana use group, gender, or their interaction (p’s>0.05).
Figure 2.
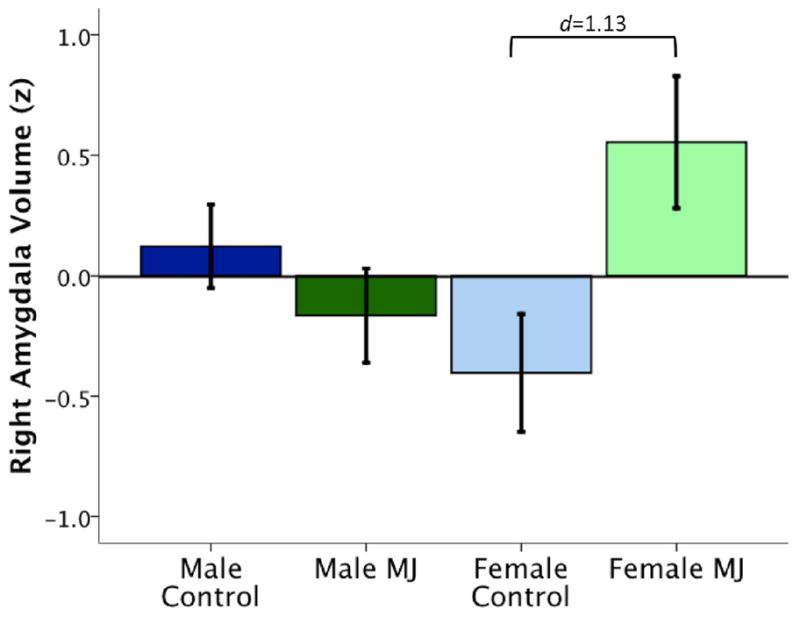
Gender moderates the relationship between right amygdala volume and marijuana user status, controlling for intracranial space, alcohol, nicotine and other drug use. Female users show significantly larger amygdala volumes than males and female non-using controls. Amygdala volume expressed as z-score ± 2 SD.
To determine whether results from the primary analysis were mediated by internalizing symptoms or family history of SUD, models of right amygdala volumes were re-run adding internalizing scores and familial SUD status as covariates, separately. Results showed no significant relationship with internalizing nor did it change the original findings (p = 0.36). No effect for family history of SUD was detected nor did it mediate the previously described effects (p = 0.72). Greater other drug use predicted smaller right amygdala volumes (R2Δ= 0.04, β= −0.23, p=0.05), above and beyond ICV, alcohol, group, gender, and group*gender interaction.
3.3. Follow-up Analyses
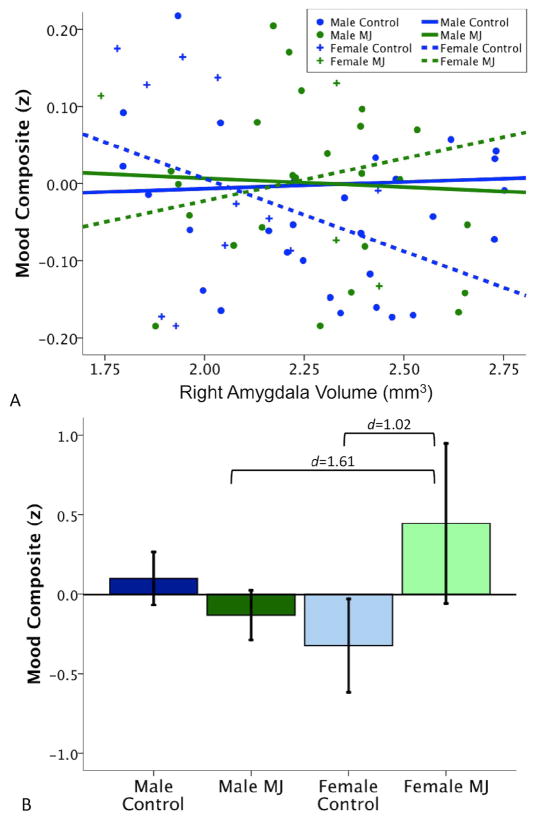
To help elucidate brain-behavior relationships linked to amygdala morphometry, multiple regressions determined how group, gender, amygdala volume (right, left or asymmetry) and centered second-order interactions were related to internalizing composite scores. Right amygdala volume (R2Δ= 0.05, β= −0.27, p=0.03), group (R2Δ= 0.07, β= 0.28, p= 0.01) and group*gender interaction (R2Δ= 0.05, β= −0.24, p=0.04) independently accounted for significant variability in mood and anxiety symptoms (see Figure 3). Specifically, smaller right amygdalas were linked to worse mood and anxiety symptomatology in the whole sample. Plotting the relationship between right amygdala volume versus internalizing symptom scores revealed that the inverse relationship between amygdala and mood/anxiety was driven by female controls and males (see Figure 3). In contrast, female marijuana users show the opposite brain-behavior pattern: larger amygdala volumes were linked to worse internalization scores. Independent of amygdala morphology, marijuana users had higher internalizing composite scores, and female marijuana users endorsed the greatest depression and anxiety symptoms. No effect for gender (p=0.62) or interactions involving amygdala volume (right amygdala*gender, p=0.18; right amygdala*group, p=0.55) were detected. Internalizing symptomatology was not linked to left amygdala volume (p=0.41) or its interactions with gender (p=0.68) and group (p=0.48). Asymmetry marginally predicted internalizing symptoms (R2Δ= 0.04, β= −0.22, p= 0.07) but not interactions with gender (p = 0.21) and group (p = 0.40).
Figure 3.
(A) Internalizing problems were linked to smaller right amygdala volume in controls and male marijuana users, but worse internalizing was associated with larger amygdala volumes in female marijuana users; (B) group*gender interaction showing higher internalizing symptoms in female users (amygdala volume expressed as z-score ± 2 SD).
Bivariate correlations were computed between amygdala volumes and internalizing measures. ICV-adjusted right amygdala volumes marginally correlated with internalizing composite scores (r=−0.19, p=0.09) but no significant links were observed with individual mood or anxiety measures (p’s>0.20). ICV-adjusted left amygdala volumes were not associated with any internalizing symptom measures (p’s>0.29). No individual depression/anxiety measure exhibited a disproportionate link with amygdala volumes.
