Abstract
Cellular cholesterol homeostasis is regulated by oxygenated cholesterol metabolites called oxysterols. While the importance of oxysterols in the acute regulation of cholesterol homeostasis is known, the precise molecular mechanisms through which oxysterols exert their effects remain to be elucidated. LY295427 (1) is a known antagonist of the cholesterol-homeostatic effects of 25-hydroxycholesterol (25-HC), a biologically active oxysterol. In order to examine the mechanism of action of this antagonism, and to further explore recent evidence suggesting that the membrane effects of 25-HC contribute to acute cholesterol regulation, we synthesized the enantiomer of LY295427 (ent-LY295427). ent-LY295427 (2) will serve as a unique probe to provide insight into the role of transcription-independent mechanisms in regulation of cholesterol homeostasis.
Keywords: enantiomer, oxysterol, LY295427
1. Introduction
Cholesterol serves a vital structural role in the cell membrane. Cholesterol alters the fluidity and permeability of membranes, forming membrane domains that regulate a number of signaling pathways [1],[2]. Tight regulation of cholesterol levels is necessary, as too little cholesterol leads to aberrant signaling and cell death, whereas too much free cholesterol is cytotoxic, and high systemic cholesterol levels can lead to atherosclerosis and heart disease [3]. While cholesterol feeds back on its own regulation, cholesterol homeostasis is also maintained by oxygenated metabolites of cholesterol termed oxysterols. Specifically, enzymatically formed side-chain oxysterols such as 25-hydroxycholesterol (25-HC) play a central role in regulating cholesterol homeostasis [4],[5]. 25-HC regulates cellular cholesterol through four major pathways that include: 1) liganding the liver × receptor (LXR) transcription factor, which activates genes responsible for cholesterol efflux; 2) increasing cholesterol movement to the endoplasmic reticulum (ER) to be esterified by the ER resident enzyme acylCoA: cholesterol acyl transferase (ACAT); 3) stimulating the degradation of HMG-CoA Reductase (HMGR), the rate limiting enzyme of de novo cholesterol synthesis; and 4) suppressing the processing of sterol regulatory element binding protein (SREBP), a transcription factor regulating cholesterol uptake and synthesis [6–8]. The overall effect of 25-HC is to decrease free cholesterol levels in the cell.
In addition to its role in regulating cholesterol homeostasis, 25-HC has unique effects in the membrane. Model membrane and computational studies both demonstrate that 25-HC has a disordering and expansive effect on membrane lipids, opposite to the condensing effect of cholesterol [5, 9–11]. 25-HC, along with other amphipathic molecules, also increases the interaction of membrane cholesterol with cholesterol oxidase, saponin, and cyclodextrin, and increases movement of plasma membrane cholesterol to the ER [12],[13]. This effect, known as cholesterol activation, is thought to work by disrupting cholesterol-phospholipid complexes. This increases cholesterol fugacity in the membrane, essentially making cholesterol more accessible for modification or transport [11],[14].
Previous work examined whether 25-HC-membrane interactions contributed to regulation of cholesterol homeostasis. The enantiomer of 25-HC (ent-25-HC) was examined in the context of its ability to regulate cholesterol homeostatic pathways [5]. These studies took advantage of the fact that enantiomers have identical physical properties, and can only be differentiated via interaction with chiral species. Thus, enantiomers have the potential to differentiate membrane effects – which are expected to be identical for the enantiomers – from specific ligand binding interactions – which are expected to be selective for only one enantiomer species [15–17]. As expected, nat and ent-25-HC had identical membrane properties, but were differentiated in their ability to activate LXR (only nat-25-HC activates LXR)[5]. Notably, this work demonstrated that both nat- and ent-25-HC regulate the SREBP and HMGR pathways, suggesting that the membrane properties of 25-HC play a role in regulating acute cholesterol homeostatic responses.
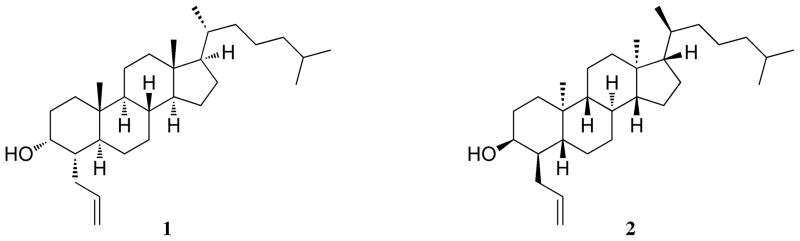
In order to further elucidate the mechanism of action of 25-HC-mediated cholesterol regulation, we synthesized the enantiomer of the compound LY295427 (ent-LY295427) (Figure 1). LY295427 (1) is a known antagonist of the cholesterol regulatory effects of 25-HC, but has no discernible effects in cells in the absence of 25-HC [18–20]. Synthesis of ent-LY295427 (2) provides us with a unique probe that can determine whether LY295427 antagonizes 25-HC by abrogating the oxysterol’s membrane effects, as nat- and ent-LY295427 are expected to have identical membrane properties. Studies with nat- and ent-LY295427 will also aid in differentiating between the various mechanisms of action used by 25-HC to control each cholesterol homeostatic pathway.
