Abstract
The gaseous mediator hydrogen sulfide (H2S) is synthesized mainly by cystathionine gamma-lyase in the heart and plays a role in the regulation of cardiovascular homeostasis. Here we first overview the state of the art in the literature on the cardioprotective effects of H2S in various models of cardiac injury. Subsequently, we present original data showing the beneficial effects of parenteral administration of a donor of H2S on myocardial and endothelial function during reperfusion in a canine experimental model of cardiopulmonary bypass. Overview of the literature demonstrates that various formulations of H2S exert cardioprotective effects in cultured cells, isolated hearts and various rodent and large animal models of regional or global myocardial ischemia and heart failure. In addition, the production of H2S plays a role in myocardial pre- and post-conditioning responses. The pathways implicated in the cardioprotective action of H2S are multiple and involve KATP channels, regulation of mitochondrial respiration, and regulation of cytoprotective genes such as Nrf-2. In the experimental part of the current article, we demonstrate the cardioprotective effects of H2S in a canine model of cardiopulmonary bypass surgery. Anesthetized dogs were subjected hypothermic cardiopulmonary bypass with 60 minutes of hypothermic cardiac arrest in the presence of either saline (control, n=8), or H2S infusion (1 mg/kg/h for 2 h). Left ventricular hemodynamic variables (via combined pressure-volume-conductance catheter) as well as coronary blood flow, endothelium-dependent vasodilatation to acetylcholine and endothelium-independent vasodilatation to sodium nitroprusside were measured at baseline and after 60 minutes of reperfusion. Ex vivo vascular function and high-energy phosphate contents were also measured. H2S led to a significantly better recovery of preload recruitable stroke work (p<0.05) after 60 minutes of reperfusion. Coronary blood flow was also significantly higher in the H2S group (p<0.05). While the vasodilatory response to sodium nitroprusside was similar in both groups, acetylcholine resulted in a significantly higher increase in coronary blood flow in the H2S-treated group (p<0.05) both in vivo and ex vivo. Furthermore, high-energy phosphate contents were better preserved in the H2S group. Additionally, the cytoprotective effects of H2S were confirmed also using in vitro cell culture experiments in H9c2 cardiac myocytes exposed to hypoxia and reoxygenation or to the cytotoxic oxidant hydrogen peroxide. Thus, therapeutic administration of H2S exerts cardioprotective effects in a variety of experimental models, including a significant improvement of the recovery of myocardial and endothelial function in a canine model of cardiopulmonary bypass with hypothermic cardiac arrest.
Keywords: cardiopulmonary bypass, ischemia/reperfusion, cardiac function, vascular reactivity, myocardial protection, hydrogen sulfide
Introduction
For many decades the conventional view has been that hydrogen sulfide (H2S) is a toxic gas that interferes with cellular functions. This viewpoint has recently been completely revised in light of three recent discoveries. First, several lines of studies demonstrated that H2S is generated from endogenous sources and is physiologically present in blood and other tissues. Second, endogenous H2S generating enzymes have been identified in mammals. H2S is synthesized from L-cysteine in mammalian tissues by two pyridoxal-5'-phosphate-dependent enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), with the latter one being the more abundant enzyme in the heart. Third, H2S was shown to inhibit neutrophil adhesion and activation [1; 2; 3]. These data demonstrate that endogenous H2S may play a role in regulation of cardiovascular function and inflammatory/immune responses as a potential endogenous gaseous transmitter.
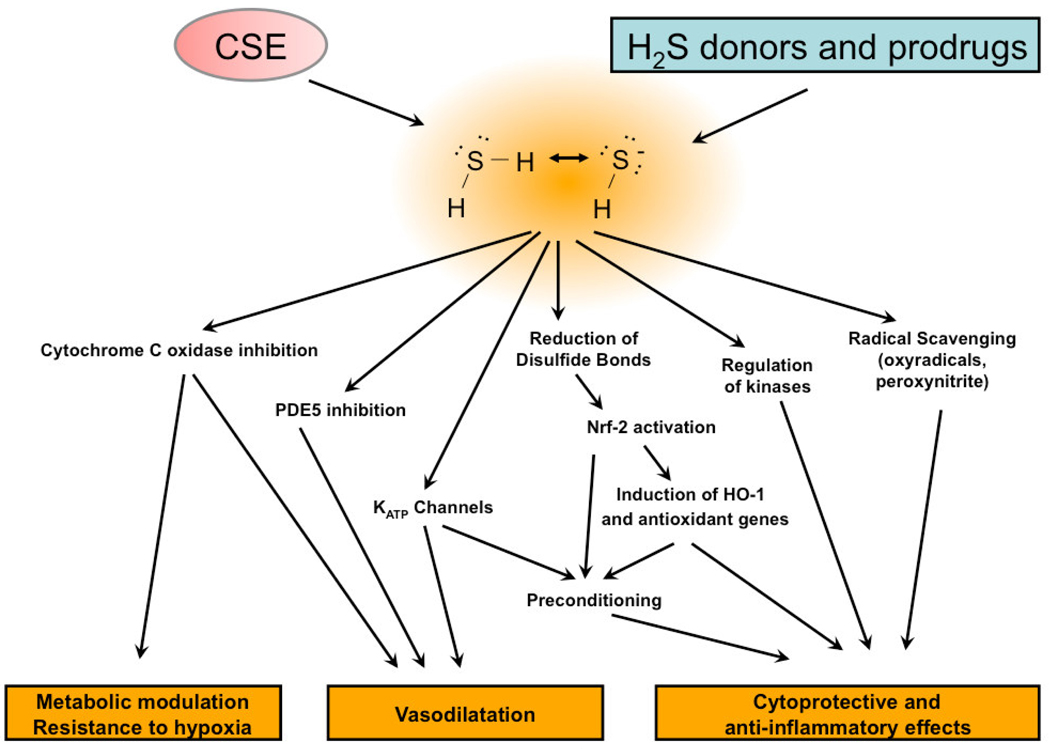
Similarly to the other two gaseous transmitters nitric oxide and carbon monoxide, administration of small amounts of H2S have been shown to exert beneficial effects in a number of experimental models of inflammatory and cardiovascular diseases. The beneficial effects of H2S in various models of cardiac injury are shown in Table 1. The cytoprotective effects of H2S were demonstrated in cultured cardiomyocytes in vitro [4; 5; 6; 7; 8; 9; 10; 11; 12], in isolated perfused heart preparations [13; 14; 15; 16; 17; 18] as well as in rodent models of cardiac dysfunction [5; 11; 17; 19; 20; 21; 22; 23; 24; 25; 26; 27; 28; 29; 30]. Many of these studies focus on acute myocardial protection, where the beneficial effects of H2S has been demonstrated in multiple rodent models of coronary artery ligation and reperfusion, homocysteine or isoproterenol induced myocardial injury and storage of hearts prior to transplantation (Table 1). A relatively smaller number of studies investigated the effects of parenteral H2S formulations in large animal models: protection against regional myocardial ischemia-reperfusion injury [31; 32; 33] and cardiopulmonary bypass [34] have been demonstrated in recent porcine studies. The endogenous production of H2S has also been shown to be required for ischemic preconditioning and post-conditioning; blockade of endogenous H2S production has been shown to inhibit these responses [8; 35]. Some of the cardioprotective pathways of H2S identified to date are depicted in Fig. 1.
Table 1.