4. Discussion
Converging lines of research suggest that adolescence represents a period of increased vulnerability to neurobehavioral consequences of marijuana use [68] and the amygdala may be a site that is particularly sensitive to marijuana exposure [8, 9]. The primary purpose of the current study was to compare amygdala volumes among adolescent marijuana users and healthy control teens. We found that female marijuana users exhibited larger right amygdala volumes relative to males and female controls. Among female marijuana using teens, larger amygdala sizes were associated with increased depression and anxiety symptomatology. As a group, marijuana using teens, especially females, endorsed greater internalizing symptoms. No relationships between left amygdala and marijuana use or internalizing scores were observed, and, although a marginal link between asymmetry and internalizing scores was observed, the relationship was more robust for right amygdala volumes.
These findings are consistent with our laboratory’s previous studies on different samples of marijuana using teens [43, 44]. For example, Medina and colleagues [42] reported larger prefrontal cortex volumes in female marijuana using adolescents, and larger volumes were linked to poorer executive function. Subclinical depression symptoms have also been reported in conjunction with reduced white matter volumes in teenage marijuana use [44], which reflects a similar pattern of neurobehavioral links with internalizing problems as in the current study. Our findings also align with previous literature connecting depression and anxiety to smaller amygdala size in both clinical samples [49, 66, 69] and in normal healthy teens [70]. However, one study of adult marijuana users found smaller amygdala volumes [27] and evidence for altered amygdala morphometry in depressed samples is mixed [72]. If replicated, these results carry important public health implications as interventions targeting teenage marijuana use may mediate risks for developing substance use disorders and comorbid internalizing problems.
Discrepancies between adult and adolescent structural MRI findings could be related to the disruption of neuromaturation by early chronic marijuana use. Brain size increases through adolescence with decreasing volumes observed over the course of adulthood [46]. Exogenous cannabinoid administration is associated with altered astrocyte functioning, and astrocytes play a critical role in eliminating weaker connections and maintaining neuronal health [73, 74]. By interfering with these support processes, marijuana exposure during adolescence may impair typical pruning, which could ultimately result in larger regional brain volumes. Chronic exposure to marijuana may also compromise neurogenesis, as reduced levels of nerve growth factor and brain derived neurotrophic factor have also been reported among adult marijuana users [75, 76], and this effect may have a greater impact after the pruning stage is primarily complete. Taken together, marijuana use may impact brain development such that interrupted pruning (i.e., maturational delay) is followed by accelerated aging (reduced neurogenesis). Therefore, larger right amygdala volumes could be expected among adolescent marijuana users, whereas continued exposure through adulthood may be related to smaller volumes eventually [47].