Figure 1.
2. Experimental
2.1 General Methods
Solvents were used as purchased or dried and purified using standard methodology. All air and/or moisture-sensitive reactions were carried out under N2 or Ar in oven-dried glassware. All extraction solvents were dried over anhydrous Na2SO4. Flash chromatography was performed using silica gel (32–63 μm) purchased from Scientific Adsorbents (Atlanta, GA). Optical rotations were measured on a Perkin-Elmer Model 341 polarimeter. Melting points were determined on a Kofler micro hot stage and are uncorrected. IR spectra were recorded as films on an NaCl plate with a Perkin-Elmer Spectrum One spectrophotometer. NMR spectra were recorded at ambient temperature in CDCl3 with a 5 mm probe on a Varian Gemini 2000 operating at 300 MHz (1H) or 75 MHz (13C). 1H and 13C NMR spectra were referenced to CDCl3 (δ 7.27) and (δ 77.00), respectively. Elemental analyses were performed by M-H-W Laboratories, Phoenix, AZ.
2.2 Synthesis of ent-LY295427
2.2.1 (3β, 5α, 8α, 9β, 10α, 13α, 14β, 17α, 20S)-3-[[(1,1-Dimethylethyl)dimethylsilyl]oxy]cholestan-24-oic acid, methyl ester (4)
Steroid 3 was prepared as described previously [21]. Acetyl chloride (0.6 ml) was added to MeOH (100 ml) to generate dry HCl, and this mixture was added to a solution of 3 (640 mg, 1.5 mmol) in MeOH (50 mL). The mixture was stirred for one h at 60 °C to completely dissolve the steroid, and then stirred at room temperature overnight to remove the acetate group and give the free alcohol. The MeOH was removed in vacuo, and the resulting white solid was dissolved in EtOAc and washed with brine (2 × 100 ml). The organic layer was dried and concentrated in vacuo to yield a white solid. Chromatography on silica (25% EtOAc in hexanes) gave the deacetylated methyl ester as a white solid (580 mg, 99%): mp 79–81 °C; [α]24D = −26.8 (c = 3.1, CHCl3); IR 3351, 2934, 2864, 1741 cm−1; 1H NMR δ 3.67 (3H, s, OMe), 3.62 (1H, m, 3-H), 2.36-0.96 (29H, m), 0.92 (3H, 19-H, and 3H, 21-H, s overlapping d, J = 5.7 Hz), 0.64 (3H, s, 18-H); 13C NMR δ 174.74, 71.81, 56.46, 55.93, 51.42, 42.71, 42.07, 40.42, 40.15, 36.44, 35.82, 35.33 (2 × C), 34.54, 31.04, 30.98, 30.52, 28.16, 27.17, 26.39, 24.18, 23.34, 20.79, 18.23, 12.00; C25H42O3: calcd. C, 76.87; H, 10.84. Found. C, 76.63; H, 11.00.
4-(Dimethylamino)pyridine (DMAP) (25 mg, 2.05 mmol), t-butyldimethylsilyl chloride (520 mg, 3.33 mmol), THF (3.5 mL), CH2Cl2 (15 mL), and distilled triethylamine (0.5 mL) were added to the deacylated methyl ester (500 mg, 1.3 mmol) and the mixture was stirred overnight at room temperature. Aqueous NH4Cl was added and stirred for 10 min, and the mixture was extracted with CH2Cl2 (3 × 150 ml), and then washed with 0.1M HCl (2 × 100) to remove the DMAP. The organic extracts were dried and concentrated in vacuo yielding a pale brown residue. The residue was passed through a silica plug using 15% EtOAc in hexanes and concentrated to yield product 4 as a white solid (640 mg, 99% yield): mp 89–90 °C; [α]D24 = −30.3 (c = 3.2, CHCl3); IR 2929, 2859, 1743 cm−1; 1H NMR δ 3.67 (3H, s, OMe), 3.59 (1H, m, 3-H), 2.36-0.97 (28H, m), 0.90 (3H, 21-H, and 9H, C(CH3)3, d, J = 6.6 Hz, overlapping s), 0.89 (3H, s, 19-H), 0.64 (3H, s, 18-H), 0.06 (6H, s, Si(CH3)2) 13C NMR δ 174.75, 72.81, 56.40, 55.96, 51.42, 42.71, 42.30, 40.22, 40.13, 36.93, 45.86, 35.58, 35.36, 34.60, 31.05, 28.17, 27.29, 26.39, 25.97 (3 × C), 25.64, 24.19, 23.37, 20.81, 18.30, 18.24, 11.99, −4.60 (2 × C); C31H56O3Si: calcd. C, 73.75; H, 11.18. Found. C, 73.86; H, 11.33.
