Abstract
RIC HSCT is a potentially curative therapeutic option for patients with advanced FL but disease relapse remains the most common cause of failure. Radioimmunoconjugates administered prior to RIC allogeneic HSCT may enhance cytoreduction and allow more time for graft versus lymphoma effect to develop without the associated toxicity of a myeloablative HSCT. We performed a retrospective study to describe the outcomes of patients with relapsed, refractory or transformed FL who received 90Y ibritumomab tiuxetan followed by fludarabine and low-dose busulfan RIC allogeneic HSCT at the Dana-Farber Cancer Institute between 2006 and 2009, inclusively. Twelve patients were identified with a median age of 55 (40–66) years and a median number of lines of therapy of 5 (2–10). Two patients (17%) had transformed to a more aggressive histology and 5 (42%) had chemorefractory FL. Cumulative incidences of grade II–IV acute GVHD at 100 days were 17% (± 11%) and chronic GVHD at 12 months were 63% (±19%). Two-year non-relapse mortality was 18% (± 12%). Two-year OS and progression-free survival (PFS) were 83% (± 11%) and 74% (± 13%), respectively. This treatment is associated with favorable outcomes including acceptable rates of GVHD and relapse in advanced FL patients and warrants prospective studies.
Keywords: Follicular lymphoma, Allogeneic hematopoietic stem cell transplantation, Radioimmunotherapy, 90Y-ibritumomab tiuxetan
INTRODUCTION
Follicular lymphoma (FL) is the most common indolent non Hodgkin lymphoma (NHL). Despite the advent of chemoimmunotherapy, FL remains incurable in the majority of cases with a median survival of 8–10 yrs [1]. During this period, 40–60% patients with advanced FL will relapse and require additional therapies. Although FL is often chemoresponsive in this setting, remissions become progressively shorter and the disease may become chemorefractory. Furthermore, FL will transform to a more aggressive histology at a rate of 3% per year, which is generally associated with a poor prognosis [2]. Improvements in the outcomes of patients with FL require effective treatment strategies for relapsed, refractory and transformed disease.
Although salvage chemotherapy followed by high-dose chemotherapy and autologous hematopoietic stem cell transplantation (HSCT) is an option for the treatment of relapsed/refractory FL [3–5], studies have also highlighted issues of relapse, long-term toxicity, and secondary malignancies. Furthermore, not all patients are candidates for autologous HSCT, as many are heavily pretreated and unable to undergo stem-cell harvesting. Lastly, salvage chemotherapy followed by autologous HSCT may not be the optimal treatment strategy in high-risk transformed FL as it may eradicate the high grade component of the lymphoma, but the low grade component often recurs.
Allogeneic HSCT offers a potentially curative approach to the treatment of FL [6]. However, reduced intensity conditioning (RIC) regimens normally used in patients with FL often fail to provide sufficient cytoreduction in high-risk patients leading to higher relapse rates [7]. Furthermore, patients with transformed FL have either been excluded or present in low numbers in most series [6, 7]. Radiotherapy has been used extensively in the treatment of lymphomas in view of their inherently radiosensitive nature [8]. The anti-CD 20 radiolabeled immunoconjugates currently available include 90Y-ibritumomab tiuxetan (Zevalin, Spectrum Pharmaceuticals, Irvine, CA, USA) and 131I-tositumomab (Bexxar, GlaxoSmithKline, Philadelphia, PA, USA). 90Y-ibritumomab tiuxetan comprises the anti-CD20 monoclonal antibody, ibritumomab, conjugated via the tiuxetan chelator to the pure beta-emitting radioisotope yttrium-90 (90Y). Prospective trials of this agent demonstrate response rates of 60 to 80 percent in previously treated disease [9–14] including patients who are refractory to rituximab.
Tumor load reduction using radioimmunoconjugates, termed radioimmunotherapy (RIT), followed by RIC allogeneic HSCT may enhance early cytoreduction, while allowing for long-term disease control through graft versus lymphoma effect (GVL) without the associated toxicity of a myeloablative HSCT in this older, often heavily pretreated patient population. Furthermore, the use of allogeneic donor stem cells renders the use of radioimmunoconjugates possible even in patients with pancytopenia or extensive bone marrow involvement. The use of RIT combined with RIC allogeneic HSCT has recently shown promising results in the management of acute myelogenous leukemia, myelodysplasia [15] and relapsed NHL [16, 17]. All series to date have reported on patients with heterogeneous histologies ranging from indolent to aggressive NHL. However, outcome is clearly related to histology [18] and the proportion of FL in prior studies was small. It is therefore difficult to determine the true impact of RIT combined with RIC HSCT on patients with relapsed, refractory or transformed FL. In this report, we describe the baseline characteristics and outcomes of 90Y ibritumomab tiuxetan followed by RIC allogeneic HSCT in patients with relapsed, refractory or transformed FL treated at the Dana-Farber Cancer Institute.
