Abstract
Several lines of evidence indicate altered trafficking of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors in schizophrenia. Previous reports have implicated alterations in the endosomal trafficking of AMPA receptors in this illness. We hypothesized that late endosome content of AMPA receptor subunits is altered in schizophrenia. Accordingly, we developed a technique to isolate and measure contents of late endosomes from postmortem human tissue. We found no changes in the expression of the AMPA subunits, GluR1-4, in late endosomes from the dorsolateral prefrontal cortex in schizophrenia. We also hypothesized that proteins involved in the sorting and trafficking of AMPA receptors between endosomal compartments would be altered in schizophrenia. We found no changes in expression of multiple proteins associated with these processes (dynamin3, Arc/ARG3.1, NEEP21, GRASP1, liprin α, and syntaxin13). Together, these data suggest that endosomal trafficking of AMPA receptors in the prefrontal cortex may be largely intact in schizophrenia.
Keywords: schizophrenia, endosome, Rab7, AMPA trafficking, postmortem
1. INTRODUCTION
Recent studies suggest a link between the pathophysiology of schizophrenia and abnormalities of glutamate receptor expression and neurotransmission (Beneyto et al., 2007; Dracheva et al., 2005; McCullumsmith et al., 2004). The glutamate hypothesis of schizophrenia posits decreased signaling through the N-methyl D-aspartate (NMDA), and possibly α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) subtypes of glutamate receptors (Coyle, 1996; Coyle et al., 2003). Some recent evidence has suggested abnormalities of trafficking of the AMPA subtype of glutamate receptor in this illness (Beneyto and Meador-Woodruff, 2006; Hammond et al., 2010; Toyooka et al., 2002).
AMPA receptor trafficking is tightly regulated from the endoplasmic reticulum (ER) to expression at the synapse (Greger and Esteban, 2007; Zhu, 2003). Mature tetrameric AMPA receptors exit the ER and Golgi and are trafficked along the shaft of the cell toward the synapse with assistance from AMPA receptor interacting proteins such as SAP97 and GRIP1 (Goldstein and Yang, 2000; Jiang et al., 2006; Sans et al., 2001; Setou et al., 2002). Upon reaching the synapse, AMPA receptor insertion into the cell membrane is activity-dependent (Greger and Esteban, 2007; Jin et al., 2006; Zhu, 2003). AMPA receptors expressed at the synapse can be inactivated through removal from the cell membrane via internalization into endosomes. Endosomes are small, spherical compartments that traffic AMPA receptors between intracellular compartments and the neuronal surface (Hirling, 2008). From early endosomes, AMPA receptors can be sorted to recycling endosomes or to late endosomes for degradation (Ehlers, 2000). AMPA receptors may also be pooled in the endosomes as a reserve (Lüscher and Frerking, 2001; Park et al., 2004). There is evidence of an increased pool of AMPA receptors in early endosomes in schizophrenia (Hammond et al., 2010).
Specific markers identify different endosomal subclasses: Rab5 is expressed in early endosomes, Rab7 in late endosomes, and Rab11 in recycling endosomes (Hanley, 2010; Ng and Tang, 2008; Wang et al., 2011). In addition to the rabs, other molecules are involved in the sorting and trafficking of AMPA receptors between these endosomal subclasses. Dynamin3 and Arc/ARG3.1 facilitate endocytosis of AMPA receptors into early endosomes, while NEEP21 and GRASP1 sort AMPA receptors from early endosomes to recycling endosomes (Bramham et al., 2008; Chowdhury et al., 2006; Hoogenraad et al., 2010; Hoogenraad and van der Sluijs, 2010; Lu et al., 2007; Steiner et al., 2005). Liprin α and syntaxin13 facilitate postsynaptic targeting and reinsertion of AMPA receptors in the plasma membrane (Ko et al., 2003; Park et al., 2006; Spangler and Hoogenraad, 2007). Dysregulated trafficking and localization of AMPA receptors may alter excitatory neurotransmission mediated by these receptors.