2.2.2 (3β, 5α, 8α, 9β, 10α, 13α, 14β, 17α, 20S)-3-[[(1,1-Dimethylethyl)dimethylsilyl]oxy]cholestan-24-ol (5)
ent-Steroid 4 (1.3 g, 2.56 mmol) was dissolved in Et2O (20 mL) and added dropwise to a solution of LAH (300 mg, 7.9 mmol) in Et2O (20 mL) at 0 °C. Reduction of the ester was complete after 30 min, and the reaction was quenched carefully with water (0.1 mL) and then 15% NaOH (0.1 mL). Additional water (0.3 mL) was added after stirring for 30 minutes. The solvent was decanted from the white aluminum salts, which were washed several times with Et2O. The solvent was removed in vacuo giving product 5 as a white solid (1.2 g, 98% yield): mp 65–67 °C; [α]D24 = −31.3 (c = 6.35, CHCl3); IR 3339, 2929, 2860 cm−1; 1H NMR δ 3.62 (1H, m, 3-H), 1.98-0.97 (31H, m), 0.94 (3H, s, 19-H), 0.90 (3H, 21-H; 9H, C(CH3)3), 0.64 (3H, s, 18-H), 0.06 (6H, s, Si(CH3)2); 13C NMR δ 72.84, 63.63, 56.42, 56.18, 42.68, 42.30, 40.21, 40.16, 36.91, 35.86, 35.59, 34.59, 31.81, 31.02, 29.43, 28.32, 27.30, 26.41, 25.98 (3 × C), 24.22, 23.39, 20.81, 18.62, 18.35, 12.00, −4.60 (2 × C); C30H56O2Si: calcd. C, 75.56; H, 11.84. Found. C, 75.37; H, 11.89.
2.2.3 (3β, 5α, 8α, 9β, 10α, 13α, 14β, 17α, 20S)-3-[[(1,1-Dimethylethyl)dimethylsilyl]oxy]cholest-24-ene (6)
Celite (200 mg), NaOAc (210 mg), and pyridiniumchlorochromate (520 mg, 1.1 mmol) were added to a solution of compound 5 (560 mg, 1.18 mmol) in CH2Cl2 (30 mL) at 0 °C. The mixture was stirred for 30 min at 0 °C, and for an additional 2 h at room temperature. The reaction was passed through a plug of silica and eluted with 20 % EtOAc in hexanes to yield a white solid. Chromatography on silica (2% EtOAc in hexanes) gave the aldehyde as a white solid (530 mg, 95% yield): mp 65–67 °C; [α]D24 = −31.3 (c = 6.35, CHCl3); IR 3339, 2929, 2860 cm−1; 1H NMR δ 9.76 (1H, s, 24-H), 3.58 (1H, m, 3-H), 2.42-0.97 (31H, m), 0.92 (3H, s, 19-H), 0.90 (3H, 21-H and 9H, C(CH3)3 overlapping) 0.63 (3H, s, 18-H), 0.06 (6H, s, Si(CH3)2); 13C NMR δ 72.84, 63.63, 56.42, 56.18, 42.68, 42.30, 40.21, 40.16, 36.91, 35.86, 35.59, 34.59, 31.81, 31.02, 29.43, 28.32, 27.30, 26.41, 25.98 (3 × C), 24.22, 23.39, 20.81, 18.62, 18.35, 12.00, −4.60 (2 × C).
Isopropyltriphenylphosphonium iodide (2.06 g) was added to a flask, which was then evacuated and flushed with Ar (3x). THF (25 mL) was added and the mixture was cooled to 0 °C for 10 min. n-BuLi (4.8 mmol, 1.9 mL of 2.5 M soln in hexanes) was added, turning the solution red. After stirring for 30 min at 0 °C, 75 μL of the phase-transfer catalyst tris[2-(2-methoxyethoxy)ethyl]amine (TDA-1) was added to the ylide mixture, followed by the steroid aldehyde (900 mg, 1.9 mmol) in THF (30 mL). After 15 min the reaction was complete and quenched by adding acetone (2 mL), and the resultant cream-colored mixture was filtered through a plug of silica and eluted with EtOAc/hexanes (1:1). After solvent removal, chromatography on silica (5% CH2Cl2 and 1% EtOAc in hexanes) gave product 6 as a white solid (900 mg, 94% yield), which was recrystallized in Et2O to give a colorless gel: [α]D24 = −31.1 (c = 5.2, CHCl3); IR 2928, 2858, 1449, 1095, 836 cm−1; 1H NMR δ 5.10 (1 H, t, J = 3.4 Hz, 24-H), 3.59 (1H, m, 3-H), 1.98-0.98 (28H, m), 1.69 (3H, s, 26-H or 27-H), 1.61 (3H, s, 26-H or 27-H), 0.94 (3H, s, 19-H), 0.90 (3H, 21-H, and 9H, C(CH3)3, d, J = 4.8 Hz, overlapping s), 0.64 (3H, s, 18-H), 0.07 (6H, s, Si(CH3)2); 13C NMR δ 130.87, 125.27, 72.86, 56.43, 56.27, 42.70, 42.32, 40.24, 40.18, 36.93, 36.06, 35.86, 35.62, 34.59, 31.02, 28.31, 27.32, 26.42, 25.98 (3 × C), 25.73, 24.74, 24.25, 23.40, 20.82, 18.58, 18.33, 17.64, 12.00, −4.60 (2 × C); C33H60O1Si1: calcd. C, 79.12; H, 12.07. Found. C, 78.89; H, 11.93.
