Abstract
Purpose
Vascular endothelial growth factor (VEGF) and infiltrating myeloid cells are known regulators of tumor angiogenesis and vascular permeability in glioblastoma. We investigated potential blood-based markers associated with radiographic changes to aflibercept, which binds VEGF and placental growth factor (PlGF) in patients with recurrent glioblastoma.
Experimental Design
In this single-arm phase II trial aflibercept was given intravenously every two weeks until disease progression. Plasma and peripheral blood mononuclear cells were collected at baseline and 24 hours, 14 days, and 28 days post-treatment. Plasma cytokines and angiogenic factors were quantified using ELISA and multiplex bead assays, and myeloid cells were assessed by flow cytometry in a subset of patients.
Results
Circulating levels of VEGF significantly decreased 24 hours after treatment with aflibercept, coincident with radiographic response observed by MRI. PlGF initially decreased 24 hours post-treatment but increased significantly by days 14 and 28. Lower baseline levels of PlGF, elevated baseline levels of CTACK/CCL27, MCP3/CCL7, MIF, and IP-10/CXCL10, and a decrease in VEGFR1+ monocytes from baseline to 24 hours were all associated with improved response. Tumor progression was associated with increases in circulating MMP9.
Conclusions
These data suggest that decreases in VEGF post-treatment are associated with radiographic response to aflibercept. Elevated baseline chemokines of monocyte lineage in responding patients supports a role for myeloid cells and chemokines as potential biomarkers and regulators of glioma angiogenesis.
INTRODUCTION
Glioblastoma is a highly angiogenic tumor type that appears to be at least partially dependent on vascular endothelial growth factor (VEGF), as evidenced by the substantial response rate of these tumors to anti-VEGF therapy in the clinic (1–4). Recent studies have suggested that solid tumor angiogenesis and growth is highly dependent upon non-tumor cells in the tumor microenvironment, particularly inflammatory cells of the myeloid lineage, including mast cells, dendritic cells, eosinophils, neutrophils, and macrophages, which are known to be important sources of VEGF and other pro-angiogenic factors, including MMP9 (5, 6). The role of these myeloid cells in promoting glioma angiogenesis, invasion, and treatment resistance is only starting to be elucidated.
In addition to their roles in tumor angiogenesis, hypoxia-regulated VEGF and placental growth factor (PlGF) are known to be important chemoattractors of VEGF receptor 1–positive (VEGFR1+) bone marrow–derived myeloid cells in glioma tumors (7, 8). Recent experimental evidence suggests that elimination of VEGFR1 signaling in bone marrow–derived cells significantly decreases tumor growth and vascularization, suggesting that VEGFR1-expressing myeloid cells play an important role in sustaining glioma angiogenesis (7). Furthermore, hypoxia promotion of neovascularization by bone marrow–derived cell recruitment to the tumor has been shown to be mediated via increased expression of the hypoxia-inducible factor -1 (HIF-1) targets SDF-1α and VEGF. Importantly, bone marrow–derived cell expression of MMP9 was essential to initiate angiogenesis in this preclinical model (9).
Intrinsic or acquired resistance limits the therapeutic efficacy of antiangiogenic therapy in patients. PlGF upregulation as a result of VEGFR inhibitor–mediated hypoxia, which may contribute to resistance by attracting bone marrow-derived cells to the tumor, is thought to be one potential mechanism of tumor resistance (9–12). Resistance to antiangiogenic therapy has been suggested to be mediated by Gr1+/CD11b+ bone marrow cells (13), although PlGF did not appear to mediate the attraction of these cells. In addition to its role in myelomonocytic chemoattraction, PlGF is thought to amplify VEGF signaling through augmenting the bioavailability of VEGF by displacing it from VEGFR1 (14). Thus, there is a strong rationale for simultaneous targeting of VEGF and PlGF.
To date, there are no validated markers for selecting patients likely to benefit from anti-VEGF therapy, optimizing drug dosage, or identifying mechanisms of therapeutic resistance. As a step towards addressing this critical unmet need, we determined the association between biomarker levels and response in patients with recurrent temozolomide-resistant glioblastoma enrolled in a multicenter phase II clinical trial of aflibercept, a potent recombinant decoy receptor that sequesters both VEGF and PlGF (NABTC 06-01). Our study shows rapid post-treatment decreases in VEGF but progressive increases in PlGF levels. Furthermore, our study indicates that circulating myeloid cells, such as VEGFR1+ monocytes, and myeloid-related cytokines are potential biomarkers for response to aflibercept in glioblastoma patients.
