Abstract
Universal newborn hearing screening (UNHS) is of paramount importance for early identification and management of hearing impairment in children. However, infants with slight/mild, progressive, or late-onset hearing impairment might be missed in conventional UNHS. To investigate whether genetic screening for common deafness-associated mutations could assist in identifying these infants, 1017 consecutive newborns in a tertiary hospital were subjected to both newborn hearing screening using a two-step distortion-product otoacoustic emissions (DPOAE) screening and newborn genetic screening (NGS) for deafness. The NGS targeted 4 deafness-associated mutations commonly found in the Taiwanese population, including p.V37I (c.109G>A) and c.235delC of the GJB2 gene, c.919-2A>G of the SLC26A4 gene, and mitochondrial m.1555A>G of the 12S rRNA gene. The results of the NGS were then correlated to the results of the NHS. Of the 1017 newborns, 16 (1.6%) had unilateral DPOAE screening failure, and 22 (2.2%) had bilateral DPOAE screening failure. A total of 199 (19.6%) babies were found to have at least 1 mutated allele on the NGS for deafness, 11 (1.1%) of whom were homozygous for GJB2 p.V37I, 6 (0.6%) compound heterozygous for GJB2 p.V37I and c.235delC, and 1 (0.1%) homoplasmic for m.1555A>G, who may potentially have hearing loss. Among them, 3 babies, 5 babies, and 1 baby, respectively, passed the NHS at birth. Comprehensive audiological assessments in the 9 babies at 3 months identified 1 with slight hearing loss and 2 with mild hearing loss. NGS for common deafness-associated mutations may identify infants with slight/mild or potentially progressive hearing impairment, thus compensating for the inherent limitations of the conventional UNHS.
Introduction
Hearing loss is one of the most common congenital disorders, with approximately 1 in 1000 newborns affected by bilateral moderate, severe or profound (i.e. >40dBHL) permanent congenital hearing loss (PCHL) [1], [2]. If the criterion of hearing loss is lowered to 15 dBHL, 0.88% of the school-aged population have slight or mild bilateral sensorineural hearing impairment (SNHI) [3]. There is solid evidence that moderate (or more severe) hearing impairment exerts a negative impact on speech, language, and cognitive development [2], and early identification and management may be of great benefit to these children, through improved language, communication, mental health, and employment prospects [4]. Although several risk factors (such as prolonged NICU admission and congenital infections) are associated with PCHL, about 50% of infants with PCHL do not have any known risk factors [5], [6], mandating the implementation of universal newborn hearing screening (UNHS) for both newborns with and without risk factors. The feasibility, cost-efficiency, and benefits of UNHS were supported by several studies [1], [7], [8], [9]. However, UNHS may suffer from 3 inherent limitations. First, since the target condition for the majority of UNHS programs is permanent hearing loss >35 dBHL, children with slight or mild hearing loss will be missed [10]. Second, children with late-onset or progressive hearing loss may not be identified by UNHS, because their hearing is normal or near-normal at birth. Third, even in countries where UNHS has been implemented, it is difficult to approach and screen specific subgroups of infants, such as those born outside of hospitals [11].
With recent advancement in the molecular genetics of hearing impairment, it has been demonstrated that more than 50% of children with SNHI have attributable genetic factors [12], making genetic testing a powerful tool for addressing hearing-impaired children. Among a plethora of deafness genes discovered in the past decade (The Hereditary Hearing Loss Homepage, http://hereditaryhearingloss.org/), mutations in certain genes, such as GJB2 (or Cx26) (MIM *121011), SLC26A4 (or PDS) (MIM *605646), and the mitochondrial 12S rRNA gene (or MTRNR1) (MIM *561000) have been shown to be much more prevalent than other genes [13]. Some common GJB2 mutations, such as p.M34T, p.V37I, and p.L90P are associated with mild-to-moderate SNHI [14]. SLC26A4 mutations contribute to Pendred syndrome (PS, MIM #274600) or non-syndromic hearing loss (DFNB4, MIM #600791), and some affected patients have progressive or fluctuating hearing loss [15], [16], [17]. Patients with the most common mitochondrial 12S rRNA mutation, m.1555A>G, also demonstrate great variation in the severity of hearing loss progression with age [18]. In other words, some common deafness-associated mutations are associated with mild and/or progressive hearing loss. Accordingly, in this study, we hypothesize that the application of newborn genetic screening for common deafness-associated mutations may compensate for the inherent limitations of UNHS.
Methods
Recruitment and study design
From 2009 to 2010, 1017 consecutive newborns in the National Taiwan University Hospital were enrolled in the study. All newborns were subjected to both newborn hearing screening (NHS) and newborn genetic screening (NGS) for deafness. The results of the NGS were then correlated to the results of the NHS. Written informed consent for participation in the project was obtained from the parents of all infants, and all procedures were approved by the Research Ethics Committee of the National Taiwan University Hospital.
Newborn hearing screening
The babies received a two-step hearing screening using distortion-product otoacoustic emissions (DPOAEs) recorded with a GSI 60 DPOAE system (Grason-Stadler Inc., Milford, NH, USA). The first step of the screening was performed at 48 hours after birth to prevent debris and vernix in the external ear canal from interfering with the DPOAE measurement [19]. Babies who failed to pass the initial screening, either for one or for both ears, were given a second chance to repeat the DPOAE screening before they were discharged from the hospital. Those failing to pass the second step of the hearing screening, either for one or for both ears, were referred to the outpatient clinic for further assessment. The referral rate of the NHS was determined by the proportion of infants who failed both steps of the DPOAE screening.
Newborn genetic screening for deafness
A bloodspot was obtained within 1 hour after birth. Sample of blood from the heel stick from each infant was spotted onto QIAcard FTA One Spot (Qiagen). For newborn screening, three 3-mm-diameter bloodspots from each blood spot on FTA paper were punched out and used. All DNA samples were extracted using the Chemagic DNA Blood Kit (Chemagen), according to the manufacturer's instructions.
Using our previously generated epidemiologic database [20], the four most common deafness-associated mutations in the Taiwanese population, p.V37I (c.109G>A) and c.235delC of GJB2, c.919-2A>G of SLC26A4, and mitochondrial m.1555A>G, were included in the NGS panel. In terms of the allele frequency in the hearing-impaired population, these 4 mutations amount to >80% of the known deafness-associated mutations in Taiwanese individuals [20], [21].
