Abstract
Inhibitor-of-apoptosis proteins (IAPs), including neuronal apoptosis inhibitory protein (NAIP), inhibit cell death. Other IAPs inhibit key caspase proteases which effect cell death, but the mechanism by which NAIP acts is unknown. Here we report that NAIP, through its third baculovirus inhibitory repeat domain (BIR3), binds the neuron-restricted calcium-binding protein, hippocalcin, in an interaction promoted by calcium. In neuronal cell lines NSC-34 and Neuro-2a, over-expression of the BIR domains of NAIP (NAIP-BIR1–3) counteracted the calcium-induced cell death induced by ionomycin and thapsigargin. This protective capacity was significantly enhanced when NAIP-BIR1–3 was co-expressed with hippocalcin. Over-expression of the BIR3 domain or hippocalcin alone did not substantially enhance cell survival, but co-expression greatly increased their protective effects. These data suggest synergy between NAIP and hippocalcin in facilitating neuronal survival against calcium-induced death stimuli mediated through the BIR3 domain. Analysis of caspase activity after thapsigargin treatment revealed that caspase-3 is activated in NSC-34, but not Neuro-2a, cells. Thus NAIP, in conjunction with hippocalcin, can protect neurons against calcium-induced cell death in caspase-3-activated and non-activated pathways.
Keywords: caspase/hippocalcin/IAP/motor neuron/NAIP
Introduction
Inhibitor-of-apoptosis proteins (IAPs) are characterized by having one or more homologous baculovirus inhibitory repeat (BIR) domains (Liston et al., 1996; Clem and Duckett, 1997; Deveraux and Reed, 1999). The BIR domain, first identified in baculovirus proteins, comprises ∼70 amino acids, the latter half forming a zinc finger structure (Hinds et al., 1999; Sun et al., 1999). Insect and mammalian homologues were later found; when over-expressed, these have anti-apoptotic properties (Liston et al., 1996; Ambrosini et al., 1997). Five human counterparts are known: XIAP, c-IAP-1 and -2, survivin and neuronal apoptosis inhibitory protein (NAIP). All have three tandem N-terminal BIR domains, except survivin which has only one BIR motif. Recently, a number of BIR-containing proteins (BIRPs) have been found in Caenorhabditis elegans and yeast; they appear to be involved in cytokinesis (Fraser et al., 1999) and chromosome segregation (Rajagopalan and Balasubramanian, 1999; Yoon and Carbon, 1999).
Functional studies indicate that human IAPs protect against a wide variety of apoptotic stimuli in various cell types (Liston et al., 1996). XIAP, c-IAP-1 and -2 and survivin interact directly with and inhibit specific caspases which are death effector proteases in the apoptosis cascade (Deveraux et al., 1997; Roy et al., 1997; Tamm et al., 1998). These IAPs inhibit caspase-3/7 by binding to the active caspase form, interfering directly with its proteolytic activity (Deveraux et al., 1997). In contrast, XIAP and c-IAP-1 and -2 inhibit caspase-9 by preventing the procaspase from adopting the active form (Deveraux et al., 1998). Recent studies on XIAP have shown that the BIR2 domain alone is sufficient to bind and inhibit active caspase-3/7, while the BIR3 region and a C-terminal RING domain mediate the interaction and suppression of procaspase-9 activation (Deveraux et al., 1999).
Compared with XIAP, c-IAP-1 and -2 have far less inhibitory capacity on caspase-3 (Roy et al., 1997), which may indicate that caspase-3 inhibition is a secondary rather than a primary property. The c-IAPs were first isolated by their ability to bind the tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2) (Rothe et al., 1995). TNF, by binding to TNF receptors, can promote either death or survival by activation of caspase-8 and NF-κB, respectively. NF-κB activation in turn induces expression of c-IAP-1 and -2, which, through their association with TRAF1 and 2, inhibit the activation of caspase-8, curbing the apoptotic signal (Wang et al., 1998).
Survivin differs from other IAPs not only in that it has just one BIR domain but also with regard to its expression. It is undetectable in terminally differentiated adult tissues, but abundantly expressed during development and, strikingly, in most human cancers (Ambrosini et al., 1997). It is expressed principally in the G2/M phase of the cell cycle, associating with the polymerized microtubules of the mitotic spindle, an interaction which, if interfered with, promotes cell death (Li et al., 1998). The interaction with the microtubules, however, is directed not through survivin’s BIR domain but rather through its C-terminal coiled-coil domain. NAIP’s vastly extended C-terminal domain is also distinct from the RING zinc-binding motif shared by XIAP and the c-IAPs, though function has yet to be assigned to this region.
Unlike the other human IAPs, the mechanism by which NAIP protects cells against apoptotic insult is not understood. The suppression of cell death afforded by NAIP has been observed in non-neuronal and neuronal cells, the latter both in vitro (Simons et al., 1999) and in vivo (Xu et al., 1997a). NAIP expression following transient forebrain ischemia is selectively upregulated in rat neurons resistant to this insult, suggesting that NAIP plays a part in conferring resistance to ischemic damage (Xu et al., 1997a). Indeed, NAIP has been associated with disease: it was first cloned as a candidate gene for involvement in the congenital neurodegenerative disorder, spinal muscular atrophy (SMA) (Roy et al., 1995). This disorder is characterized by a depletion of motor neurons from the ventral horn of the spinal cord, which degenerate in a manner consistent with apoptosis. Mutations and deletions of NAIP are observed in patients with SMA, suggesting a role for NAIP in the disease. The high frequency of alterations within the BIR domains of NAIP suggests that it is these regions which play a crucial role in protection of neurons against degeneration.
In this study, we demonstrate that NAIP, through its BIR3 domain, specifically binds the neuron-restricted calcium-binding protein hippocalcin, in an interaction promoted by calcium. Co-expression of hippocalcin with the BIR domains of NAIP in neuronal cells markedly enhanced protection against calcium-induced cell death compared with expression of NAIP’s BIR domains alone. Our results indicate a synergic protective effect between NAIP and hippocalcin within neurons against calcium-induced cell death, which may have significant implications in neurodegenerative diseases.
Results
NAIP BIR domains protect against calcium-induced motor neuron death
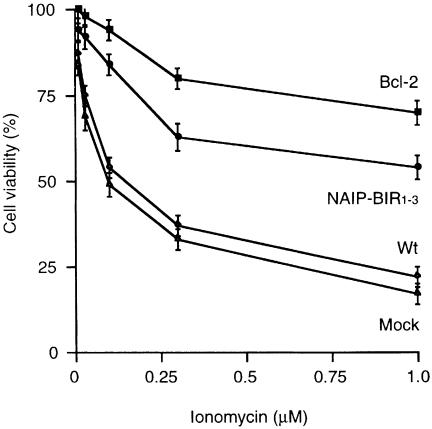
To determine whether the N-terminal BIR domains of NAIP can protect neurons from death, we stably transfected the spinal cord motor neuron-like cell line, NSC-34 (Cashman et al., 1992), with a construct encoding the BIR domains of NAIP (NAIP-BIR1–3). Expression of exogenous NAIP-BIR1–3 mRNA was confirmed by northern blotting. Treatment of the NSC-34 cells with ionomycin induced cell death in a concentration- dependent manner, but NSC-34-transfected NAIP-BIR1–3 cells were more resistant to ionomycin than control or mock transfected cells, as determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Figure 1). When we compared an enhanced green fluorescence protein (EGFP)-tagged NAIP-BIR1–3 (EGFP–NAIP-BIR1–3) construct with NAIP-BIR1–3 alone, we observed a similar protective effect (data not shown), suggesting that the additional EGFP does not interfere with function. Although over-expression of NAIP-BIR1–3 counteracted calcium-induced cell death, the effect was not complete, and was less than that afforded by Bcl-2 (Figure 1), which has been shown previously to inhibit various types of cell death (Adams and Cory, 1998).
Fig. 1. Effect of NAIP-BIR1–3 on survival of NSC-34 cells after ionomycin treatment. NSC-34 cells were stably transfected with expression vectors encoding NAIP-BIR1–3 or human Bcl-2. Control and transfected cell lines were treated with different concentrations of ionomycin and kept in culture for 24 h. Cell viability was analyzed using the MTT assay and survival in the absence of ionomycin was set at 100%. Values represent the mean ± SEM of three independent experiments. *p <0.001 for NAIP-BIR1–3 and Bcl-2 versus mock transfected, and p <0.005 for NAIP-BIR1–3 versus Bcl-2. Three cell lines over-expressing the various cDNA constructs were tested, in each case giving similar results.