2.2.4 ent-Epicoprostanol; (3β, 5α, 8α, 9β, 10α, 13α, 14β, 17α, 20S)-Cholestan-3-ol (7)
10% Pd on carbon (340 mg) was added to a solution of compound 6 (900 mg, 1.8 mmol) in MeOH/EtOAc (200 mL), and hydrogenated in a Parr hydrogenator at 60 psi for 30 min. The reaction solution was passed through a plug of silica gel with EtOAc and concentrated. The resulting yellowish solid was chromatographed on silica (100% hexanes) to yield the saturated hydrogenation product as a white solid (840 mg, 93%): mp 78–81 °C; [α]D24 = −32.1 (c = 4.6, CHCl3); IR 2929, 2864, 1095 cm−1; 1H NMR δ 3.59 (1H, m, 3-H) 1.98-0.92 (31H, m), 0.91 (3H, s, 19-H), 0.90 (9H, s, C(CH3)3), 0.88 (6H, d, J = 6.9 Hz, 26-H and 27-H), 0.86 (3H, d, J = 6.6 Hz, 21-H), 0.64 (3H, s, 18-H), 0.05 (6H, s, Si(CH3)2); 13C NMR δ 72.87, 56.46, 56.39, 42.70, 42.35, 40.27, 40.21, 30.52, 36.96, 36.18, 35.90, 35.82, 35.61, 34.61, 1.05, 28.34, 28.00, 27.34, 26.44, 25.98 (3 × C), 24.25, 23.89, 23.40, 22.81, 20.84, 18.67, 18.33, 12.00, −4.59 (2 × C); C33H62OSi: calcd. C, 78.81; H, 12.43. Found. C, 79.00; H, 12.22.
Tetrabutylammonium fluoride (anhydrous) (7 mL) was added to a solution of the saturated steroid (480 mg, 0.95 mmol) in THF (50 mL) and was stirred at room temperature overnight. The reaction was quenched with a saturated NH4Cl solution and extracted with EtOAc. The organic extracts were washed with brine, dried, and concentrated in vacuo to give a cream-colored solid. Chromatography on silica (15% EtOAc in hexanes) gave product 7 as a white solid (370 mg, 99% yield): mp 102–104 °C; [α]D24 = −28.9 (c = 4.6, CHCl3); IR 3339, 2933, 2865 cm−1; 1H NMR δ 3.63-3.62 (1H, m, 3-H), 2.00-0.96 (32H, m), 0.92 (3H, s, 19-H), 0.90 (3H, d, J = 6.9 Hz, 21-H), 0.87 (6H, d, J = 6.6 Hz, 26H and 27-H), 0.65 (3H, s, 18-H); 13C NMR δ 71.90, 56.52, 56.33, 42.68, 42.10, 40.45, 40.21, 39.51, 36.46, 36.15, 35.85, 35.80, 35.33, 34.58, 30.55, 28.31, 28.00, 27.21, 26.44, 24.24, 23.83, 23.38, 22.81, 22.55, 20.82, 18.65, 12.02; C27H48O: calcd. C, 83.43; H, 12.45. Found. C, 83.43; H, 12.61.
2.2.5 ent-Coprostanone; (5α, 8α, 9β, 10α, 13α, 14β, 17α, 20S)-cholestan-3-one (8)
Jones reagent was added dropwise to a solution of ent-steroid 7 (370 mg, 0.95 mmol) in acetone (40 mL), changing the reaction mixture from colorless to a cloudy green/blue and finally to red/yellow, indicating complete oxidation of the steroid. After 5 min, excess Jones reagent was quenched with 2-propanol until the reaction remained green. The solution was evaporated in vacuo, dissolved in EtOAc and washed with water and brine. The organic layer was dried and concentrated in vacuo to yield product 8 as a white low-melting solid (300 mg, 82%): [α]D24 = −33.7 (c = 3.5, CHCl3); IR 2932, 2867, 1717 cm−1; 1H NMR δ 2.75-1.04 (31H, m), 1.02 (3H, s, 19-H), 0.91 (3H, d, J = 6.6 Hz, 21-H), 0.86 (6H, d, J = 6.6 Hz, 26-H and 27-H), 0.68 (3H, s, 18-H); 13C NMR δ 213.44, 56.45, 56.30, 44.34, 42.70, 42.36, 40.72, 40.07, 39.48, 37.20, 37.00, 36.12, 35.76, 35.52, 34.88, 28.26, 27.99, 26.62, 25.77, 24.17, 23.80, 22.80, 22.64, 22.54, 21.19, 18.65, 12.04; C27H46O1: calcd. C, 83.87; H, 11.99. Found. C, 83.80; H, 11.89.