Cardioprotective effects of H2S and its donors in various in vitro and in vivo models of cardiac injury.
| Experimental model |
Effect of sulfide/sulfide donor | Proposed mechanism(s) | Ref. |
|---|---|---|---|
| Cultured cardiomyocytes | |||
| Primary adult rat cardiomyocytes. | NaHS (10–100 µM) protected against the loss of cell viability during metabolic inhibition elicited by sodium cyanide and 2-deoxyglucose | Activation of protein kinase C and activation of sarcolemmal KATP channels. | [4] |
| Isolated mitochondria from mouse cardiac myocytes. | Na2S (10 µM) improved the functional recovery of mitochondrial function after hypoxia and reoxygenation. | Regulated partial inhibition of mitochondrial respiration. | [5] |
| Primary adult rat cardiomyocytes. | Preconditioning with NaHS (100 µM), reduced the degree of cardiac injury and arrhythmias during the subsequent ischemic period. | Activation of KATP channels; activation of PKC; induction of COX-2; activation of Erk and Akt pathways. | [6; 7; 8; 9] |
| Primary neonatal rat cardiomyocytes. | NaHS (100 µM) decreased the number of apoptotic cells in an in vitro model of hypoxia and reoxygenation. | Inhibition of JNK phosphorylation, decreased cytochrome C release and enhanced Bcl-2 expression. | [10] |
| Primary neonatal rat cardiomyocytes. | S-propargyl-cysteine (10 µM) improved cell viability during ischemia. | [46] | |
| Cultured H9c2 myoblasts. | Nuclear and mitochondrial ultrastructural changes in response to homocysteine were reduced by NaHS (100 µM – 1 mM). | Inhibition of endoplasmatic retuculum stress (GRP78/CHOP activation), inhibition of caspase 12 activation. | [11] |
| Primary neonatal rat cardiomyocytes. | NaHS (10 – 50 µM) pretreatment protected against cardiomyocyte apoptosis induced by hypoxia/reoxygenation. | Activation of Akt; subsequent phosphorylation of GSK-3beta (Ser9) and consequent inhibition on mPTP opening, of Bax translocation, and of caspase-3 activation and induction of survivin. | [12] |
| Cultured H9c2 myoblasts. | Na2S (1–600 µM) protected against the decline in cell viability in response to hydrogen peroxide, but not in response to hypoxia. | Current study | |
| Isolated hearts | |||
| Myocardial ischemia-reperfusion in isolated perfused rat hearts. | NaHS (0.1 – 1 µM) infusion reduced myocardial infarct size and reduced the duration and severity of arrhythmias. NaHS also protected against the decreases in intracellular calcium transients during hypoxia. | Activation of KATP channels. | [16] |
| Hypothermic heart storage and transplantation | Improved functional recovery of the hearts after storage in the presence of NaHS (1 µM) infusion; reduced apoptosis. | Preservation of ATP in the hearts (regulated metabolic inhibition). | [14] |
| Myocardial ischemia-reperfusion in isolated perfused rat hearts. | NaHS (40 µM) infusion improved the contractile function of the hearts during reperfusion and reduced the incidence of arrhyhtmias. | Activation of KATP channels. | [18] |
| Myocardial ischemia-reperfusion in isolated perfused rat hearts. | NaHS (1–10 µM) infusion reduced infarct size and decreased the biochemical markers of myocyte necrosis. | Activation of KATP channels. | [15] |
| Myocardial ischemia-reperfusion in isolated perfused rat hearts. | NaHS (40 µM) infusion tended to decrease infarct size. | Metabolic effects (elevation of ADP levels in the heart). | [13] |
| Hypoxia/reoxygenat ion in isolated perfused mouse hearts. | Na2S (10 µM) infusion improved the recovery of the hearts in the reoxygenation stage. | Preservation of mitochondrial function, inhibition of mitochondrial permeability transition, reduction in oxidant production. | [17] |
| Rodent models | |||
| Isoproterenol-induced myocardial injury in the rat. | NaHS (160 or 800 µg/kg/day) attenuated the release of cardiac enzymes, reduced conjugated diene formation and malon dialdehyde formation. | Direct antioxidant effects. | [22] |
| Myocardial ischemia-reperfusion in the mouse | NaHS (50–200 µg/kg i.v.) reduced myocardial infarct size. | Regulated partial inhibition of mitochondrial function, inhibition of neutrophil infiltration, inhibition of pro-inflammatory mediator production. | [5] |
| Myocardial ischemia-reperfusion in the rat | NaHS (0.8 mg/kg/day) reduced myocardial infarct size. | [29] | |
| Myocardial ischemia-reperfusion in the rat. | NaHS (3 mg/kg i.v.) reduced myocardial infarct size. | Inhibition of p38MAPK activation, inhibition of activation of NF-κB, inhibition of neutrophil infiltration, inhibition of peroxidation. | [24; 25] |
| Myocardial ischemia-reperfusion in the rat. | NaHS (1.1 mg/kg/day) reduced infarct size. | Upregulation of survivin. | [30] |
| Cardiopulmonary arrest model in the mouse. | Na2S (0.55 mg/kg i.v.) improved survival, protected against the development of left ventricular dysfunction and improved neurological function. | Reduction of oxidative stress; activation of Akt and AMPK; induction of the phosphorylation and consequent activation of NO synthase 3. | [17] |
| Adriamicin-induced cardiotoxicity in rats | Na2S (1.1 mg/kg/day i.p.) improved cardiac function and cardiac histopathology. | Upregulation of the antioxidants SOD and GSH-Px ; reduction in oxidant stress. | [26] |
| Myocardial ischemia-reperfusion in the mouse | Na2S preconditioning (0.1 mg/kg, given as a pretreatment 24h prior to ischemia) reduced infarct size and improved myocardial contractility. | Nrf2 activation, leading to increased the expression of antioxidants (heme oxygenase-1 and thioredoxin 1) and upregulation of Hsp90 and Hsp70, Bcl-2, Bcl-xL, and COX-2. | [20] |
| Myocardial ischemia-reperfusion in the rat. | NaHS (0.08–8 mg/kg) pretreatment reduced infarct size and decreased the number of apoptotic cells in the reperfused myocardium. | Activation of Akt; subsequent phosphorylation of GSK-3beta (Ser9) and consequent inhibition on mPTP opening, of Bax translocation, and of caspase-3 activation and induction of survivin. | [12] |
| Hyperhomocysteine mia-induced myocardial injury in the rat. | NaHS (1.1 mg/kg/day i.p.) protected against the homocysteine-induced deterioration of myocardial ultrastructure. | Inhibition of endoplasmatic reticulum stress: decreased expression of ER stress-associated proteins, including GRP78, CHOP, and caspase 12. | [11] |
| Myocardial ischemia/reperfusio n in the rat. | S-propyl-l-cysteine, S-allyl-l-cysteine and S-propargyl-l-cysteine (50 mg/kg) release H2S and protected against the histopathological alterations. | Preservation of SOD and GPx activities and tissue GSH levels; reduction of lipid peroxidation, preservation of SOD and GPx activities and tissue GSH levels. | [21; 27] |
| Chronic heart failure in the rat induced by aorta-to-vena cava shunting. | NaHS (2.4 mg/l in the drinking water) improved cardiac histology, as evidenced by reduced fibrosis and reduced apoptosis. | Reduction in oxidative stress, reduction in nitrosative stress (nitrotyrosine staining), inhibition of matrix metalloproteinase activation, reversal of the alterations in TIMP expression, leading to a protection against remodelling. | [23] |
| Chronic heart failure in the rat induced by permanent ligation of the left anterior descending coronary artery. | NaHS (3.2 mg/kg/day) improved survival, maintained cardiac function, protected against the heart failure-induced fibrosis, preserved cardiac morphological ultrastructure and attenuated apoptosis. | Reduction of the mitochondrial release of cytochrome c; protection against the activation of caspase-3 and bax, an induction of an increase in bcl-2 levels in the myocardium. | [27] |
| Chronic heart failure in the mouse induced by permanent ligation of the left anterior descending coronary artery. | Na2S (100 µg/kg/day i.v.) improved left ventricular contractile function and ultrastructure. | Induction of Nrf-2 and NRF-1 in the cardiac myocytes, Akt phosphorylation (Ser 473), reduction of oxidative stress, improvement of mitochondrial function and preservation of cardiac ATP levels. | [19] |
| Large animal models | |||
| Myocardial ischemia-reperfusion in the pig | Na2S (100 µg/kg bolus + 1 mg/kg/h infusion for 2 hours) reduced myocardial infarct size, improved myocardial contractility and reduced neutrophil infiltration. Na2S also improved coronary microvascular reactivity. | Reduction in caspase-3 activation and PARP cleavage. Reduction of poly(ADP-ribosylation) and TUNEL staining in the reperfused heart. Reduction in Hsp70. Reduction of multiple pro-inflammatory cytokines. | [31; 32; 33] |
| Cardiopulmonary bypass in the pig | Na2S (100 µg/kg bolus + 2 mg/kg/h infusion for 3 hours) improved coronary microvascular reactivity. | Reduction in Hsp70, upregulation of HO-1, upregulation of bcl-2, downregulation of bad; activation of phospho-Erk; inhibition of AIF translocation. Phosphorylation of NOS-3. | [34] |
| Cardiopulmonary bypass induced myocardial and endothelial injury in the dog | Na2S, (1 mg/kg/hour infusion) improved cardiac contractility and vascular function. | Maintenance of cardiac ATP levels, protection of endothelial function. | Current study |
Abbreviations: AIF; apoptosis-inducing factor; AMPK: adenosine monophosphate-activated protein kinase; ADP: adenosine diphosphate; ATP: adenosine triphosphate; CHOP: CAAt/enhancer binding protein homolohous transcription factor; COX: cyclooxygenase; erk: extracellular signal-regulated kinase; ER: endoplasmatic reticulum; GPx: glutathione peroxidase; GRP78: glucose-regulated protein 78 kDa; GSH: glutathione; GSH-Px: glutathione peroxidase; GSK-3beta: glucogen synthase kinase 3 beta; hsp: heat shock protein; HO-1: heme oxygenase-1; jnk: c-Jun N-terminal kinase; KATP ATP-dependent potassium channel; Na2S: disodium sulfide; NaHS: sodium hydrogen sulfide; NF-κB: nuclear factor κB; NO: nitric oxide; MAPK: nrf-1: nuclear respiratory factor-1; MAP kinase; mPTP: mitochonrial permeability transition pore; PARP: poly(ADP-ribose) polymerase; PKC: protein kinase C; SOD: superoxide dismutase; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
Figure 1.