The BIR domains of NAIP interact with hippocalcin
While it is clear that XIAP, c-IAP-1 and -2 and survivin inhibit specific caspases through direct interaction (Deveraux et al., 1997; Roy et al., 1997; Tamm et al., 1998), this has not been observed with NAIP (Roy et al., 1997). This suggests that the BIR domains of NAIP act elsewhere or require additional molecules to protect cells against harmful stimuli. To investigate this, we used the yeast two-hybrid system to identify potential interactors of the BIR domains of NAIP which may be involved in NAIP’s protective ability. When a human fetal brain library was screened with the BIR domains of NAIP as bait, the most potent interactor found was hippocalcin, a member of a neuron-specific family of calcium binding proteins. Within the two-hybrid system, hippocalcin interacted strongly with NAIP-BIR1–3, but neither with XIAP nor with itself (Table I).
Table I. Specific interaction between NAIP-BIR1–3 and hippocalcin.
| DNA-binding domain construct | Activation domain construct | β-galactosidase activity |
|---|---|---|
| NAIP (aa 46–344) | hippocalcin | ++ |
| NAIP (aa 46–344) | NAIP (aa 46–421) | – |
| NAIP (aa 46–344) | XIAP | – |
| Hippocalcin | hippocalcin | – |
| Hippocalcin | NAIP (aa 46–421) | + |
| Hippocalcin | XIAP | – |
| XIAP | hippocalcin | – |
| XIAP | NAIP (aa 46–421) | – |
Yeast cells (Y190) were sequentially transformed with expression vectors encoding various GAL4 DNA-binding domain and activation domain fusion proteins as indicated. Filter assays for β-galactosidase activity were performed on double transformants streaked out and grown on Trp– Leu– plates for 2 days. Time taken for development of strong blue colour: ++, within 2 h; +, within 4 h; –, never developed.
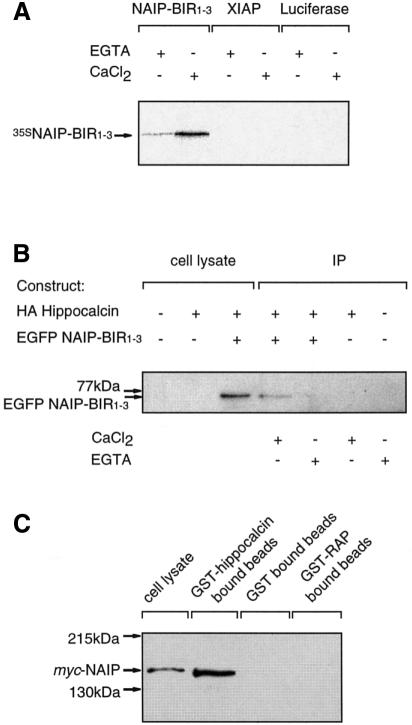
To confirm the interaction found and to determine whether calcium influences the binding, hippocalcin was expressed as a glutathione S-transferase (GST) fusion protein, immobilized on glutathione beads and incubated with radiolabeled in vitro translated NAIP-BIR1–3 in the presence of 1 mM CaCl2 or 1 mM EGTA. Figure 2A shows that NAIP-BIR1–3 bound specifically to hippocalcin and that this interaction was enhanced by calcium. This was also evident in co-immunopreciptiation experiments using COS-7 cells transfected with hemagglutinin (HA)-tagged hippocalcin (HA–hippocalcin) and EGFP–NAIP-BIR1–3. When the constructs were co-expressed, EGFP–NAIP-BIR1–3 was detected by western blot analysis in anti-HA monoclonal antibody (12CA5) immune complexes in the presence but not the absence of 1 mM calcium (Figure 2B). These data show that the BIR domains of NAIP interact specifically with hippocalcin and that binding is enhanced by calcium.
Fig. 2. The BIR domains of NAIP interact with hippocalcin. (A) GST-tagged hippocalcin immobilized on glutathione beads was incubated for 1 h with 35S-labelled NAIP-BIR1–3 generated in vitro. Following centrifugation and washing, the beads were boiled in SDS–PAGE sample buffer and the proteins were resolved by SDS–PAGE and visualized by autoradiography. (B) COS-7 cells were transiently transfected with expression vectors encoding HA-tagged hippocalcin and EGFP–NAIP-BIR1–3. Cells were lysed 48 h after transfection, and anti-HA immunoprecipitates or total cell lysates were analyzed for the presence of EGFP–NAIP-BIR1–3 by western blotting. Immunopreciptiation was performed in the absence (1 mM EGTA) or presence of 1 mM CaCl2. (C) Lysates from COS-7 cells over-expressing myc-tagged full-length NAIP were incubated with GST-tagged hippocalcin, GST alone and control GST-tagged RAP, immobilized on glutathione beads. The presence of bound NAIP after washing was determined by western blot analysis using an anti-Myc antibody.
To ensure that not only the BIR domains of NAIP but also the entire NAIP protein can interact with hippocalcin, lysates containing myc-tagged full-length NAIP were incubated with GST–hippocalcin immobilized on glutathione beads or as controls, immobilized GST protein and GST fused to low density lipoprotein receptor-related protein-associated protein 1 (RAP). Western blot analysis of the proteins attached to the beads following extensive washing shows that full-length NAIP bound to hippocalcin but not to the control proteins (Figure 2C).
Hippocalcin is expressed in spinal cord motor neurons
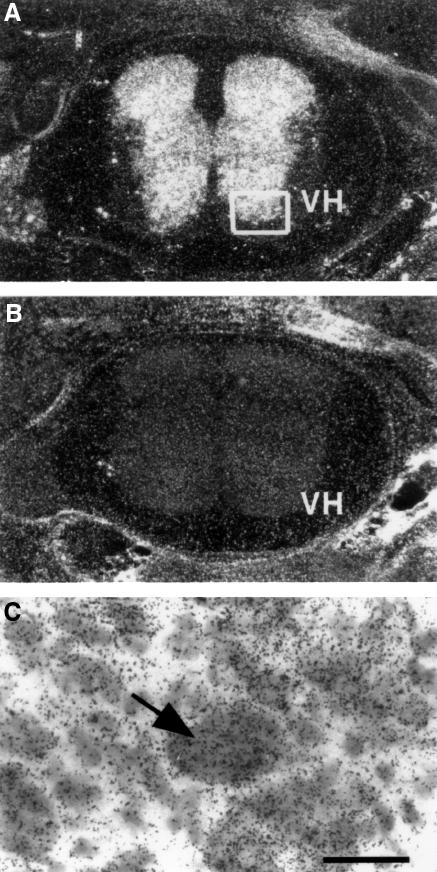
NAIP is expressed within large motor neurons of the spinal cord (Xu et al., 1997b), consistent with an important function for NAIP in these cells. To determine whether hippocalcin is also present in the spinal cord, we performed in situ hybridization (Skoglosa et al., 1999) using a probe specific for rat hippocalcin mRNA. Hippocalcin mRNA was abundantly expressed in neonatal rat spinal cord (Figure 3A), particularly in the cell bodies of the large neurons of lamina 9, the size of which is indicative of α-motor neurons (Figure 3C). This suggests that hippocalcin is present in motor neurons of the spinal cord together with NAIP.
Fig. 3. Presence of hippocalcin mRNA in neonatal rat spinal cord. (A) A synthetic oligonucleotide probe specific for rat hippocalcin was radiolabeled and in situ hybridization was performed as described in Materials and methods. (B) Control hybridization performed with a 200-fold excess of ‘cold’ probe. (C) High magnification of boxed region shown in (A). Arrow indicates motor neuron in lamina 9. Scale bar represents 50 µM. VH denotes ventral horn.
