Abstract
Magnetic isolation is a promising method for separating and concentrating pancreatic islets of Langerhans for transplantation in Type 1 Diabetes patients. We are developing a continuous magnetic islet sorter to overcome the restrictions of current purification methods that result in limited yield and viability. In Quadrupole Magnetic Sorting (QMS) islets are magnetized by infusing superparamagnetic microbeads into islets’ vasculature via arteries that serve the pancreas. The performance of the islet sorter depends on the resulting speed of the islets in an applied magnetic field, a property known as magnetophoretic mobility. Essential to the design and successful operation of the QMS is a method to measure the magnetophoretic mobilities of magnetically infused islets. We have adapted a Magnetic Particle Tracking Velocimeter (MPTV) to measure the magnetophoretic mobility of particles up to 1000 microns in diameter. Velocity measurements are performed in a well-characterized uniform magnetic energy gradient using video imaging followed by analysis of the video images with a computer algorithm that produces a histogram of absolute mobilities. MPTV was validated using magnetic agarose beads serving as islet surrogates and subjecting them to QMS. Mobility distributions of labeled porcine islets indicated that magnetized islets have sufficient mobility to be captured by the proposed sorting method, with this result confirmed in test isolations of magnetized islets.
Keywords: Particle tracking velocimetry, magnetic flow sorter, pancreatic islets isolation, magnetic particles
INTRODUCTION
Magnetic isolation of islets is an attractive alternative to the current state of the art due to its ease of use, speed and selectivity. Current islet isolation procedures depend on centrifugation in density gradients. These procedures are limited in their throughput and separation efficiency and subject islets to potentially detrimental physical stresses (London et al., 1998; Soon-Shiong et al., 1989). To address the problems with throughput and efficiency, continuous flow quadrupole magnetic islet sorting was tested (Shenkman et al. 2009), and a separator was developed (Kennedy et al., 2007). Essential to the design and operation of such a device is a method to measure the magnetophoretic mobilities of magnetically infused islets.
The magnetophoretic mobility of a particle indicates how responsive the particle is to an applied magnetic field. Paramagnetic particles have positive susceptibility and move toward increasing magnetic field intensity. Islets are rendered paramagnetic by infusing the donor pancreas with paramagnetic microspheres such as Dynabeads®. Bead capture efficiency varies with the age of the donor and pancreas weight and affects the magnetophoretic mobility of the islets.
Several different techniques have been used to measure the magnetophoretic mobility of paramagnetic particles, including the use of visual microscopic observation of the movement of the labeled particle with stopwatch and magneto-cytometry (Gill et al., 1960; Davis et al., 1993). Magnetic Particle Tracking Velocimetry (MPTV) measures cell surface antigen counts through the quantification of antibody binding capacities (ABC) (McCloskey et al., 2001; Melnik et al., 2001; McCloskey et al., 2000; McCloskey et al., 2001a) and can be used to characterizes magnetophoretic mobilities of nano and micro-particles (Häfeli et al., 2002; Zhang et al., 2002; Chalmers et al., 1999) and living cells (Chalmers et al., 1999). Two important features of the MPTV apparatus set it apart from other magnetic tracking technologies: 1) the use of an isodynamic (i. e., constant-force) magnetic field as indicated by “Sm = constant”, and 2) capacity to track a large number of individual particles, up to 10,000, in a fraction of an hour. Both features are essential for high accuracy and precision of the MPTV analysis (Sridhra Redy et al., 1996; Moore et al., 2004; Zborowski et al., 2003). Those features are at the basis of the competitive advantage of the MPTV over other magnetic motion analyzers that are based on single-particle analysis in high-gradient magnetic fields which is difficult to measure (Watarai et al., 2001; Watanabe et al., 2004; Suwa et al., 2004).
MPTV leverages current technologies in video microscopy, computer processing speed and finite element analysis for magnetic fields to measure the induced motion of cells and particles in the highly characterized magnetic and gravity field. The motion of the particles in the known field is then translated into a characteristic parameter called magnetophoretic mobility in a magnetic field and sedimentation rate in a gravity field. MPTV measurements involve videotaping the movement of immunomagnetically labeled particles through a known viscosity medium and magnetic susceptibility in a well-defined magnetic energy density gradient. The velocity of each particle along with its location within the magnetic energy gradient is recorded. From this information, the magnetophoretic mobility of each particle is calculated. A significant advantage of this method over other techniques is that large numbers of cells or particles can be processed in a very short time.
Present MPTV devices developed to measure magnetophoretic mobility of single cells and magnetic microspheres were found unable to accommodate the large, rapidly sedimenting, rapidly moving islets and particles used as islet surrogates The MPTV described herein measures magnetophoretic mobilities and sedimentation rates of cells and large particles like islets with reliable software and variable magnetic field strength. The software developed for MPTV also facilitates the selection of optimum parameters to use with QMS to achieve isolation with maximum purity and yield. This paper explains the features of a refined MPTV system with results obtained with large particles including porcine pancreatic islets of Langerhans.