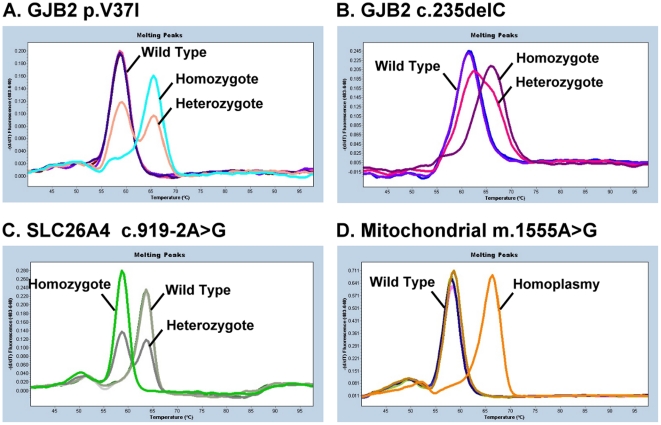
The four mutations were screened using the real-time polymerase chain reaction (PCR) assay with fluorescence resonance energy transfer (FRET) hybridization probes in a LightCycler 480 instrument (Roche) (Fig. 1). The real-time PCR was performed using two primers and two adjacent fluorescent hybridization probes. One probe was labeled with fluorescein at the 3′-end as the donor, and the other probe (acceptor) was labeled with LightCycler (LC) Red fluorophore at the 5′-end, which was phosphorylated at the 3′-end. Real-time PCR was performed in a total volume of 10 µL containing 1 µL of DNA, 3 mM of MgCl2, 0.25 µM of each primer, 0.25 of µM fluorescein probe, 0.25 µM of LC Red fluorophore probe, and 1× LightCycler FastStart DNA Master Hybridization Probes Mix (Roche), as provided by the manufacturer. The cycling conditions for real-time PCR in LiC480 were as follows: 95°C for 10 min followed by 50 cycles of denaturation at 95°C for 10 s with a temperature transition rate of 4.4°C/s, annealing at 56°C for 10 s with a temperature transition rate of 2.2°C/s, and extension at 72°C for 10 s, with a temperature transition rate of 4.4°C/s.
Figure 1. Melting curve analysis of real-time hybridization with FRET probes for the four most common deafness-associated mutations in the Taiwanese population.
A, p.V37I of the GJB2 gene; B, c.235delC of the GJB2 gene; C, c.919-2A>G of the SLC26A4 gene; and D, mitochondrial m.1555A>G.
Audiological assessments after discharge
For infants who failed the NHS, another DPOAE test was performed within 1 month after discharge, and those failing to pass the test again were referred to a pediatric otologist for comprehensive audiological assessments at 3 months. The comprehensive audiological assessments included a behavioral observation audiometry (BOA) in a sound field using warble tones, narrow band noise, and live voice; DPOAE testing; and a diagnostic auditory brainstem response (ABR, Nicolet, Bravo, Madison, WI, USA) under sedation to determine the hearing thresholds at 0.5, 1, 2, and 4 kHz [22]. The hearing level of the better ear calculated by four-tone average (0.5, 1, 2, and 4 kHz) was labeled as slight (16∼25 dBHL), mild (26∼40 dBHL), moderate (41∼70 dBHL), severe (71∼95 dBHL), or profound (>95 dBHL) (GENDEAF: http://audiology.unife.it/www.gendeaf.org/index.html) [3].
For infants who passed the NHS but segregated for 2 mutated GJB2 alleles (i.e., with the p.V37I/p.V37I, p.V37I/c.235delC, and c.235delC/c.235delC genotypes), 2 mutated SLC26A4 alleles (i.e., with the c.919-2A>G/c.919-2A>G genotype), or the m.1555A>G mutation (i.e., homoplasmic or heteroplasmic), comprehensive audiological assessments including BOA, DPOAE, and ABR were also performed at 3 months to confirm their audiological phenotypes.
Genetic examination in hearing impaired infants and heterozygous infants
For hearing-impaired infants who revealed an inconclusive genotype in NGS (i.e., segregating 0 or only 1 mutated GJB2 or SLC26A4 allele), mutation screening of both exons of GJB2, all of the 21 exons of SLC26A4, and the whole mitochondrial 12S rRNA gene was completed by direct sequencing [20]. For infants who passed the NHS but carried heterozygous GJB2 mutations, direct sequencing of the coding region of GJB2 was also performed.
Results
Results of newborn hearing screening
Results of the two-step NHS in the 1017 subjects are summarized in Table 1. Among the 1017 babies, 979 (96.3%) were screened as normal when they bilaterally passed both steps of the DPOAE testing. Of the remaining 38 babies, 16 (1.6%) had unilateral DPOAE screening failure, and 22 (2.2%) had bilateral DPOAE screening failure. The referral rate of the two-step NHS was 3.7%.
Table 1. Newborn hearing screening in the 1017 subjects.
| OAE results | No. of subjects (%) |
| Bilateral pass | 979 (96.3) |
| Unilateral failure | 16 (1.6) |
| Bilateral failure | 22 (2.2) |
| Total | 1017 (100) |
| Referral rate | 38 (3.7) |
Results of newborn genetic screening for deafness
Of the 1017 subjects, a total of 199 (19.6%) were screened as having at least 1 mutated allele according to the NGS for deafness. Table 2 summarizes their genotypes. As for the GJB2 mutations, 17 subjects (1.7%) had two mutated alleles, 11 (1.1%) of whom were homozygous for p.V37I and 6 (0.6%) compound heterozygous for p.V37I and c.235delC. None (0%) were homozygous for c.235delC. Nineteen subjects (1.9%) were shown to have 1 c.235delC allele, and 156 subjects (15.3%) were heterozygous for p.V37I. The high carrier rate of GJB2 p.V37I in this newborn cohort was compatible to that observed in a normal control population in our previous report [23]. In relation to the SLC26A4 c.919-2A>G mutation, 6 subjects (0.6%) were heterozygous for c.919-2A>G and none (0%) were homozygotes. One subject (0.1%) was found to have the m.1555A>G mutation, with the mutation load detected as “homoplasmy” in hybridization probe testing, which was later confirmed using a restriction enzyme digestion method [24]. None of the 1017 babies segregated for mutations in 2 different genes for deafness, according to NGS.
Table 2. Newborn genetic screening in the 1017 subjects.
| Genotype | No. of subjects (%) |
| GJB2 | |
| p.V37I/p.V37I | 11 (1.1) |
| p.V37I/c.235delC | 6 (0.6) |
| c.235delC/c.235delC | 0 (0) |
| p.V37I/wt | 156 (15.3) |
| c.235delC/wt | 19 (1.9) |
| SLC26A4 | |
| c.919-2A>G/c.919-2A>G | 0 (0) |
| c.919-2A>G/wt | 6 (0.6) |
| Mitochondrial 12S rRNA | |
| m.1555A>G | 1 (0.1) |
| No mutation identified | 818 (80.4) |
wt, wild type.