METHODS
Patients
We evaluated all consecutive patients with relapsed, refractory or transformed FL who received 90Y ibritumomab tiuxetan followed by RIC allogeneic HSCT at the Dana-Farber Cancer Institute (Boston, MA, USA) between 2006 and 2009, inclusively. Only patients suspected of transformed FL on clinical grounds underwent repeat lymph node biopsy. Patients were selected to receive 90Y ibritumomab tiuxetan prior to RIC allogeneic HSCT on the basis of refractoriness to other lines of therapy for FL including, chemoimmunotherapy and radiotherapy, as judged by the treating physician. Patients refractory to 90Y ibritumomab tiuxetan did not proceed to allogeneic HSCT. The database was closed for analysis in May 2010 and chart review was performed for all data. This study was approved by the institutional review board was conducted in accordance with the principles of the Declaration of Helsinki.
Radioimmunotherapy
Patients received 0.4 mCi/kg of 90Y-Ibritumomab tiuxetan prior to a planned RIC allogeneic HSCT. The radioimmunotherapy regimen was administered in two distinct steps. The first step involved an infusion of rituximab (250 mg/m2) following premedication. Within 4 hours of rituximab, an infusion of 111In ibritumomab tiuxetan (5 mCi over 10 minutes) was administered. A biodistribution assessment was performed 48 to 72 hr after 111In ibritumomab tiuxetan. Seven days later patients with acceptable biodistribution received rituximab (250 mg/m2) followed within 4 hours by 90Y-Ibritumomab tiuxetan 0.4 mCi/kg (14.8 MBq/kg).
Reduced intensity conditioning
The conditioning regimen consisted of intravenous fludarabine and low-dose intravenous busulfan. Fludarabine (30 mg/m2) was administered as an intravenous infusion over 30 minutes on days -5, -4, -3 and -2. Busulfan (0.8 mg/kg) was administered by intravenous infusion over 3 hours on days -5, -4, -3 and -2 for a total dose of 3.2 mg/kg based on actual body weight [19]. In one recipient of umbilical cord stem cells, the conditioning regimen consisted of fludarabine (30 mg/m2) as an intravenous infusion over 30 minutes on days -8, -7, -6 and -5, -4, -3, melphalan (100 mg/m2 on day -2) and anti-thymocyte globulin (1.5 mg/kg on days -7, -5, -3, -1).
Stem cell collection and administration
Patients received peripheral-blood stem cells or umbilical cord blood from matched or mismatched, related or unrelated donors. HLA-matched sibling or unrelated donors underwent filgrastim mobilization for 5 consecutive days from Day –4 pre-HSCT to Day 0. Peripheral blood stem cells were collected by large volume apheresis. Unrelated donors were managed and mobilized following the procedures of the local donor centers. Plasma and red cell depletion were allowed for ABO incompatibility but no other form of graft manipulation was performed. A minimum dose of 2.0 × 106 CD34+ cells/kg was targeted.
GVHD prophylaxis and supportive care
Most patients received a graft-versus-host disease (GVHD) prophylaxis regimen consisting of tacrolimus ± sirolimus starting on day -3 ± low-dose methotrexate on days 1, 3, 6 as previously described [20]. In 3 patients, bortezomib was added to a tacrolimus-based GVHD prophylaxis regimen as part of a study protocol. Two patients received sirolimus and mycophenolate mofetil without a calcineurin inhibitor. Tacrolimus and sirolimus taper usually commenced between day 60 to 100, with the goal to be off all immune suppression by 6–9 months post transplant in the absence of GVHD. Acute GVHD (aGVHD) was graded according to the consensus scoring system [21]. Supportive care for all patients consisted of Pneumocystis jiroveci prophylaxis and varicella zoster virus/herpes simplex virus prophylaxis. Cytomegalovirus viral load was closely monitored by DNA-based assay and pre-emptive therapy was initiated in cases of reactivation.
Statistics
Overall survival (OS) was defined as the time from the date of allogeneic HSCT to the date of death from any cause; those alive or lost to follow-up were censored at the date last known alive. Progression-free survival (PFS) was defined as the time from the date of allogeneic HSCT to the date of relapse or death; those alive were censored at the date last known alive and relapse-free. OS and PFS were calculated using the Kaplan Meier (KM) method. Cumulative incidence curves for grade II–IV acute GVHD (aGVHD) and chronic GVHD (cGVHD) with death as a competing risk were also constructed and were calculated from the date of allogeneic HSCT.