2.2.6 ent-Cholestenone; (8α, 9β, 10α, 13α, 14β, 17β, 20S)-cholest-4-en-3-one (9)
Pyridinium tribromide (190 mg, 0.5 mmol) was dissolved in acetic acid (20 mL) at 55 °C and added dropwise to a solution of compound 8 (200 mg) in acetic acid (10 mL) at 20 °C. After 30 min the reaction was complete, EtOAc (20 mL) was added, and the solution was concentrated in vacuo. The resulting residue was dissolved in EtOAc and washed with a saturated solution of NaHCO3 and brine. The organic layer was dried and concentrated in vacuo to give the 4-brominated steroid as a brown oil, which was used immediately. LiCl (75 mg, 1.8 mmol) was added to a solution of the brominated steroid in DMF (20 mL) and stirred for 3 hrs at 100 °C. The DMF was removed in vacuo, and the residue was extracted with EtOAc and water and washed with brine. Chromatography on silica (0–5% EtOAc in hexane) gave the product 9 as a white solid (100 mg, 50% yield): mp 75–79 °C; [α]D24 = −87.4 (c = 1.35, CHCl3); 1H NMR δ 5.72 (1H, s, 4-H), 2.43-0.85 (28H, m), 1.18 (3H, s, 19-H), 0.91 (3H, d, J = 6.3 Hz, 21-H), 0.86 (6H, d, J = 6.6 Hz, 26-H and 27-H), 0.71 (3H, s, 18-H); 13C NMR δ 199.64, 171.68, 123.74, 56.11, 55.87, 53.82, 42.38, 39.63, 39.48, 38.60, 36.11, 35.73, 35.68, 35.62, 33.97, 32.94, 32.04, 28.16, 27.99, 24.16, 23.80, 22.78, 22.52, 21.02, 18.62, 17.38, 11.93; C27H44O1: calcd. C, 84.31; H, 11.53. Found. C, 84.43; H, 11.66.
2.2.7 (4β, 5β, 8α, 9β, 10α, 13α, 14β, 17β, 20S)-4-(2-Propenyl)cholestan-3-one (10)
Li metal (4.5 mg, 0.6 mmol) was added to a dry flask, which was subsequently evacuated, flushed with N2, and cooled to −78 °C. NH3 (~ 10 mL) was condensed into the flask, dissolving the Li to yield a deep blue solution. THF (5 mL) was added. t-BuOH (25 μL) was added to a solution of compound 9 (100 mg. 0.3 mmol) in THF (5 mL), and the steroid solution was added dropwise to the blue Li solution. After 15 min, excess Li was quenched with dropwise addition of isoprene (~ 0.1 mL) turning the solution pale orange. Allyl iodide (50 μL) was then added to the mixture and stirred at −78 °C. After 1 hr, additional allyl idodie (25 μL) was added, and the reaction was stirred an additional 1.5 h. The reaction was quenched with sat. NH4Cl solution (20 mL), and allowed to warm to room temperature. The mixture was extracted with EtOAc, and the organic extracts were washed with brine, dried, and concentrated in vacuo. Chromatography on silica (0–6% EtOAc in hexanes) gave product 10 as a pale-yellow solid (100 mg, 90% yield), which was recrystallized from MeOH/Et2O: mp 91–94 °C; [α]D24 = −47.0 (c = 1.0, CHCl3); IR 3369, 2931, 2849, 1705 cm−1; 1H NMR δ 5.82-5.73 (1H, m, -CH=), 5.04-4.96 (2H, m, -CH2), 2.48–0.68 (32H, m), 1.06 (3H, s, 19-H), 0.89 (3H, d, J = 6.3 Hz, 21-H), 0.86 (6H, d, J = 6.6 Hz, 26-H and 27-H), 0.68 (3H, s, 18-H); 13C NMR δ 212.50, 136.59, 115.98, 56.33, 56.28, 54.11, 50.24, 49.89, 42.54, 39.96, 39.51, 38.80, 38.20, 36.26, 36.15, 35.79, 34.92, 31.78, 29.84, 28.26, 28.00, 25.36, 24.18, 23.84, 22.81, 22.55, 21.38, 18.65, 12.76, 12.06; C30H50O1: calcd. C, 84.44; H, 11.81. Found C, 84.29; H, 11.60