Pathways implicated in the cardioprotective effects of H2S.
Although there is a significant body of evidence demonstrating the protective effects of H2S in various models of cardiac injury, most of the studies are focusing on focal ischemia-reperfusion (see above). Less attention has been paid to global ischemia, such as the one that occurs in conjunction to cardiopulmonary bypass. This condition, nevertheless, is highly significant, given the fact that the majority of the cardiac surgical procedures done today is performed with aortic cross-clamping and cardioplegic arrest. Despite improvements in cardioplegic techniques, ventricular dysfunction following cardioplegic arrest is a major cause of perioperative morbidity and mortality [36]. Even if cardiac dysfunction is not clinically evident, a reduction of myocardial contractility is apparent, as demonstrated in humans by the measurement of pressure-volume relationships [36; 37]. In addition, coronary endothelial and peripheral vascular dysfunction may further complicate the postoperative course [38; 39]. Extracorporal circulation is also known to induce a systemic inflammatory reaction with free radical release leading to secondary organ injury [39]. The aim of the experimental component of the present study was to test the hypothesis that H2S improves myocardial and endothelial function after hypothermic cardioplegic arrest and reperfusion in a clinically relevant canine model of cardiopulmonary bypass.
Materials and methods
In vivo canine model of cardiopulmonary bypass
Animals
20 dogs (foxhounds) weighing 19 to 34 kg were used in this experiment. In 4 animals without cardiopulmonary bypass the hearts were explanted and the coronary arteries were prepared for in vitro isolated vessel experiments (controls without cardiopulmonary bypass). 16 animals were used for the cardiopulmonary bypass studies, which were conducted according to our previously described experimental protocols [40; 41; 42]. All animals received humane care in compliance with the ”Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the ”Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 1996). The experiments were approved by the Ethical Committee of the Land Baden-Württemberg for Animal Experimentation.
General management and cardiopulmonary bypass
The dogs were premedicated with propionylpromazine and anesthetized with pentobarbital (15 mg/kg initial bolus and then 0.5 mg/kg/h i.v.), paralyzed with pancuronium bromide (0.1 mg/kg as a bolus and then 0.2 mg/kg/h i.v.) and endotracheally intubated. The dogs were ventilated with a mixture of room air and O2 (FiO2=60%) at a frequency of 12–15/min and a tidal volume starting at 15 ml/kg per minute. The settings were adjusted to maintain arterial partial carbon dioxide pressure levels between 35–40 mmHg. The femoral artery and vein were cannulated to record aortic pressure and to take blood samples for biochemical analysis. Basic intravenous volume substitution was carried out with Ringer´s solution (1 ml/min/kg). According to the values of potassium, bicarbonate and base excess, substitution included administration of potassium chloride and sodium bicarbonate (8.4%). Neither catecholamines nor other hormonal or pressor substances were administered.
After left anterolateral thoracotomy in the fourth intercostal space and pericardiotomy, the great vessels were dissected. After systemic anticoagulation with sodium heparin (300 U/kg) the left subclavian artery was cannulated for arterial perfusion. The venous cannula was placed in the right atrium. The extracorporeal circuit consisted of a heat exchanger, a venous reservoir, a roller pump and a membrane oxygenator primed with Ringer lactate solution (1000 ml) supplemented with heparin (150 U/kg) and 20 ml sodium bicarbonate (8,4%). After initiation of CPB, the body temperature was cooled to 28°C. After crossclamping of the aorta, the heart was arrested with a single dose of 25 ml/kg HTK solution (in mM: 15 NaCl, 9 KCl, 4 MgCl2, 6 H2O, 18 histidine hydrochloride monohydrate, 180 histidine, 2 tryptophan, 30 mannitol, 0.015 CaCl2, 1 potassium-hydrogen-2-oxopentandioate, H2O). During cardiac arrest the pump flow was set at 100 ml/kg/min to maintain perfusion pressure above a value of 35–40 mmHg at any time point and alpha-stat management was applied. Twenty minutes prior to cross-clamp removal, rewarming was initiated. After 60 minutes of cardiac arrest, the aorta was declamped and the heart was reperfused with normothermic blood in the bypass circuit. If necessary, ventricular fibrillation was counteracted with DC cardioversion of 40 J. Ventilation was restarted with 100% oxygen. All animals were weaned from CPB without inotropic support 20 min after the release of the aortic cross clamp. Each animal underwent 90 minutes of CPB with 60 minutes of cardiac arrest. Functional measurements were performed before cardiopulmonary bypass and after one hour of reperfusion. In addition myocardial probes were collected for high-energy phosphate analysis at the end of experiments.
Cardiac Function
Left ventricular systolic and diastolic pressures and volumes were measured by a combined 6F Millar pressure-conductance catheter with 6 mm spacing, inserted via the apex. In addition, dP/dt was derived from the pressure signal. Stroke volume was calculated from the integrated flow signal measured by an aortic ultrasonic flow probe and was used to calibrate the volume signal from the conductance catheter. Parallel conductance was estimated by rapid injection of one ml of hypertonic saline into the pulmonary artery or superior vena cava, respectively. Vena cava occlusions were performed to obtain a series of pressure-volume loops. Preload recruitable stroke work (PRSW) was calculated as load-independent index of myocardial contractility (Sigma 5 and Software CONDUCT, Leycom, Leiden, The Netherlands).
Coronary blood flow and vascular function
Coronary blood flow was measured on the left anterior descendent artery with a perivascular ultrasonic flow probe. Coronary endothelium-dependent vasodilatation was assessed after intracoronary administration of a single bolus of acetylcholine (ACh, 10−7 mol) and endothelium-independent vasodilatation after sodium-nitroprusside (SNP, 10−4 mol). The vascular response was expressed as percent change of baseline coronary vascular resistance.