MATERIALS AND METHODS
Study Design
Patients were enrolled in a multicenter phase II study of aflibercept for treatment of recurrent glioblastoma at first relapse conducted through the North American Brain Tumor Consortium (NABTC 06-01; NCT00369590). All patients had histologically confirmed glioblastoma or gliosarcoma with unequivocal evidence of progression after chemoradiation and no more than one adjuvant temozolomide-containing regimen. Eligible patients were treated with aflibercept (VEGF Trap, Regeneron Pharmaceuticals, Tarrytown, NJ) at the phase II dose of 4 mg/kg intravenously every 2 weeks until tumor progression, development of excessive toxicity, or consent withdrawal. At four weeks following the first treatment dose, radiographic response using magnetic resonance imaging (MRI) was determined using criteria established by Macdonald et al. (15). More information about the phase II study and preliminary clinical results are detailed elsewhere (16). The study was approved by the Cancer Treatment Experimental Program (CTEP) and the Institutional Review Board of each participating NABTC site.
A total of 32 patients with recurrent glioblastoma were enrolled in the phase II study. One patient refused to participate in specimen collection and one patient withdrew consent after the first dose of aflibercept. Not all requested samples were collected by all centers: baseline plasma biomarker levels were available on 26 patients, and baseline circulating cells were collected from only 16 patients. Samples were analyzed by laboratory personnel blinded to clinical response and outcome.
Measurement of plasma biomarkers
Ethylenediaminetetraacetic acid (EDTA) plasma was obtained at baseline, 24 hours, and every 14 days following the first dose of aflibercept and was stored at −80°C. Samples were batched, and each patient’s samples were analyzed simultaneously. Circulating levels of plasma and urine VEGF, PlGF, soluble VEGFR2, carbonic anhydrase 9 (CA9), and basic fibroblast growth factor (bFGF) were measured using ELISA assays according to the manufacturer’s instructions (R & D Systems, Minneapolis, MN). The VEGF ELISA used in this study does not measure VEGF bound to aflibercept (personal communication with Regeneron Pharmaceuticals) and thus represents “free” VEGF, similar to the concept described using an immunodepletion protocol, as previously described (17, 18). MMP-9 and e-selectin were measured using a Lincoplex Assay (LINCO Research, Millipore, St. Charles, MO). Plasma samples from baseline and days 1, 14 and 28 were analyzed for cytokines and angiogenic factors using commercially available multiplex suspension arrays (Bio-Rad Laboratories Hercules, CA), as previously described(19, 20). A list of analytes is included in Supplementary Table 1. All samples were run in duplicate, and the analysis was repeated if the median coefficient of variance (CV) for all the analytes was greater than 25% for a given sample.
Flow cytometry analysis of circulating mononuclear and endothelial cells
Peripheral blood mononuclear cells (PBMCs) were isolated using cell preparation tubes (CPTs) with sodium citrate (BD Biosciences, Franklin Lakes, NJ). Cell preparation, freezing, and staining were performed as previously described (21, 22) with the modifications described below. Baseline and subsequent follow-up samples from each patient were thawed and analyzed within a single batch to minimize inter-assay variability. The nuclear stain Syto16 was used to identify nucleated cells, and staining was performed using a panel of established antibodies for circulating endothelial and myeloid cells, including CD45, CD31, CD146, CD133, CD14, and VEGFR-1 (21, 22). Monocytes were defined as positive for CD14 (23).. The percentage of stained cells was determined by comparison with appropriate isotype controls. The volume of blood analyzed was determined using the lymphocyte and monocyte numbers obtained from the patient’s differential blood count. Antibodies were purchased from the following sources: anti–human VEGFR-1-APC, R&D Systems; CD14-PE, CD31-FITC, and CD45-PerCp, BD Biosciences; CD133-APC and CD14-FITC, Miltenyi Biotec (Auburn, CA).; P1H12-PE (CD146-PE), Chemicon (Temecula, CA); Syto-16 and CD14-Alexa Fluor 700, Invitrogen, (Carlsbad, CA). Flow cytometry was done using 8-color FACSCanto (BD Biosciences) and data analyzed using FlowJo (Tree Star, CA).