We postulate that altered endosomal trafficking of AMPA receptors may be associated with the pathophysiology of schizophrenia. Consistent with this hypothesis, we have previously reported increased GluR1 in an early endosome compartment isolated from postmortem cortex samples in schizophrenia (Hammond et al., 2010). To further test this hypothesis, in the present study we isolated and characterized intact late endosomes from postmortem brain tissue and measured the expression of AMPA receptor subunits and trafficking molecules in these endosomes from subjects with schizophrenia and a comparison group. To assess the fidelity of the endosomal system, we also measured expression of proteins associated with endosomal handling of AMPA receptors in brains from these same subjects.
2. MATERIALS AND METHODS
2.1 Subjects and Tissue Preparation
Subjects from the Mount Sinai Medical Center brain bank were recruited prospectively and underwent extensive antemortem diagnostic and clinical assessment (Table 1). Patients were considered to be “off” medication if they had not taken antipsychotics for 6 weeks or more at the time of death. Exclusion criteria included a history of alcoholism, substance abuse, death by suicide, or coma for >6 h before death. Consent was obtained from next of kin for each subject. Brains were collected and cut coronally in 10 mm slabs. The dorsolateral prefrontal cortex was dissected from the coronal slabs, snap frozen, and stored at −80 °C. This tissue was pulverized, adding small amounts of liquid nitrogen as necessary, and stored at −80 °C until used.
Table 1.
Subject Demographics
| Homogenate studies | Endosome studies | |||
|---|---|---|---|---|
| comparison | schizophrenia | comparison | schizophrenia | |
| N | 28 | 35 | 20 | 20 |
| Sex | 11m/17f | 23m/12f | 8m/12f | 15m/5f |
| Tissue pH | 6.4 ± 0.2 | 6.4 ± 0.3 | 6.4 ± 0.3 | 6.4 ± 0.3 |
| PMI (hours) | 7.8 ± 7.0 | 12.5 ± 6.6 | 8.5 ± 7.8 | 13.3 ± 6.3 |
| Age (years) | 78 ± 14 | 74 ± 12 | 78 ± 12 | 73 ± 11 |
| Medication (on/off) | 0/28 | 24/11 | 0/21 | 12/8 |
Values presented as mean ± standard deviation. Male (m), female (f), postmortem interval (PMI). “Off” medication indicates no antipsychotics for six weeks or more prior to death.
Tissue was prepared for western blots as previously described (Funk et al., 2009). Tissue was reconstituted in 5 mM Tris-HCl pH 7.4, 0.32 M sucrose, and a protease inhibitor tablet (Complete Mini, Roche Diagnostics, Mannheim, Germany). Tissue was homogenized using a Power Gen 125 homogenizer (Thermo Fisher Scientific, Rockford, Illinois) at speed 5 for 60 s. Homogenates were assayed for protein concentration using a BCA protein assay kit (Thermo Scientific, Rockford, Illinois), and stored at −80 °C.
2.2 Western Blot Analysis
Commercially available antibodies were used for the western blot analyses with antisera dilutions determined empirically (Table 2). Samples for western blots were placed in reducing buffer-containing β-mercaptoethanol and heated at 70 °C for 10 min. Samples for each subject were then run in duplicate by SDS-polyacrylamide gel electrophoresis on Invitrogen (Carlsbad, California) 4–12% gradient gels, and transferred to polyvinylidene fluoride membrane using Bio-Rad semi-dry transblotter (Hercules, California). The membranes were blocked in LiCor (Lincoln, Nebraska) blocking buffer or 1% BSA for 1 h at room temperature, and probed with primary antibody in 0.1% Tween LiCor blocking buffer or 0.1% Tween in 1% BSA at the dilutions and for the durations indicated in Table 2. Membranes were then washed four times for 5 min each with 0.01% Tween phosphate-buffered saline. Membranes were probed with IR-dye labeled secondary antibody in 0.1% Tween, 0.01% SDS LiCor blocking buffer or 0.1% Tween, 0.01% SDS 1% BSA for 1 h at room temperature in the dark. Membranes were washed again with 0.01% Tween phosphate-buffered saline four times for 5 min each and then briefly rinsed three times in distilled water. The blots were stored in distilled water at 4 °C until scanned using the LI-COR Odyssey laser-based image detection method (Bond et al., 2008). We tested each antibody using varying concentrations of total protein from homogenized human cortical tissue to confirm we were in the linear range of the assay.
Table 2.