Analysis of NHS results according to genotypes in NGS
The results of NHS in subjects with different genotypes in NGS are shown in Table 3. Among the 11 babies homozygous for the GJB2 p.V37I mutation and the 6 babies compound heterozygous for GJB2 p.V37I and c.235delC, 8 (73%) and 1 (17%), respectively, failed to pass the NHS. In other words, 3 babies homozygous for p.V37I and 5 babies compound heterozygous for p.V37I and c.235delC, with potential hearing problems, were not detected by NHS, indicating that NGS could identify an additional subgroup of affected newborns outside the target area of NHS.
Table 3. Newborn hearing screening results in subjects with different genotypes.
| No. of subjects | ||||
| Genotype | Total (n) | NHS pass (n) | NHS failure (n) | Failure rate (%) |
| GJB2 | ||||
| p.V37I/p.V37I | 11 | 3 | 8 | 73 |
| p.V37I/c.235delC | 6 | 5 | 1 | 17 |
| p.V37I/wt | 156 | 148 | 8 | 5 |
| c.235delC/wt | 19 | 18 | 1 | 5 |
| SLC26A4 | ||||
| c.919-2A>G/wt | 6 | 6 | 0 | 0 |
| Mitochondrial 12S rRNA | ||||
| m.1555A>G | 1 | 1 | 0 | 0 |
NHS, newborn hearing screening; wt, wild type.
The failure rates of NHS in the 156 GJB2 p.V37I heterozygotes and 19 c.235delC heterozygotes were 5% (n = 8) and 5% (n = 1), respectively. Both exons of GJB2 were then sequenced in the 9 heterozygous babies who failed to pass NHS, yet we did not identify any mutation in the second GJB2 allele. Likewise, a second GJB2 mutation was not detected in the other 166 heterozygous babies who passed NHS, indicating that they were probably coincidental carriers of the mutations.
None (0%) of the 6 babies heterozygous for SLC26A4 c.919-2A>G failed to pass NHS. Probably these babies were only carriers. However, the possibility of Pendred syndrome or non-syndromic DFNB4 could not be completely excluded, because PS or DFNB4 patients might be born with normal hearing or minimal hearing loss, with their hearing deteriorating later during childhood or adolescence [25]. The only subject who was homoplasmic for the mitochondrial m.1555A>G mutation passed the NHS, implying that the hearing of this baby at birth might still be normal or near normal and could not be detected by NHS.
Audiological assessments after discharge
Among the 38 infants who failed the NHS, 9 (24%) segregated for 2 mutated GJB2 alleles (including 8 p.V37I homozygotes and 1 p.V37I/c.235delC compound heterozygote), 9 (24%) segregated for 1 mutated GJB2 allele (including 8 p.V37I heterozygotes and 1 c.235delC heterozygote), and 20 (53%) segregated for none of the 4 deafness-associated mutations (Table 4). Six of the 9 babies with 2 mutated GJB2 alleles revealed bilateral hearing loss at 3 months, including 2 with moderate hearing loss, 3 with mild hearing loss and 1 with slight hearing loss. The other 3 babies with 2 mutated GJB2 alleles revealed normal hearing at 3 months. Nevertheless, a close observation of hearing is still warranted in these 3 babies because progressive hearing loss is a common feature in patients with p.V37I [26]. One of the 9 babies with 1 mutated GJB2 allele demonstrated bilateral moderate hearing loss at 3 months, and 1 of the 20 babies with no mutation detected by NGS showed unilateral profound hearing loss.
Table 4. Audiological results (at 3 m) in infants failing NHS.
| Genotype | Total (n) | Loss F/U (n) | Normal hearing (n) | Unilateral (n) | Bilateral (n) |
| 2 mutated GJB2 alleles | 9 | 0 | 3 | 0 | 6a |
| 1 mutated GJB2 allele | 9 | 1 | 7 | 0 | 1b |
| No mutation detected | 20 | 3 | 16 | 1c | 0 |
Including 2 babies with moderate hearing loss, 3 with mild hearing loss and 1 with slight hearing loss.
The baby revealed bilateral moderate hearing loss.
The baby revealed unilateral profound hearing loss.
Comprehensive audiological assessments were also completed in the 9 infants who passed the NHS but segregated for 2 mutated GJB2 alleles or homoplasmic m.1555A>G mutation (Table 5). One baby (NGS0071) showed slight hearing loss at 3 months, whereas 2 babies (NGS0032 and NGS0379) showed mild hearing loss at 3 months.
Table 5. Audiological results (at 3 m) in infants passing NHS but segregating for an abnormal genotype.
| ABR | threshold | (dBHL) | ||||||||
| Subject no. | Sex | Genotype | Laterality | DPOAE results | 0.5 k Hz | 1 k Hz | 2 k Hz | 4 k Hz | average | Audiometry shape |
| NGS0032 | M | GJB2 p.V37I/c.235delC | bil. symmetric | bil. pass | 25 | 30 | 30 | 30 | 28.8 | flat |
| NGS0071 | F | GJB2 p.V37I/c.235delC | bil. symmetric | bil. pass | 15 | 15 | 15 | 20 | 16.3 | flat |
| NGS0072 | F | GJB2 p.V37I/p.V37I | bil. symmetric | bil. pass | 15 | 10 | 15 | 15 | 13.8 | flat |
| NGS0379 | F | GJB2 p.V37I/c.235delC | bil. symmetric | bil. pass | 30 | 35 | 35 | 35 | 33.8 | flat |
| NGS0586 | F | m.1555A>G | bil. symmetric | bil. pass | 15 | 10 | 10 | 15 | 12.5 | flat |
| NGS0598 | F | GJB2 p.V37I/c.235delC | bil. symmetric | bil. pass | 10 | 10 | 10 | 15 | 11.3 | flat |
| NGS0736 | M | GJB2 p.V37I/p.V37I | bil. symmetric | bil. pass | 10 | 5 | 5 | 10 | 7.5 | flat |
| NGS0830 | F | GJB2 p.V37I/c.235delC | bil. symmetric | bil. pass | 10 | 10 | 10 | 10 | 10 | flat |
| NGS0961 | M | GJB2 p.V37I/p.V37I | bil. symmetric | bil. pass | 10 | 10 | 5 | 10 | 8.75 | flat |
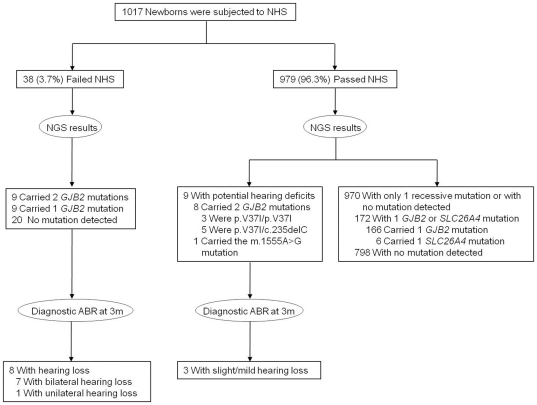
The clinical utility of NGS was summarized in the flow from NHS and NGS to identification of hearing loss (Fig. 2). Among the 979 newborns who passed NHS, 9 were identified to have potential hearing deficits by NGS. Of these 9, 3 were identified with slight/mild hearing loss at 3 months with diagnostic ABR. In other words, NGS might be useful for identifying slight/mild hearing loss that was not detected by conventional NHS.