NAIP and hippocalcin synergically protect against calcium-induced neuronal death
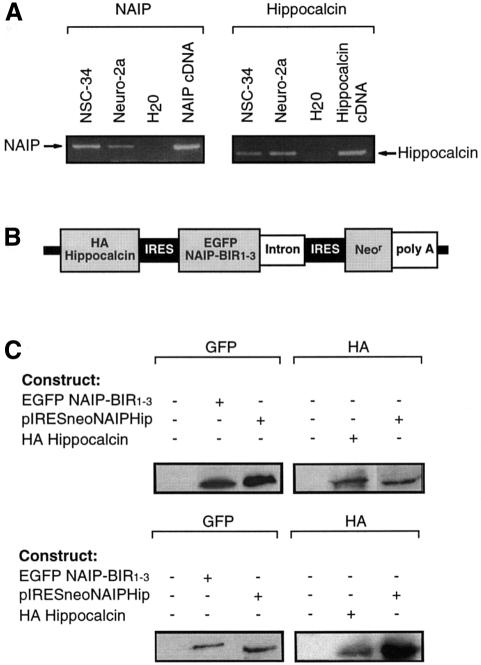
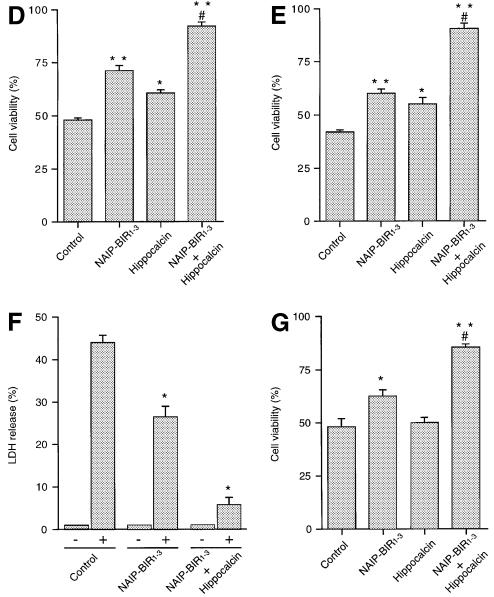
To understand the function of the NAIP–hippocalcin interaction and its effects on motor neuron death, we studied the NSC-34 motor neuron-like cell line which exhibits properties of spinal cord motor neurons (Cashman et al., 1992). These cells, expressing both NAIP and hippocalcin endogenously (Figure 4A), were stably transfected with a vector construct containing cDNAs encoding EGFP–NAIP-BIR1–3 and HA–hippocalcin in tandem, separated by an internal ribosomal entry site (IRES), permitting co-translation of the two proteins (Jackson et al., 1990) (Figure 4B and C). Over-expression of this hybrid vector construct was significantly more effective in counteracting cell death induced by ionomycin than NAIP-BIR1–3 alone, as determined by MTT assay (Figure 4D). Over-expression of hippocalcin alone in NSC-34 cells was neuroprotective to a certain extent against ionomycin but less so than NAIP-BIR1–3. These data show that the combination of NAIP-BIR1–3 and hippocalcin afforded the most robust protection of NSC-34 cells in this experimental setup.
Fig. 4. Inhibition of calcium-induced cell death by NAIP-BIR1–3 and hippocalcin in neuronal cells. (A) Expression of NAIP and hippocalcin in NSC-34 and Neuro-2a cells. mRNA was detected by RT–PCR on total RNA. Specific bands for NAIP (283 bp) and hippocalcin (166 bp) were seen after agarose gel electrophoresis. Empty lanes represent water blank without cDNA. cDNA corresponding to mouse NAIP (1–1100 bp) cloned into pBluescript and human hippocalcin in pACT2 (primers specific for both human and mouse) were used as positive controls. (B) Tricistronic cDNA used for transfection. cDNA encoding HA-tagged hippocalcin and EGFP–NAIP-BIR1–3, separated by an IRES sequence, were cloned into pIRESneo expression vector. (C) Western blot analysis shows the presence of EGFP–NAIP-BIR1–3 and HA–hippocalcin fusion proteins in stably transfected NSC-34 (upper panels) and Neuro-2a (lower panels) cells. (D and E), Wild-type NSC-34 cells (control) and cells stably transfected with EGFP–NAIP-BIR1–3 (NAIP-BIR1–3), HA–hippocalcin (Hippocalcin) and EGFP–NAIP-BIR1–3/HA–hippocalcin (NAIP-BIR1–3 + Hippocalcin) were treated for 24 h with 0.3 µM ionomycin (D) or 0.75 µM thapsigargin (E). Cell viability was determined by MTT assay. Values represent the means ± SEM of three independent experiments. **p <0.001 for NAIP-BIR1–3 and NAIP-BIR1–3 + hippocalcin versus control; *p <0.002 for hippocalcin versus control. #, p <0.001 for NAIP-BIR1–3 + hippocalcin versus NAIP-BIR1–3. (F) Release of LDH after treatment of wild-type and transfected cells with 0.75 µM thapsigargin. Values represent the mean ± SEM (n = 4). *p <0.001 for transfected versus control cells. (G) Wild-type Neuro-2a cells (control) and cells stably transfected with EGFP–NAIP-BIR1–3, HA–hippocalcin and EGFP–NAIP-BIR1–3 + HA–hippocalcin were treated for 24 h with 0.75 µM thapsigargin. Cell viability was determined by MTT assay. Values represent the means ± SEM of three independent experiments. *p <0.006 for NAIP-BIR1–3 versus control; **p <0.001 for NAIP-BIR1–3 + hippocalcin versus control; #, p <0.001 for NAIP-BIR1–3 + hippocalcin versus NAIP-BIR. Three different cell lines over-expressing the various cDNA constructs were tested, in each case giving similar results.
Besides ionomycin, we also studied the effects of thapsigargin, which releases calcium from intracellular stores by blocking uptake (Wei et al., 1998). NSC-34 cells died when treated with sub-micromolar concentrations of thapsigargin; this effect which was inhibited by co-expression of NAIP-BIR1–3 and hippocalcin, and to a lesser degree, by NAIP-BIR1–3 alone (Figure 4E). These results show that over-expression of NAIP-BIR1–3 with hippocalcin renders the NSC-34 motor neurons resistant to calcium-induced cell death.
The data obtained using the MTT assay were substantiated by measuring the amount of lactate dehydrogenase (LDH) released from cells into the medium as a marker for cell viability. Thapsigargin increased LDH release from control NSC-34 cells by ∼45%, compared with 5% in cells co-expressing NAIP-BIR and hippocalcin (Figure 4F). Using NAIP-BIR1–3 over-expressing cells, the LDH effect was intermediate (25% increase), suggesting that both NAIP and hippocalcin are needed for maximal protection against cell death following calcium elevation.
To determine the intracellular calcium levels before and after treatment with ionomycin and thapsigargin, the fura-2 method was used. Control, NAIP-BIR1–3 and NAIP-BIR1–3/hippocalcin transfected NSC-34 cells did not differ with respect to basal Ca2+ concentrations, the maximum peak or sustained (3 min after addition) responses to 0.3 µM ionomycin, and there was no difference in peak and sustained Ca2+ responses to 1 µM thapsigargin in control and NAIP-BIR1–3/hippocalcin cells (data not shown). This suggests that the protective effect of NAIP-BIR1–3 or NAIP-BIR1–3/hippocalcin against NSC-34 cell death is not caused directly by changes in the Ca2+ concentration, as proposed for Bcl-2-induced protection (Lam et al., 1994), though it is not possible here to rule out a difference in long-term Ca2+ response.
The protective synergy of NAIP-BIR1–3 and hippocalcin was also observed in the neuroblastoma cell line, Neuro-2a, stably transfected with NAIP-BIR1–3 and hippocalcin. Neuro-2a cells over-expressing both NAIP-BIR1–3 and hippocalcin significantly abrogated death induced by thapsigargin treatment (0.75 µM) compared with untransfected, NAIP-BIR1–3 and hippocalcin transfected cell lines, as determined by MTT assay (Figure 4G).
NAIP binds hippocalcin via its BIR3 domain
Recent studies have shown that the individual BIR domains of XIAP bind and inhibit different caspase proteins to suppress cell death. The BIR2 domain binds caspase-3/7, while BIR3 combined with the RING domain interacts with caspase-9 (Deveraux et al., 1999).
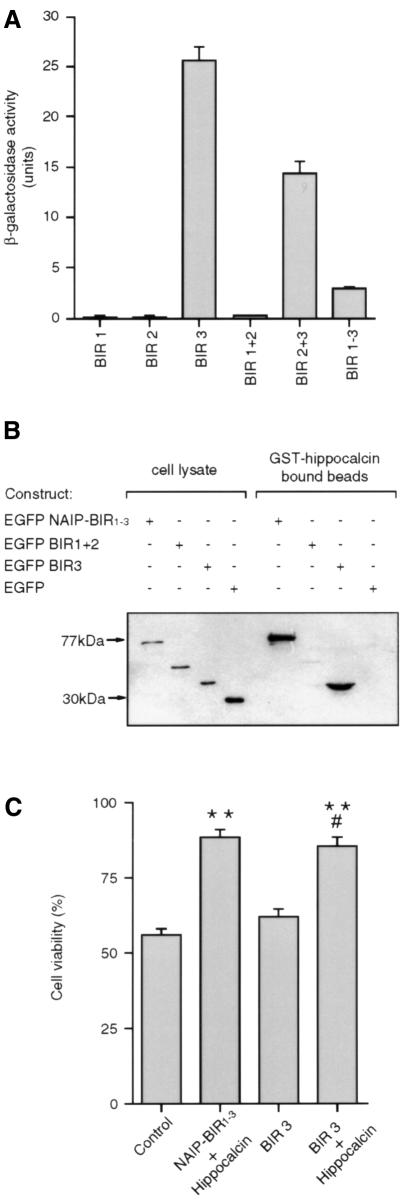
To determine whether a single BIR domain or a combination of BIR domains is required for the binding of NAIP to hippocalcin, deletion constructs of the BIR domains were analysed for the efficiency with which they bound hippocalcin in the yeast two-hybrid system. Figure 5A shows that BIR1 and BIR2 did not bind hippocalcin, while all constructs containing the BIR3 domain bound hippocalcin. Indeed, a construct encoding just the BIR3 domain interacted strongly with hippocalcin.