2.2.8 ent-LY295427 (2) (3β, 4β, 5β, 8α, 9β, 10α, 13α, 14β, 17α, 20S)-4-(2-Propenyl)cholestan-3-ol
K-selectride (0.5 mmol, 0.45 mL, 1M in THF) was added to a solution of compound 10 (100 mg, 0.2 mmol) in THF (5 mL) at −78 °C. After completing addition, the cold bath was removed and the mixture was stirred for 2 h. The mixture was then cooled to 0 °C, and after 10 minutes MeOH (0.54 mL), 5 M NaOH (0.48 mL), and 30% H2O2 (0.18 mL) were added sequentially. The cold bath was removed and the reaction mixture was stirred vigorously for 2 h as a white precipitate formed. Half-saturated NaCl was added, and the reaction mixture was extracted with EtOAc. The organic extracts were washed with half-saturated NaCl, dried, and concentrated in vacuo. The resulting cream-colored solid was chromatographed on silica (0–3% EtOAc in hexanes) yielding ent-LY295427 as a white solid (70 mg, 70% yield) which was recrystallized in Et2O/MeOH: mp 116–118 °C; [α]D24 = −5.8 °C (c = 5, CHCl3); IR 3496, 2933, 2867, 1736 cm−1; 1H NMR δ 5.92–5382 (1H, m, -CH=), 5.13-5.01(2H, m, =CH2), 3.90 (1H, br s, CH(OH), 2.30-2.25 (1H, m), 2.05-0.65 (32H, m), 0.90 ( 3H, d, J = 6.6 Hz, 21-H), 0.87 (6H, d, J = 6.6 Hz, 26-H and 27-H), 0.82 (3H, s, 19-H), 0.65 (3H, s, 18-H); 13C NMR δ 138.39, 115.80, 67.88, 56.60, 56.24, 54.48, 43.58, 42.47, 40.27, 40.10, 39.52, 36.52, 36.18, 35.80, 34.91, 34.38, 32.22, 32.13, 29.02, 28.26, 28.00, 24.13, 23.83, 22.81, 22.55, 20.98, 18.65, 12.34, 12.06; C30H52O: calcd. C, 84.04; H, 12.22. Found. C, 83.77; H, 11.96.
Results
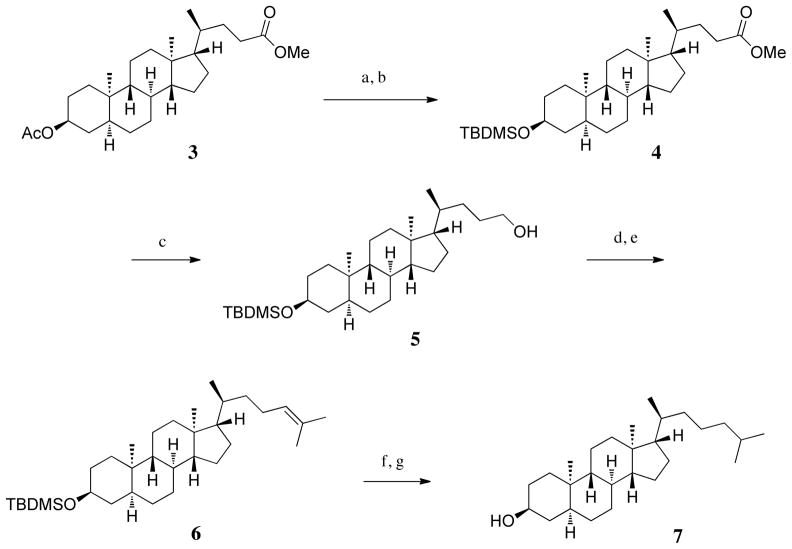
The strategy used to prepare ent-LY295427 (2) (Figure 1) was analogous to that used previously to prepare ent-cholesterol, with the steroid nucleus synthesized first followed by construction of the side-chain. Thus, ent-testosterone was prepared from 2-methyl-1,3-cyclopentanedione, as described previously [22]. In a divergence from the earlier ent-cholesterol synthesis, the Δ4 double bond of testosterone was hydrogenated to give 5β-dihydrotestosterone. While the Δ4 double bond would have to be regenerated after completion of side-chain synthesis, this strategy avoided problems from the multiple hydrogenation steps required for construction of the side chain. Following this strategy, ent-testosterone was converted to ent-steroid 3 as described previously [21].
At this point the acetate protecting group, which was necessary for synthesis of compound 3, was not compatible with the conditions necessary to complete synthesis of the side-chain (Scheme 1). Thus, the acetate group was removed via transesterification with methanol, and the resulting free alcohol was re-protected as a tert-butyldimethylsilyl ether (4).