Figure 2. From newborn hearing screening (NHS) and newborn genetic screening (NGS) to identification of hearing loss.
Among the 979 newborns who passed NHS, 9 were identified to have potential hearing deficits by NGS. Of these 9, 3 were identified with slight/mild hearing loss at 3 months with diagnostic ABR. ABR, auditory brainstem response.
Genetic examination in hearing impaired infants
As shown in Table 4, 1 baby with bilateral moderate hearing loss harbored only a mutated GJB2 allele, and 1 baby with unilateral profound hearing loss had none of the 4 deafness-associated mutations. Mutation screening of both exons of GJB2, all 21 exons of SLC26A4, and the whole mitochondrial 12S rRNA gene, was performed in the 2 babies, yet no mutation was identified.
Discussion
In the present study, we developed a high throughput genetic screening tool to screen 4 common deafness-associated mutations in the Taiwanese population, and applied it to a prospective cohort of 1017 newborns. Our preliminary results revealed that NGS for deafness might compensate for the inherent limitations of conventional UNHS, including failure to identify infants with slight or mild hearing loss, as well as failure to identify infants who may potentially have late-onset or progressive hearing loss during childhood or adolescence.
There have been disputes with regard to whether the goal of UNHS should be to find out infants with slight or mild hearing loss [10], [27]. Several studies demonstrated that slight/mild bilateral SNHI might also have a negative impact on academic performance [28], attention capacity [29], and language skills [30] of children. In a study arguing that slight/mild bilateral SNHI might not affect language, reading, behavior, or health-related quality of life, phonological short-term memory and phonological discrimination were reported to be poorer in children with slight/mild bilateral SNHI [3]. However, including slight/mild losses within the target population of UNHS indicates that the screening program should be altered to achieve higher sensitivity, which inevitably will reduce the specificity of UNHS and greatly increase referral and false-positive rates [27]. To circumvent this difficulty, efforts have been made to ascertain the risk factors for slight/mild SNHI, disclosing that conventional risk factors for neonatal hearing loss, such as craniofacial anomaly, family history of childhood hearing loss and perinatal illness, were not strongly predictive of slight/mild SNHI [31]. On the other hand, the present study provides evidence that NGS, by detecting subjects with genetic mutations associated with mild-to-moderate SNHI, might help in identifying slight/mild SNHI not targeted by conventional UNHS. Although there is still no unanimous consensus concerning the standard management of infants with slight/mild SNHI, referral to early intervention, speech and language monitoring, and provision of resources to parents are recommended [32], [33]. Infants diagnosed as having slight/mild SNHI in the present cohort were referred to rehabilitation facilities for further consultation with an audiologist and a speech pathologist. Even if parents chose not to have their baby fit with a hearing aid immediately, they were provided with information about how to optimize the listening environment for their baby.
To identify infants and children with late-onset or progressive hearing loss at the earliest possible time is also considered important and has been included in the national Early Hearing Detection and Intervention (EHDI) goals (http://www.cdc.gov/ncbddd/ehdi/nationalgoals.htm). However, there is still no efficient way to achieve this goal [11]. In a recent report, progressive hearing impairment was noted in 7 (39%) of the 18 patients who were either homozygous for p.V37I mutation or segregated for a p.V37I allele in compound heterozygosity with a truncating allele, indicating that progression is a common feature for p.V37I [26]. In a Japanese cohort, it was also reported that p.V37I was found mainly in patients diagnosed at a higher age [34]. Progressive hearing loss has also been reported among patients with other GJB2 mutations, such as p.M34T [35], [36]. These lines of evidence highlight the clinical utility of NGS for certain GJB2 mutations in identifying infants who are likely to have late-onset or progressive hearing loss, necessitating a close observation of hearing in these affected individuals. The potential benefits of identifying subjects with SLC26A4 mutations or the mitochondrial m.1555A>G mutation might even be greater. For subjects with SLC26A4-mutation-associated Pendred syndrome or non-syndromic DFNB4, attacks of acute sensorineural hearing loss could be prevented by avoiding head trauma or abrupt barometric pressure changes [25], thus halting the progression or fluctuation of hearing impairment. The severity of hearing loss due to m.1555A>G mutation is modulated by several factors [24]. Especially, the use of aminoglycosides should be avoided in individuals with the mutation, because m.1555A>G is well established as a predisposing factor for aminoglycoside ototoxicity [37].
Interestingly, a recent study reported 32 of 108 cochlear implant (CI) recipients (29.6%) born in Illinois during or after 2003 passed UNHS, demonstrating the limitation of the current UNHS in early identification of delayed-onset SNHI [38]. The authors also pointed out that this problem could not be solved by recent JCIH guidelines, which suggest reevaluation by 24 to 30 months of age in children with known risk factors who pass UNHS [38]. Repeated hearing screening in all children provides a solution, but it might be rather costly as well as unfeasible once the babies are discharged from hospitals or clinics. On the other hand, NGS might be helpful in identifying a group of children with increased risk in developing SNHI for whom a close audiological assessment should be implemented. In the Illinois series, a substantial proportion of CI recipients would have been identified by NGS, since among the 32 CI recipients who passed UNHS, 6 were diagnosed as having connexin mutations, and 7 were found to have cochlear malformations, which might be associated with SLC26A4 mutations.
With regard to the difficulty in approaching and screening specific subgroups of infants such as those born outside of hospitals, NGS might also serve as an alternative solution. Obtaining a few drops of blood from a heel stick within the first 2 or 3 days of life is a minimally invasive procedure, and can be conducted by a nurse or a midwife with basic training. In addition, the new generation of DNA cards, such as filter blotters, Guthrie cards, and FTA cards, are easy to collect, transport, and store.
Offering parents reproductive choices (prenatal diagnosis) has been considered a benefit of expanded newborn screening programs [39]. Although this might be true for certain rare diseases, its clinical implications in deafness should be scrutinized with caution. Some deaf advocates argue that deafness is not a disability, and are against screening for hearing defects [40]. Even for hearing parents of deaf children, it has been documented that few of them would use genetic information to terminate an affected pregnancy, although most recorded a positive attitude toward prenatal genetic testing for deafness [41], [42].