Fig. 5. The BIR3 domain of NAIP binds hippocalcin to protect against calcium-induced cell death. (A) Yeast two-hybrid assay for binding of the various BIR domains of NAIP to hippocalcin. Yeast cells (Y190) were co-transformed with pACT2 expression vector encoding hippocalcin fused to the activation domain of GAL4, and one of six pYTH6 expression vector constructs encoding one or more of the BIR domains of NAIP each fused to the GAL4 DNA-binding domain. β-galactosidase activity of the double transformants was determined in a liquid assay using ONPG as substrate. (B) COS-7 cells were transiently transfected with EGFP–NAIP-BIR1–3, EGFP–BIR1+2, EGFP–BIR3 and EGFP. Cells were lysed and samples analysed by western blot (lanes 1–4). Lysates containing approximately equivalent amounts of EGFP (fusion) protein were incubated, in the presence of 1 mM CaCl2, with GST–hippocalcin immobilized on glutathione beads. After washing, the presence of bound EGFP fusion proteins was determined by western blot analysis using antibody specific for EGFP (lanes 5–8). (C) Wild-type Neuro-2a cells (control) and cells stably transfected with EGFP–NAIP-BIR1–3 + HA–hippocalcin, EGFP–BIR3 and EGFP–BIR3 + HA–hippocalcin were treated for 24 h with 0.75 µM thapsigargin and cell viability was determined by MTT assay. Values represent the mean ± SEM (n = 4). **p <0.001 for NAIP-BIR1–3 + hippocalcin and BIR3 + hippocalcin versus control. #, p <0.001 for BIR3 + hippocalcin versus BIR3.
To confirm that the BIR3 region alone is sufficient to bind hippocalcin, deletion constructs of the BIR domains of NAIP fused to EGFP were over-expressed in COS-7 cells and their lysates incubated with GST–hippocalcin immobilized on glutathione beads. The beads were washed and bound proteins were analyzed by western blotting. Hippocalcin bound only EGFP–NAIP-BIR1–3 and EGFP–BIR3, not EGFP–BIR1+2 or EGFP alone, indicating that the BIR3 domain is crucial for binding these two proteins (Figure 5B).
The BIR3 domain of NAIP and hippocalcin synergically protect against neuronal death
To determine whether the synergic protective effect of NAIP-BIR1–3 and hippocalcin is mediated through the BIR3 domain, EGFP–BIR3 was expressed in Neuro-2a cells alone and co-expressed with HA–hippocalcin. In parallel, NAIP-BIR1–3 and hippocalcin were also co-expressed. Expression of EGFP–BIR3 did not protect cells treated with thapsigargin but, when co-expressed with hippocalcin, the protection was comparable to that afforded by NAIP-BIR1–3 and hippocalcin (Figure 5C).
The NAIP–hippocalcin interaction protects cells against death mediated through both caspase-3-dependent and -independent pathways
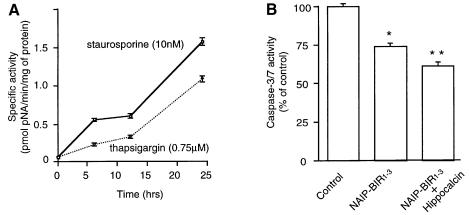
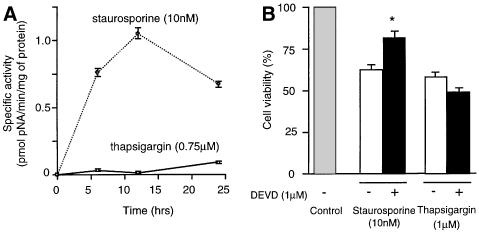
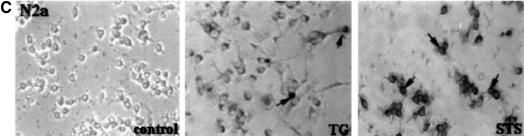
Caspase-3 has a key role in apoptosis (Slee et al., 1999), but recent reports indicate that cell death with the morphological characteristics of apoptosis can occur without caspase-3 activation (Borner and Monney, 1999). To study this, we monitored caspase-3 activity in the NSC-34 cell line at various times after thapsigargin and staurosporine application. Caspase-3-like activity was detectable 6 h after treatment, as determined by cleavage of the caspase-3/7-specific substrate, N-acetyl-Asp-Glu-Asp-p-nitroanilide (Ac-DEVD-pNA) (Figure 6A). The presense of the active form of caspase-3 in the treated cells at 24 h was confirmed using immunocytochemistry with an antibody specific for the active form of caspase-3 (Figure 6C). To determine whether the over-expression of NAIP-BIR1–3 and hippocalcin depressed caspase-3/7 activation in these cells, stable cell lines were assayed for caspase-3/7 activity after 24 h thapsigargin treatment. The results show that NAIP-BIR significantly reduced caspase-3/7 activity, but this was augmented by co-expression of hippocalcin, consistent with a synergic protective effect of these two proteins (Figure 6B).
Fig. 6. Caspase-3 is activated in NSC-34 cells after staurosporine or thapsigargin treatment. (A) Staurosporine (10 nM) or thapsigargin (0.75 µM) was added to NSC-34 and, at indicated times, cells were lysed and caspase-3/7 activity was measured as described in Materials and methods. (B) Wild-type NSC-34 cells (control) and cells stably transfected with EGFP–NAIP-BIR1–3 (NAIP-BIR1–3) and EGFP–NAIP-BIR1–3/HA–hippocalcin (NAIP-BIR1–3 + Hippocalcin) were treated for 24 h with thapsigargin (0.75 µM) and the lysates were assayed for caspase-3/7 activity. Values represent the mean ± SEM (n = 3). *p <0.001 for NAIP-BIR1–3 versus control. **p <0.001 for NAIP-BIR1–3 + hippocalcin versus control. (C) NSC-34 cells were treated with thapsigargin (TG) or staurosporine (STS) for 24 h at the concentrations indicated above and stained for activated caspase-3.
To substantiate these data, caspase-3-like activity was also assayed in Neuro-2a cells after staurosporine and thapsigargin treatment. As in NSC-34 cells, caspase-3/7 activity was readily detectable 6–24 h after staurosporine addition (Figure 7A). In contrast, caspase-3/7 activity was virtually undetectable after 24 h of thapsigargin treatment, after which time >50% of cells were dead (Figure 7A). To clarify these results, Neuro-2a cells were treated with staurosporine or thapsigargin in the presence or absence of the specific caspase-3/7 inhibitor, z-DEVD-fmk (fmk, fluoromethyl ketone). z-DEVD-fmk was partially protective against staurosporine, but not against thapsigargin (Figure 7B). Immunocytochemical analysis of treated Neuro-2a cells at 24 h using the active caspase-3 specific antibody showed many positive cells after staurosporine treatment, but very few after thapsigargin treatment (Figure 7C). Analysis of the caspase-3 substrate poly(ADP–ribose) polymerase (PARP) (Tewari et al., 1995) in lysates from Neuro-2a cells treated with staurosporine revealed the 89 kDa breakdown product associated with caspase-3-induced cell death. In contrast, lysates of control and thapsigargin-treated cells indicated no cleavage of PARP, in line with caspase-3-independent cell death.