Scheme 1.
a) MeOH, AcCl, 60 °C, 1h; b) DMAP, TBDMSCl, THF, CH2Cl2, TEA, overnight; c) Et2O, LAH, 30 min 0 °C, 2 h; d) CH2Cl2, NaOAc, Celite, PCC, 30 min 0 °C, 2 h; e) Isopropyltriphenylphosphosium iodide, n-BuLi, TDA-1, THF, 0 °C, 15 min; f) 10% Pd/C, MeOH/EtOAc, 60 psi H2, 30 min; g) Tetrabutylammonium fluoride (anhydrous), THF, 24h.
The focus then returned to completion of the side-chain. The side-chain methyl ester of ent-steroid 4 was reduced to yield the alcohol 5. Oxidation of this alcohol gave an aldehyde which was used immediately in the next reaction. Wittig reaction with isopropyltriphenylphosphonium iodide completed the addition of the final three carbons to the side-chain to yield ent-steroid 6. Hydrogenation followed by removal of the TBDMS ether gave ent-epicoprostanol 7.
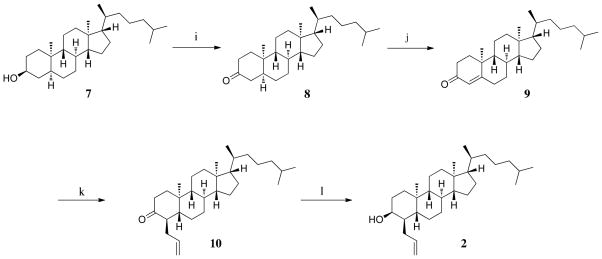
With side-chain synthesis complete, focus returned to the sterol nucleus and the regeneration of the Δ4 double bond. In order to regenerate the Δ4double bond, 7 was oxidized to the ketone 8, which was then brominated at the C-4 position, taking advantage of the cis-ring fusion to preferentially direct bromination at that site (Scheme 2) [23]. The unstable brominated steroid was immediately treated with LiCl in DMF to eliminate the bromine and form the enone 9. The allylic group was added at the C-5 position via reductive addition using lithium in liquid ammonia and allyl iodide to form ent-steroid 10 [18]. Selective reduction to the 3β alcohol using K-Selectride completed synthesis of ent-LY295427 (2).
Scheme 2.
i) Jones reagent, acetone; j) 1. Pyridinium tribromide, acetic acid, 2. LiCl, DMF, 100 °C, 4 h; k) Li, NH3, THF, t-BuOH, allyl iodide, −78 °C, 2.5h; l) K-selectride, THF, −78 °C, 2 h.
Discussion
We have successfully synthesized ent-LY295427 in 28 steps starting from 2-methyl-1,3-cyclopentanedione. The compound LY295427 is a known antagonist of the cholesterol-regulatory effects of 25-HC, but the mechanism of that antagonism is unknown. This is particularly interesting, as 25-HC regulates several cholesterol-homeostatic pathways through different mechanisms. Recent work using the enantiomers of 25-HC demonstrated that the membrane properties of 25-HC contribute to its cholesterol regulatory effects.
The successful synthesis of ent-LY295427 provides the cholesterol field with a unique probe for examination of the molecular mechanism through which the LY295427 compound antagonizes the effects of 25-HC. This chemical biology approach takes advantage of the fact that enantiomers have identical physical properties, and thus should have identical membrane-effects. Future biophysical and cell-based studies using nat- and ent-LY295427 may also aid in differentiating between mechanisms used by 25-HC to acutely regulate distinct cholesterol homeostatic pathways.
Acknowledgments
The authors gratefully acknowledge funding from the NIH Training Grant 5T32HL007275-30 (AAB), HL67773 (DSO, DFC) and GM47969 (DFC).