The present study’s primary finding of gender moderating the relationship between amygdala volume and marijuana use may reflect neurodevelopmental differences between boys and girls. Our finding of similar amygdala volumes among male users and controls could be based on boys initiating marijuana use at a different developmental stage than girls. For example, male and female users in our sample began using marijuana at similar ages (M=14.19, 14.25 years of age, respectively); however, brains volumes of teen boys peak at around this age whereas girls exhibit decreasing cortical volumes starting at age 11 [46]. Female-specific gender differences may be related to the interruption of pruning at more advanced stages whereas boys’ brains may reflect greater resiliency to deleterious effects of marijuana if exposure occurs when pruning is at an earlier stage. Alternatively, male-specific macrostructural effects may not be measurable until young adulthood when developmental factors are less transitional. This may be reflected our observation of greater variance in amygdala volumes among this study’s male subgroups.
Pubertal steroids may contribute to altered amygdala morphometry among adolescent female marijuana users [77]. Cannabinoid receptor density and affinity change with sex steroid distributions across the estrous cycle [78]. Animal studies have shown that intact female rodents self-administer cannabinoid agonists and display greater drug seeking and reinstatement behaviors than do male or ovariectomized female rats [79, 80], suggesting that females may be particularly sensitive to the reinforcing effects of marijuana. With adolescent females expressing more CB1 receptors than adults and male peers [48], the cannabinoid system may have a stronger regulatory role in teenaged girls. Thus, negative marijuana effects during adolescence may leave teenage females particularly vulnerable to changes in brain volume.
Larger amygdala volumes found in female marijuana users may be due, in part, to their elevated internalizing symptoms. Indeed, increased amygdala volumes have been related to pediatric depression and anxiety [81, 82]. Here, female marijuana users, who endorsed the most depression and anxiety symptoms, exhibited larger right amygdala volumes and a unique pattern of increasing amygdala size with greater internalizing problems was observed in female users.
There are some weaknesses of the study that need to be considered. It is possible that morphologic changes associated with marijuana use may be influenced by factors predating drug exposure, such as familial risk for substance use or sub-clinical mood and anxiety symptoms. Children of alcoholics demonstrate reduced right amygdala volumes irrespective of personal alcohol use [83], which suggests that biological factors, such as genetic loading, may account for variability in amygdala morphometry, particularly with respect to substance use. However, we observed larger amygdala in female users even after statistically controlling for family history of SUD. Abnormal amygdala volumes in female marijuana users may also reflect an early marker for developing clinically significant mood or anxiety symptoms. A recent meta-analysis of amygdala morphometry and depression reported mixed findings for larger and smaller amygdala volumes in depressed patients [72], though the results indicated larger amygdala volumes was linked to first major depressive episode. Therefore, larger right amygdala sizes among female marijuana users may represent premorbid risk for internalizing disorders. Our lack of significant findings for left amygdala volumes and asymmetry may relate to marijuana use in the midst of developments unique to adolescence [84]. For example, amygdala asymmetry may be a more robust biomarker in adult samples after neuromaturation is complete. Finally, while the overall sample is relatively large compared to other morphometric studies, the number of females in each group was small. Larger samples balanced for gender are necessary to confirm these findings.
5. Conclusions
In summary, the current study provides evidence that adolescent marijuana users show abnormalities of amygdala morphometry that are moderated by gender. Female marijuana users exhibited larger right amygdala volumes, reported worse depression and anxiety and greater amygdala size was linked with higher internalizing symptomatology among this subgroup. No differences in amygdala size were observed in marijuana using teens compared to non-using peers. Despite no group-level differences in amygdala volumes, being a marijuana user was associated with worse mood and anxiety symptoms. In conclusion, adolescents, especially females, may be more vulnerable to the negative consequences of marijuana on amygdala structure and function. Future studies should assess the interrelationships of marijuana use, gender and internalizing symptoms prospectively as teens enter young adulthood. Longitudinal investigations are essential to characterize and confirm the impact of these factors on brain morphology and neurobehavioral outcomes.