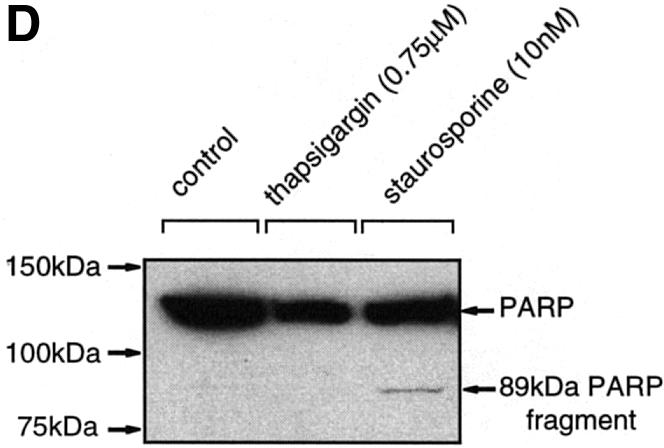
Fig. 7. Caspase-3 is activated in Neuro-2a cells after staurosporine treatment but not after thapsigargin treatment. (A) Staurosporine (10 nM) or thapsigargin (0.75 µM) was added to Neuro-2a and, at the indicated times, cells were lysed and caspase-3/7 activity was measured as described in Materials and methods. (B) Staurosporine (10 nM) or thapsigargin (10 µM) was applied for 24 h to Neuro-2a cells in the presence or absence of z-DEVD-fmk (1 µM) and cell viability was determined by MTT assay. Values indicate the mean ± SEM (n = 7) and are representative of three independent experiments. *p <0.001 for staurosporine + z-DEVD-fmk versus staurosporine. (C) Neuro-2a cells were treated with thapsigargin (TG) or staurosporine (STS) for 24 h at the concentrations indicated above and stained for activated caspase-3. (D) Lysates prepared from untreated Neuro-2a cells and cells treated with staurosporine (10 nM) or thapsigargin (0.75 µM) for 24 h were subjected to western blotting using an antibody specific for PARP (116 kDa) and its 89 kDa fragment (CII10).
Discussion
This study was undertaken to examine how NAIP protects neuronal cells. The results show that the N-terminal part of NAIP, containing the BIR domains, significantly protected against cell death induced by calcium overload of the motor neuron-like NSC-34 cell line. The effect of NAIP-BIR1–3 in these cells was not as robust as that observed with over-expression of the anti-apoptotic protein, Bcl-2, in line with a different mechanism of action for NAIP in cell death protection involving different proteins.
Using the two-hybrid system and co-immunoprecipitation studies, we have identified hippocalcin as a protein specifically binding to the BIR domains of NAIP in a calcium-promoted interaction. Co-expression of hippocalcin with NAIP’s BIR domains markedly enhanced its protective capacity against calcium insult in both the NSC-34 motor neuron-like and Neuro-2a neuroblastoma cell lines, indicating synergy between the two proteins in neurons.
Previously, it was shown that NAIP, in addition to XIAP and the c-IAPs, protect against a wide variety of death-inducing agents in cultured non-neuronal cells such as Chinese hamster ovary (CHO) and fibroblast cells (Liston et al., 1996). Subsequent investigations showed that over-expression of NAIP is able to counteract neuronal cell death in hippocampus after ischemia, though the mechanism involved is not understood (Xu et al., 1997a). The anti-death potential of other mammalian IAPs is derived from their inhibition of specific caspases (Deveraux et al., 1997; Roy et al., 1997; Tamm et al., 1998), but the inability of NAIP to bind any of the caspases tested to date (Roy et al., 1997) points to alternative mechanisms. Neuronal cells are, in many respects, more intricate than non-neuronal cells, especially with regard to cell excitability and handling of calcium ions. Neuronal survival is crucially dependent on intracellular calcium concentration, which is regulated by neurotrophic factors, different uptake and release processes, and buffering molecules (Berridge et al., 1998). Whereas low intracellular calcium is detrimental, increasing the calcium concentration to a certain extent enhances cell viability (Koike et al., 1989; Yano et al., 1998). Large increases in calcium concentrations, however, trigger death by various mechanisms in different cells (McConkey and Orrenius, 1997; Kruman et al., 1998). Motor neurons are particularly vulnerable to increased calcium concentrations caused, for example, by excessive glutamate receptor stimulation (Rothstein et al., 1993; Berridge et al., 1998). Recently, it was shown that excitotoxicity of cultured forebrain neurons by glutamate involves uptake of calcium into mitochondria, which can trigger neuronal death (Stout et al., 1998). However, so far there are no data on the role of IAP proteins in protecting against calcium-induced neuronal death.
To study the role of NAIP in neurons, we challenged NSC-34 (Cashman et al., 1992) and Neuro-2a (Olmsted et al., 1970) cell lines with agents that increase intracellular calcium concentrations. Within 24 h after ionomycin or thapsigargin treatment, many NSC-34 cells died. Over-expression of NAIP-BIR1–3 and, to a lesser extent, hippocalcin, rendered the NSC-34 cells more resistant to calcium-induced cell death. Co-expression of NAIP-BIR1–3 with hippocalcin almost completely abrogated death induced by both agents. This finding indicates a synergic effect of NAIP-BIR1–3 and hippocalcin in protecting NSC-34 cells against increased calcium concentrations. This synergy was also found in Neuro-2a cells in response to thapsigargin treatment. The enhanced binding of hippocalcin to NAIP-BIR1–3 observed in vitro upon increased calcium concentrations is consistent with an interaction between the two proteins in vivo when intracellular calcium concentrations are increased. It should be noted that there was no significant difference in calcium concentrations between control and NAIP-BIR1–3, and/or hippocalcin over-expressing NSC-34 cells under these conditions, suggesting that the regulation of calcium per se was not changed by the transfection. However, long-term effects on calcium concentrations, not revealed in this study, may still occur in NAIP-BIR1–3 or NAIP-BIR1–3/hippocalcin over-expressing cells. Alternatively, the local concentration of calcium, for example within mitochondria, may differ between control and NAIP-BIR1–3/hippocalcin over-expressing cells.
Hippocalcin is a member of family of neuron-specific proteins characterized by four EF-hand regions of which either two or three can bind calcium ions (Takamatsu and Noguchi, 1993). Previous studies have revealed the expression patterns of hippocalcin in the developing and adult brain (Saitoh et al., 1993, 1994), but there are no data on the functional role of the molecule. Recoverin, which belongs to the same family, is involved in regulating the half-life of activated rhodopsin in the retina (Chen et al., 1995). Intriguingly, recoverin has been identified as an autoantigen in cancer-associated retinopathy (CAR), a degenerative disease of the retina (Polans et al., 1995). Anti-recoverin antibodies from sera of CAR patients can penetrate retina photoreceptor and bipolar cells after intravitreal injection, causing widespread apoptosis (Adamus et al., 1998). The connection between recoverin and CAR, and now of hippocalcin with NAIP, suggests that this family of proteins may play a significant role in neuronal survival.
Recent studies have shown that both XIAP and the c-IAPs inhibit specific caspases (Roy et al., 1997; Deveraux et al., 1999). For XIAP, it has been determined that the second of its three BIR domains inhibits the activated form of caspase-3/7 (Takahashi et al., 1998). In contrast, the mechanism of caspase-9 inhibition involves prevention of autoproteolytic processing into its active conformation by the BIR3 domain in conjunction with the C-terminal RING domain (Deveraux et al., 1999). NAIP, like XIAP, has three BIR domains, but no C-terminal RING motif. The absence of the latter in NAIP suggests that its BIR3 domain does not inhibit the processing of caspase-9, but has an alternative function. Data from both the yeast two-hybrid system and in vitro binding studies show that the BIR3 domain of NAIP, but not BIR1 or 2, interacts with hippocalcin. XIAP did not bind hippocalcin, indicating specificity for NAIP. Expression of the BIR3 domain of NAIP in Neuro-2a cells had no protective effect against calcium-induced cell death, but co-expression with hippocalcin suppressed cell death. This demonstrates that the BIR3 domain of NAIP both binds hippocalcin and, through the interaction, exerts a protective effect.
Caspase-3 is a key protein promoting apoptosis in most cells studied. It is activated by cleavage of its proform by the upstream caspases-8 and -9, which are activated by different stimuli (reviewed in Slee et al., 1999). Cells can die in an apoptotic manner independently of this enzyme activity, though (Borner and Monney, 1999). Our results indicate that thapsigargin, which increases intracellular calcium, can kill Neuro-2a cells through a pathway not involving caspase-3 activation. Staurosporine readily activated caspase-3 in these cells, showing that the enzyme was present. The lack of an effect of thapsigargin on caspase-3 indicates that, in these cells, an alternative pathway is used for the protection by NAIP and hippocalcin. Stress, elicited by thapsigargin, on the endoplasmic reticulum has recently been implicated in cell death, drawing attention to a novel pathway involving caspase-12 (Nakagawa et al., 2000). It remains to be studied whether NAIP in conjunction with hippocalcin can influence other caspases such as caspase-12 in neurons.