RESULTS
Database search
Between 2006 and 2009, 41 patients underwent allogeneic HSCT at DFCI for FL. During this period, 13 were assessed to receive 90Y ibritumomab tiuxetan followed by RIC allogeneic HSCT. One patient progressed despite 90Y ibritumomab tiuxetan and did not undergo a planned HSCT. Of the 29 patients who did not receive 90Y ibritumomab tiuxetan prior to conditioning, 3 underwent myeloablative allogeneic HSCT and the remainder (n=26) achieved good disease control with other agents prior to RIC allogeneic HSCT. The outcomes of the 12 patients who received 90Y ibritumomab tiuxetan followed by RIC allogeneic HSCT are reported in this study.
Patient and Disease Characteristics
Baseline characteristics of the 12 patients included in this study are listed in table 1. The median age was 55 years (range: 40–66) and 6 patients (50%) were female. Ten patients (83%) had relapsed FL and two patients (17%) had transformed FL confirmed by lymph node biopsy findings. The median number of therapies was 5 (range: 2–10) and 1 patient (8%) had undergone a prior autologous HSCT. Prior to receiving RIT, 7 (52%) patients were in PR and 5 (47%) were refractory to their last treatment. There were no cases of inadequate biodistribution and all patients received 90Y-Ibritumomab tiuxetan 0.4 mCi/kg. The median time from RIT to allogeneic HSCT was 1 month (range: 0.4–5.8). Greater than 5 months separated RIT from allogeneic HSCT in 2 patients due to donor delays. Eight patients (67%) received their grafts from unrelated donors, 4 of which were antigen mismatched. One patient received double umbilical cord blood transplantation.
Table 1.
Baseline characteristics
| N (%) | |
|---|---|
| Number of Pts. | 12 |
| Age, median (range) | 55 (40, 66) |
| Sex, Female | 6 (50) |
| Type of Follicular Lymphoma | |
| Relapsed | 10 (83) |
| Transformed | 2 (17) |
| FL Stage at Diagnosis | |
| I | 0 (0) |
| II | 1 (8) |
| III | 4 (33) |
| IV | 7 (58) |
| FL Stage at Transplant | |
| I | 0 (0) |
| II | 1 (8) |
| III | 4 (33) |
| IV | 7 (58) |
| Status at Transplant Conditioning | |
| CR | 0 (0) |
| PR | 7 (58) |
| Refractory | 5 (42) |
| No. of Prior Therapies before RIT, median (range) | 5 (2, 10) |
| Prior Autologous Transplant | 1 (8) |
| Karnofsky Performance Status | |
| 70 | 2 (16) |
| 80 | 5 (42) |
| 90 | 4 (32) |
| 100 | 1 (8) |
| Time from RIT to Allotransplant in mos., median (range) | 1.0 (0.4, 5.8) |
| HLA Matched (A, B, DR)/Donor Type | |
| Matched Unrelated | 4 (33) |
| Matched Related | 3 (25) |
| Mismatched Unrelated* | 4 (33) |
| Double umbilical cord blood | 1(8) |
| CMV | |
| Donor −/Recipient − | 4 (33) |
| Donor −/Recipient + | 1 (8) |
| Donor +/Recipient − | 4 (33) |
| Donor +/Recipient + | 3 (25) |
| Stem Cell Source | |
| PBSC | 11 (92) |
| Cord Blood | 1 (8) |
| Type of Conditioning | |
| Busulfan/Fludarabine | 11 (92) |
| Melphalan/Fludarabine/ATG | 1 (8) |
| GVHD Prophylaxis | |
| Tacrolimus/mini-methotrexate | 4 (33) |
| Tacrolimus/Sirolimus/mini-methotrexate | 1 (8) |
| Tacrolimus/Sirolimus | 2 (16) |
| Tacrolimus/Bortezomib/mini-methotrexate | 3 (25) |
| Sirolimus/Mycophenolate mofetil | 2 (16) |
| CD34 dose × 106/kg (median, range). n = 9 | 6.42 (5.81) |
Three patients had one antigen mismatch and one had two antigen mismatches
Engraftment and chimerism
The median (range) dose of CD34 cell dose infused was 6.42 (3.29 – 9.09) × 106/kg (n=9). Ten patients (83%) had a nadir absolute neutrophil count (ANC) below 500 cells/mL, and 8 (67%) a platelet nadir below 20,000 cells/mL. All patients engrafted, with a median time to neutrophil recovery of 14 days (range: 11–35), and a median time to platelet recovery of 20 days (range: 11–163). Among 12 patients, 11 had chimerism measurements available at day 30, 9 at day 100 and 6 at day 365. The median (range) percentage donor whole blood chimerism achieved at these time points was 97% (88–100%), 98% (93–100%) and 100% (99–100%) (Table 2).