New ethical questions might emerge with the institution of NGS for deafness, including risks of discrimination or stigmatization, respect for autonomy of persons to make their own decisions, and parental anxiety resulting from a false positive test or the carrier status of a recessive mutation, as other newborn screening panels [39]. For hereditary hearing impairment, of which the majority of cases are inherited in an autosomal recessive manner, identification of healthy carriers could be of special concern because it might lead to unjustified parental anxiety about the health of their baby [43]. In the present study, 175 babies were found to carry 1 GJB2 mutation, and 6 babies were found to carry 1 SLC26A4 mutation; among them, 9 babies and no baby failed the NHS at birth, respectively. These 9 babies were managed as other newborns who failed the NHS according to the national guidelines, i.e., another DPOAE test within 1 month after discharge, followed by comprehensive audiological assessments at 3 months if indicated. Seven of the 9 babies had normal hearing at 3 months (Table 4), and were regarded as coincidental carriers, given the high frequency of the GJB2 p.V37I allele in the Taiwanese population and the absence of a second GJB2 mutation in these babies. One baby revealed bilateral moderate hearing loss at 3 months, and genetic counseling was performed as hearing-impaired patients with only 1 GJB2 mutated allele detected [44]. By contrast, for carriers who passed the NHS, no further audiological studies were performed. The great majority of these babies were p.V37I heterozygotes, and were regarded as coincidental carriers given the high frequency of the GJB2 p.V37I allele in the Taiwanese population. The parents were assured that their babies were not at an increased risk of developing hearing impairment, and access to genetic counseling was provided whenever necessary to minimize potential stress for families.
Of special note, deafness-associated mutations should be adjusted if NGS for deafness is to be implemented in other populations because different ethnic groups show different mutation spectra for each deafness gene. Although mutations of the connexin 26-encoding GJB2 gene are the most common cause of hereditary hearing loss in most world populations, they occur at different frequencies across populations. To screen GJB2 mutations in Caucasians, c.35delG, p.M34T, and p.L90P should be included in the screening panel instead of p.V37I and c.235delC, since these mutations are more common in Caucasians [14]. Likewise, to screen GJB2 mutations in Ashkenazi Jews, c.167delT should be included [45]. Leading to Pendred syndrome, the most common form of syndromic deafness [46], as well as to DFNB4, a common form of non-syndromic deafness with enlarged vestibular aqueduct (EVA), mutations of SLC26A4 might be the second most frequent cause of hereditary hearing loss worldwide. Common SLC26A4 mutations also differ across populations. To screen SLC26A4 mutations, p.T416P and c.1001+1G>A should be covered in populations of northern European ethnicity [47], [48], [49], [50], whereas p.H723R should be included if screening is to be performed in Japanese [51] or Koreans [52]. The m.1555A>G mutation appears to be a common cause of hearing impairment worldwide, and has been reported in 2–5% of sensorineural hearing-impaired Caucasians [53], [54], Japanese [55], and Southeast Asians [56]. In a European birth cohort unselected for hearing loss, the prevalence of m.1555A>G was as high as 1 in 520 [57], attesting to the necessity for conducting national genetic screening prior to aminoglycoside administration. For Spanish subjects, the p.Q829X mutation of the OTOF gene (MIM *603861) should be added due to its high prevalence in patients with prelingual SNHI [58].
Some limitations of the present study deserve discussion. First, only common, known deafness-associated mutations are included in the current screening panel. This might lead to a false assurance in individuals with rare or novel mutations because of normal NGS results. This limitation, however, might be overcome with the advent of the next-generation sequencing technology, as massively parallel sequencing has recently been proven as an effective tool for addressing hereditary hearing loss [59]. Second, as a preliminary report, the present study documented the audiological results in 9 infants passing NHS, but segregating for an abnormal genotype, until 3 months. Although 3 of them were confirmed as having slight/mild SNHI, the follow-up period of 3 months is apparently too short to elucidate the frequency of late-onset hearing loss. Long-term follow-up of the present cohort is thus necessary to delineate correlations between NHS and NGS findings. Third, genetic heterogeneity of hereditary hearing loss might preclude a precise interpretation of NGS results. For instance, genetic diagnosis is complicated by the fact that 10%–50% of affected subjects with GJB2 mutations carry only 1 mutant allele. Although the del(GJB6-D13S1830) mutation, through eliminating a cis-regulatory element of GJB2, provided an explanation for the deafness in as many as 30%–70% of affected GJB2 heterozygotes in some populations [60], [61], the etiology remained unclear for the others. These affected heterozygotes pose a diagnostic dilemma: their hearing loss might be attributed to an unrecognized GJB2 mutation, or they might be merely coincidental carriers with hearing loss unrelated to GJB2. Similarly, it is also difficult to interpret the genetic results in babies who passed NHS and segregated only 1 mutated GJB2 allele on NGS, although it can be inferred that a greater proportion of these babies are coincidental carriers as compared to “affected” heterozygotes. Nonetheless, NGS for deafness is still useful in identifying babies with 2 mutated GJB2 alleles, for whom the genetic diagnosis is more straightforward. These babies require a close audiological observation or intervention because their genotypes are associated with mild-to-moderate, progressive, or late-onset hearing impairment.
In a retrospective report, it was demonstrated that some patients with 2 GJB2 mutations could not be identified with UNHS [62]. By contrast, the present study might be the first prospective study to have conducted hearing screening in conjunction with genetic screening in all newborns and longitudinal follow-up of these infants. For babies with a definite genetic diagnosis on NGS (i.e., with 2 mutated GJB2 alleles, 2 mutated SLC264 alleles, homoplasmic or heteroplasmic m.1555A>G mutation) who reveal normal hearing at 3 months, comprehensive audiological evaluation will be repeated at 1 year, whereas for normal hearing babies with 1 mutated GJB2 or SLC264 allele, adequate genetic counseling will be implemented, and additional genetic study (such as sequencing of the whole gene) will be provided whenever necessary. Once hearing impairment develops in these babies, the management protocol will be switched to the conventional management protocol for pediatric SNHI regardless of their genotypes. Long-term follow-up of this cohort might provide insight into how and to what extent genetic mutations exert their influence on the development of pediatric hearing impairment, as well as provide important audiological information about the natural history of specific genetic mutations, such as the GJB2 p.V37I mutation.