In this study, we show that the BIR domains of NAIP are sufficient to protect the NSC-34 and Neuro-2a cell lines against calcium-induced cell death. NAIP, through its BIR domains, binds hippocalcin in an interaction enhanced by calcium. The increased affinity of NAIP-BIR1–3 for hippocalcin under high calcium ion concentrations indicates that the two proteins may interact in cells after intracellular calcium is increased. At low calcium concentrations, hippocalcin remains calcium-free and is predominantly cytosolic (Saitoh et al., 1993), whereas at higher concentration hippocalcin binds calcium, inducing a conformational change which promotes membrane association (Kobayashi et al., 1993; Takamatsu and Noguchi, 1993). It remains to be determined whether the calcium-mediated switch in configuration prompts an alteration in the cellular distribution of NAIP, which in turn, may influence NAIP’s protective properties.
Our results identify the BIR3 region of NAIP as responsible for the binding of hippocalcin and for neuroprotection. Previously the BIR2 domain of XIAP was shown to inhibit caspase-3/7. Taken together, the data suggest that the individual BIR regions may have different roles in cell death. The insights into the function of the BIR3 domain may be of importance for designing potentially novel therapeutic measures against neurodegenerative disorders, such as SMA.
This study provides the first evidence that an IAP can protect against calcium-induced cell death and that this effect can be substantially enhanced within neurons by an interaction with a small calcium-binding protein. In addition to hippocalcin, there are other members of this neuron-specific family whose functions have yet to be characterized. NAIP’s interaction with hippocalcin may represent a general mechanism by which the protective effect of IAPs can be augmented within neurons by members of the hippocalcin family.
Materials and methods
Cloning of NAIP and XIAP
cDNA corresponding to amino acids (aa) 6–421 of NAIP was synthesized from human placenta cDNA using PCR primers and conditions as previously described (Roy et al., 1995), and cloned into pBluescript. Full-length XIAP cDNA was obtained by screening from a human fetal brain library (Clontech). PCR was used to generate constructs with appropriate restriction sites for cloning into expression vectors. The sequences of all constructs were verified by automated sequencing.
Expression constructs
cDNA encompassing the BIR domains of NAIP was subcloned in-frame into the GAL4 DNA-binding domain vector pYTH6 (aa 46–344) and activation domain vector, pACT2 (aa 46–421). Full-length XIAP cDNA was subcloned into two-hybrid vectors pGBT9 and pGAD424, and hippocalcin into pYTH6 and into the bacterial GST expression vector pGEX-6P. For expression in mammalian cells, NAIP cDNA (aa 6–421) was subcloned into pEGFPC1 and pCDNA3.1HisC. Full-length hippocalcin was subcloned into pCDNA3.1HisC, pEGFPC3 and pIRESneo behind an in-frame HA epitope tag. A tricistronic construct was generated in pIRESneo by inserting an IRES sequence downstream of HA-tagged hippocalcin, followed by EGFP–NAIP-BIR1–3 (aa 6–421), a synthetic intron, IRES sequence and neomycin resistance gene of the vector. Deletion constructs of NAIP’s BIR domains were cloned into pYTH6 (BIR1aa 46–157; BIR2aa 161–268; BIR3aa 264–362; BIR1+2aa 46–233; BIR2+3aa 161–344) and pEGFPC1 (BIR1+2aa 46–233; BIR3aa 264–362). Myc-tagged full-length human NAIP cDNA in pCDNA3, human Bcl-2 cDNA in pSFFV and GST–RAP in pGEX-2T were kindly provided by Alex MacKenzie, Stanley Korsmeyer and Mårten Larsson, respectively.
Yeast two-hybrid system
Screening. pYTH6 NAIP-BIR (aa 46–344) was linearized and integrated into the yeast genome of yeast strain Y190 through a lithium acetate/polyethylene glycol (PEG)-based transformation method and used to screen a human fetal brain library (Clontech) essentially as previously described (Aspenstrom and Olson, 1995). Transformants were grown on Trp– Leu– His– plates supplemented with 25 mM 3-aminotriazole for 4–8 days and streaked on to Trp– Leu– His– and Trp– Leu– plates. Colonies were assayed for β-galactosidase after 2 days using a filter lift procedure (Aspenstrom and Olson, 1995).
ONPG assay. Double transformants were streaked on to Trp– Leu– plates and grown for 2 days. Three individual colonies for each double transformant type were picked and grown overnight at 30°C in 5 ml Trp– Leu– liquid medium. On the next day, culture volumes were increased to 25 ml and the yeast grown to an OD600nm of ∼1.0. The yeast cells were harvested and resuspended in Z-buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4) and the suspension was divided into three. Following repeated freeze–thawing in liquid nitrogen and a 30°C water bath, Z-buffer containing β-mercaptoethanol and the substrate O-nitrophenyl-β-D-galactopyranoside (ONPG) were added to the samples at a final concentration of 2% (v/v) and 2.2 mM, respectively. The samples were incubated at 30°C until a yellow colour developed; the reaction was then terminated by addition of Na2CO3 and cleared by centrifugation. The OD420nm was determined for each sample and the β-galactosidase activity determined (Aspenstrom and Olson, 1995).
In vitro binding assays
GST–hippocalcin, GST–RAP and GST were expressed, purified and immobilized on glutathione beads according to standard methodology. 35S-labeled NAIP-BIR (aa 6–421), XIAP and luciferase proteins were generated from cDNAs by in vitro transcription and translation using the TNT T7-coupled reticulocyte lysate system (Promega), but with the addition of 1 mM ZnCl2. 35S-labeled NAIP-BIR1–3 (15 µl), 35S-labeled XIAP (30 µl) or 35S-labeled luciferase (30 µl) were diluted in 700 µl of binding buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 1% NP-40) supplemented with 1 mM CaCl2 or 1 mM EGTA and incubated for 1 h with GST–hippocalcin immobilized on glutathione beads (50 µl). The beads were recovered by centrifugation, washed eight times in the appropriate binding buffer and boiled in SDS–PAGE sample buffer, and the supernatant was retrieved by centrifugation. Samples were resolved by SDS–PAGE and visualized by autoradiography.
COS-7 cells were transfected at 75% confluency using the calcium phosphate method (30 µg DNA per 10 cm plate). The cells were washed in phosphate-buffered saline (PBS) after 48 h transfection and lysed for 40 min in 1 ml of ice-cold lysis buffer [50 mM HEPES pH 7.5, 250 mM NaCl, 0.2% NP-40 and protease inhibitors (Boehringer Complete, EDTA-free)], supplemented with 1 mM CaCl2 or 1 mM EGTA and incubated for 1 h with GST–hippocalcin immobilized on glutathione beads (50 µl). As controls, GST alone and GST–RAP were used. The beads were recovered by centrifugation, washed three times in the appropriate binding buffer and boiled in SDS–PAGE sample buffer, and the supernatant was retrieved by centrifugation. Samples were resolved by SDS–PAGE and transferred to nitrocellulose membrane for western blot analysis. For analysis, rabbit anti-GFP (Clontech), monoclonal anti-Myc epitope, goat anti-rabbit IgG conjugated to horseradish peroxidase (HRP) and rabbit anti-mouse IgG conjugated to HRP antibodies were used. Bound antibody was detected by enhanced chemiluminescence.
Co-immunoprecipitation and western blot analysis
COS-7 cells were transfected and lysed as described above in lysis buffer supplemented with 1 mM CaCl2 or 1 mM EGTA. Monoclonal anti-HA epitope antibody (3.5 µg) (clone 12CA5) was added to the cleared lysate (800 µl) and the mixture rotated for 2 h at 4°C. Complexes were immunoprecipitated with protein G–Sepharose (30 µl) for 1 h at 4°C and washed three times with the appropriate lysis buffer. The Sepharose beads were boiled in SDS–PAGE sample buffer and the supernatant was retrieved by centrifugation. Samples were resolved by SDS–PAGE and transferred to nitrocellulose membrane for western blot analysis. For analysis, rabbit anti-GFP (Clontech), monoclonal anti-HA epitope, goat anti-rabbit IgG conjugated to HRP and rabbit anti-mouse IgG conjugated to HRP were used. Bound antibody was detected by enhanced chemiluminescence.
In situ hybridization
In situ hybridization of hippocalcin was performed using neonatal Wistar rats on spinal cord sections. The oligonucleotide probe was 47 nucleotides in length, complementary to nucleotides 58–104 of the rat hippocalcin coding sequence (Skoglosa et al., 1999) (Scandinavian Gene Synthesis). A 200-fold excess of cold probe was used as a negative control. Expression patterns of hippocalcin mRNA in the brain corresponded to previously described work (Saitoh et al., 1993).