Antibodies used for Western blot studies
| Antibody | Species | Concentration | Blocking buffer | Incubation | Company |
|---|---|---|---|---|---|
| Dynamin3 | rabbit | 1:1000 | Licor | 16 hours | Abcam Inc., Cambridge, MA |
| NEEP21 | mouse | 1:100 | Licor | 16 hours | Santa Cruz Biotechnology, Santa Cruz, CA |
| GRASP1 | rabbit | 1:1000 | Licor | 16 hours | Abcam Inc., Cambridge, MA |
| Arc/Arg3.1 | rabbit | 1:1000 | 1% BSA | 16 hours | Abcam Inc., Cambridge, MA |
| Syntaxin13 | mouse | 1:1000 | 1% BSA | 16 hours | Abcam Inc., Cambridge, MA |
| Liprin α | rabbit | 1:1000 | Licor | 16 hours | Abcam Inc., Cambridge, MA |
| GluR1 | rabbit | 1 : 1000 | Licor | 16 hours | Millipore, Bellarica, MA |
| GluR2 | rabbit | 1 : 250 | 1% BSA | 16 hours | Abcam Inc., Cambridge, MA |
| GluR3 | rabbit | 1 : 1000 | Licor | 16 hours | Cell Signaling, Danvers, MA |
| GluR4 | rabbit | 1 : 500 | Licor | 16 hours | Millipore, Bellarica, MA |
| GRIP1 | rabbit | 1 : 500 | Licor | 16 hours | Abcam Inc., Cambridge, MA |
| NSF | rabbit | 1 : 2000 | Licor | 16 hours | Abcam Inc., Cambridge, MA |
| SAP97 | rabbit | 1 : 1000 | Licor | 16 hours | Abcam Inc., Cambridge, MA |
| EEA1 | rabbit | 1 : 1000 | Licor | 2 hours | Abcam Inc., Cambridge, MA |
| PSD95 | mouse | 1 : 1000 | Licor | 1 hour | Millipore, Bellarica, MA |
| GRP78/BiP | mouse | 1 : 250 | Licor | 1 hour | BD Transduction, San Jose, CA |
| GS | mouse | 1 : 5000 | Licor | 1 hour | BD Transduction, San Jose, CA |
| VCP | mouse | 1:5000 | Licor | 2 hours | Abcam Inc., Cambridge, MA |
Abbreviations: Neuron Enriched Endosomal Protein 21 (NEEP21), GRIP Associated Protein1 (GRASP1), Glutamate Receptor (GluR), Glutamate Receptor Interacting Protein 1 (GRIP1), N-ethylmalemide Sensitive Factor (NSF), Synapse Associated Protein 97 (SAP97), Early Endosome Antigen 1 (EEA1), Post-synaptic Density 95 (PSD95), Glucose Regulated Protein 78/Binding Protein (GRP78/BiP), Glutamine Synthetase (GS), Valosin-containing protein (VCP).
2.3 Immunoisolation of Late Endosomes
A subset of subjects (Table 1) was used for late endosome isolation because of the large amounts of tissue required for this technique. For each subject, isolation was performed in duplicate. In total, 80 μl (6.7 × 108 beads/ml) of sheep anti-mouse Dynabead M280 magnetic beads (Invitrogen) were washed three times with ice-cold phosphate-buffered saline. All washes consisted of 5 min rotating at 4 °C and 2 min on the magnet (Dynal MPC-S, Invitrogen). Beads were then resuspended in 70 μl of phosphate-buffered saline and 20 μg of mouse anti-Rab7 antibody (Abcam, Cambridge, Massachusetts). The bead-antibody solution was incubated while rotating at 4 °C for 16–18 h to form a bead-antibody complex. Subsequently, 80 μl of fresh beads were chilled on ice and washed three times with ice-cold phosphate-buffered saline. We added 260 μg of homogenized tissue in 5 mM Tris-HCl (final volume 200 μl) to the freshly washed beads, and precleared the tissue for 1 h while rotating at 4 °C. The bead-antibody complex was washed three times with ice-cold phosphate-buffered saline. After the 1-h incubation, the precleared tissue homogenate was collected and incubated with the bead-antibody complex for 3 h while rotating at 4 °C to isolate late endosomes. The supernatant of the bead-antibody-endosome complex was collected and saved, and the bead-antibody-endosome complex was washed three times with ice-cold phosphate-buffered saline. This complex was reconstituted in 20 μl of distilled Milli-Q water and samples were prepared for western blot analysis or electron microscopy. Samples for western blot analysis were heated in reducing buffer-containing β-mercaptoethanol at 70 °C for 10 min. Samples were placed in the Dynal magnet for 2 min before loading on the gel.