Statistical analysis
Descriptive statistics were used to characterize cell counts and percentage changes. The Wilcoxon signed rank test was used to access changes from baseline to various time points. The Mann-Whitney test was used to compare the percentage change between responding patients and non-responding patients. Kruskal Wallis tests were applied to compare the expression of plasma protein concentrations and circulating cell counts among patients with progressed disease, stable disease, and partial response. Because the plasma protein concentrations and circulating cell counts were highly skewed, these were log-transformed in the analyses. Cumulative logistic regression models were used to explore the association between biomarkers and response. We estimated the odds ratio (OR) with 95% confidence interval for each biomarker. The time to disease progression were calculated from the date of registration. The Cox proportional hazard regression model was used to assess the effect of biomarkers on disease progression. All tests were two-sided. The statistically significant level was P = 0.05. Due to the exploratory nature of these analyses, we did not control for multiple analyses. Observations which are missing were excluded from the analysis. Observations, which were below the limit of quantitation were conservatively set at 0. All computations were carried out using SAS 9.13 (SAS Institute, Cary, NC).
RESULTS
Aflibercept rapidly sequesters VEGF
After treatment with aflibercept, VEGF levels significantly decreased compared to baseline at 24 hours (p<0.0001) and remained significantly lower than baseline at 14 days (p<0.0001) (Figure 1A). The ELISA did not measure VEGF bound to aflibercept and thus the decrease in VEGF levels demonstrates that aflibercept rapidly sequesters circulating free VEGF. Over time, there was a rise in VEGF levels from their 24 hour nadir such that VEGF levels at 28 days were not significantly different from baseline. This may reflect an accumulation of VEGF in the circulation or possibly the dissociation of VEGF from aflibercept during the ELISA assay, although the latter may be less likely given the high affinity of aflibercept for VEGF (Kd ~0.5 picomole/L).
Figure 1.
Modulation of VEGF, PlGF and CA9 levels in patients treated with aflibercept. Samples were taken at baseline and then 1, 14 and 28 days following the first dose of aflibercept as described in the Materials and Methods section. Box-whisker plots: horizontal line in the middle portion of the box, mean. Bottom and top boundaries of boxes, 25th and 75th percentiles, respectively. Lower and upper whiskers, 5th and 95th percentiles, respectively. A, plasma VEGF (pg/mL) B, urinary VEGF normalized to creatinine (pg/mg Cr), C, plasma PlGF (pg/mL) D, CA9 (pg/mL) in patients treated with aflibercept. *, p<0.05 compared to baseline.
PlGF levels were lower at baseline compared to VEGF. Low levels of PlGF at baseline were associated with response (OR=0.15, 95% CI 0.02 to 0.97, p=0.046), as shown in Figure 3. Following initiation of aflibercept treatment, PlGF had a dramatic rise compared to baseline, increasing within 24 hours (p<0.0001) and remaining significantly elevated at 14 days (p<0.0001) and 28 days (p<0.0001) (Figure 1C). The dramatic and sustained increase in PlGF levels suggests that aflibercept treatment induced the expression of PlGF, as described in previous reports of other anti-VEGF agents (1, 20, 24).
Figure 3.
Change in VEGF and cytokine levels associated with radiographic response to aflibercept. A, maximum tumor reduction in target lesion (n = 26; excludes patients without baseline biomarker studies). #, greater than 100% increase in tumor size. Nineteen patients had a measurable decrease in radiographic contrast enhancement following 28 days of treatment with aflibercept. B, the ratio of change in VEGF concentrations (pg/mL) from baseline to 28 days (p=0.41 for trend) demonstrating a relative increase in VEGF in patients with progressive disease (PD) and stable disease (SD) compared to patients with partial response (PR) who had smaller increases in VEGF at 28 days. Data shown as median ± SEM. C, changes in MMP9 and TIMP1 are associated with progressive disease (p=0.07 and p=0.03, respectively). Data are shown as median ± SEM.
Total urine VEGF levels normalized to patient creatinine levels increased in a fashion similar to that of plasma VEGF. Urine VEGF levels were marginally significantly different from baseline at 24 hours (p=0.053) and 28 days (p=0.058) (Figure 1B). There was no correlation, however, between total urine and plasma VEGF levels using a Spearman test for correlation.