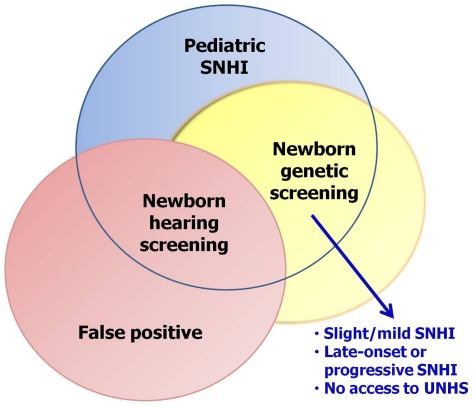
In conclusion, the present study revealed that NGS for common deafness-associated mutations, by detecting subjects with mutations associated with mild-to-moderate, progressive, or late-onset hearing impairment, might compensate for the inherent limitations of conventional UNHS (Fig. 3). For infants born outside of hospitals and who do not have access to UNHS at birth, NGS might also serve as an alternative. The benefits of NGS for deafness would be maximized with the construction of a well-designed infrastructure to support testing, counseling, education, treatment, and follow-up. Despite its clinical utility, the authors would like to emphasize that the role of NGS for deafness is to augment the armamentarium of UNHS instead of replacing UNHS, given that a genetic cause could not be identified in many hearing-impaired children. To our knowledge, this pilot report might be among the first to demonstrate the clinical utility of NGS for deafness. A nation-wide screening is currently underway to confirm the long-term benefits of NGS for the detection of deafness.
Figure 3. Clinical utility of newborn genetic screening (NGS) for deafness.
NGS for common deafness-associated mutations, by detecting subjects with mutations associated with mild-to-moderate, progressive, or late-onset hearing impairment, may compensate for the inherent limitations of conventional universal newborn hearing screening (UNHS), including failure to identify infants with slight or mild hearing loss, as well as failure to identify infants who potentially have late-onset or progressive hearing loss during their childhood or adolescence. For infants born outside of hospitals and who do not have an access to UNHS at birth, NGS may also serve as an alternative. SNHI, sensorineural hearing impairment.
Acknowledgments
We wish to thank all subjects and their parents for participating in the study.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by a research grant from the National Science Council of the Executive Yuan of the Republic of China (NSC-97-2314-B-002-092-MY3; NSC-99-2314-B-002-063-MY3; 99-2622-B-002-002-CC2; 100-2622-B-002-003-CC2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kennedy CR, McCann DC, Campbell MJ, Law CM, Mullee M, et al. Language ability after early detection of permanent childhood hearing impairment. N Engl J Med. 2006;354:2131–2141. doi: 10.1056/NEJMoa054915. [DOI] [PubMed] [Google Scholar]
- 2.Thompson DC, McPhillips H, Davis RL, Lieu TL, Homer CJ, et al. Universal newborn hearing screening: summary of evidence. JAMA. 2001;286:2000–2010. doi: 10.1001/jama.286.16.2000. [DOI] [PubMed] [Google Scholar]
- 3.Wake M, Tobin S, Cone-Wesson B, Dahl HH, Gillam L, et al. Slight/mild sensorineural hearing loss in children. Pediatrics. 2006;118:1842–1851. doi: 10.1542/peds.2005-3168. [DOI] [PubMed] [Google Scholar]
- 4.Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early- and later-identified children with hearing loss. Pediatrics. 1998;102:1161–1171. doi: 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]
- 5.Wessex Universal Neonatal Hearing Screening Trial Group. Controlled trial of universal neonatal screening for early identification of permanent childhood hearing impairment. Lancet. 1998;352:1957–1964. [PubMed] [Google Scholar]
- 6.Kennedy C, McCann D, Campbell MJ, Kimm L, Thornton R. Universal newborn screening for permanent childhood hearing impairment: an 8-year follow-up of a controlled trial. Lancet. 2005;366:660–662. doi: 10.1016/S0140-6736(05)67138-3. [DOI] [PubMed] [Google Scholar]
- 7.Barsky-Firkser L, Sun S. Universal newborn hearing screenings: a three-year experience. Pediatrics. 1997;99:E4. doi: 10.1542/peds.99.6.e4. [DOI] [PubMed] [Google Scholar]
- 8.Vohr BR, Carty LM, Moore PE, Letourneau K. The Rhode Island Hearing Assessment Program: experience with statewide hearing screening (1993–1996). J Pediatr. 1998;133:353–357. doi: 10.1016/s0022-3476(98)70268-9. [DOI] [PubMed] [Google Scholar]
- 9.Mason JA, Herrmann KR. Universal infant hearing screening by automated auditory brainstem response measurement. Pediatrics. 1998;101:221–228. doi: 10.1542/peds.101.2.221. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JL, White KR, Widen JE, Gravel JS, James M, et al. A multicenter evaluation of how many infants with permanent hearing loss pass a two-stage otoacoustic emissions/automated auditory brainstem response newborn hearing screening protocol. Pediatrics. 2005;116:663–672. doi: 10.1542/peds.2004-1688. [DOI] [PubMed] [Google Scholar]
- 11.White KR, Forsman I, Eichwald J, Munoz K. The evolution of early hearing detection and intervention programs in the United States. Semin Perinatol. 2010;34:170–179. doi: 10.1053/j.semperi.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Smith RJ, Bale JF, Jr, White KR. Sensorineural hearing loss in children. Lancet. 2005;365:879–890. doi: 10.1016/S0140-6736(05)71047-3. [DOI] [PubMed] [Google Scholar]
- 13.Hilgert N, Smith RJ, Van Camp G. Forty-six genes causing nonsyndromic hearing impairment: which ones should be analyzed in DNA diagnostics? Mutat Res. 2009;681:189–196. doi: 10.1016/j.mrrev.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snoeckx RL, Huygen PL, Feldmann D, Marlin S, Denoyelle F, et al. GJB2 mutations and degree of hearing loss: a multicenter study. Am J Hum Genet. 2005;77:945–957. doi: 10.1086/497996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 16.Li XC, Everett LA, Lalwani AK, Desmukh D, Friedman TB, et al. A mutation in PDS causes non-syndromic recessive deafness. Nat Genet. 1998;18:215–217. doi: 10.1038/ng0398-215. [DOI] [PubMed] [Google Scholar]
- 17.Mori T, Westerberg BD, Atashband S, Kozak FK. Natural history of hearing loss in children with enlarged vestibular aqueduct syndrome. J Otolaryngol Head Neck Surg. 2008;37:112–118. [PubMed] [Google Scholar]