2.4 Electron Microscopy
Immediately after immunoisolation and reconstitution in Milli-Q water, bead-antibody-endosome complexes were embedded in agarose and then fixed with 4% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) overnight at room temperature. The complexes were then washed and treated with 1% osmium tetroxide for 1 h, mordanted with 0.25% uranyl acetate in acetate buffer for 30 min to overnight, washed and dehydrated with a graded series of ethanol washes and propylene oxide. Finally, the samples were embedded in epoxy resin, thin sectioned and counterstained with uranyl acetate and lead citrate. Images were captured using an FEI Tecnai Spirit 20–120kv Transmission Electron Microscope.
2.5 Data Analysis
Near-infrared fluorescent signals obtained from the LiCor Odyssey scanner were expressed as raw integrated intensity with top–bottom median intralane background subtraction using Odyssey 3.0 analytical software (LiCor) (Bond et al, 2008). For homogenate protein studies, duplicate lanes of protein expression from each subject were normalized to valosin-containing protein (VCP) as an in-lane loading control. VCP was chosen because no changes have previously been detected in subjects with schizophrenia compared with control subjects (Bauer et al., 2009; Hammond et al., 2010). For immunoisolation studies, duplicate lanes of protein expression from each subject were normalized to Rab7 as an in-lane loading control.
To confirm the immunocapture of endosomes and to assess capture efficiency, 1650X direct magnification electron micrograph images of preclear, negative control, and immunoisolation samples, were printed, coded, and randomly sorted. Counts were made by an observer blind to condition. Beads or endosomes on the borders of each image were not included in the counts.
Data were analyzed using Statistica (Statsoft, Tulsa, Oklahoma). Correlation analyses were carried out to identify any associations between the dependent variables and pH, age, and postmortem interval. One-way analysis of covariance was performed if significant correlations were found. If no correlations were present, data were analyzed with one-way analysis of variance. Secondary analyses were performed using sex and medication status (receiving antipsychotics at the time of death vs. off medication for six weeks or more at the time of death) as the independent measure.
3. RESULTS
3.1 Late Endosome Enrichment
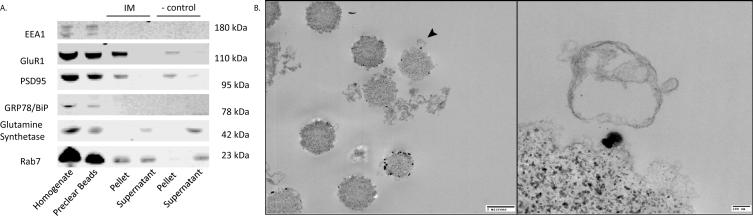
To analyze alterations in late endosome content in schizophrenia, we used magnetic beads bound to a late endosome-specific antibody to obtain an enriched late endosome fraction from postmortem tissue (Figure 1). To verify the enrichment of late endosomes in our immunoisolation, we used western blot analysis to measure expression of proteins not expected to be expressed in late endosomes, including those found in early endosomes (EEA1), endoplasmic reticulum (GRP78/BiP), and astrocytes (glutamine synthetase). As anticipated, we did not find any of these markers in our late endosome preparation (Figure 1 – IM, pellet). We do have evidence of minor non-specific PSD95 remnants in our late endosome enrichment that also occurs in our negative control preparation (Figure 1A – IM, pellet; - control, pellet). Additionally, as expected we have evidence of an AMPA receptor subunit (GluR1) expressed in our late endosome enriched fraction (Figure 1A – IM, pellet).
Figure 1.
Characterization and visualization of late endosomes. A. Proteins not expressed in late endosomes (IM – Pellet) include those found in early endosomes (EEA1), endoplasmic reticulum (GRP78/BiP), and astrocytes (glutamine synthetase). Expression of the AMPA receptor subunit (GluR1) is present in early endosomes (IM – Pellet). Immunoisolation (IM). Negative control (-ctrl). Early Endosome Antigen 1 (EEA1). Post-synaptic density 95 (PSD95). Glucose Regulated Protein 78/Binding immunoglobulin protein (GRP78/BiP). Ionotropic Glutamate Receptor 2 (GluR2). B. Electron micrograph of late endosome immunoisolation. Late endosomes were isolated using magnetic beads and Rab7 capture antibody and imaged using electron microscopy. The panel on the right is an enlarged view of the area indicated by the arrow on the left.