RT–PCR
Total RNA extracted from NSC-34 and Neuro-2a cells was used to generate cDNA using a cDNA synthesis kit (Stratagene, La Jolla, CA). For PCR analysis of NAIP and hippocalcin expression, primers corresponding to base pairs 220–243 and 483–503 and base pairs 46–66 and 185–211 were used, respectively, in 25 cycles of amplification.
Northern blot analysis
Total RNA from NSC-34 cells was prepared, subjected to gel electrophoresis and transferred to a nylon filter (Amersham). The filters were hybridized overnight with a [32P]dCTP-labelled (Rediprime, Amersham) NAIP-BIR1–3 probe in hybridization solution (Korhonen et al., 1997). After washing, the amount of NAIP-BIR1–3 transcripts was determined by autoradiography.
Cell culture
NSC-34 cells (kindly given by Dr Cashman) and Neuro-2a cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and transfected with different expression vectors as previously described. Stable clones were selected using G418 (600 µg/ml, Life Technologies). The amounts of NAIP-BIR1–3 and hippocalcin fusion proteins were determined by western blot analysis. Stably expressing NSC-34 and Neuro-2a cell lines were treated for 24 h with ionomycin (Sigma) or thapsigargin (Sigma). The extent of cell death was determined by MTT assay (Skoglosa et al., 1999) and an enzymatic assay for LDH (Boehringer-Mannheim). Three different cell lines over-expressing the various cDNA constructs were tested in each case. Statistical analysis was performed using ANOVA and a two-tailed Student’s t-test.
The survival of wild-type Neuro-2a cells after treatment for 24 h with ionomycin (1 µM), staurosporine (10 nM) or thapsigargin (0.75 µM) in the presence or absence of the caspase-3/7 inihibitor z-DEVD-fmk (Clontech) at 1 µM was determined by MTT assay.
Calcium assay
Intracellular calcium ion concentrations were measured using fura-2 essentially as previously described (Kukkonen et al., 1997), except that 1 mM probenecid [p-(dipropylsulfamoyl)benzoic acid; Sigma] was added to the medium during the fura-2 loading and measurement to inhibit the leak of fura-2.
Caspase-3 assay
The caspase-3 assay was performed using a Caspase-3 Cellular Activity Assay kit (BioMol). In brief, thapsigargin (0.75 µM) or staurosporine (10 nM) in DMEM was applied to NSC-34 and Neuro-2a cells deprived of serum for 4 h. At the times indicated, the cells were washed once in PBS, lysed in cell lysis buffer supplemented with 0.1% NP-40 and centrifuged for 10 min at 10 000 g. In a reaction volume of 100 µl, 10 µl of the lysate supernatant (1–3 µg/µl) was added to assay buffer and the caspase-3/7 substrate Ac-DEVD-pNa (200 µM) and incubated at 37°C. Release of p-nitroaniline was monitored by recording the OD405nm at 30 min intervals. Assays were performed in duplicate.
Immunocytochemistry
NSC-34 and Neuro-2a cells were treated with thapsigargin (0.75 µM) or staurosporine (1 nM) for 24 h then fixed for 10 min with acetone:methanol (1:1) at –20°C. After fixation, endogenous peroxidases were quenched with H2O2 and the cells blocked with TNB buffer (NEN). Primary anti-active caspase-3 antibody (Pharmingen) was used at 1:100 dilution and secondary anti-rabbit antibody (Vector laboratories) at 1:200 dilution. Specific signal was visualized using diaminobenzidine as a chromogen.
PARP cleavage
Neuro-2a cells were treated for 24 h with staurosporine (10 nM) or thapsigargin (0.75 µM) in serum-free DMEM, and as control with DMEM containing 5% serum. To prepare samples for western blot analysis, cells were resuspended in medium and pelleted at 1000 g for 2 min. After two washes in ice-cold PBS supplemented with protease inhibitors (Boehringer Complete, EDTA-free), from which a small portion has been taken for determination of protein concentration, the cells were lysed in denaturing lysis buffer (62.5 mM Tris–Cl pH 6.8, 6 M urea, 10% glycerol, 2% SDS, 5% β-mercaptoethanol). The samples were subjected to two 15 s sonication pulses while on ice; they were then incubated for 15 min at 65°C and Bromophenol blue was added to 0.01%. Samples (25 µg) were resolved by SDS–PAGE and transferred to nitrocellulose membrane for western blot analysis. For analysis, C2-10 monoclonal anti-PARP antibody (BioMol) and rabbit anti-mouse IgG conjugated to HRP were used. Bound antibody was detected by enhanced chemiluminescence.
Acknowledgments
Acknowledgements
We thank Dr N.Cashman for the NSC-34 cell line, Dr Alex MacKenzie for full-length NAIP cDNA, Dr Stanley Korsmeyer for the human Bcl-2-expressing vector, Mårten Larsson for the GST–RAP-expressing vector and Professor Karl E.O.Åkerman for scientific advice. This work was supported by the Swedish Cancer Foundation (Cancerfonden), a European Biotechnology Grant and the Swedish Foundation for Strategic Research.
References
- Adams J.M. and Cory,S. (1998) The Bcl-2 protein family: arbiters of cell survival. Science, 281, 1322–1326. [DOI] [PubMed] [Google Scholar]
- Adamus G., Machnicki,M., Elerding,H., Sugden,B., Blocker,Y.S. and Fox,D.A. (1998) Antibodies to recoverin induce apoptosis of photoreceptor and bipolar cells in vivo. J. Autoimmun., 11, 523–533. [DOI] [PubMed] [Google Scholar]
- Ambrosini G., Adida,C. and Altieri,D.C. (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nature Med., 3, 917–921. [DOI] [PubMed] [Google Scholar]
- Aspenstrom P. and Olson,M.F. (1995) Yeast two-hybrid system to detect protein–protein interactions with Rho GTPases. Methods Enzymol., 256, 228–241. [DOI] [PubMed] [Google Scholar]
- Berridge M.J., Bootman,M.D. and Lipp,P. (1998) Calcium—a life and death signal. Nature, 395, 645–648. [DOI] [PubMed] [Google Scholar]
- Borner C. and Monney,L. (1999) Apoptosis without caspases: an inefficient molecular guillotine? Cell Death Differ., 6, 497–507. [DOI] [PubMed] [Google Scholar]
- Cashman N.R., Durham,H.D., Blusztajn,J.K., Oda,K., Tabira,T., Shaw,I.T., Dahrouge,S. and Antel,J.P. (1992) Neuroblastoma × spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev. Dyn., 194, 209–221. [DOI] [PubMed] [Google Scholar]
- Chen C.K., Inglese,J., Lefkowitz,R.J. and Hurley,J.B. (1995) Ca2+-dependent interaction of recoverin with rhodopsin kinase. J. Biol. Chem., 270, 18060–18066. [DOI] [PubMed] [Google Scholar]
- Clem R.J. and Duckett,C.S. (1997) The IAP genes: unique arbitrators of cell death. Trends Cell Biol., 7, 337–339. [DOI] [PubMed] [Google Scholar]
- Deveraux Q.L., Leo,E., Stennicke,H.R., Welsh,K., Salvesen,G.S. and Reed,J.C. (1999) Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J., 18, 5242–5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux Q.L. and Reed,J.C. (1999) IAP family proteins—suppressors of apoptosis. Genes Dev., 13, 239–252. [DOI] [PubMed] [Google Scholar]
- Deveraux Q.L., Roy,N., Stennicke,H.R., Van Arsdale,T., Zhou,Q., Srinivasula,S.M., Alnemri,E.S., Salvesen,G.S. and Reed,J.C. (1998) IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J., 17, 2215–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux Q.L., Takahashi,R., Salvesen,G.S. and Reed,J.C. (1997) X-Linked IAP is a direct inhibitor of cell-death proteases. Nature, 388, 300–304. [DOI] [PubMed] [Google Scholar]
- Fraser A.G., James,C., Evan,G.I. and Hengartner,M.O. (1999) Caenorhabditis elegans inhibitor of apoptosis protein (IAP) homologue BIR-1 plays a conserved role in cytokinesis. Curr. Biol., 9, 292–301. [DOI] [PubMed] [Google Scholar]
- Hinds M.G., Norton,R.S., Vaux,D.L. and Day,C.L. (1999) Solution structure of a baculoviral inhibitor of apoptosis (IAP) repeat. Nature Struct. Biol., 6, 648–651. [DOI] [PubMed] [Google Scholar]