High-energy phosphate content
Adenosine triphosphate (ATP), adenosine diphosphate (ADP) and adenosine monophosphate (AMP) contents were assessed with standard photometry using an enzyme-kinetic assay [40; 41; 42].
Ex vivo vascular function
In addition endothelium-dependent and –independent relaxation was investigated in isolated coronary rings. After the end of the in vivo experiments the hearts were excised and the left anterior descendent coronary arteries were isolated and placed in cold (+4 °C) Krebs-Henseleit solution (118 mM NaCl, 4,7 mM KCl, 1,2 mM KH2PO4, 1,2 mM MgSO4, 1,77 mM CaCl2, 25 mM NaHCO3, 11,4 mM glucose; pH=7,4). The coronary arteries were prepared and cleaned from periadventitial fat and surrounding connective tissue and cut transversely into 4-mm width rings using an operation microscope.
Isolated coronary rings were mounted on stainless steel hooks in individual organ baths (Radnoti Glass Technology, Monrovia, CA, USA), containing 25 ml of Krebs-Henseleit solution at 37 °C and aerated with 95% O2 and 5% CO2. Isometric contractions were recorded using isometric force transducers (Radnoti Glass Technology, Monrovia, CA, USA), digitized, stored and displayed with the IOX Software System (EMKA Technologies, Paris, France). The rings were placed under a resting tension of 2 g and equilibrated for 60 minutes. U46619 (5× 10−7 M) was used to precontract the rings until a stable plateau was reached, and relaxation responses were examined by adding cumulative concentrations of endothelium-dependent dilator acetylcholine (ACh, 10−9–10−4 M) and endothelium-independent dilator sodium nitroprusside (SNP, 10−10–10−5 M). Relaxation was expressed as percent of contraction induced by U46619.
Study groups
Cardiopulmonary bypass was performed in control animals (n=8) and animals treated with sodium sulfide for intravenous injection (n=8). Sodium sulfide for parenteral injection was produced and formulated to pH neutrality and iso-osmolarity by Ikaria Inc. (Seattle, WA) using H2S gas (Mattheson, Newark, CA) as the starting material. The start of sulfide infusion was at 30 min prior to the start of cardioplegia. Infusion continued until the end of the experiments (2 hours infusion in total, at a dose of 1 mg/kg/h, i.v.). The dose of sulfide is based on previous small and large animal studies (10–12). All animals underwent 1 hour of cold cardioplegic arrest and a total cardiopulmonary bypass time of 90 minutes. In addition coronary artery rings were explanted from dogs (n=4) without cardiopulmonary bypass for ex vivo vascular function measurements.
In vitro cell culture experiments
Cell culture
H9c2 rat heart myoblast cells were obtained form the European Collection of Cell Cultures and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (Invitrogen), 4 mM glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin. 7 days prior to the assay 10 000 cells/well were plated into 96-well tissue culture plates and cultured at 37 °C at 5% CO2 atmosphere. Cardiomyoblasts from passage numbers from 30 to 36 were used for the assays.
Oxidant- and hypoxia induced cell death models
Cell death was either induced in confluent H9c2 cultures by hydrogen peroxide (oxidant model) or oxygen-glucose depletion (hypoxia model). In the oxidant model fresh culture medium without phenol red was added to the cells prior to the assay and H2S was applied to the cells at 1–600 µM final concentration in 5% of the culture volume. 30 min later cardiomyoblasts were challenged with 700 µM H2O2 and cultured at 37 °C for 3 or 24 hours. Cells treated with vehicle only and served as control.
In the hypoxia model culture medium was replaced with DMEM containing 0 g/l glucose (Biochrom AG, Berlin, Germany) and H2S was applied in 1–600 µM final concentration in 5% of the culture volume 30 min prior to hypoxia induction. Culture plates were placed in gas-tight incubation chambers (Billups-Rothenberg Inc, Del Mar) and the chamber atmosphere was replaced by flushing the chamber with 95% N2: 5% CO2 mixture at 25 l/min flow rate for 5 min. The hypoxic chamber was sealed and incubated at 37 °C for 10–14 hours. After hypoxia the culture medium was removed and fresh DMEM containing 4.5g/l glucose supplemented with 10 % FBS and MTT was added to measure cell viability. Cells exposed to hypoxia in complete culture medium served as positive controls, as no reduction was detected in cell viability compared to cells maintained at normal culture conditions (complete culture medium, 5% CO2 atmosphere, 37 °C), if H9c2 cells were exposed to oxygen depletion with culture medium containing 4.5g/l glucose and 10% serum or cells were serum and glucose deprived at standard culture atmosphere for the above noted culture periods. Adenosine (100 µM) was used as a pharmacological control in these experiments, as it has been recently shown to serve as an alternative source of ATP and to improve cell viability under conditions of severe combined oxygen/glucose deprivation [43].
MTT cell viability assay
Cell viability was estimated by the MTT method. Shortly, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) was added to the cells at a final concentration of 0.5 mg/ml and cells were cultured at 37 °C for 1 hour. The monolayer was washed with PBS and the formazan dye was dissolved in isopropanol. The amount of converted formazan dye was measured at 570 nm with background measurement at 690 nm on a Powerwave reader (Biotek). A calibration curve was created by measuring the MTT converting capacity of serial dilutions of H9c2 cells, and viable cell count was calculated using Gen5 data reduction software.
LDH assay
Lactate dehydrogenase (LDH) release was determined as a measure of cell death. 30 µl of supernatant was saved before addition of MTT and stored at 4 °C until assayed. Cell culture supernatant (30 µl) was mixed with 100 µl freshly prepared LDH assay reagent to reach final concentrations of 85 mM lactic acid, 1040 mM nicotinamide adenine dinucleotide (NAD+), 224 mM N-methylphenazonium methyl sulfate (PMS), 528 mM 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium chloride (INT) and 200 mM Tris (pH 8.2). The changes in absorbance were read kinetically at 492 nm for 15 min (kinetic LDH assay) on a monochromator based reader (Powerwave HT, Biotek) at 37 °C. LDH activity was expressed in mOD/min or as percent values of Vmax of control wells receiving hydrogen peroxide (100%), with controls receiving vehicle only as blank (0%).
Statistics
All values were expressed as mean ± standard error of the mean (SEM). Paired t-test was used to compare two means within groups. Individual means between the groups were compared by unpaired t-test. In the case of cell culture experiments, means were compared by one-way ANOVA followed by an unpaired t-test with Bonferroni correction for multiple comparisons. A probability value less than 0.05 was considered statistically significant. Because of technical reasons one animal was excluded in the H2S-treated group. High-energy phosphate contents were assessed from 6 animals/group.
Results
Effect of H2S in a canine model of cardiopulmonary bypass
The infusion of the H2S donor did not lead to any changes of hemodynamic parameters before CPB (data nor shown). Heart rate, mean arterial pressure, cardiac output and coronary blood flow are shown in Table 2. Baseline heart rate tended to be higher in the treatment groups without reaching the level of significance, otherwise no differences were noted. Mean arterial pressure showed a decreasing tendency in both groups after CPB, without reaching the level of significance. Cardiac output showed no major differences between the groups and over the time. Coronary blood flow was comparable in both groups at baseline. It decreased significantly in the control group after CPB while it remained unchanged in the H2S-treated group. Left ventricular endsystolic pressure decreased significantly in the control group but not in the H2S-treated group (Table 2).
Table 2.