Using electron microscopy, we confirmed the isolation of intact late endosomes in our immunoisolation. Further, we measured the number of endosomes per bead in preclear, negative control, and immunoisolation samples. We found a 1.7-fold increase in the endosome to bead ratio in our immunoisolation samples relative to our preclear beads samples and a 17.3-fold increase in the endosome to bead ratio in our immunoisolation samples relative to our negative control samples.
3.2 Protein Expression in Late Endosomes
We examined the expression of the AMPA receptor subunits, GluR1-4, in Rab7 positive late endosomes samples. All protein expression was measured relative to Rab7 expression in the same lane. We found no significant differences in GluR1, GluR2, GluR3, or GluR4 in the enriched endosome fraction (Figure 2 and Table 3). We found no significant correlations between protein expression and age, pH, or PMI in our isolated endosome samples. In addition, we found no influence of sex or medication status in our isolated endosome studies.
Figure 2.
Representative western blots of proteins in late endosomes (left panel) and total homogenate (right panel). Expression of markers for quantified proteins is shown in duplicate. Comparison (comp). Schizophrenia (scz). . Ionotropic Glutamate Receptor 1 (GluR1). Ionotropic Glutamate Receptor 2 (GluR2). Ionotropic Glutamate Receptor 3 (GluR3). Ionotropic Glutamate Receptor 4 (GluR4). N-ethylmaleimide Sensitive Factor (NSF). Synapse Associated Protein 97 (SAP97). Neuron-Enriched Endosomal Protein 21 (NEEP21). GRIP-Associate Protein 1 (GRASP1).
Table 3.
Statistical Analysis of Dependent Measures
| Late Endosomes Isolation Studies | |||
|---|---|---|---|
| Dependent Measure | F statistic | df | p value |
| GluR1 | 0.186 | 1, 36 | 0.67 |
| GluR2 | 0.020 | 1, 38 | 0.89 |
| GluR3 | 0.00019 | 1, 38 | 0.99 |
| GluR4 | 0.508 | 1, 38 | 0.48 |
| GRIP1 | 0.00010 | 1, 38 | 0.99 |
| NSF | 0.238 | 1, 38 | 0.63 |
| SAP97 | 0.00011 | 1, 38 | 0.99 |
Abbreviations: degrees of freedom (df). Statistical values presented from ANOVA calculation.
We also examined the enriched fraction to determine if there were alterations in the expression of the AMPA receptor-interacting proteins, GRIP1, NSF, or SAP97. We found no significant change in the expression of GRIP1, NSF or SAP97 in our late endosome-enriched fraction (Table 3). We found no significant associations with GRIP1/Rab7, NSF/Rab7, or SAP97/Rab7 and medication status or sex.
3.3 Protein Expression in Tissue Homogenates
We have examined the expression of proteins involved in the sorting of AMPA receptors in endosomes as well as those involved in the fusion of endosome subtypes using VCP as a loading control (Figure 3). As previously reported (Bauer et al., 2009; Hammond et al., 2010), we found no changes in VCP (non-normalized) in schizophrenia. We found no significant correlations between protein expression and PMI, pH, or age in our samples. In addition, we found no significant effect of either sex or medication status in these samples. We found no significant difference in the expression of Arc/ARG3.1, NEEP21, Liprin α, dynamin3, GRASP1 or syntaxin13 (Figure 2 and Table 4). Additionally, we found no significant difference in the expression of Rab7, the marker we used to isolate late endosomes (Figure 2 and Table 4).
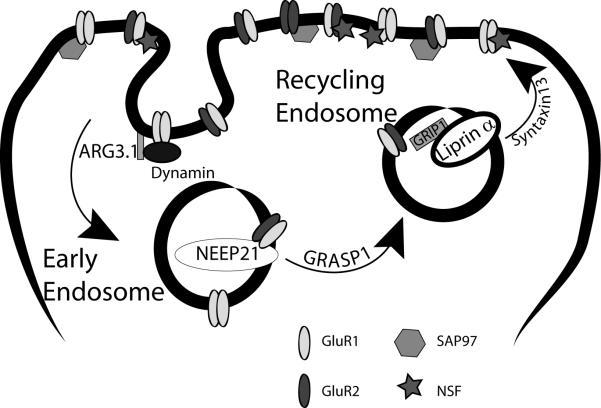
Figure 3.