Examined amygdala volumes of teen marijuana users and controls.
Gender moderates effect of marijuana use and amygdala morphometry.
Female marijuana users showed abnormally large right amygdala volumes.
Worse depression/anxiety linked to larger right amygdalas in female users, who reported more internalizing problems.
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse to S.F.T. (R21 DA15228; R01 DA21182) and to K.L.M. (F32 DA20206; R03 DA27457) and by the University of Cincinnati URC Graduate Research Fellowship (T.M.). This study was used to satisfy requirements for the first author’s master’s thesis and portions were presented at the 2009 meeting of the College on Problems of Drug Dependence in Reno, NV. Gratitude is expressed to the staff of the UCSD Adolescent Brain Imaging Project and UC Brain Imaging and Neuropsychology Labs, to Drs. Paula Shear and Jim Eliassen, to the participants and to their families.
Role of the Funding Source
Data collection for this work was supported by grants from the National Institute on Drug Abuse to S.F.T. (DA15228; DA21182). Partial salary support during the preparation of this manuscript was provided by a National Institute on Drug Abuse grant to K.L.M. (DA27457) and by the University of Cincinnati URC to T.M. (Graduate Research Fellowship). All funding sources had no involvement in this study’s analysis and interpretation of data, in the writing of this report, or in the decision to submit this paper for publication.
Footnotes
Disclosure Statement
All authors contributed conceptually to the creation, analysis, and writing of this manuscript, and provided final approval for its submission. No author has a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. NIH Publication No. 09–7401. Bethesda, MD: National Institute on Drug Abuse; 2009. Monitoring the Future national results on adolescent drug use: An overview of key findings, 2008. [Google Scholar]
- 2.Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:465–70. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anavi-Goffer S, Mulder J. The polarised life of the endocannabinoid system in CNS development. Chembiochem. 2009;10:1591–8. doi: 10.1002/cbic.200800827. [DOI] [PubMed] [Google Scholar]
- 4.Fride E. The endocannabinoid-CB receptor system: Importance for development and in pediatric disease. Neuro Endocrinol Lett. 2004;25:24–30. [PubMed] [Google Scholar]
- 5.Hayatbakhsh MR, Najman JM, Jamrozik K, Mamun AA, Alati R, Bor W. Cannabis and anxiety and depression in young adults: a large prospective study. J Am Acad Child Adolesc Psychiatry. 2007;46:408–17. doi: 10.1097/chi.0b013e31802dc54d. [DOI] [PubMed] [Google Scholar]
- 6.Wadsworth EJ, Moss SC, Simpson SA, Smith AP. Cannabis use, cognitive performance and mood in a sample of workers. J Psychopharmacol. 2006;20:14–23. doi: 10.1177/0269881105056644. [DOI] [PubMed] [Google Scholar]
- 7.Reitan RM, Wolfson D. Emotional disturbances and their interaction with neuropsychological deficits. Neuropsychol Rev. 1997;7:3–19. doi: 10.1007/BF02876970. [DOI] [PubMed] [Google Scholar]
- 8.Fattore L, Melis M, Fadda P, Pistis M, Fratta W. The endocannabinoid system and nondrug rewarding behaviours. Exp Neurol. 2010 doi: 10.1016/j.expneurol.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–52. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–6. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 12.Tan H, Lauzon NM, Bishop SF, Bechard MA, Laviolette SR. Integrated cannabinoid CB1 receptor transmission within the amygdala-prefrontal cortical pathway modulates neuronal plasticity and emotional memory encoding. Cereb Cortex. 2010;20:1486–96. doi: 10.1093/cercor/bhp210. [DOI] [PubMed] [Google Scholar]
- 13.Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–18. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rammes G, Eder M, Dodt HU, Kochs E, Zieglgansberger W. Long-term depression in the basolateral amygdala of the mouse involves the activation of interneurons. Neuroscience. 2001;107:85–97. doi: 10.1016/s0306-4522(01)00336-0. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–8. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) 2002;159:379–87. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- 17.Degroot A, Nomikos GG. Genetic deletion and pharmacological blockade of CB1 receptors modulates anxiety in the shock-probe burying test. Eur J Neurosci. 2004;20:1059–64. doi: 10.1111/j.1460-9568.2004.03556.x. [DOI] [PubMed] [Google Scholar]