THEORY
The following analysis applies to the use of MPTV applied to the isolation of magnetically labeled pancreatic islets of Langerhans by quadrupole magnetic sorting. The magnetophoretic mobility (μm) of a particle is defined as the ratio of the velocity of the particle in the magnetic field, uf, to the magnetic field energy gradient, Sm
| (1) |
Any population of magnetically labeled particles exhibits a statistical distribution of μm based on several characteristics. Mobility of the pancreatic islets depends on the number of magnetic Dynabeads® infused into each islet. The following equation relates the Dynabeads and islet properties to the magnetophoretic mobility of a magnetically labeled islet.
| (2) |
where nD is the number of Dynabeads, Δχ is the difference in magnetic susceptibility between the medium and the beads, Vi is the volume of a bead, η is the viscosity of the medium, and Di is the diameter of the islet.
The magnetophoretic mobility distribution measured using MPTV is used to predict the QMS output fractions based on the defined flow rate parameters. QMS is a split flow type continuous magnetic sorter developed to separate single cells by labeling specific cells with magnetic beads. QMS was designed to isolate Dynabead-infused islets from exocrine tissue based on their magnetophoretic mobility (μm) (Kennedy et al., 2007). Figure 1 describes the basic QMS mechanism for isolation of magnetic cells from nonmagnetic cell populations and defines the variables used for its analysis. QMS can be operated continuously with pancreatic digest containing labeled islets entering at inlet a’ (sample) and carrier buffer at inlet b’ (buffer) and isolated islets exiting the system at outlet b (positive), unlabeled tissue at outlet a (negative), some of the tissue fragments (especially islets) which are labeled heavily enough to reach the wall of the flow channel will be stopped by the wall and can be collected at the end of the isolation process as the “wall” (W) fraction. There are two possible ways to collect magnetic islets: in fraction b (as a dilute suspension in several liters of fresh fluid) or at the channel wall (at high concentration in less than one liter of fresh fluid). The latter delivers the product at a more desirable concentration. MPTV analysis facilitates either of these modes by providing critical data for adjusting QMS flow rates. The boundary between inlet flows is defined as the Inner Splitting Surface (ISS) and the boundary between outlet flows is called the Outer Splitting Surface (OSS). The distance between ISS and OSS is called the transport lamina. There are four critical mobilities in the separation of islets by QMS. These are
Figure 1.
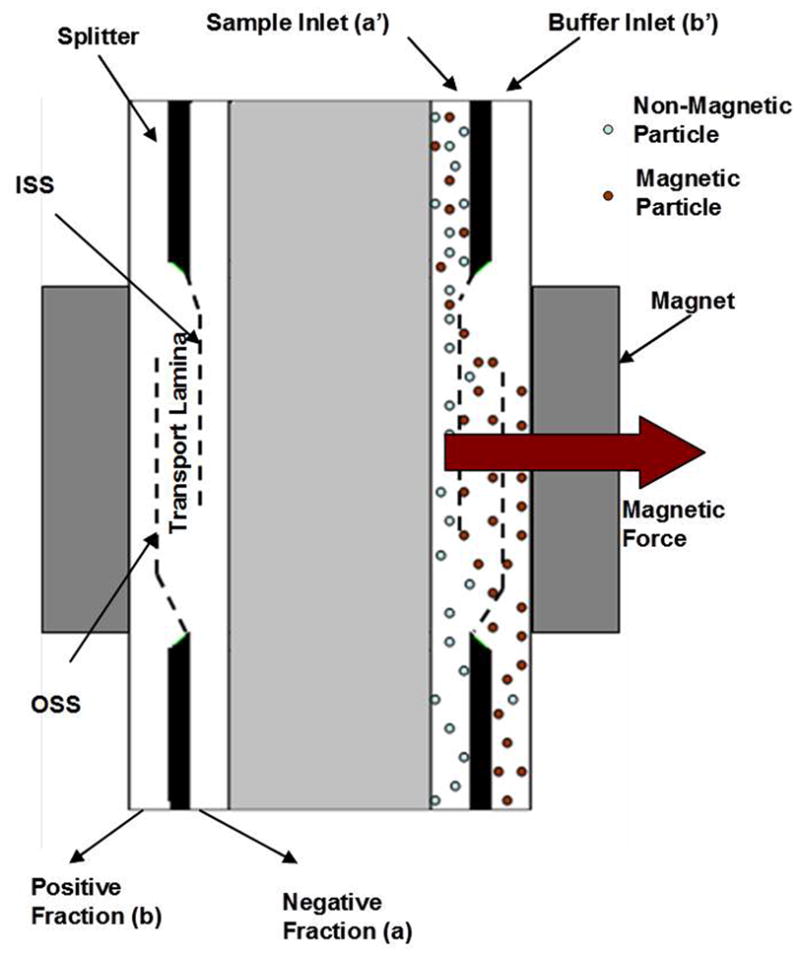
Schematic diagram of the Quadrupole Magnetic cell Sorter (QMS)
μm0 = mobility whereby a particle entering the flow channel at the ISS just reaches the OSS and is eluted into the b (positive) fraction,
μm1 = mobility whereby a particle entering the flow channel at the wall of the core reaches the OSS and can exit with the b fraction,
μm2 = mobility whereby a particle entering the flow channel at the ISS reaches the wall of the shell and may be trapped in the flow channel,
μm3 = mobility whereby a particle entering the flow channel at the core wall reaches the wall of the shell and remains on the wall.