Representation of multiple proteins involved in the endosomal trafficking of AMPA receptors. Arc/ARG3.1 and dynamin assist in the endocytosis of AMPA receptors from the cell membrane. NEEP21 associates with AMPA receptors in early endosomes. GRASP1 is involved in the sorting of AMPA receptors from the early endosomes to recycling endosomes. Liprin α and syntaxin13 assist in the postsynaptic localization and reinsertion of AMPA receptors into the cell membrane. Ionotropic Glutamate Receptor 1 (GluR1). Ionotropic Glutamate Receptor 2 (GluR2). Neuron-Enriched Endosomal Protein 21 (NEEP21). GRIP-Associate Protein 1 (GRASP1). Glutamate Receptor Interacting Protein 1 (GRIP1). Synapse Associated Protein 97 (SAP97). N-ethylmaleimide Sensitive Factor (NSF).
Table 4.
Statistical Analysis of Dependent Measures
| Total Homogenate Studies | |||
|---|---|---|---|
| Dependent Measure | F statistic | df | p value |
| Arc/ARG3.1 | 0.063 | 1, 61 | 0.80 |
| NEEP21 | 0.142 | 1, 54 | 0.71 |
| Liprin α | 0.009 | 1, 57 | 0.92 |
| dynamin3 | 0.210 | 1, 54 | 0.65 |
| GRASP1 | 0.019 | 1, 61 | 0.89 |
| syntaxin13 | 0.115 | 1, 52 | 0.74 |
| Rab7 | 0.00035 | 1, 54 | 0.99 |
Abbreviations: degrees of freedom (df). Statistical values presented from ANOVA calculation.
4. DISCUSSION
We have previously reported increased expression of GluR1 in isolated early endosomes in frontal cortex of schizophrenia (Hammond et al., 2010). Our interpretation of these results was that the increase in GluR1 in early endosomes reflected an increase in the forward trafficking of AMPA receptors. From the early endosome, AMPA receptors may be sorted to late endosomes. An increase in AMPA receptors in early endosomes may indicate an increase in AMPA receptors in other endosomal compartments. To extend this hypothesis, we isolated late endosomes and examined expression of the AMPA receptor subunits, GluR1-4, and AMPA receptor interacting proteins, GRIP1, NSF, and SAP97, in this fraction. We predicted that we would find an increase in AMPA receptor subunits in the late endosomes, consistent with our previous finding in early endosomes, further implicating altered endosomal handling and trafficking of AMPA receptors in schizophrenia.
Based on western blot protein characterization, our late endosome isolation is free from contaminants of other subcellular fractions with the exception of the postsynaptic density (Figure 1A – IM, pellet lane). The postsynaptic density expressed in our isolation is no greater than the expression in our negative control (Figure 1A – negative (-) control, pellet lane) and intact postsynaptic densities were not visualized by electron microscopy. While the early endosome (EEA1) expression is not as prominent as late endosome (Rab7) expression in the homogenate lane (Figure 1A – homogenate), we did not detect cross-contamination with early endosomes (EEA1) by western blot (Figure 1A – IM, pellet lane) or morphological profile in electron microscopy. Furthermore, our isolated late endosomes have similar morphologic features to previously published reports on late endosomes in the preclinical literature (Figure 1B) (Kamsteeg et al., 2006; Kobayashi et al., 1998). Using this enriched fraction of late endosomes, we found no significant changes in the expression of any of the AMPA receptor subunits, GluR1-4, or the three AMPA receptor interacting proteins in schizophrenia. These results suggest that AMPA receptor trafficking in late endosomes is not altered in schizophrenia. Taken together with our earlier data, in two separate endosomal compartments, early and late, we have only identified increased GluR1 expression in early endosomes (Hammond et al., 2010).