- 18.Bambico FR, Nguyen NT, Katz N, Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol Dis. 2010;37:641–55. doi: 10.1016/j.nbd.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- 20.Viveros MP, Marco EM, Llorente R, Lopez-Gallardo M. Endocannabinoid system and synaptic plasticity: implications for emotional responses. Neural Plast. 2007;2007:52908. doi: 10.1155/2007/52908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubino T, Guidali C, Vigano D, Realini N, Valenti M, Massi P, Parolaro D. CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology. 2008;54:151–60. doi: 10.1016/j.neuropharm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, Manuck SB. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornelius JR, Aizenstein HJ, Hariri AR. Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addict Behav. 2010;35:644–6. doi: 10.1016/j.addbeh.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: an FMRI study. Drug Alcohol Depend. 2009;105:139–53. doi: 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, Seal M, Surguladze SA, O’Carrol C, Atakan Z, Zuardi AW, McGuire PK. Distinct effects of delta-9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66:95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- 26.Scallet AC. Neurotoxicology of cannabis and THC: a review of chronic exposure studies in animals. Pharmacol Biochem Behav. 1991;40:671–6. doi: 10.1016/0091-3057(91)90380-k. [DOI] [PubMed] [Google Scholar]
- 27.Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- 28.Pedraza O, Bowers D, Gilmore R. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. J Int Neuropsychol Soc. 2004;10:664–78. doi: 10.1017/S1355617704105080. [DOI] [PubMed] [Google Scholar]
- 29.Goncalves-Pereira PM, Oliveira E, Insausti R. Quantitative volumetric analysis of the hippocampus, amygdala and entorhinal cortex: normative database for the adult Portuguese population. Revista de neurologia. 2006;42:713–22. [PubMed] [Google Scholar]
- 30.Brierley B, Shaw P, David AS. The human amygdala: a systematic review and meta-analysis of volumetric magnetic resonance imaging. Brain research Brain research reviews. 2002;39:84–105. doi: 10.1016/s0165-0173(02)00160-1. [DOI] [PubMed] [Google Scholar]
- 31.Freeman HD, Cantalupo C, Hopkins WD. Asymmetries in the hippocampus and amygdala of chimpanzees (Pan troglodytes) Behavioral neuroscience. 2004;118:1460–5. doi: 10.1037/0735-7044.118.6.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Brain Res Rev. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Lanteaume L, Khalfa S, Regis J, Marquis P, Chauvel P, Bartolomei F. Emotion induction after direct intracerebral stimulations of human amygdala. Cereb Cortex. 2007;17:1307–13. doi: 10.1093/cercor/bhl041. [DOI] [PubMed] [Google Scholar]
- 34.Barnes J, Whitwell JL, Frost C, Josephs KA, Rossor M, Fox NC. Measurements of the amygdala and hippocampus in pathologically confirmed Alzheimer disease and frontotemporal lobar degeneration. Archives of neurology. 2006;63:1434–9. doi: 10.1001/archneur.63.10.1434. [DOI] [PubMed] [Google Scholar]
- 35.Niu L, Matsui M, Zhou SY, Hagino H, Takahashi T, Yoneyama E, Kawasaki Y, Suzuki M, Seto H, Ono T, Kurachi M. Volume reduction of the amygdala in patients with schizophrenia: a magnetic resonance imaging study. Psychiatry research. 2004;132:41–51. doi: 10.1016/j.pscychresns.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vainio P, Partanen K, Partanen J, Tiihonen J, Viinamaki H, Karjalainen AK, Lehtonen J. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychological medicine. 2000;30:117–25. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- 37.Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, Wu H, Bogerts B. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Archives of general psychiatry. 1999;56:913–9. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- 38.Sarac-Hadzihalilovic A, Dilberovic F, Kucukalic A. Observing the asymmetry of amygdaloid complex in patients with complex partial attacks. Bosnian journal of basic medical sciences/Udruzenje basicnih mediciniskih znanosti = Association of Basic Medical Sciences. 2007;7:21–8. doi: 10.17305/bjbms.2007.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. 2007;13:807–20. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009;173:228–37. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009;31:349–55. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol. 2009 doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Res. 2010;182:152–9. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry. 2007;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kloos A, Weller RA, Chan R, Weller EB. Gender differences in adolescent substance abuse. Curr Psychiatry Rep. 2009;11:120–6. doi: 10.1007/s11920-009-0019-8. [DOI] [PubMed] [Google Scholar]
- 46.Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72:46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of cannabinoid CB receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. Br J Pharmacol. 2010;161:103–12. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–8. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 50.Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–83. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hollingshead AB. Two-factor index of social position. New Haven, CT, USA: Yale University Press; 1965. [Google Scholar]