The equations predicting these four critical mobilities are given in the Appendix A. All the particles with μm< μm0 exit in the a fraction, those having μm0 ≤ μm ≤ μm1 will exit either in a or b fraction, μm1 ≤ μm ≤ μm2 will exit in b fraction, μm2 ≤ μm ≤ μm3 will exit in b fraction or become trapped on the flow channel wall and μm ≥ μm3 with be trapped on the flow channel wall.
MATERIALS AND METHODS
Particles and Viscous Liquid
Three types of magnetic particles with similar size ranges and different magnetization were used for this study. Agarose magnetic beads (Bioscience Beads, RI) with diameters 300 to 500 microns and magnetite loading of 0.5%, 1% and 6% by volume were used for testing. As the particles used for testing settled rapidly in the aqueous solution, high-viscosity, high-density liquid was prepared by dissolving Ficoll® (400,000 MW) (Sigma-Aldrich) to maintain the particles in suspension while measuring their mobilities.
QMS System
QMS is a split flow type continuous magnetic sorter originally developed to separate single cells by labeling specific cells with magnetic beads. QMS was improved to isolate Dynabead-infused islets from exocrine tissue based on their magnetophoretic mobility (μm) (Kennedy et al., 2007). Figure 1 describes the basic QMS mechanism, presented in detail above in the THEORY section. .
Magnetic Particle Tracking Velocimetry
The MPTV technology is comprised mainly of five components (Figure 2): a sample channel containing the suspension of cells and particles, a magnet capable of providing a constant magnetic force in the sample channel zone, a pump for introducing fluid and sample, a video microscope capable of imaging the sample with various degrees of magnification, and a computer with software capable of capturing and processing the video images for particle mobility analysis.
Figure 2.
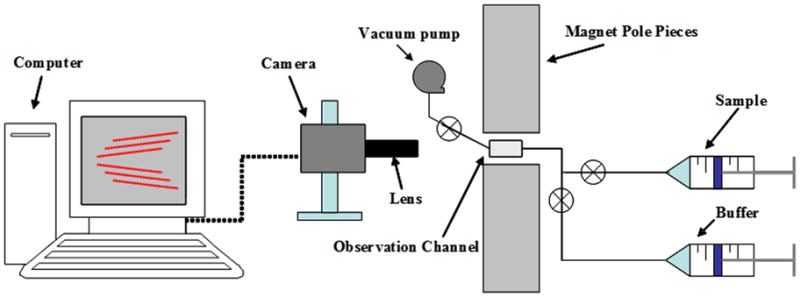
A simplified diagrammatic representation of an MPTV system
Magnet Assembly
The custom designed magnet assembly is comprised of a base plate, two neodymium-iron-boron (NeFeB) magnets and two 1018 carbon steel pole pieces. The pole pieces are shaped to match a very specific modified hyperbolic profile. The magnetic force is perpendicular to the direction of gravitational force so magnetophoretic mobility measurements are independent of sedimentation velocity, which can be used independently to estimate particle dimensions.
Fluid System
The stopped-flow channel consists of a borosilicate glass channel with square (2 mm) cross-section. One end of the 6 cm long channel is connected by a pair of solenoid pinch valves to a disposable 50 ml syringe for sample injection and a 50ml syringe for priming buffer while the outlet end connects into a waste vessel. The observation channel is positioned within the magnet assembly and is sandwiched between the video lens and the backlight. An automated vacuum pump and pinch valves were used to control the sample flow into and out of the channel during programmed or operator-controlled sample changes.
Imaging System
The selection of the video microscope system components and their operational settings is critical, as the pixel size, field of view, magnification, and frame capture speed dictate the maximum and minimum velocities and particle sizes that can be accurately analyzed. Particle movement in the stopped-flow channel was recorded with a grasshopper 2.0MP B&W, 1394b, and 1/1.8 inch CCD camera (Point Gray, AZ) operating at 30 frames per second with 1x objective (Edmund Optics, NJ) mounted on a inch Manual translation stage (Thor Labs, NJ). Light was supplied by dark field illumination using a white light LED source (Edmund Optics, NJ).
Analysis of Video Data
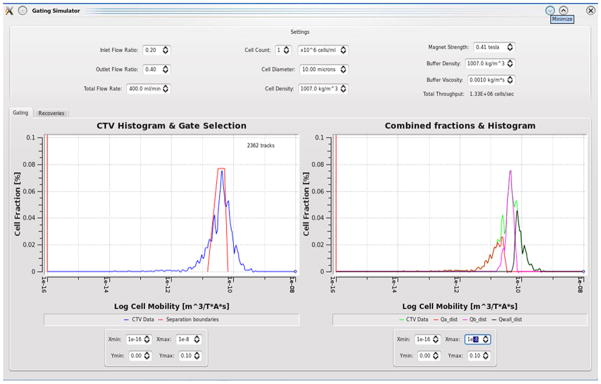
Image processing software converts the video data into useful velocity data and corresponding property data such as hydrodynamic radius and magnetophoretic mobility. A program named “Cytotest” first thresholds the images into binary black or white images based on a user-defined gray-level threshold. This eliminates pixels that are below certain brightness, leaving the light-reflecting particles visible in the image records. It then identifies particles in each frame of the video file using a program named “OpenCV” in which cells & particles outside a specific size range are rejected. It then searches successive frames in the video record to identify particle movement by connecting the tracks of each identified particle to its match in a successive frame. Proprietary predictive and adaptive algorithms are employed to improve the accuracy of particle matching across frames and eliminate bad tracks. The track lengths are then converted to a rate of travel in pixels per millisecond, and that rate is converted to a magnetophoretic mobility measurement based on the magnet strength and image pixel size. That value is recorded in a histogram with other tracks delimited by logarithmic bin sizes and plotted by the software.