Since chronic anti-VEGF therapy is known to increase tumor hypoxia (9, 10, 25) and higher tissue levels of the hypoxia marker CA9 were recently reported to be associated with poor outcome in patients with glioblastoma (26), we explored changes in plasma levels of soluble CA9. As shown in Figure 1D, there was a marginally significant increase in CA9 at 24 hours, with a non-significant trend towards increasing levels with continued aflibercept therapy, suggesting that anti-VEGF therapy modulation of tumor hypoxia may be monitored in plasma over time.
Cytokine and angiogenic factor modulation following aflibercept treatment
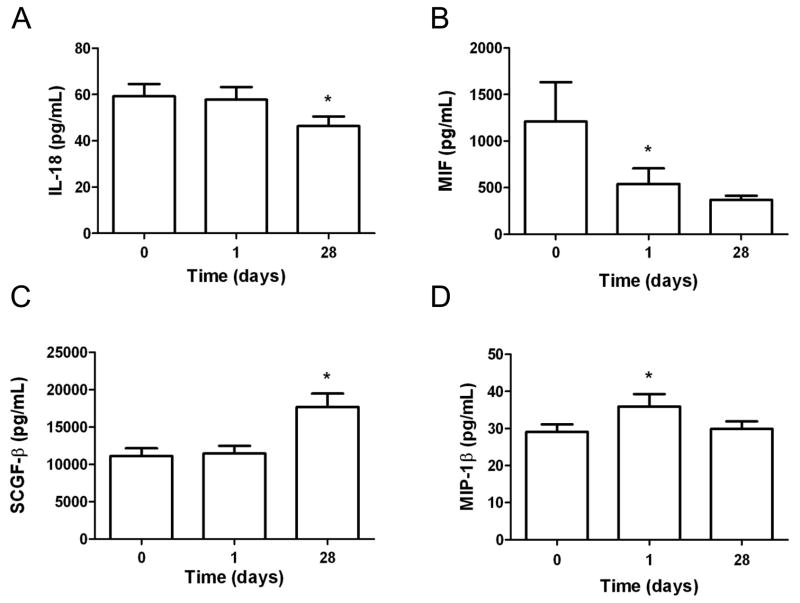
In addition evaluation of the primary aflibercept targets, we also explored the modulation of cytokine and angiogenic factors following aflibercept treatment. Plasma concentrations of interleukin (IL)-18 significantly decreased at 28 days (p=0.02) and macrophage migratory inhibitory factor (MIF) significantly decreased at 1 day (p=0.001) following aflibercept (Figure 2A, B). In contrast, an increase in macrophage inhibitory factor (MIP)-1β was observed at 1 day (p=0.002) and stem cell growth factor (SCGF)-β significantly increased at 28 days (p=0.003) following aflibercept treatment (Figure 2C, D). These chemokines are related to the attraction, differentiation and proliferation of bone marrow derived cells. Their change is consistent with the hypothesis that antiangiogenic therapy may promote the attraction and proliferation of myeloid cells as a mechanism of tumor escape from antiangiogenic therapy.
Figure 2.
Cytokine and angiogenic factor modulation following aflibercept treatment. Changes in A, IL-18, B, MIF, C, SCGF-β and MIP-1β (all in pg/mL) were observed on day 1 and 28 following treatment with aflibercept. Data are shown as median ± SEM. *, p<0.05 compared to baseline.
Change in VEGF and cytokine levels associated with radiographic changes to aflibercept
Nineteen of 26 patients with baseline biomarker levels had some reduction in tumor size (16). The best radiographic response at 28 days is shown in Figure 3A. Three patients (11.5%) had progressive disease (PD), 16 patients (61.5%) had stable disease (SD), and 7 patients (27%) had a partial response (PR) at the day 28 MRI. Two patients did not have sustained responses at the second imaging evaluation but were analyzed as PR at 28 days. These radiographic response data are fairly representative of the entire 32 patient cohort, compare favorably to historical controls (27), and are similar to that seen with other anti-VEGF agents (3, 28).
Levels of plasma or urine VEGF and plasma PlGF were not significantly different at baseline between response groups. There was no correlation between pre-treatment tumor size and baseline VEGF levels. Using a cumulative logistic regression model of the change in log-transformed levels, no significant association was found between the decrease in VEGF from baseline to 28 days and radiographic response (PD, SD and PR; p=0.41 for trend), Figure 3B. An increase in MMP9 (p=0.07) and its endogenous inhibitor TIMP1 (p=0.03) at 28 days compared to baseline is significantly associated with a risk of tumor progression during aflibercept treatment, Figure 3C.