- 18.Kokotas H, Petersen MB, Willems PJ. Mitochondrial deafness. Clin Genet. 2007;71:379–391. doi: 10.1111/j.1399-0004.2007.00800.x. [DOI] [PubMed] [Google Scholar]
- 19.Chang KW, Vohr BR, Norton SJ, Lekas MD. External and middle ear status related to evoked otoacoustic emission in neonates. Arch Otolaryngol Head Neck Surg. 1993;119:276–282. doi: 10.1001/archotol.1993.01880150024004. [DOI] [PubMed] [Google Scholar]
- 20.Wu CC, Chen PJ, Chiu YH, Lu YC, Wu MC, et al. Prospective mutation screening of three common deafness genes in a large Taiwanese Cohort with idiopathic bilateral sensorineural hearing impairment reveals a difference in the results between families from hospitals and those from rehabilitation facilities. Audiol Neurootol. 2008;13:172–181. doi: 10.1159/000112425. [DOI] [PubMed] [Google Scholar]
- 21.Wu CC, Lu YC, Chen PJ, Liu AY, Hwu WL, et al. Application of SNaPshot multiplex assays for simultaneous multigene mutation screening in patients with idiopathic sensorineural hearing impairment. Laryngoscope. 2009;119:2411–2416. doi: 10.1002/lary.20621. [DOI] [PubMed] [Google Scholar]
- 22.Wu CC, Lee YC, Chen PJ, Hsu CJ. Predominance of genetic diagnosis and imaging results as predictors in determining the speech perception performance outcome after cochlear implantation in children. Arch Pediatr Adolesc Med. 2008;162:269–276. doi: 10.1001/archpediatrics.2007.59. [DOI] [PubMed] [Google Scholar]
- 23.Hwa HL, Ko TM, Hsu CJ, Huang CH, Chiang YL, et al. Mutation spectrum of the connexin 26 (GJB2) gene in Taiwanese patients with prelingual deafness. Genet Med. 2003;5:161–165. doi: 10.1097/01.GIM.0000066796.11916.94. [DOI] [PubMed] [Google Scholar]
- 24.Wu CC, Chiu YH, Chen PJ, Hsu CJ. Prevalence and clinical features of the mitochondrial m.1555A>G mutation in Taiwanese patients with idiopathic sensorineural hearing loss and association of haplogroup F with low penetrance in three families. Ear Hear. 2007;28:332–342. doi: 10.1097/AUD.0b013e318047941e. [DOI] [PubMed] [Google Scholar]
- 25.Jackler RK, De La Cruz A. The large vestibular aqueduct syndrome. Laryngoscope. 1989;99:1238–1242; discussion 1242–1233. doi: 10.1288/00005537-198912000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Chan DK, Schrijver I, Chang KW. Connexin-26-associated deafness: Phenotypic variability and progression of hearing loss. Genet Med. 2010;12:174–181. doi: 10.1097/GIM.0b013e3181d0d42b. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy C, Kimm L, Thornton R, Davis A. False positives in universal neonatal screening for permanent childhood hearing impairment. Lancet. 2000;356:1903–1904. doi: 10.1016/S0140-6736(00)03267-0. [DOI] [PubMed] [Google Scholar]
- 28.Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 1998;19:339–354. doi: 10.1097/00003446-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Hick CB, Tharpe AM. Listening effort and fatigue in school-age children with and without hearing loss. J Speech Lang Hear Res. 2002;45:573–584. doi: 10.1044/1092-4388(2002/046). [DOI] [PubMed] [Google Scholar]
- 30.Davis JM, Elfenbein J, Schum R, Bentler RA. Effects of mild and moderate hearing impairments on language, educational, and psychosocial behavior of children. J Speech Hear Disord. 1986;51:53–62. doi: 10.1044/jshd.5101.53. [DOI] [PubMed] [Google Scholar]
- 31.Cone BK, Wake M, Tobin S, Poulakis Z, Rickards FW. Slight-mild sensorineural hearing loss in children: audiometric, clinical, and risk factor profiles. Ear Hear. 2010;31:202–212. doi: 10.1097/AUD.0b013e3181c62263. [DOI] [PubMed] [Google Scholar]
- 32.McKay S, Gravel JS, Tharpe AM. Amplification considerations for children with minimal or mild bilateral hearing loss and unilateral hearing loss. Trends Amplif. 2008;12:43–54. doi: 10.1177/1084713807313570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joint Committee on Infant Hearing. Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898–921. doi: 10.1542/peds.2007-2333. [DOI] [PubMed] [Google Scholar]
- 34.Tsukada K, Nishio S, Usami S. A large cohort study of GJB2 mutations in Japanese hearing loss patients. Clin Genet. 2010;78:464–470. doi: 10.1111/j.1399-0004.2010.01407.x. [DOI] [PubMed] [Google Scholar]
- 35.Pollak A, Skorka A, Mueller-Malesinska M, Kostrzewa G, Kisiel B, et al. M34T and V37I mutations in GJB2 associated hearing impairment: evidence for pathogenicity and reduced penetrance. Am J Med Genet A. 2007;143A:2534–2543. doi: 10.1002/ajmg.a.31982. [DOI] [PubMed] [Google Scholar]
- 36.Marlin S, Feldmann D, Blons H, Loundon N, Rouillon I, et al. GJB2 and GJB6 mutations: genotypic and phenotypic correlations in a large cohort of hearing-impaired patients. Arch Otolaryngol Head Neck Surg. 2005;131:481–487. doi: 10.1001/archotol.131.6.481. [DOI] [PubMed] [Google Scholar]
- 37.Hutchin T, Haworth I, Higashi K, Fischel-Ghodsian N, Stoneking M, et al. A molecular basis for human hypersensitivity to aminoglycoside antibiotics. Nucleic Acids Res. 1993;21:4174–4179. doi: 10.1093/nar/21.18.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young NM, Reilly BK, Burke L. Limitations of universal newborn hearing screening in early identification of pediatric cochlear implant candidates. Arch Otolaryngol Head Neck Surg. 2011;137:230–234. doi: 10.1001/archoto.2011.4. [DOI] [PubMed] [Google Scholar]
- 39.Dhondt JL. Expanded newborn screening: social and ethical issues. J Inherit Metab Dis. 2010;33:S211–217. doi: 10.1007/s10545-010-9138-y. [DOI] [PubMed] [Google Scholar]
- 40.Levy N. Deafness, culture, and choice. J Med Ethics. 2002;28:284–285. doi: 10.1136/jme.28.5.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunger JW, Murray GS, O'Riordan M, Matthews AL, Smith RJ, et al. Parental attitudes toward genetic testing for pediatric deafness. Am J Hum Genet. 2000;67:1621–1625. doi: 10.1086/316901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Withrow KA, Burton S, Arnos KS, Kalfoglou A, Pandya A. Consumer motivations for pursuing genetic testing and their preferences for the provision of genetic services for hearing loss. J Genet Couns. 2008;17:252–260. doi: 10.1007/s10897-007-9143-y. [DOI] [PubMed] [Google Scholar]