Hemodynamic variables in a canine model of cardiopulmonary bypass; effects of H2S.
| Before CBP | After CPB | |||
|---|---|---|---|---|
| control | H2S | control | H2S | |
| HR (min−1) | 114±8 | 135± 7 | 130±4 | 139 ± 8 |
| MAP (mmHg) | 104±10 | 91±9 | 86±4 | 75±16 |
| CO (l/min) | 3.22±0.29 | 2.82±0.27 | 2.73±0.34 | 3.02±0.21 |
| CBF (ml/min) | 40±5 | 46±5 | 24±3# | 58±17* |
| LVEDP (mmHg) | 10±2 | 11±1 | 11±1 | 13±2 |
| LVESP (mmHg) | 104±9 | 113±19 | 87±3# | 95±11 |
HR heart rate, MAP mean aortic pressure, CO cardiac output, CBF, coronary blood flow, LVEDP left ventricular end-diastolic pressure, LVESP left ventricular endsystolic pressure. All values are given as mean ± SEM,
p<0.05 vs. baseline,
p<0.05 control vs. H2S.
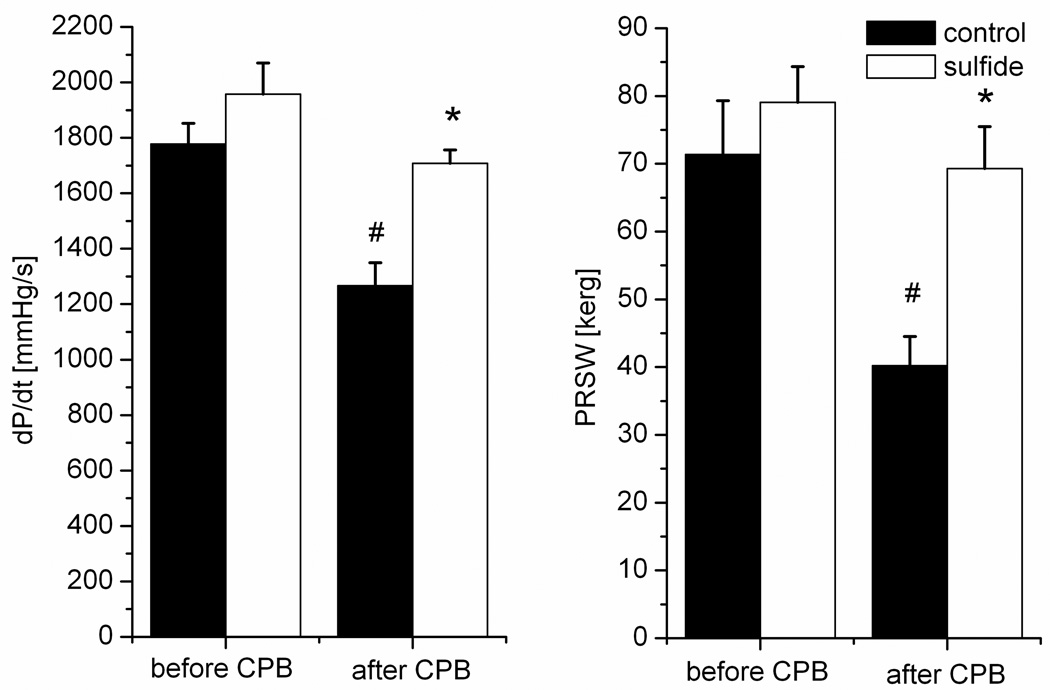
Figure 2 shows the changes of the indices of myocardial contractility. Baseline values did not differ between the groups. After CPB, both left ventricular dP/dt and PRSW decreased significantly (by 30% and by 46 % respectively) in the control group, and remained at baseline levels in the H2S group (Figure 2).
Figure 2.
The changes of left ventricular peak positive dP/dt and of the slope of preload recruitable stroke work (PRSW) at baseline and after cardiopulmonary bypass (CPB) at 60 minutes of reperfusion. All values are given as mean±SEM, #p<0.05 vs. baseline, *p<0.05 H2S vs. control.
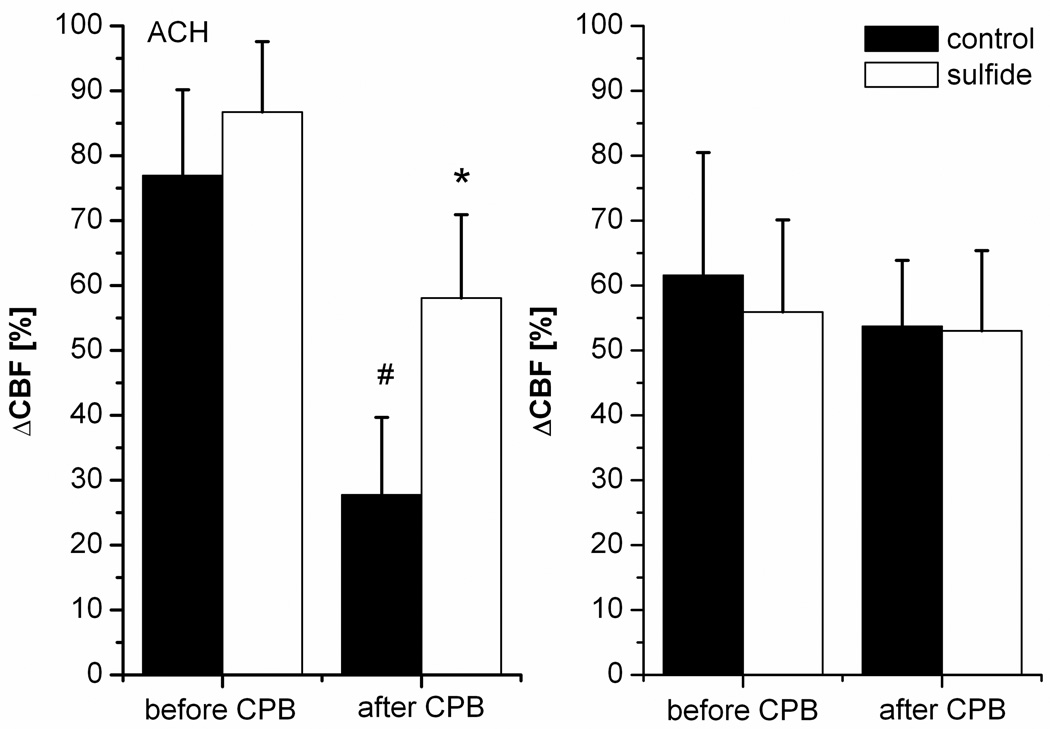
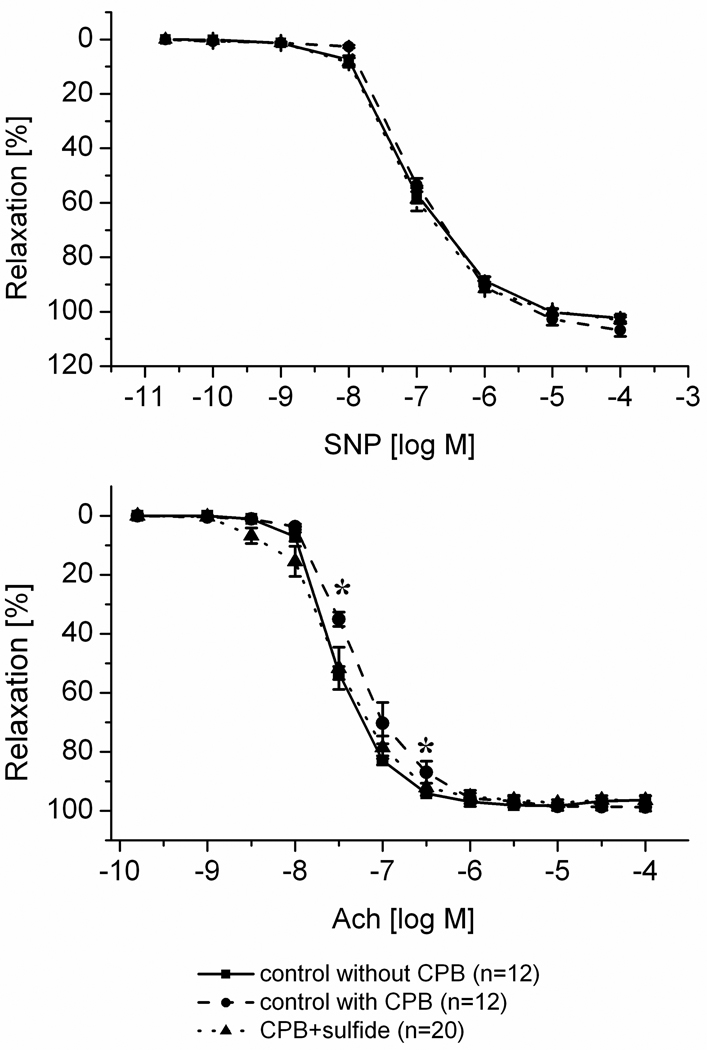
Endothelium dependent vasorelaxation of the coronary arteries is shown on Figure 3. Before CPB no differences have been observed. After CPB the response to acetylcholine was slightly but significantly reduced in the control group, which was partly abolished by H2S (Figure 3). The response to sodium nitroprusside did not differ between the groups and over the time. Ex vivo assessment of the coronary artery rings showed a similar phenomenon (Figure 4): the response to acetylcholine was diminished in the control group in comparison to coronary rings explanted from animals which did not underwent cardiopulmonary bypass. Treatment with H2S significantly improved the response to acetylcholine. In contrast, no difference was observed in the response to sodium nitroprusside.