Baseline cytokine and angiogenic factors associated with radiographic changes to aflibercept
The variability in the degree and durability of response led us to explore the potential role of other cytokine and angiogenic factors in tumor response to anti-VEGF and anti-PlGF therapy. Baseline levels of several markers predicted response to aflibercept (Figure 4A, B). Using a cumulative logistic regression model, high expression of several monocyte-associated factors, including cutaneous T-cell-attracting chemokine (CTACK/CCL27), macrophage chemotactic protein-3 (MCP-3/CCL7), macrophage migratory inhibitory factor (MIF), and interferon-(IFN)-gamma-inducible protein 10 (IP-10/CXCL10) were all significantly associated with response: CTACK/CCL27: OR=15.49 (95% CI: 1.49–164.2), p=0.02; MCP3/CCL7: OR=9.3 (95% CI: 1.08–79.84), p=0.04; MIF: OR=2.59 (95% CI: 1.06–6.30), p=0.035; IP-10/CXCL10: OR=4.90 (95% CI: 1.19–20.09), p=0.028. As monocytes are known to localize to sites of hypoxia (29), we evaluated soluble CA9 levels as an indirect surrogate of tissue hypoxia. Elevated levels of baseline CA9 was borderline significantly associated with response (OR 6.33, 95%CI 0.97–41.2, p=0.054). Thus, tumors with high baseline levels of hypoxia may be more sensitive to aflibercept.
Figure 4.
Baseline levels of cytokines predict response to aflibercept. A, elevated levels of CTACK/CCL27, MCP3/CCL7, MIF, IP-10/CXCL10, and CA9 predict response to aflibercept. Box-whisker plots: horizontal line in the middle portion of the box, mean. Bottom and top boundaries of boxes, 25th and 75th percentiles, respectively. Lower and upper whiskers, 5th and 95th percentiles, respectively. B, odds ratio and 95% confidence intervals for individual chemokines.
Baseline and dynamic changes in chemokines predict tumor progression
Given the known regulation of VEGF and PlGF by tumor hypoxia, and their potential association with response, we then explored the association between circulating levels of VEGF and CA9. We found an association between baseline levels of VEGF and CA9 (Spearman correlation coefficient r=0.485, p=0.026; Figure 5A). No association was seen between levels of PlGF and CA9. Consistent with the idea that hypoxic tumors may be more vulnerable to VEGF inhibition, elevated baseline levels of CA9 were marginally significantly associated with a longer time to progression using a Cox proportional hazards model (HR=0.36, 95% CI 0.13–1.13, p=0.081; Figure 5B).
Figure 5.
Relationship between baseline VEGF and CA9 levels. A, correlation between baseline plasma VEGF and CA9. Association between CA9, MMP9 and radiographic tumor progression. B, effect of baseline CA9 on time to progression. C, effect of change in MMP9 concentration from baseline to 28 days on time to progression. D, effect of change in biomarker expression on risk of progression (hazard ratio with 95% confidence interval).
Since tumor growth might be heralded by concomitant increases in circulating factors, we next sought to determine whether dynamic changes in markers correlated with tumor progression (Figure 5C, D). A statistically significant correlation was seen between an increase in MMP9 levels from baseline to 28 days and tumor progression (HR 3.90, 95% CI 1.34 to 11.3, p=0.013). Tissue inhibitor of metalloproteinase (TIMP)-1, an endogenous inhibitor of MMP9, showed a trend toward significant (HR 1.34, 95%CI 0.97–1.85, p=0.077). There was also borderline significance between progression and PDGF-β (HR 0.84, 95%CI 0.67–1.04, p=0.012).
VEGFR1-expressing peripheral blood monocytes predict radiographic response to aflibercept
Recent experimental evidence supports the role of VEGF and PlGF as critical mediators of bone marrow–derived VEGFR1+ myeloid cell accumulation in glioma tumor tissue (7). We observed VEGFR1 staining mainly on monocytes expressing the monocyte marker CD14; 19% of cells expressing CD14 were positive for VEGFR1 compared to only 9.9% of CD14-negative cells. Baseline levels of VEGFR1 monocyte populations, CECs, and CEPs were not significantly associated with radiographic disease status following treatment with aflibercept. Using a Cox proportional hazards model of progression, we found that a low baseline level of VEGFR1-/CD14+ monocytes was significantly correlated with shorter time to progression (HR 0.37, 95% CI 0.14–0.97, p=0.04; Figure 6A). We also found an association between greater decreases in VEGFR1+/CD14+ monocytes from baseline to day 1 and response to aflibercept (OR=0.1, 95% CI 0.01–0.77, p=0.03; Figure 6B). Changes in CECs/CEPs were not associated with response (data not shown). These findings support the recently described role of VEGFR1-expressing bone marrow–derived cells in mediating the pro-angiogenic phenotype in glioblastoma (7).