Experimental Procedures
The camera lens was adjusted to visualize the central region of the sample cell at constant magnetic energy gradient using the MPTV translation stage. Ficoll® solution was prepared by mixing 25.2 gm of Ficoll powder in 100 milliliters of distilled water which helps to keep the magnetic particles suspended. Magnetic particle suspensions were injected into the sample cell using a syringe after priming the cell with buffer using a vacuum pump. Fixed volumes of the particle suspension, typically 1 to 5 milliliters were processed by repeated injections into the field of view to observe the magnetic deflection of the particles. Particle samples were pumped in a direction opposite to the direction in which the magnetic energy gradient operates on the particles then stopped. Particle deflection was observed with no fluid velocity so that particle motion was only due to magnetic and gravitational force. Successive volumes of the suspensions were injected to obtain several series of deflection images at a rate of 30 frames per second. After analyzing the samples in MPTV, the optimized parameters given by software were used to run QMS with magnetic particles.
The sample consisting of magnetic particles was introduced into the a’ inlet flow stream and carrier buffer was introduced into the b’ inlet flow stream by dual head Watson-Marlow peristaltic pumps, and the positive fraction outlet was controlled by a peristaltic pump while leaving the negative fraction outlet to exit at atmospheric pressure to maintain equilibrium of flow in the flow channel. Experiments were carried out at total flow rates (Q), inlet flow ratio (Qa’/Q) and outlet flow ratios predicted by MPTV. Turbidity sensors are connected to positive and negative fraction outlets to detect absorbance. Magnetic particles stacked to the wall were collected by removing the flow channel from magnet assembly and counted under microscope and results were compared with MPTV predictions.
Pancreas Procurement and Labeling
Pancreata were harvested from juvenile pigs (25–32Kg) using a previously published technique established for procurement of the intact pancreas for perfusion preservation prior to islet isolation (Taylor et al., 2010; Taylor et al., 2011). In brief, small farm pigs (Domestic Yorkshire, male, 25 to 32 kg, Hambone Farms, SC) were used as pancreas donors. Following induction of general anesthesia with ketamine (22mg/kg), acepromazine (0.2 mg/kg), and atropine (0.025mg/kg), and anesthesia maintenance with isoflurane in oxygen, the animals were incubated and connected to a ventilator. The abdominal cavity was opened and the descending aorta was cannulated below the kidneys. The inferior vena cava and aorta were close-clamped above the diaphragm. An in-situ gravity-driven flushing of the pancreas was initiated using 2L of cold Lactated Ringer’s solution while for blood flow the inferior vena cava was cut open above the diaphragm, downstream from the clamp. The pig was euthanized through exsanguinations and a lethal dose of 5% sodium pentobarbital administered intravenously. The latter is an accepted form of euthanasia according to the latest guidelines from the American Veterinary Medical Association Panel on Euthanasia (AVMA). All animal care and handling complied with policies and approval of the Institutional Animal Care and Use Committee (IACUC) at the Medical University of South Carolina, where the organ procurements were carried out.
Organ exposure to warm ischemia was kept below 3 minutes by using the cold solution vascular flush and by placing ice inside the abdominal cavity during surgical excision of the pancreas. The pancreas was dissected with a segment of proximal duodenum starting near pylorus and inclusive of most of the duodenum’s second descending loop to protect the superior and inferior pancreaticoduodenal arteries. The common bile duct and pancreatic duct openings were included as part of the duodenum segment to facilitate pancreatic duct cannulation, necessary for subsequent enzyme infusion for islet isolation. The splenic vein and artery were ligated prior to detachment of the spleen. A 5–7cm long aortic segment was left attached to the pancreas for subsequent organ cannulation and included the openings of both superior mesenteric artery (SMA) and celiac trunk (CT) vessels. The pancreas was removed from the body, immersed in cold University of Wisconsin solution (UW, Viaspan, and Fisher Scientific) and placed on ice for subsequent and transportation to the research laboratory and infusion of magnetic beads. Upon arrival at the lab all exposed arterial branches on the margin of gastro duodenal and hepatic sides of the pancreas were meticulously identified and ligated to ensure uniform perfusion throughout the gland and allow the effluent to emerge only from the portal vein by avoiding leaks from the many arterial branches as previously described (Taylor et al., 2010; Taylor et al., 2011).
Infusion of the magnetic beads into the pancreas was achieved via cannulation of the SMA and CT on the aortic patch. Paramagnetic Magnetic Particles (4.5 microns in diameter, Dynabead M450, Invitrogen, Carlsbad, CA) suspended in 1 liter of KPS1 organ preservation solution (Organ Recovery Systems, Itasca, IL.) and 500ml was infused into the CT and SMA by hand-syringe. Infusion of MP suspension was immediately followed by 2x 60ml flush with KPS1 at 0 to 4 °C prior to processing for islet isolation.