We also tested the hypothesis that there are abnormalities of endosomal machinery in schizophrenia. Specifically, we focused on proteins that interact with AMPA receptors, such as dynamin3, NEEP21, and liprin α, as well as proteins that are more directly involved in endosome machinery, Arc/ARG3.1, GRASP1, and syntaxin13. Disruption of any of these proteins may alter the endosomal trafficking of AMPA receptors. Together, the necessity of these proteins in the trafficking of AMPA receptors near the synapse and the hypothesis that endosomal trafficking may be altered in schizophrenia made these six proteins ideal targets to measure. We found no significant changes in any of these endosomal trafficking proteins, suggesting that AMPA receptor-associated trafficking machinery is intact in schizophrenia.
It is conceivable that the magnitude of changes in these proteins in schizophrenia is too small to detect with our current sample size. We feel that this is unlikely given that in our total homogenate studies, we analyzed protein expression from 63 subjects, and in our endosome isolation studies we analyzed protein expression from 40 subjects. There is also the possibility that our in-lane normalizing proteins, VCP in total homogenate and Rab7 in endosome studies, masked any differences that may be present. However, our data were also analyzed without normalization and no significant changes were found.
While there are reports detailing changes in AMPA receptor interacting protein expression in schizophrenia, there are no previous reports that have examined multiple proteins involved in a localized but complex trafficking system as presented in this report. Our lack of significant findings suggests that with the exception of our earlier GluR1 finding in early endosomes, the endosomal trafficking system of AMPA receptors is largely intact in schizophrenia. Though we did not find distal changes in AMPA receptor trafficking, there may be abnormalities in AMPA receptor trafficking proximally in the endoplasmic reticulum or Golgi apparatus, an area we are currently pursuing.
Acknowledgement
This work is supported by MH086257 (JCH), MH53327 (JMW), MH064673 & MH066392 (VH) and MH074016 & Doris Duke Clinical Scientist Award (REM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bauer D, Haroutunian V, McCullumsmith R, Meador-Woodruff J. Expression of four housekeeping proteins in elderly patients with schizophrenia. J Neural Transm. 2009;116:487–91. doi: 10.1007/s00702-008-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff J. Lamina-specific abnormalities of AMPA receptor trafficking and signaling molecule transcripts in the prefrontal cortex in schizophrenia. Synapse. 2006;60:585–98. doi: 10.1002/syn.20329. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Bond D, Primrose D, Foley E. Quantitative evaluation of signaling events in Drosophila s2 cells. Biol Proced Online. 2008;10:20–8. doi: 10.1251/bpo139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–7. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–59. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3:241–53. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- Coyle J, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann N Y Acad Sci. 2003;1003:318–27. doi: 10.1196/annals.1300.020. [DOI] [PubMed] [Google Scholar]
- Dracheva S, McGurk S, Haroutunian V. mRNA expression of AMPA receptors and AMPA receptor binding proteins in the cerebral cortex of elderly schizophrenics. J Neurosci Res. 2005;79:868–78. doi: 10.1002/jnr.20423. [DOI] [PubMed] [Google Scholar]
- Ehlers M. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–25. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Funk A, Rumbaugh G, Harotunian V, McCullumsmith R, Meador-Woodruff J. Decreased expression of NMDA receptor-associated proteins in frontal cortex of elderly patients with schizophrenia. Neuroreport. 2009;20:1019–22. doi: 10.1097/WNR.0b013e32832d30d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L, Yang Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci. 2000;23:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- Greger I, Esteban J. AMPA receptor biogenesis and trafficking. Curr Opin Neurobiol. 2007;17:289–97. doi: 10.1016/j.conb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Hammond JC, McCullumsmith RE, Funk AJ, Haroutunian V, Meador-Woodruff JH. Evidence for abnormal forward trafficking of AMPA receptors in frontal cortex of elderly patients with schizophrenia. Neuropsychopharmacology. 2010;35:2110–9. doi: 10.1038/npp.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley JG. Endosomal sorting of AMPA receptors in hippocampal neurons. Biochem Soc Trans. 2010;38:460–5. doi: 10.1042/BST0380460. [DOI] [PubMed] [Google Scholar]