Figure 6.
VEGFR1-expressing peripheral blood monocytes predict radiographic response to aflibercept. A, low levels of VEGFR1−/CD14+ cells correlate with shorter time to progression. B, decrease in VEGFR1+/CD14+ cells from 24 hours to baseline correlates with radiographic response to aflibercept in sixteen patients (n=4 for the responding cohort). Data presented in box-whisker plots: horizontal line in the middle portion of the box, mean. Bottom and top boundaries of boxes, 25th and 75th percentiles, respectively. Lower and upper whiskers, 5th and 95th percentiles, respectively.
DISCUSSION
In this study, we report for the first time serial assessment of plasma VEGF and PlGF in patients receiving aflibercept for recurrent glioblastoma as a means to address changes in drug targets VEGF and PlGF. To address other pathways that may be contributing to response or resistance to aflibercept, and to identify potential biomarkers, we have also conducted a comprehensive profiling of plasma cytokines and angiogenic factors and as well as circulating cellular populations including CECs/CEPs and VEGFR1+ monocytes.
A recent retrospective tissue analysis of patients with recurrent glioblastoma treated with bevacizumab demonstrated a positive correlation between tumor VEGF levels and response to bevacizumab (26). Circulating VEGF did not show a significant association with radiographic response to aflibercept although there was no significant association between radiographic response and a decline in VEGF levels from baseline to 28 days. Based on this finding, one may speculate that patients who do not achieve a sufficient drop in VEGF levels may benefit from a higher dose of the drug, a possibility that merits further investigation.
PlGF also is thought to play an important role in tumor angiogenesis. Tumor growth is reduced in PlGF knockout mice (30), and overexpression of PlGF in preclinical tumor models increases tumor growth (31). PlGF is thought to recruit VEGFR1+ macrophages and other bone marrow–derived cells to the tumor, where they release pro-angiogenic factors in a paracrine manner (8). The recruitment of bone marrow–derived cells to tumor is thought to be a major mechanism of resistance to antiangiogenic therapy (32) and, thus, targeting PlGF is an attractive approach (12), although in some preclinical models PlGF does not appear to play a dominant role in resistance (13). Surprisingly, patients in the current phase II trial did not have many durable responses (16). Although the reasons are not clear, it is possible that dual targeting of angiogenic growth factors or more potent VEGF inhibition does not provide added benefit. It is also possible that since aflibercept can bind to either VEGF or PlGF, an abundance of PlGF might compete with VEGF for binding to aflibercept. However, inhibition of PlGF remains an attractive approach to blocking angiogenic escape and the attraction of VEGFR1+ myeloid cells. Higher doses of aflibercept may be needed to remove excess VEGF in the face of increasing levels of PlGF.
Hypoxia, a pathologic hallmark of glioblastoma, is a known trigger of VEGF, PlGF, and other cytokine production (8, 33) and may be an important mediator of resistance to antiangiogenic therapy (10, 25, 32). Carbonic anhydrase (CA) is a hypoxia-inducible transmembrane enzyme that is a reliable marker of hypoxia (34) and was recently shown to be an independent prognostic factor for malignant astrocytoma (35, 36). We found an association between VEGF and soluble CA9 levels at baseline, although PlGF levels were not similarly correlated. Anti-VEGF therapy has been associated with an increase in tumor hypoxia, and we demonstrated an increase in soluble CA9 during anti-VEGF treatment. The use of CA9 as a non-invasive marker of tumor hypoxia, together with imaging and blood-based biomarkers, may provide valuable information regarding the biologic impact of anti-VEGF therapy on an important factor thought to be driving tumor resistance to antiangiogenic therapy.