- 43.Lewis S, Curnow L, Ross M, Massie J. Parental attitudes to the identification of their infants as carriers of cystic fibrosis by newborn screening. J Paediatr Child Health. 2006;42:533–537. doi: 10.1111/j.1440-1754.2006.00917.x. [DOI] [PubMed] [Google Scholar]
- 44.Lipan M, Ouyang X, Yan D, Angeli S, Du LL, et al. Clinical comparison of hearing-impaired patients with DFNB1 against heterozygote carriers of connexin 26 mutations. Laryngoscope. 2011;121:811–814. doi: 10.1002/lary.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morell RJ, Kim HJ, Hood LJ, Goforth L, Friderici K, et al. Mutations in the connexin 26 gene (GJB2) among Ashkenazi Jews with nonsyndromic recessive deafness. N Engl J Med. 1998;339:1500–1505. doi: 10.1056/NEJM199811193392103. [DOI] [PubMed] [Google Scholar]
- 46.Batsakis JG, Nishiyama RH. Deafness with sporadic goiter. Pendred's syndrome. Arch Otolaryngol. 1962;76:401–406. doi: 10.1001/archotol.1962.00740050413004. [DOI] [PubMed] [Google Scholar]
- 47.Coyle B, Reardon W, Herbrick JA, Tsui LC, Gausden E, et al. Molecular analysis of the PDS gene in Pendred syndrome. Hum Mol Genet. 1998;7:1105–1112. doi: 10.1093/hmg/7.7.1105. [DOI] [PubMed] [Google Scholar]
- 48.Campbell C, Cucci RA, Prasad S, Green GE, Edeal JB, et al. Pendred syndrome, DFNB4, and PDS/SLC26A4 identification of eight novel mutations and possible genotype-phenotype correlations. Hum Mutat. 2001;17:403–411. doi: 10.1002/humu.1116. [DOI] [PubMed] [Google Scholar]
- 49.Pryor SP, Madeo AC, Reynolds JC, Sarlis NJ, Arnos KS, et al. SLC26A4/PDS genotype-phenotype correlation in hearing loss with enlargement of the vestibular aqueduct (EVA): evidence that Pendred syndrome and non-syndromic EVA are distinct clinical and genetic entities. J Med Genet. 2005;42:159–165. doi: 10.1136/jmg.2004.024208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi BY, Stewart AK, Madeo AC, Pryor SP, Lenhard S, et al. Hypo-functional SLC26A4 variants associated with nonsyndromic hearing loss and enlargement of the vestibular aqueduct: genotype-phenotype correlation or coincidental polymorphisms? Hum Mutat. 2009;30:599–608. doi: 10.1002/humu.20884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsukamoto K, Suzuki H, Harada D, Namba A, Abe S, et al. Distribution and frequencies of PDS (SLC26A4) mutations in Pendred syndrome and nonsyndromic hearing loss associated with enlarged vestibular aqueduct: a unique spectrum of mutations in Japanese. Eur J Hum Genet. 2003;11:916–922. doi: 10.1038/sj.ejhg.5201073. [DOI] [PubMed] [Google Scholar]
- 52.Park HJ, Shaukat S, Liu XZ, Hahn SH, Naz S, et al. Origins and frequencies of SLC26A4 (PDS) mutations in east and south Asians: global implications for the epidemiology of deafness. J Med Genet. 2003;40:242–248. doi: 10.1136/jmg.40.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hutchin TP, Thompson KR, Parker M, Newton V, Bitner-Glindzicz M, et al. Prevalence of mitochondrial DNA mutations in childhood/congenital onset non-syndromal sensorineural hearing impairment. J Med Genet. 2001;38:229–231. doi: 10.1136/jmg.38.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kupka S, Toth T, Wrobel M, Zeissler U, Szyfter W, et al. Mutation A1555G in the 12S rRNA gene and its epidemiological importance in German, Hungarian, and Polish patients. Hum Mutat. 2002;19:308–309. doi: 10.1002/humu.9017. [DOI] [PubMed] [Google Scholar]
- 55.Usami S, Abe S, Akita J, Namba A, Shinkawa H, et al. Prevalence of mitochondrial gene mutations among hearing impaired patients. J Med Genet. 2000;37:38–40. doi: 10.1136/jmg.37.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malik SG, Pieter N, Sudoyo H, Kadir A, Marzuki S. Prevalence of the mitochondrial DNA A1555G mutation in sensorineural deafness patients in island Southeast Asia. J Hum Genet. 2003;48:480–483. doi: 10.1007/s10038-003-0056-9. [DOI] [PubMed] [Google Scholar]
- 57.Bitner-Glindzicz M, Pembrey M, Duncan A, Heron J, Ring SM, et al. Prevalence of mitochondrial 1555A→G mutation in European children. N Engl J Med. 2009;360:640–642. doi: 10.1056/NEJMc0806396. [DOI] [PubMed] [Google Scholar]
- 58.Migliosi V, Modamio-Hoybjor S, Moreno-Pelayo MA, Rodriguez-Ballesteros M, Villamar M, et al. Q829X, a novel mutation in the gene encoding otoferlin (OTOF), is frequently found in Spanish patients with prelingual non-syndromic hearing loss. J Med Genet. 2002;39:502–506. doi: 10.1136/jmg.39.7.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shearer AE, DeLuca AP, Hildebrand MS, Taylor KR, Gurrola J, 2nd, et al. Comprehensive genetic testing for hereditary hearing loss using massively parallel sequencing. Proc Natl Acad Sci U S A. 2010;107:21104–21109. doi: 10.1073/pnas.1012989107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Del Castillo I, Moreno-Pelayo MA, Del Castillo FJ, Brownstein Z, Marlin S, et al. Prevalence and evolutionary origins of the del(GJB6-D13S1830) mutation in the DFNB1 locus in hearing-impaired subjects: a multicenter study. Am J Hum Genet. 2003;73:1452–1458. doi: 10.1086/380205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez-Paris J, Schrijver I. The digenic hypothesis unraveled: the GJB6 del(GJB6-D13S1830) mutation causes allele-specific loss of GJB2 expression in cis. Biochem Biophys Res Commun. 2009;389:354–359. doi: 10.1016/j.bbrc.2009.08.152. [DOI] [PubMed] [Google Scholar]
- 62.Norris VW, Arnos KS, Hanks WD, Xia X, Nance WE, et al. Does universal newborn hearing screening identify all children with GJB2 (Connexin 26) deafness? Penetrance of GJB2 deafness. Ear Hear. 2006;27:732–741. doi: 10.1097/01.aud.0000240492.78561.d3. [DOI] [PubMed] [Google Scholar]