Pancreas Digestion and QMS Islets Isolation
The infused pancreas was digested for islets isolation using an adaptation of the Ricordi method (Ricordi et al., 1998) as previously described (Taylor et al 2010). Before performing an actual isolation using QMS, the QMS column was filled with HBSS with serum solution. Once the digested tissue collection started from the Ricordi chamber, the collection tube from the Ricordi chamber was connected to a reservoir bag, partially filled with HBSS with 10% serum and placed in between the QMS flow channel a’ inlet and the Ricordi chamber. This sends the tissue to the reservoir at a flow rate equal to QMS inlet flow rate. Outlet fractions from QMS were collected for MPTV analysis.
RESULTS AND DISCUSSION
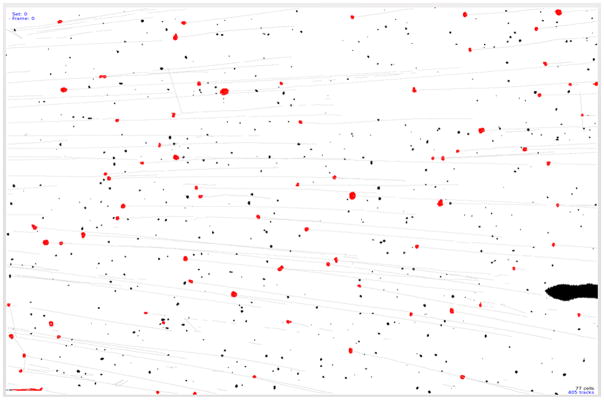
Several improvements were made on cell tracking velocimetry to facilitate measurement of the magnetophoretic mobility of much larger particles such as pancreatic islets and to obtain essential data to set the parameters of the QMS to isolate the magnetic fraction (islets) from non-magnetic fraction in QMS. A newly built MPTV was initially tested with Dynabeads® to evaluate performance. Figure 3 shows a histogram of the magnetophoretic mobility of Dynabeads® developed by MPTV. The magnetophoretic mobility was obtained by dividing the MPTV-determined particle velocity by the magnitude of the magnetic energy gradient, Sm, 6.275 TA/mm2. Particles with mobility less than 10−16 m3/TAs are considered as underflows (unlabeled). Figure 4 shows how, using QMS software, the data of Figure 3 are used to predict the mobility distributions of the three fractions (a, b and W) produced in a QMS separation. Figure 5 displays the tracks developed by MPTV for one set of Dynabeads moving magnetically in the sample cell. Dark spots in Figure 5 are blemishes on the sample cell wall, which, for example, were neglected during tracking calculations by thresholding the images with respect to intensity and spot size. Any of these blemishes measured as tracks were characterized as “underflows” (particles with zero mobility). These are seen catalogued in Figure 3 as a vertical line at 10−16 on the mobility axis.
Figure 3.
Mobility histogram given by MPTV for Dynabeads
Figure 4.
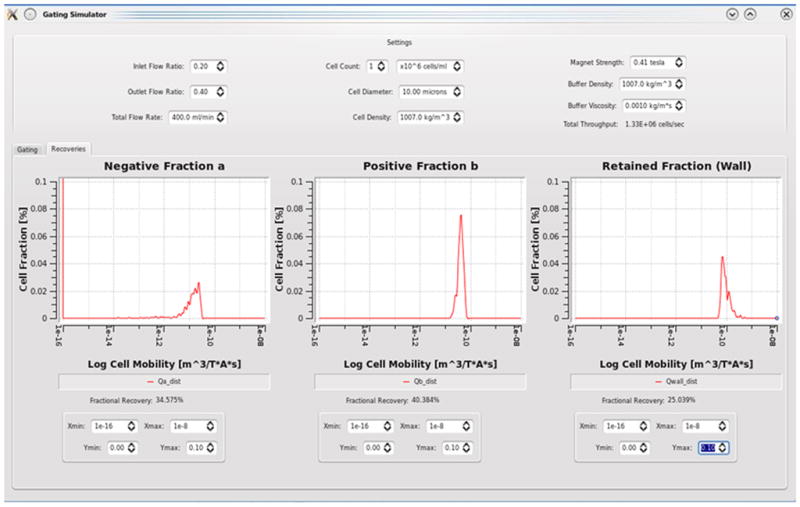
Histograms of Dynabead mobilities predicted in each fraction of the QMS output based on data of Figure 3
Figure 5.
Particle tracks developed by MPTV analysis software. Red dots are the particles tracked and black spots are disturbances or blemishes discriminated out of the analysis
The important development made to the MPTV is the ability to predict the QMS flow parameters required to get the desired isolation based on the mobilities measured using MPTV. Figure 4 shows the histograms developed by MPTV for the three outlet fractions from QMS for Dynabeads based on the mobility predictions for the total flow rate in the QMS of 400 ml/min, inlet flow ratio of 0.2 and outlet flow ratio of 0.4. Underflows are neglected while selecting the flow parameters.
The expression for determining particle magnetization from their motion in a magnetic field is a compound quantity depending on particle size, magnetic velocity and field gradient. The product of field and gradient in the measurement region is assumed to be constant. Three different magnetic particles similar in size were used in this study, and their variation in magnetophoretic mobility is due to differences in magnetite loading. Thus three different magnetic particles with magnetite loading 0.5%, 1% and 6% were used for surrogate islets in the MPTV studies.