Figure 3.
In vivo coronary vascular function. Endothelium dependent vasodilatation after acetylcholine (ACh, 10−7 mol, left panel) and endothelium-independent vasodilatation after sodium-nitroprusside (SNP, 10−4 mol, right panel). Measurements were taken at baseline and after cardiopulmonary bypass (CPB) at 60 minutes of reperfusion. All values are given as mean±SEM, #p<0.05 vs. baseline, *p<0.05 H2S vs. control.
Figure 4.
Ex vivo coronary vascular function. Response to acetylcholine on the bottom and the response to sodium nitroprusside on the top. “n” indicates the number of coronary rings used in these experiments. Percent relaxation were measured after pre-contraction with U46619 at each doses of acetylcholine and sodium nitroprusside. All values are given as mean±SEM, #p<0.05 vs. baseline, *p<0.05 H2S vs. control.
Myocardial high-energy phosphate content values are shown in Table 3. ATP values were significantly higher in the H2S-treated group after cardiopulmonary bypass.
Table 3.
High-energy phosphates at the end of experiments upon one hour of reperfusion in a canine model of cardiopulmonary bypass; effect of H2S.
| control | H2S | |
|---|---|---|
| ATP (µmol/g drw) | 9.1 ± 1.2 | 15.8 ± 0.9* |
| ADP (µmol/g drw) | 5.8 ± 0.4 | 5.4 ± 0.3 |
| AMP (µmol/g drw) | 1.4 ± 0.3 | 1.3±0.2 |
All values are given as mean ± SEM,
p<0.05 control vs. H2S.
Effect of H2S in cardiomyocyte cell cultures
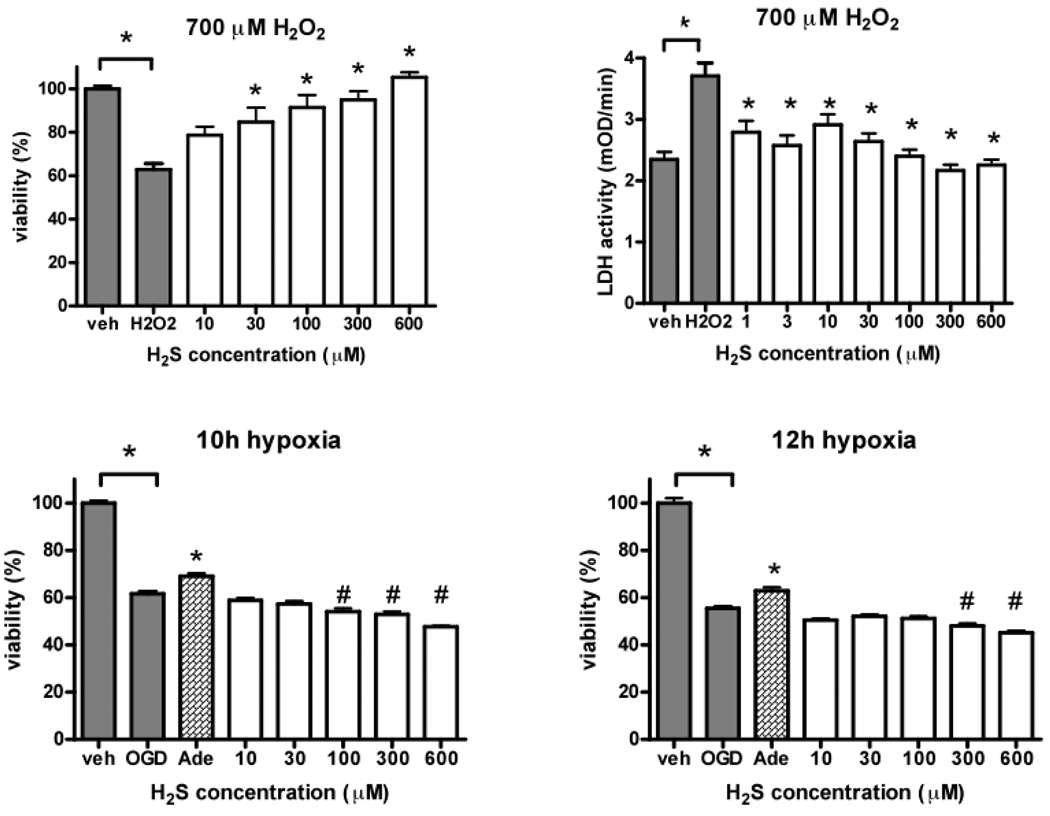
H2S dose-dependently improved cell viability (Figure 5, left, top) and reduced cell necrosis (Figure 5, right top) after oxidative injury by hydrogen-peroxide exposition. Hypoxia/glucose deprivation led to decreased cell viability after both 10 and 12 hours of hypoxia, which, however, was unaffected by H2S administration (Figure 5, bottom).
Figure 5.
Myoblast cells viability (Figure 4, left, top) and LDH release (Figure 4, right top) after oxidative injury by 700 µM hydrogen peroxide exposition in the presence of various concentrations of H2S. Cell viability after hypoxia/glucose (OGD) deprivation after 10 (left bottom) and 12 (right bottom) hours of hypoxia. Adenosine (Ade) served as positive control. All values are given as mean±SEM, *p<0.05 H2S vs. control, #p<0.05 vs. adenosine.
Discussion
In the experimental section of the current article, the benefit of the therapeutic supplementation of the endogenous gaseotransmitter H2S was assessed after cardioplegic arrest and reperfusion in a canine model of cardiopulmonary bypass. In accordance with the literature [36; 37], hypothermic cardioplegic arrest and reperfusion resulted in a decline in ventricular contractile and endothelial function. We showed for the first time in a clinically relevant large animal model that therapeutic administration of H2S improves ventricular function and improves endothelial recovery and leads to a better preservation of ATP pools.
There are a number of potential mechanisms through which H2S may exert cardioprotective effects in the present model. Our cell culture experiments clearly demonstrate, that H2S protects against free radical mediated injury, but not against hypoxia itself. This is in accordance with novel findings which demonstrate in a pig model of infarction and reperfusion that H2S reduces free radical damage, DNA strand breaks and PARP activation and subsequently apoptosis and necrosis [32]; events that occur during the reperfusion stage. Also in various other models [22], H2S was shown to exert antioxidant effects. Whether additional anti-inflammatory properties of H2S contribute to its beneficial effects in this model remains to be clarified. However, H2S has been shown to inhibit neutrophil adhesion and activation in response to inflammatory stimuli [44], as well as suppress the release of the pro-inflammatory mediator tumor necrosis factor-α [45]. Additionally, vasodilatory effects of H2S, and KATP-channel activating effects of the molecule (which are known to induce cardiac protection) may contribute to its protective actions. Recently it was also shown that H2S may exert preconditioning-like effects in a rodent model of ischemia/reperfusion involving both the activation of sarcolemmal KATP channels and the increase of intracellular nitric oxide synthesis [8].