In addition to their role in angiogenesis, VEGF and PlGF attract VEGFR1+ myeloid cells to hypoxic, avascular sites as mediators of glioma angiogenesis, progression, and invasion (7, 8). In this study, we show for the first time that baseline levels of myeloid-associated cytokines are predictive of glioblastoma response to anti-VEGF therapy. Hypoxia is a strong regulator of MIF expression and secretion (37), while VEGF augments IFN-γ-mediated expression of IP-10 (38). VEGF and IP-10alone can mediate immune inflammation and have a major impact on leukocyte trafficking (38). These chemokines are thought to be chemotactic and modulatory for immune cells, including monocytes and macrophages. Although the specific role of these chemokines in glioma angiogenesis is unknown, it is intriguing to speculate that tumor-infiltrating myeloid cells may be partially responsible for VEGF-mediated angiogenesis. In support of this theory, we show that an early decrease in VEGFR1+/CD14+ cells was associated with tumor response to VEGF inhibition. Furthermore, we show that increases in MMP9 levels were associated with tumor progression. Although the source of MMP9 is unknown, preclinical evidence points towards myeloid cells as a major source of MMP9, which mediates pro-invasive and pro-angiogenic escape from anti-VEGF therapy in glioma (9). Furthermore, targeting these pathways may be a potential strategy for blocking or delaying the emergence of therapeutic resistance to VEGF pathway blockade.
There as several limitations of this study. Multiple markers were analyzed for this small trial which may have led to the discovery of false positive markers. Notably, this trial was a single arm study without a control arm. Although these data provide valuable information about potential mediators of disease status, the absence of a control arm and randomization of study subjects limits the interpretation of these markers, which could be prognostic.
This study describes the onset and duration of aflibercept-mediated changes in plasma levels of circulating VEGF and PlGF in patients with recurrent glioblastoma. Following an initial rapid decrease, VEGF levels continue to increase over time. PlGF levels increase following aflibercept treatment, suggesting that PlGF expression may be induced in response to continuous anti-VEGF therapy. Furthermore, we provide compelling evidence that elevated levels of myeloid-associated chemokines such as MIF, IP-10, MCP3/CCL7, and CTACK/CCL27 are predictive of radiographic response. In agreement with the role of circulating myeloid cells in tumor angiogenesis, a decrease in VEGFR1+ monocytes from baseline to 24 hours was associated with response. These findings suggest not only that bone marrow–derived cells may drive the angiogenic phenotype in a subset of glioblastoma patients but also that MMP9-expressing myeloid cells may mediate resistance to antiangiogenic therapy.
Supplementary Material
TRANSLATIONAL RELEVANCE.
There is a critical need to develop biomarkers to identify patients most likely to benefit from antiangiogenic therapy. We describe our analyses of plasma cytokine and angiogenic factors and peripheral blood monocytes from a phase II clinical trial of aflibercept (VEGF and PlGF) sequestering agent) in patients with recurrent glioblastoma. Our results have several potentially important implications. A rapid decrease in circulating levels of free VEGF corresponded with tumor response on MRI. Patients with high baseline levels of chemokines of the monocytic lineage may derive the greatest benefit from antiangiogenic therapy. Similarly, patients with early decreases in circulating VEGFR1+ monocytes may be more likely to have a response to this therapy. Conversely, MMP9 may be a predictor of tumor progression on this therapy. These results provide important insight into potential mediators of angiogenesis in glioblastoma and provide potential circulating biomarkers of response and progression to be integrated into future clinical trials in glioma.
Acknowledgments
The clinical trial was funded by the National Institutes of Health: NABTC # U01-CA62399. Correlative studies supported in part by 1R21A126127 and an ASCO Career Development Award, both to J. de Groot.