Abbreviations
- 25-HC
25-hydroxycholesterol
- LXR
liver × receptor
- ACAT
acylCoA: cholesterol acyl transferase
- HMGR
HMG-CoA Reductase
- SREBP
sterol regulatory element binding protein
- ent
enantiomer
- nat
natural
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silvius JR. Role of cholesterol in lipid raft formation: lessons from lipid model systems. Biochim Biophys Acta. 2003;1610:174–183. doi: 10.1016/s0005-2736(03)00016-6. [DOI] [PubMed] [Google Scholar]
- 2.Pike LJ. Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim Biophys Acta. 2005;1746:260–273. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 4.Gill S, Chow R, Brown AJ. Sterol regulators of cholesterol homeostasis and beyond: the oxysterol hypothesis revisited and revised. Prog Lipid Res. 2008;47:391–404. doi: 10.1016/j.plipres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Gale SE, Westover EJ, Dudley N, Krishnan K, Merlin S, Scherrer DE, Han X, Zhai X, Brockman HL, Brown RE, Covey DF, Schaffer JE, Schlesinger P, Ory DS. Side-chain oxygenated cholesterol regulates cellular cholesterol homeostasis through direct sterol-membrane interactions. J Biol Chem. 2009;284:1755–1764. doi: 10.1074/jbc.M807210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu X, Menke J, Chen Y, Zhou G, MacNaul K, Wright S, Sparrow C, Lund E. 27-Hydroxycholesterol is an endogenous ligand for liver × receptor in cholesterol-loaded cells. J Biol Chem. 2001;276:38378–38387. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- 7.Du X, Pham YH, Brown AJ. Effects of 25-hydroxycholesterol on cholesterol esterification and sterol regulatory element-binding protein processing are dissociable: implications for cholesterol movement to the regulatory pool in the endoplasmic reticulum. J Biol Chem. 2004;279:47010–47016. doi: 10.1074/jbc.M408690200. [DOI] [PubMed] [Google Scholar]
- 8.Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc Natl Acad Sci. 2007;104:6511–6518. doi: 10.1073/pnas.0700899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massey JB. Membrane and protein interactions of oxysterols. Curr Opin Lipidol. 2006;17:296–301. doi: 10.1097/01.mol.0000226123.17629.ab. [DOI] [PubMed] [Google Scholar]
- 10.Massey JB, Pownall HJ. Structures of biologically active oxysterols determine their differential effects on phospholipid membranes. Biochemistry. 2006;45:10747–10758. doi: 10.1021/bi060540u. [DOI] [PubMed] [Google Scholar]
- 11.Olsen BN, Schlesinger PH, Baker NA. Perturbations of membrane structure by cholesterol and cholesterol derivatives are determined by sterol orientation. JACS. 2009;131:4854–4865. doi: 10.1021/ja8095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lange Y, Ye J, Duban M, Steck TL. Activation of membrane cholesterol by 63 amphipaths. Biochem. 2009;48:8505–8515. doi: 10.1021/bi900951r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange Y, Ye J, Steck TL. Activation of membrane cholesterol by displacement from phospholipids. J Biol Chem. 2005;280:36126–36131. doi: 10.1074/jbc.M507149200. [DOI] [PubMed] [Google Scholar]
- 14.Lange Y, Steck TL. Cholesterol homeostasis and the escape tendency (activity) of plasma membrane cholesterol. Prog Lipid Res. 2008;47:319–332. doi: 10.1016/j.plipres.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westover EJ, Covey DF. The enantiomer of cholesterol. J Membr Biol. 2004;202:61–72. doi: 10.1007/s00232-004-0714-7. [DOI] [PubMed] [Google Scholar]
- 16.Zitzer A, Westover EJ, Covey DF, Palmer M. Differential interaction of the two cholesterol-dependent, membrane-damaging toxins, streptolysin O and Vibrio cholerae cytolysin, with enantiomeric cholesterol. FEBS Lett. 2003;553:229–231. doi: 10.1016/s0014-5793(03)01023-8. [DOI] [PubMed] [Google Scholar]
- 17.Westover EJ, Covey DF, Brockman HL, Brown RE, Pike LJ. Cholesterol depletion results in site-specific increases in epidermal growth factor receptor phosphorylation due to membrane level effects. Studies with cholesterol enantiomers. J Biol Chem. 2003;278:51125–51133. doi: 10.1074/jbc.M304332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin HS, Rampersaud AA, Archer RA, Pawlak JM, Beavers LS, Schmidt RJ, Kauffman RF, Bensch WR, Bumol TF. Synthesis and biological evaluation of a new series of sterols as potential hypocholesterolemic agents. J Med Chem. 1995;38:277–288. doi: 10.1021/jm00002a010. [DOI] [PubMed] [Google Scholar]
- 19.Janowski BA, Shan B, Russell DW. The hypocholesterolemic agent LY295427 reverses suppression of sterol regulatory element-binding protein processing mediated by oxysterols. J Biol Chem. 2001;276:45408–45416. doi: 10.1074/jbc.M108348200. [DOI] [PubMed] [Google Scholar]
- 20.Janowski BA. The hypocholesterolemic agent LY295427 up-regulates INSIG-1, identifying the INSIG-1 protein as a mediator of cholesterol homeostasis through SREBP. Proc Natl Acad Sci. 2002;99:12675–12680. doi: 10.1073/pnas.202471599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katona BW, Cummins CL, Ferguson AD, Li T, Schmidt DR, Mangelsdorf DJ, Covey DF. Synthesis, characterization, and receptor interaction profiles of enantiomeric bile acids. J Med Chem. 2007;50:6048–6058. doi: 10.1021/jm0707931. [DOI] [PubMed] [Google Scholar]
- 22.Rychnovsky SA, Mickus DE. Synthesis of ent-cholesterol, the unnatural enantiomer. J Org Chem. 1992;57:2732–2736. [Google Scholar]
- 23.Tochtrop GP, DeKoster GT, Cistola DP, Covey DF. Synthesis of [3,4-13C2]-enriched bile salts as NMR probes of protein-ligand interactions. J Org Chem. 2002;67:6764–6771. doi: 10.1021/jo0259109. [DOI] [PubMed] [Google Scholar]