As reviewed in the Introduction section, over the last five years, a number of independent groups have reported the beneficial effects of H2S donor compounds in a number of animal models of disease (Table 1). Many of these studies focus on myocardial protection, where the cardioprotective effects of H2S have been demonstrated in cultured myocytes, isolated perfused hearts, as well as in rodent and large animal models of coronary artery ligation and reperfusion. The current study, which demonstrates the beneficial effect of H2S treatment in the setting of cardioplegic arrest and reperfusion confirms and extends these previous findings into a clinically relevant large animal preparation.
The prevention of left ventricular contractile dysfunction by H2S treatment is in accordance with the previous literature showing improved cardiac contractility after myocardial ischemia after H2S therapy, as demonstrated in beating cardiomyocytes and isolated perfused hearts (e.g. [16]). In the present study we extended these findings and demonstrated in a large animal model of cardiopulmonary bypass in vivo, and demonstrated that therapeutic administration of H2S results in a marked improvement of left ventricular contractility after cardiac preservation and reperfusion. Cardiac injury with temporary myocardial dysfunction is a well-described phenomenon in the context of cardiac surgery [36; 37]. Hearts undergoing coronary bypass surgery or other surgical procedures requiring cardiopulmonary bypass and elective cardioplegia undergo repetitive episodes of ischemia and reperfusion, which leads to endothelial injury as well as contractile dysfunction and morphological injury, despite the use of cardioprotective cardioplegic solutions and other strategies of myocardial protection. In cardiac surgery, as in coronary occlusion, myocardial and endothelial injury appears to occur upon reperfusion with unmodified blood. Even blood cardioplegia, which is viewed as the most protective cardioplegic approach, does not prevent this surgical reperfusion injury. Several concepts have been tested to reduce ischemia/reperfusion injury such as pharmacological and non-pharmacological preconditioning, improvement of ischemic tolerance by additives to the cardioplegic solution and application of substances, which reduces reperfusion injury such as radical scavengers or vasodilators. The current study supports the view that H2S may have a potential protective role in this context.
Coronary blood flow was reduced and endothelial function was impaired, while smooth muscle function remained unaffected after hypothermic cardiac arrest and reperfusion. Potential mechanisms of this selective endothelial dysfunction include free radical mediated cytotoxicity, neutrophil-endothelium interactions and impaired NO-synthesis [38]. As H2S has its own vasodilatory properties, the prevention of the decrease of coronary blood flow and the significantly improved endothelial function may be, in part, related to this effect. In addition and importantly, there is a strong evidence that free radical production and oxidative stress is a major contributing factor to endothelial dysfunction after cardioplegic arrest and reperfusion and direct antioxidant effects of H2S may have contributed to the protective effects observed. The recently reported inhibitory effect of H2S on phosphodiesterase-5 (PDE-5) [46] may also have contributed to the protective effects; in fact, PDE-5 inhibitors have recently been demonstrated to be of therapeutic benefit in the same experimental model of cardiopulmonary bypass [41].
In summary, a substantial body of literature (Table 1) demonstrates the cardioprotective effects of H2S in various models of cardiac injury. We have further confirmed and extended these findings in the experimental section of our report, demonstrating that hypothermic cardiac arrest followed by reperfusion leads to a significant impairment of myocardial and endothelial function, which can be ameliorated by administration of H2S. Based on the present data and other emerging studies in the literature, additional preclinical and clinical studies with therapeutic administration of H2S may be warranted in various forms of acute and chronic cardiac dysfunction.
Acknowledgement
The contribution of Paul Hill (Ikaria Inc., Seattle, WA) in producing the injectable formulation of hydrogen sulfide used in the current study is appreciated. We thank Ms. Karin Sonnenberg and Lutz Hoffman for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010;12:1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- 2.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 3.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 4.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther. 2006;316:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- 5.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu LF, Pan TT, Neo KL, Yong QC, Bian JS. Cyclooxygenase-2 mediates the delayed cardioprotection induced by hydrogen sulfide preconditioning in isolated rat cardiomyocytes. Pflugers Arch. 2008b;455:971–978. doi: 10.1007/s00424-007-0346-8. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Chen X, Pan TT, Neo KL, Lee SW, Khin ES, Moore PK, Bian JS. Cardioprotection induced by hydrogen sulfide preconditioning involves activation of ERK and PI3K/Akt pathways. Pflugers Arch. 2008a;455:607–616. doi: 10.1007/s00424-007-0321-4. [DOI] [PubMed] [Google Scholar]
- 8.Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol. 2006;40:119–130. doi: 10.1016/j.yjmcc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Pan TT, Neo KL, Hu LF, Yong QC, Bian JS. H2S preconditioning-induced PKC activation regulates intracellular calcium handling in rat cardiomyocytes. Am J Physiol Cell Physiol. 2008;294:C169–C177. doi: 10.1152/ajpcell.00282.2007. [DOI] [PubMed] [Google Scholar]
- 10.Shi S, Li QS, Li H, Zhang L, Xu M, Cheng JL, Peng CH, Xu CQ, Tian Y. Anti-apoptotic action of hydrogen sulfide is associated with early JNK inhibition. Cell Biol Int. 2009;33:1095–1101. doi: 10.1016/j.cellbi.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 11.Wei H, Zhang R, Jin H, Liu D, Tang X, Tang C, Du J. Hydrogen sulfide attenuates hyperhomocysteinemia-induced cardiomyocytic endoplasmic reticulum stress in rats. Antioxid Redox Signal. 2010;12:1079–1091. doi: 10.1089/ars.2009.2898. [DOI] [PubMed] [Google Scholar]
- 12.Yao LL, Huang XW, Wang YG, Cao YX, Zhang CC, Zhu YC. Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3beta-dependent opening of mPTP. Am J Physiol Heart Circ Physiol. 2010;298:H1310–H1319. doi: 10.1152/ajpheart.00339.2009. [DOI] [PubMed] [Google Scholar]
- 13.Bliksoen M, Kaljusto ML, Vaage J, Stenslokken KO. Effects of hydrogen sulphide on ischaemia-reperfusion injury and ischaemic preconditioning in the isolated, perfused rat heart. Eur J Cardiothorac Surg. 2008;34:344–349. doi: 10.1016/j.ejcts.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Hu X, Li T, Bi S, Jin Z, Zhou G, Bai C, Li L, Cui Q, Liu W. Possible role of hydrogen sulfide on the preservation of donor rat hearts. Transplant Proc. 2007;39:3024–3029. doi: 10.1016/j.transproceed.2007.05.086. [DOI] [PubMed] [Google Scholar]
- 15.Ji Y, Pang QF, Xu G, Wang L, Wang JK, Zeng YM. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur J Pharmacol. 2008;587:1–7. doi: 10.1016/j.ejphar.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 16.Johansen D, Ytrehus K, Baxter GF. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury--Evidence for a role of K ATP channels. Basic Res Cardiol. 2006;101:53–60. doi: 10.1007/s00395-005-0569-9. [DOI] [PubMed] [Google Scholar]