- 52.Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–23. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 53.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 54.APA. Diagnostic and statistical manual of mental disorders-IV-Text Revision. 4. Washington, D.C.: American Psychiatric Association; 2000. [Google Scholar]
- 55.Wilkinson GS. The wide range achievement test-3 administration manual. Wilmington, DE, USA: Jastak Associates; 1993. [Google Scholar]
- 56.Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 57.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–38. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 58.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 59.Beck AT. Beck Depression Inventory. San Antonio, TX, USA: Psychological Corporation; 1978. [Google Scholar]
- 60.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 61.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spielberger CD, Gorusch RL, Lushene RE. Manual for the state-trait anxiety inventory. Palo Alto, CA, USA: Consulting Psychologists Press; 1970. [Google Scholar]
- 63.Lahey BB, Applegate B, Waldman ID, Loft JD, Hankin BL, Rick J. The structure of child and adolescent psychopathology: generating new hypotheses. Journal of Abnormal Psychology. 2004;113:358–85. doi: 10.1037/0021-843X.113.3.358. [DOI] [PubMed] [Google Scholar]
- 64.Verona E, Javdani S, Sprague J. Comparing factor structures of adolescent psychopathology. Psychological assessment. 2011 doi: 10.1037/a0022055. [DOI] [PubMed] [Google Scholar]
- 65.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 66.Richardson EJ, Griffith HR, Martin RC, Paige AL, Stewart CC, Jones J, Hermann BP, Seidenberg M. Structural and functional neuroimaging correlates of depression in temporal lobe epilepsy. Epilepsy Behav. 2007;10:242–9. doi: 10.1016/j.yebeh.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 67.Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 68.Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacol Biochem Behav. 2009;92:559–65. doi: 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA. Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry. 2005;57:21–6. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 70.van der Plas EA, Boes AD, Wemmie JA, Tranel D, Nopoulos P. Amygdala volume correlates positively with fearfulness in normal healthy girls. Soc Cogn Affect Neurosci. 2010 doi: 10.1093/scan/nsq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: gender and psychopathology. Annu Rev Clin Psychol. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- 72.Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bindukumar B, Mahajan SD, Reynolds JL, Hu Z, Sykes DE, Aalinkeel R, Schwartz SA. Genomic and proteomic analysis of the effects of cannabinoids on normal human astrocytes. Brain Res. 2008;1191:1–11. doi: 10.1016/j.brainres.2007.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 75.Angelucci F, Ricci V, Spalletta G, Pomponi M, Tonioni F, Caltagirone C, Bria P. Reduced serum concentrations of nerve growth factor, but not brain-derived neurotrophic factor, in chronic cannabis abusers. Eur Neuropsychopharmacol. 2008;18:882–7. doi: 10.1016/j.euroneuro.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 76.D’Souza DC, Pittman B, Perry E, Simen A. Preliminary evidence of cannabinoid effects on brain-derived neurotrophic factor (BDNF) levels in humans. Psychopharmacology (Berl) 2009;202:569–78. doi: 10.1007/s00213-008-1333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–7. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martin M, Fernandez-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54:159–70. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- 79.Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol. 2007;152:795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fattore L, Spano MS, Altea S, Fadda P, Fratta W. Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: influence of ovarian hormones. Br J Pharmacol. 2010;160:724–35. doi: 10.1111/j.1476-5381.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, Axelson DA, Frustaci K, Boring AM, Hall J, Ryan ND. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48:51–7. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- 82.MacMillan S, Szeszko PR, Moore GJ, Madden R, Lorch E, Ivey J, Banerjee SP, Rosenberg DR. Increased amygdala: hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J Child Adolesc Psychopharmacol. 2003;13:65–73. doi: 10.1089/104454603321666207. [DOI] [PubMed] [Google Scholar]
- 83.Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- 84.Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29:141–52. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]