References
- 1.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stark-Vance V. Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma. World Federation of Neuro-Oncology; Edinburgh, United Kingdom: 2005. [Google Scholar]
- 3.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 4.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 5.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 6.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–33. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerber M, Reiss Y, Wickersheim A, et al. Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res. 2008;68:7342–51. doi: 10.1158/0008-5472.CAN-07-6241. [DOI] [PubMed] [Google Scholar]
- 8.Loges S, Schmidt T, Carmeliet P. Antimyeloangiogenic” therapy for cancer by inhibiting PlGF. Clin Cancer Res. 2009;15:3648–53. doi: 10.1158/1078-0432.CCR-08-2276. [DOI] [PubMed] [Google Scholar]
- 9.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–75. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 13.Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–20. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 14.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994;269:25646–54. [PubMed] [Google Scholar]
- 15.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–80. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 16.de Groot J, Wen PY, Lamborn K, Chang S, Cloughesy TF, Chen AP, DeAngelis LM, Mehta MP, Gilbert MR, Yung WK, Prados MD. Phase II single arm trial of aflibercept in patients with recurrent temozolomide-resistant glioblastoma: NABTC 0601. J Clin Oncol. 2008 May 26;20(suppl) abstr 2020. [Google Scholar]
- 17.Bocci G, Man S, Green SK, et al. Increased plasma vascular endothelial growth factor (VEGF) as a surrogate marker for optimal therapeutic dosing of VEGF receptor-2 monoclonal antibodies. Cancer Res. 2004;64:6616–25. doi: 10.1158/0008-5472.CAN-04-0401. [DOI] [PubMed] [Google Scholar]
- 18.Loupakis F, Falcone A, Masi G, et al. Vascular endothelial growth factor levels in immunodepleted plasma of cancer patients as a possible pharmacodynamic marker for bevacizumab activity. J Clin Oncol. 2007;25:1816–8. doi: 10.1200/JCO.2006.10.3051. [DOI] [PubMed] [Google Scholar]
- 19.Hanrahan EO, Lin HY, Kim ES, et al. Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 28:193–201. doi: 10.1200/JCO.2009.22.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopetz S, Hoff PM, Morris JS, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 28:453–9. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norden-Zfoni A, Desai J, Manola J, et al. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:2643–50. doi: 10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- 22.Mancuso P, Antoniotti P, Quarna J, et al. Validation of a standardized method for enumerating circulating endothelial cells and progenitors: flow cytometry and molecular and ultrastructural analyses. Clin Cancer Res. 2009;15:267–73. doi: 10.1158/1078-0432.CCR-08-0432. [DOI] [PubMed] [Google Scholar]
- 23.Wu H, Zurita AJ, Drevs J, Zirrgiebel U, Jürgensmeier JM, Robertson J, Puchalski TA, McKee KS, Heymach JV. Cediranib targeting of circulating VEGFR-1+ monocyte subpopulations. J Clin Oncol. 2008 May 26;20(Supplement):14687. [Google Scholar]
- 24.Willett CG, Boucher Y, Duda DG, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23:8136–9. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 25.Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:4589–99. doi: 10.1158/1078-0432.CCR-09-0575. [DOI] [PubMed] [Google Scholar]
- 26.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26:271–8. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–8. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 28.Desjardins ADPB, Herndon JE, II, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Friedman HS, Vredenburgh JJ. Effect of bevacizumab (BEV) and irinotecan (CPT-11) on dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in glioblastoma (GBM) patients. J Clin Oncol. 2008:26. [Google Scholar]
- 29.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: Effectors of angiogenesis and tumor progression. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–83. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 31.Adini A, Kornaga T, Firoozbakht F, Benjamin LE. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res. 2002;62:2749–52. [PubMed] [Google Scholar]
- 32.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol. 2005;7:134–53. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potter C, Harris AL. Hypoxia inducible carbonic anhydrase IX, marker of tumour hypoxia, survival pathway and therapy target. Cell Cycle. 2004;3:164–7. [PubMed] [Google Scholar]
- 35.Haapasalo JA, Nordfors KM, Hilvo M, et al. Expression of carbonic anhydrase IX in astrocytic tumors predicts poor prognosis. Clin Cancer Res. 2006;12:473–7. doi: 10.1158/1078-0432.CCR-05-0848. [DOI] [PubMed] [Google Scholar]
- 36.Korkolopoulou P, Perdiki M, Thymara I, et al. Expression of hypoxia-related tissue factors in astrocytic gliomas. A multivariate survival study with emphasis upon carbonic anhydrase IX. Hum Pathol. 2007;38:629–38. doi: 10.1016/j.humpath.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Rendon BE, Willer SS, Zundel W, Mitchell RA. Mechanisms of macrophage migration inhibitory factor (MIF)-dependent tumor microenvironmental adaptation. Exp Mol Pathol. 2009;86:180–5. doi: 10.1016/j.yexmp.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boulday G, Haskova Z, Reinders ME, Pal S, Briscoe DM. Vascular endothelial growth factor-induced signaling pathways in endothelial cells that mediate overexpression of the chemokine IFN-gamma-inducible protein of 10 kDa in vitro and in vivo. J Immunol. 2006;176:3098–107. doi: 10.4049/jimmunol.176.5.3098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.