- 17.Minamishima S, Bougaki M, Sips PY, Yu JD, Minamishima YA, Elrod JW, Lefer DJ, Bloch KD, Ichinose F. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation. 2009;120:888–896. doi: 10.1161/CIRCULATIONAHA.108.833491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Huang H, Liu P, Tang C, Wang J. Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening K ATP channels. Can J Physiol Pharmacol. 2007;85:1248–1253. doi: 10.1139/Y07-120. [DOI] [PubMed] [Google Scholar]
- 19.Calvert JW, Elston M, Nicholson CK, Gundewar S, Jha S, Elrod JW, Ramachandran A, Lefer DJ. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation. 2010;122:11–19. doi: 10.1161/CIRCULATIONAHA.109.920991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chuah SC, Moore PK, Zhu YZ. S-allylcysteine mediates cardioprotection in an acute myocardial infarction rat model via a hydrogen sulfide-mediated pathway. Am J Physiol Heart Circ Physiol. 2007;293:H2693–H2701. doi: 10.1152/ajpheart.00853.2007. [DOI] [PubMed] [Google Scholar]
- 22.Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, Du J, Tang C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 23.Mishra PK, Tyagi N, Sen U, Givvimani S, Tyagi SC. H2S ameliorates oxidative and proteolytic stresses and protects the heart against adverse remodeling in chronic heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H451–H456. doi: 10.1152/ajpheart.00682.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sivarajah A, Collino M, Yasin M, Benetti E, Gallicchio M, Mazzon E, Cuzzocrea S, Fantozzi R, Thiemermann C. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock. 2009;31:267–274. doi: 10.1097/SHK.0b013e318180ff89. [DOI] [PubMed] [Google Scholar]
- 25.Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock. 2006;26:154–161. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- 26.Su YW, Liang C, Jin HF, Tang XY, Han W, Chai LJ, Zhang CY, Geng B, Tang CS, Du JB. Hydrogen sulfide regulates cardiac function and structure in adriamycin-induced cardiomyopathy. Circ J. 2009;73:741–749. doi: 10.1253/circj.cj-08-0636. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Wang XL, Liu HR, Rose P, Zhu YZ. Protective effects of cysteine analogues on acute myocardial ischemia: novel modulators of endogenous H(2)S production. Antioxid Redox Signal. 2010;12:1155–1165. doi: 10.1089/ars.2009.2947. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Wang Q, Guo W, Zhu YZ. Hydrogen sulfide attenuates cardiac dysfunction in a rat model of heart failure: a mechanism through cardiac mitochondrial protection. Biosci Rep. 2010 doi: 10.1042/BSR20100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC, Huang SH, Tan CS, Whiteman M, Lu J, Moore PK. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol. 2007;102:261–268. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]
- 30.Zhuo Y, Chen PF, Zhang AZ, Zhong H, Chen CQ, Zhu YZ. Cardioprotective effect of hydrogen sulfide in ischemic reperfusion experimental rats and its influence on expression of survivin gene. Biol Pharm Bull. 2009;32:1406–1410. doi: 10.1248/bpb.32.1406. [DOI] [PubMed] [Google Scholar]
- 31.Osipov RM, Robich MP, Feng J, Liu Y, Clements RT, Glazer HP, Sodha NR, Szabo C, Bianchi C, Sellke FW. Effect of hydrogen sulfide in a porcine model of myocardial ischemia-reperfusion: comparison of different administration regimens and characterization of the cellular mechanisms of protection. J Cardiovasc Pharmacol. 2009;54:287–297. doi: 10.1097/FJC.0b013e3181b2b72b. [DOI] [PubMed] [Google Scholar]
- 32.Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, Szabo C, Sellke FW. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur J Cardiothorac Surg. 2008;33:906–913. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, Szabo C, Stahl GL, Sellke FW. Hydrogen sulfide therapy attenuates the inflammatory response in a porcine model of myocardial ischemia/reperfusion injury. J Thorac Cardiovasc Surg. 2009;138:977–984. doi: 10.1016/j.jtcvs.2008.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osipov RM, Robich MP, Feng J, Chan V, Clements RT, Deyo RJ, Szabo C, Sellke FW. Effect of hydrogen sulfide on myocardial protection in the setting of cardioplegia and cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2010;10:506–512. doi: 10.1510/icvts.2009.219535. [DOI] [PubMed] [Google Scholar]
- 35.Yong QC, Lee SW, Foo CS, Neo KL, Chen X, Bian JS. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am J Physiol Heart Circ Physiol. 2008;295:H1330–H1340. doi: 10.1152/ajpheart.00244.2008. [DOI] [PubMed] [Google Scholar]
- 36.Wallace A, Lam HW, Nose PS, Bellows W, Mangano DT. Changes in systolic and diastolic ventricular function with cold cardioplegic arrest in man. The Multicenter Study of Perioperative Ischemia (McSPI) Research Group. J Card Surg. 1994;9:497–502. doi: 10.1111/jocs.1994.9.3s.497. [DOI] [PubMed] [Google Scholar]
- 37.Gorcsan J, 3rd, Gasior TA, Mandarino WA, Deneault LG, Hattler BG, Pinsky MR. Assessment of the immediate effects of cardiopulmonary bypass on left ventricular performance by on-line pressure-area relations. Circulation. 1994;89:180–190. doi: 10.1161/01.cir.89.1.180. [DOI] [PubMed] [Google Scholar]
- 38.Sellke FW, Shafique T, Ely DL, Weintraub RM. Coronary endothelial injury after cardiopulmonary bypass and ischemic cardioplegia is mediated by oxygen-derived free radicals. Circulation. 1993;88:II395–II400. [PubMed] [Google Scholar]
- 39.Szabo G, Soos P, Mandera S, Heger U, Flechtenmacher C, Seres L, Zsengeller Z, Sack FU, Szabo C, Hagl S. Mesenteric injury after cardiopulmonary bypass: role of poly(adenosine 5'-diphosphate-ribose) polymerase. Crit Care Med. 2004;32:2392–2397. doi: 10.1097/01.ccm.0000148009.48919.6a. [DOI] [PubMed] [Google Scholar]
- 40.Radovits T, Korkmaz S, Miesel-Groschel C, Seidel B, Stasch JP, Merkely B, Karck M, Szabo G. Pre-conditioning with the soluble guanylate cyclase activator Cinaciguat reduces ischaemia-reperfusion injury after cardiopulmonary bypass. Eur J Cardiothorac Surg. 2010 doi: 10.1016/j.ejcts.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 41.Szabo G, Radovits T, Veres G, Krieger N, Loganathan S, Sandner P, Karck M. Vardenafil protects against myocardial and endothelial injuries after cardiopulmonary bypass. Eur J Cardiothorac Surg. 2009;36:657–664. doi: 10.1016/j.ejcts.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 42.Veres G, Radovits T, Otila G, Hirschberg K, Haider H, Krieger N, Knoll A, Weigang E, Szabolcs Z, Karck M, Szabo G. Efficacy of the non-adenosine analogue A1 adenosine receptor agonist (BR-4935) on cardiovascular function after cardiopulmonary bypass. Thorac Cardiovasc Surg. 2010;58:86–92. doi: 10.1055/s-0029-1186271. [DOI] [PubMed] [Google Scholar]
- 43.Módis K, Gero D, Nagy N, Szoleczky P, Tóth ZD, Szabó C. Cytoprotective effects of adenosine and inosine in an in vitro model of acute tubular necrosis. Br J Pharmacol. 2009;158:1565–1578. doi: 10.1111/j.1476-5381.2009.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 45.Hu LF, Wong PT, Moore PK, Bian JS. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem. 2007;100:1121–1128. doi: 10.1111/j.1471-4159.2006.04283.x. [DOI] [PubMed] [Google Scholar]
- 46.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 47.Wang Q, Liu HR, Mu Q, Rose P, Zhu YZ. S-propargyl-cysteine protects both adult rat hearts and neonatal cardiomyocytes from ischemia/hypoxia injury: the contribution of the hydrogen sulfide-mediated pathway. J Cardiovasc Pharmacol. 2009;54:139–146. doi: 10.1097/FJC.0b013e3181ac8e12. [DOI] [PubMed] [Google Scholar]