- Hirling H. Endosomal trafficking of AMPA-type glutamate receptors. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Hoogenraad C, Popa I, Futai K, Sanchez-Martinez E, Wulf P, van Vlijmen T, Dortland B, Oorschot V, Govers R, Monti M, Heck A, Sheng M, Klumperman J, Rehmann H, Jaarsma D, Kapitein L, van der Sluijs P. Neuron specific Rab4 effector GRASP-1 coordinates membrane specialization and maturation of recycling endosomes. PLoS Biol. 2010;8:e1000283. doi: 10.1371/journal.pbio.1000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad CC, van der Sluijs P. GRASP-1 regulates endocytic receptor recycling and synaptic plasticity. Commun Integr Biol. 2010;3:433–5. doi: 10.4161/cib.3.5.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Suppiramaniam V, Wooten M. Posttranslational modifications and receptor-associated proteins in AMPA receptor trafficking and synaptic plasticity. Neurosignals. 2006;15:266–82. doi: 10.1159/000105517. [DOI] [PubMed] [Google Scholar]
- Jin W, Ge W, Xu J, Cao M, Peng L, Yung W, Liao D, Duan S, Zhang M, Xia J. Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking, and synaptic plasticity. J Neurosci. 2006;26:2380–90. doi: 10.1523/JNEUROSCI.3503-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamsteeg EJ, Hendriks G, Boone M, Konings IB, Oorschot V, van der Sluijs P, Klumperman J, Deen PM. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc Natl Acad Sci U S A. 2006;103:18344–9. doi: 10.1073/pnas.0604073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Kim S, Valtschanoff JG, Shin H, Lee JR, Sheng M, Premont RT, Weinberg RJ, Kim E. Interaction between liprin-alpha and GIT1 is required for AMPA receptor targeting. J Neurosci. 2003;23:1667–77. doi: 10.1523/JNEUROSCI.23-05-01667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–7. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- Lu J, Helton TD, Blanpied TA, Rácz B, Newpher TM, Weinberg RJ, Ehlers MD. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55:874–89. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Frerking M. Restless AMPA receptors: implications for synaptic transmission and plasticity. Trends Neurosci. 2001;24:665–70. doi: 10.1016/s0166-2236(00)01959-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullumsmith R, Clinton S, Meador-Woodruff J. Schizophrenia as a disorder of neuroplasticity. Int Rev Neurobiol. 2004;59:19–45. doi: 10.1016/S0074-7742(04)59002-5. [DOI] [PubMed] [Google Scholar]
- Ng EL, Tang BL. Rab GTPases and their roles in brain neurons and glia. Brain Res Rev. 2008;58:236–46. doi: 10.1016/j.brainresrev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Park M, Penick E, Edwards J, Kauer J, Ehlers M. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–5. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–30. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Racca C, Petralia R, Wang Y, McCallum J, Wenthold R. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J Neurosci. 2001;21:7506–16. doi: 10.1523/JNEUROSCI.21-19-07506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setou M, Seog D, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–7. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- Spangler SA, Hoogenraad CC. Liprin-alpha proteins: scaffold molecules for synapse maturation. Biochem Soc Trans. 2007;35:1278–82. doi: 10.1042/BST0351278. [DOI] [PubMed] [Google Scholar]
- Steiner P, Alberi S, Kulangara K, Yersin A, Sarria JC, Regulier E, Kasas S, Dietler G, Muller D, Catsicas S, Hirling H. Interactions between NEEP21, GRIP1 and GluR2 regulate sorting and recycling of the glutamate receptor subunit GluR2. EMBO J. 2005;24:2873–84. doi: 10.1038/sj.emboj.7600755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka K, Iritani S, Makifuchi T, Shirakawa O, Kitamura N, Maeda K, Nakamura R, Niizato K, Watanabe M, Kakita A, Takahashi H, Someya T, Nawa H. Selective reduction of a PDZ protein, SAP-97, in the prefrontal cortex of patients with chronic schizophrenia. J Neurochem. 2002;83:797–806. doi: 10.1046/j.1471-4159.2002.01181.x. [DOI] [PubMed] [Google Scholar]
- Wang T, Ming Z, Xiaochun W, Hong W. Rab7: role of its protein interaction cascades in endolysosomal traffic. Cell Signal. 2011;23:516–21. doi: 10.1016/j.cellsig.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Zhu J. Mechanisms of synaptic plasticity: from membrane to intracellular AMPAR trafficking. Mol Interv. 2003;3:15–8. doi: 10.1124/mi.3.1.15. [DOI] [PubMed] [Google Scholar]