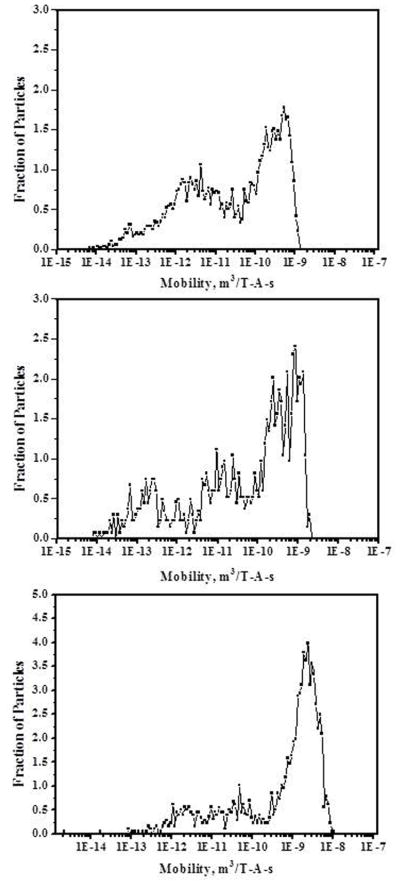
Figure 6a shows the mobility histogram of the magnetic micro beads BSI. The x-axis represents the magnetophoretic mobility values of the magnetic bead on a log scale and y-axis represents the fraction of the beads with specific magnetophoretic mobility. Mobilities were measured in a high viscosity Ficoll® medium to avoid settling of the micro beads while measuring the velocity due to magnetic field. The required density of the medium was 1.36 g/cm3 corresponding to Ficoll® 400 having viscosity of 10.8 Pa-s. Particle velocities were corrected for viscosity when calculating magnetophoretic mobilities. Histograms are plotted with mobilities calculated for water based on the viscosities. Mobilities are measured for the beads with minimum size of 300 microns and maximum size of 500 microns by omitting smaller and larger beads from the histogram analysis. In this case 126 beads were tracked with 2030 tracks. Beads with mobility less than 10−16 are grouped as underflows.
Figure 6.
a) Magnetophoretic mobility histogram of BSI magnetic particles b) Magnetophoretic mobility histogram of BSII magnetic particles c) Magnetophoretic mobility histogram of BSIII magnetic particles
Figure 6b and 6c show the mobility histograms for magnetic micro beads BSII and BSIII. Results generated with 147 beads with 2672 tracks for BSII and 186 beads with 3276 tracks for BSIII were plotted. MPTV can analyze large numbers of particles in a small amount of time on a particle-by-particle basis. As the iron oxide concentration varies in beads BSI, BSII and BSIII, which changes the density of the beads, measurements were done with Ficoll® solution with appropriate density to avoid the settling of the particles in the sample cell. Mobility histograms show the increase in the average and maximum mobility of the beads with the increase in the magnetite concentration from beads BSI to beads BSIII, and these histograms indicate that significant differences in magnetophoretic mobility can be detected between magnetic beads.
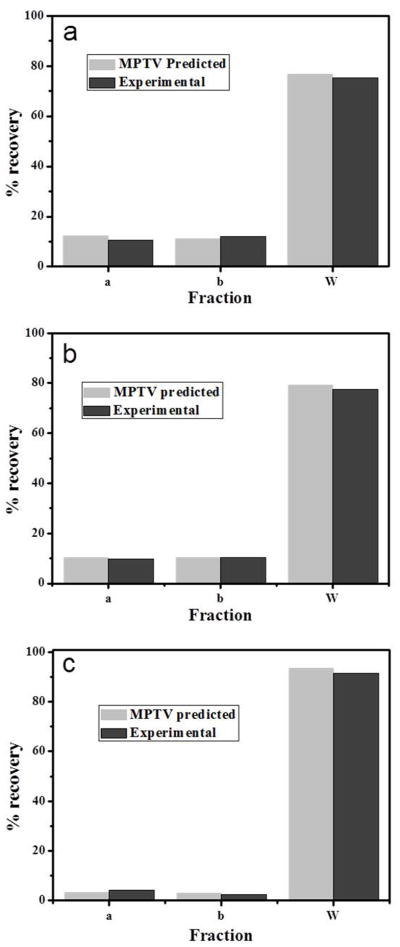
Magnetophoretic mobility measurements and flow rate parameter predictions made by MPTV software were tested by conducting the experiments in QMS using the pure magnetic beads. Figure 7a shows the comparison of the predicted and experimental results for the three outlet fractions from QMS for magnetic particles BSI. The QMS isolation process was controlled by three flow parameters: total flow rate (Q), inlet flow ratio (Ri=Qa’/Q), and Outlet flow ratio (Ro=Qa/Q). These flow rates were selected based on the measured magnetophoretic mobility of the magnetic beads. The critical mobilities μm0 and μm2 were selected to maximize the amount of beads in the b fraction. The flow rates predicted were Q = 400 milliliters per minute, Ri = 0.25 and Ro = 0.4 corresponding to critical mobilities for beads BSI of μm0 = 14.79 × 10−12 m3/TAs and μm2 = 1.355 × 10−11 m3/TAs.