- Jackson R.J., Howell,M.T. and Kaminski,A. (1990) The novel mechanism of initiation of picornavirus RNA translation. Trends Biochem. Sci., 15, 477–483. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Takamatsu,K., Saitoh,S. and Noguchi,T. (1993) Myristoylation of hippocalcin is linked to its calcium-dependent membrane association properties. J. Biol. Chem., 268, 18898–18904. [PubMed] [Google Scholar]
- Koike T., Martin,D.P. and Johnson,E.M.,Jr (1989) Role of Ca2+ channels in the ability of membrane depolarization to prevent neuronal death induced by trophic-factor deprivation: evidence that levels of internal Ca2+ determine nerve growth factor dependence of sympathetic ganglion cells. Proc. Natl Acad. Sci. USA, 86, 6421–6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen L., Hamner,S., Olsson,P.A. and Lindholm,D. (1997) Bcl-2 regulates the levels of the cysteine proteases ICH and CPP32/Yama in human neuronal precursor cells. Eur. J. Neurosci., 9, 2489–2496. [DOI] [PubMed] [Google Scholar]
- Kruman I., Guo,Q. and Mattson,M.P. (1998) Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J. Neurosci. Res., 51, 293–308. [DOI] [PubMed] [Google Scholar]
- Kukkonen J.P., Shariatmadari,R., Courtney,M.J. and Akerman,K.E. (1997) Localization of voltage-sensitive Ca2+ fluxes and neuropeptide Y immunoreactivity to varicosities in SH-SY5Y human neuroblastoma cells differentiated by treatment with the protein kinase inhibitor staurosporine. Eur. J. Neurosci., 9, 140–150. [DOI] [PubMed] [Google Scholar]
- Lam M., Dubyak,G., Chen,L., Nunez,G., Miesfeld,R.L. and Distelhorst,C.W. (1994) Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc. Natl Acad. Sci. USA, 91, 6569–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Ambrosini,G., Chu,E.Y., Plescia,J., Tognin,S., Marchisio,P.C. and Altieri,D.C. (1998) Control of apoptosis and mitotic spindle checkpoint by survivin. Nature, 396, 580–584. [DOI] [PubMed] [Google Scholar]
- Liston P. et al. (1996) Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature, 379, 349–353. [DOI] [PubMed] [Google Scholar]
- McConkey D.J. and Orrenius,S. (1997) The role of calcium in the regulation of apoptosis. Biochem. Biophys. Res. Commun., 239, 357–366. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Zhu,H., Morishima,N., Li,E., Xu,J., Yankner,B.A. and Yuan,J. (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature, 403, 98–103. [DOI] [PubMed] [Google Scholar]
- Olmsted J.B., Carlson,K., Klebe,R., Ruddle,F. and Rosenbaum,J. (1970) Isolation of microtubule protein from cultured mouse neuroblastoma cells. Proc. Natl Acad. Sci. USA, 65, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polans A.S., Witkowska,D., Haley,T.L., Amundson,D., Baizer,L. and Adamus,G. (1995) Recoverin, a photoreceptor-specific calcium-binding protein, is expressed by the tumor of a patient with cancer-associated retinopathy. Proc. Natl Acad. Sci. USA, 92, 9176–9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S. and Balasubramanian,M.K. (1999) S. pombe Pbh1p: an inhibitor of apoptosis domain containing protein is essential for chromosome segregation. FEBS Lett., 460, 187–190. [DOI] [PubMed] [Google Scholar]
- Rothe M., Pan,M.G., Henzel,W.J., Ayres,T.M. and Goeddel,D.V. (1995) The TNFR2–TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell, 83, 1243–1252. [DOI] [PubMed] [Google Scholar]
- Rothstein J.D., Jin,L., Dykes-Hoberg,M. and Kuncl,R.W. (1993) Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc. Natl Acad. Sci. USA, 90, 6591–6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N. et al. (1995) The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell, 80, 167–178. [DOI] [PubMed] [Google Scholar]
- Roy N., Deveraux,Q.L., Takahashi,R., Salvesen,G.S. and Reed,J.C. (1997) The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J., 16, 6914–6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh S., Takamatsu,K., Kobayashi,M. and Noguchi,T. (1993) Distribution of hippocalcin mRNA and immunoreactivity in rat brain. Neurosci. Lett., 157, 107–110. [DOI] [PubMed] [Google Scholar]
- Saitoh S., Takamatsu,K., Kobayashi,M. and Noguchi,T. (1994) Expression of hippocalcin in the developing rat brain. Dev. Brain Res., 80, 199–208. [DOI] [PubMed] [Google Scholar]
- Simons M. et al. (1999) Adenovirus-mediated gene transfer of inhibitors of apoptosis protein delays apoptosis in cerebellar granule neurons. J. Neurochem., 72, 292–301. [DOI] [PubMed] [Google Scholar]
- Skoglosa Y., Lewen,A., Takei,N., Hillered,L. and Lindholm,D. (1999) Regulation of pituitary adenylate cyclase activating polypeptide and its receptor type 1 after traumatic brain injury: comparison with brain-derived neurotrophic factor and the induction of neuronal cell death. Neuroscience, 90, 235–247. [DOI] [PubMed] [Google Scholar]
- Slee E.A., Adrain,C. and Martin,S.J. (1999) Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ., 6, 1067–1074. [DOI] [PubMed] [Google Scholar]
- Stout A.K., Raphael,H.M., Kanterewicz,B.I., Klann,E. and Reynolds,I.J. (1998) Glutamate-induced neuron death requires mitochondrial calcium uptake. Nature Neurosci., 1, 366–373. [DOI] [PubMed] [Google Scholar]
- Sun C. et al. (1999) NMR structure and mutagenesis of the inhibitor-of-apoptosis protein XIAP. Nature, 401, 818–822. [DOI] [PubMed] [Google Scholar]
- Takahashi R., Deveraux,Q., Tamm,I., Welsh,K., Assa-Munt,N., Salvesen,G.S. and Reed,J.C. (1998) A single BIR domain of XIAP sufficient for inhibiting caspases. J. Biol. Chem., 273, 7787–7790. [DOI] [PubMed] [Google Scholar]
- Takamatsu K. and Noguchi,T. (1993) Hippocalcin: a calcium-binding protein of the EF-hand superfamily dominantly expressed in the hippocampus. Neurosci. Res., 17, 291–295. [DOI] [PubMed] [Google Scholar]
- Tamm I., Wang,Y., Sausville,E., Scudiero,D.A., Vigna,N., Oltersdorf,T. and Reed,J.C. (1998) IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases and anticancer drugs. Cancer Res., 58, 5315–5320. [PubMed] [Google Scholar]
- Tewari M., Quan,L.T., O’Rourke,K., Desnoyers,S., Zeng,Z., Beidler,D.R., Poirier,G.G., Salvesen,G.S. and Dixit,V.M. (1995) Yama/CPP32β, a mammalian homolog of CED-3, is a Crm-inhibitable protease that cleaves the death substrate poly(ADP–ribose) polymerase. Cell, 81, 801–809. [DOI] [PubMed] [Google Scholar]
- Wang C.Y., Mayo,M.W., Korneluk,R.G., Goeddel,D.V. and Baldwin,A.S.,Jr (1998) NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science, 281, 1680–1683. [DOI] [PubMed] [Google Scholar]
- Wei H., Wei,W., Bredesen,D.E. and Perry,D.C. (1998) Bcl-2 protects against apoptosis in neuronal cell line caused by thapsigargin-induced depletion of intracellular calcium stores. J. Neurochem., 70, 2305–2314. [DOI] [PubMed] [Google Scholar]
- Xu D.G. et al. (1997a) Elevation of neuronal expression of NAIP reduces ischemic damage in the rat hippocampus. Nature Med., 3, 997–1004. [DOI] [PubMed] [Google Scholar]
- Xu D.G., Korneluk,R.G., Tamai,K., Wigle,N., Hakim,A., Mackenzie,A. and Robertson,G.S. (1997b) Distribution of neuronal apoptosis inhibitory protein-like immunoreactivity in the rat central nervous system. J. Comp. Neurol., 382, 247–259. [PubMed] [Google Scholar]
- Yano S., Tokumitsu,H. and Soderling,T.R. (1998) Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature, 396, 584–587. [DOI] [PubMed] [Google Scholar]
- Yoon H.J. and Carbon,J. (1999) Participation of Bir1p, a member of the inhibitor of apoptosis family, in yeast chromosome segregation events. Proc. Natl Acad. Sci. USA, 96, 13208–13213. [DOI] [PMC free article] [PubMed] [Google Scholar]