Figure 7.
a) Comparison of MPTV-predicted fractional recovery of BSI particles b) BSII particles c) BSIII particles in the three outlet fractions of the QMS with experimental results
Figure 7a shows the recovery of the particles in all fractions. Total recovery of the magnetic particles during the experimental isolation with QMS was 98%. The loss of some magnetic particles was due to the settling of the particles in the inlet tubes and at the bottom of the flow channel. The MPTV-predicted ‘a’ fraction recovery was high compared to the experimental isolation as the MPTV predicted ‘a’ fraction also contains some non-particle-related tracks. Predicted and experimental ‘b’ fraction recovery is very low because the magnet strength of the QMS is high enough to capture most of the magnetic particles on to the wall of the flow channel. The difference in the wall fraction recoveries is due to the difference in the ‘a’ fractions which affected the total recovery. Figures 7b and 7c show the experimental outlet fractions compared with MPTV predictions and recoveries for beads BSII and BSIII.
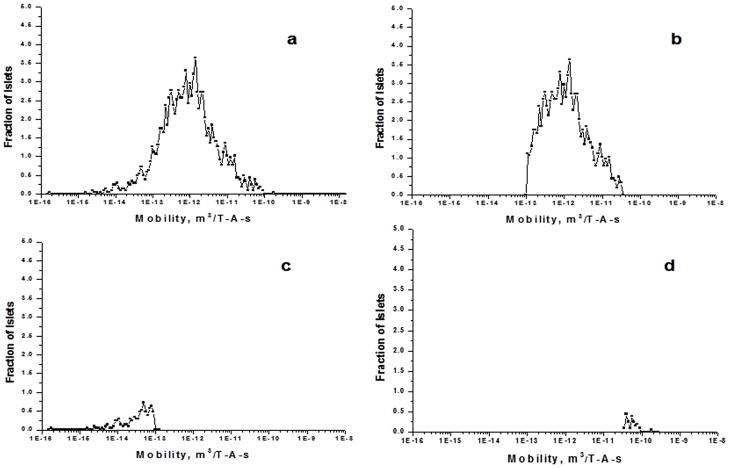
Figure 8a shows the histogram of magnetophoretic mobilities of the porcine islets isolated from acinar tissue with QMS. These are the islets collected from the b fraction of the QMS operated at a total flow rate of 400 ml/min and inlet ratio of 0.4 and outlet ratio of 0.6. Islets were infused with Dynabeads. After MPTV developed the mobility histogram, QMS flow parameters in MPTV gate window for the islets isolation were set to Q= 400 milliliters per minute, Qa’/Q = 0.4 and Qa/Q = 0.6 and fractional recovery histograms were generated. Figure 8b, 8c and 8d show the MPTV-predicted fractional recovery of each outlet of the QMS. These histograms show that 98% of the islets used to measure the mobility exit in the b fraction. Islets that are in the ‘a’ fraction histogram show the nonspecific crossover in the islet isolation experiments. This result also confirmed the ability of the MPTV to predict the QMS parameters based on the measured mobilities.
Figure 8.
a) Magnetophoretic mobility histogram of pancreatic islets isolated with QMS. b) MPTV predicted histogram for the b fraction c) a fraction, and d) wall fraction of the islets at a total flow rate of 400ml/min and Ri = 0.25 and Ro = 0.6
CONCLUSIONS
The purpose of this study was to develop MPTV to measure the mobility of magnetic particles up to 1000 microns in size and to predict optimized flow parameters based on the magnetophoretic mobilities to isolate magnetic particles from nonmagnetic particles using QMS. The capability of the newly developed MPTV in measuring magnetophoretic mobility was confirmed with the measurements of the mobilities of standard Dynabeads®. MPTV was successfully used to measure the mobility of magnetic particles up to 500 microns in size with different mobilities. MPTV’s ability to predict the optimized flow parameters was also tested successfully with magnetic particles and isolated islets of Langerhans, the target application of MPTV. MPTV can be used online to analyze the mobilities of the magnetically infused islets and to predict the flow parameters before sending the tissue through a magnetic field for purification using QMS.
Supplementary Material
Acknowledgments
This research was funded in part by the U.S. Department of Health and Human Services under SBIR grant 5R44DK072647-03 from the National Institute of Diabetes and Digestive and Kidney Disease Research (NIDDK) awarded to Techshot, Inc., Greenville, Indiana, USA.
References
- Chalmers JJ, Zhao Y, Nakamura M, Melnik K, Lasky L, Moore L, Zborowski M. An Instrument to Determine the Magnetophoretic Mobility of Labeled, Biological Cells and Paramagnetic Particles. Journal of Magnetism and Magnetic Materials. 1999;194:231–241. [Google Scholar]
- Chalmers JJ, Haam S, Zhao Y, McCloskey K, Moore L, Zborowski M, Williams PS. Quantification of Cellular Properties from External Fields and Resulting Induced Velocity: Cellular Hydrodynamic Diameter. Biotechnology and Bioengineering. 1999;64:509–518. doi: 10.1002/(sici)1097-0290(19990905)64:5<509::aid-bit1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Davis RS. New method to measure magnetic susceptibility. Measurement Science and Technology. 1993;4(2):141–147. [Google Scholar]
- Gill SJ, Malone CJ, Downing M. Magnetic susceptibility of single small particles. The review of scientific instruments. 1960;31(12):1299–1303. [Google Scholar]
- Häfeli UO, Ciocan R, Dailey JP. Characterization of Magnetic Particles and Microspheres and Their Magnetophoretic Mobility Using a Digital Microscopy Method. European Cells and Materials. 2002;3(2):24–27. [Google Scholar]
- Kennedy DJ, Todd P, Logan S, Becker M, Papas KK, Moore LR. Engineering quadrupole magnetic flow sorting for the isolation of pancreatic islets. Journal of Magnetism and Magnetic Materials. 2007;311:388–395. [Google Scholar]
- London NJM, Swift SM, Clayton HA. Isolation, culture and functional evaluation of islets of Langerhans. Diabetes & Metabolism. 1998;23:200–207. [PubMed] [Google Scholar]
- McCloskey KE, Zborowski M, Chalmers JJ. Measurement of CD2 Expression Levels of IFN-α-Treated Fibrosarcomas Using Cell Tracking Velocimetry. Cytometry. 2001;44:137–147. [PubMed] [Google Scholar]
- McCloskey KE, Chalmers JJ, Zborowski M. Magnetophoretic Mobilities Correlate to Antibody Binding Capacity. Cytometry. 2000;40:307–315. doi: 10.1002/1097-0320(20000801)40:4<307::aid-cyto6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- McCloskey KE, Comella K, Chalmers JJ, Margel S, Zborowski M. Mobility Measurements of Immunomagnetically Labeled Cells Allow Quantitation of Secondary Antibody Binding Amplification. Biotechnology and Bioengineering. 2001a;75(6):642–655. doi: 10.1002/bit.10040. [DOI] [PubMed] [Google Scholar]
- Melnik K, Nakamura M, Comella K, Lasky LC, Zborowski M, Chalmers JJ. Evaluation of Eluents from Separations of CD34+ Cells from Human Cord Blood Using a Commercial, Immunomagnetic Cell Separation System. Biotechnology. 2001;17:907–916. doi: 10.1021/bp010079r. [DOI] [PubMed] [Google Scholar]
- Moore LR, Milliron S, Williams PS, Chalmers JJ, Margel S, Zborowski M. Control of Magnetophoretic Mobility by Susceptibility-Modified Solutions as Evaluated by Cell Tracking Velocimetry and Continuous Magnetic Sorting. Analytical Chemistry. 2004;76:3899–3907. doi: 10.1021/ac049910f. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Zborowski M, Lasky LC, Margel S, Chalmers JJ. Theoretical and Experimental Analysis of the Accuracy and Reproducibility of Cell Tracking Velocimetry. Experiments in Fluids. 2001;30:371–380. [Google Scholar]
- Shenkman RM, Chalmers JJ, Hering BJ, Kirchhof N, Papas KK. Quadrupole Magnetic Sorting of Porcine Islets of langerhans. Tissue Engineering: Part C. 2009;15(2):147–156. doi: 10.1089/ten.tec.2008.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon-Shiong P, Heintz R, Terasaki P. Identification of novel blood group reactive monoclonal antibodies cytotoxic to human acinar cells but not islets. Transplantation Proceedings. 1989;21(1):2622–2623. [PubMed] [Google Scholar]
- Reddy Sridhar, Moore Lee R, Sun Liping, Zborowski Maciej, Chalmers JJ. Determionation of the magnetic susceptibility of labeled particles by video imaging. Chemical Engineering Science. 1996;51(6):947–956. [Google Scholar]
- Suwa M, Watarai H. Magnetophoretic Detection of Photo-Induced Spin Transition. Chemistry Community. 2004:1656–1657. doi: 10.1039/b403386h. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Baicu SC, Greene E, Vazquez A, Brassil J. Islet Isolation From Juvenile Porcine Pancreas After 24-Hour Hypothermic Machine Perfusion Preservation. Cell Transplantation. 2010;19:613–628. doi: 10.3727/096368910X486316. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Baicu S. Hypothermic Perfusion of Pancreas: Emphasis on Preservation prior to Islet Isolation. In: Uygun K, Lee CY, Yarmush Martin L, Langer Robert S, editors. Organ Preservation and Reengineering. Series “Methods In Bioengineering”. Boston: Artech House Publisher; 2011. [Google Scholar]
- Watanabe K, Suwa M, Watarai H. New Principles of Magnetophoretic Velocity Mass Analysis. Analytical Sciences. 2004;20:1483–1485. doi: 10.2116/analsci.20.1483. [DOI] [PubMed] [Google Scholar]
- Watarai H, Namba M. Magnetophoretic Behavior of Single Polystyrene Particles in Aqueous Manganese (II) Chloride. Analytical Sciences. 2001;17:1233–1236. doi: 10.2116/analsci.17.1233. [DOI] [PubMed] [Google Scholar]
- Zborowski M, Ostera GR, Moore LR, Milliron S, Chalmers JJ, Schechter AN. Red Blood Cell Magnetophoresis. Biophysics Journal. 2003;84:2638–2645. doi: 10.1016/S0006-3495(03)75069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Nakamura M, Comella K, Moore L, Zborowski M, Chalmers J. Characterization/Quantification of the Factors Involved in the Imparting a Magnetophoretic Mobility on Cells and Particles. European Cells and Materials. 2002;3(2):34–36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.