Table 2.
Patient Outcomes
| Outcome | N |
|---|---|
| No. of Pts. with ANC ≤ 500 | 10 |
| Time to Neutrophil Engraftment in days, median (range) | 14 (11, 35) |
| No. of Pts. with Platelets ≤ 20 000 | 8 |
| Time to Platelet Engraftment in days, median (range) | 20 (11, 163) |
| Day 30 donor chimerism % - median (range). n = 11 | 97 (12) |
| Day 100 donor chimerism % - median (range). n = 9 | 98 (7) |
| Day 365 donor chimerism % - median (range). n = 6 | 100 (1) |
| gr. II–IV aGVHD | 4 |
| gr. III–IV aGVHD | 1 |
| cGVHD | 7 |
| No. of Relapses | 1 |
| No. of Deaths | 2 |
| Sepsis/GVHD | 1 |
| Infection | 1 |
| Outcome | % Estimate (SE) |
| Cum. Inc. gr. II–IV aGVHD at 100 days | 17% (11%) |
| Cum. Inc. gr. II–IV aGVHD at 200 days | 25% (13%) |
| Cum. Inc. cGVHD at 12 months | 63% (19%) |
| Cum. Inc. NRM at 12 months | 18% (12 %) |
| PFS 2-year | 74% (13 %) |
| OS 2-year | 83% (11%) |
Toxicity and GVHD
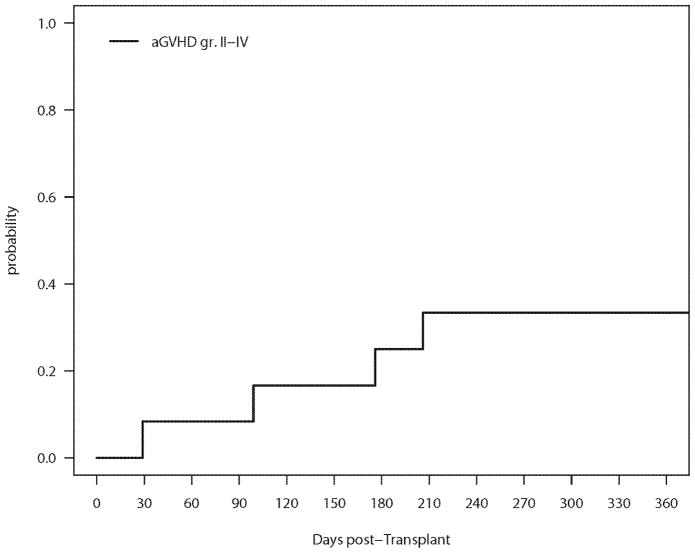
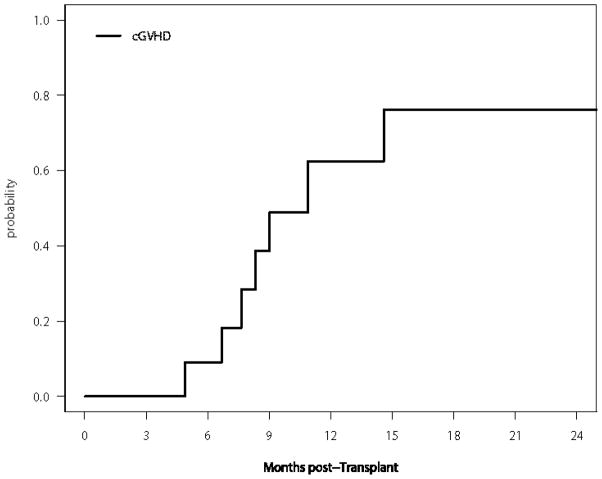
Administration of the RIT was not associated with any grade 3–4 non-hematologic toxicity. The cumulative incidences of aGVHD (grade II–IV) and cGVHD are shown in Figures 1 and 2, respectively and table 2. The incidence of grade II–IV acute GVHD (SE) was 17% (11%) at 100 days and 25% (13%) at 200 days. Only one patient developed grade III–IV aGVHD. Seven patients developed cGVHD, one of which was severe. The 1-year cumulative incidence of cGVHD (SE) was 63% (19%). Two patients died of infectious causes, one of which as a direct consequence of severe aGVHD of the skin, resulting in a 2- year cumulative incidence (SE) of non-relapse mortality (NRM) of 18% (12%).
Figure 1.
Cumulative Incidence of Grade II–IV aGVHD
Figure 2.
Cumulative Incidence of cGVHD
Treatment Response and Survival
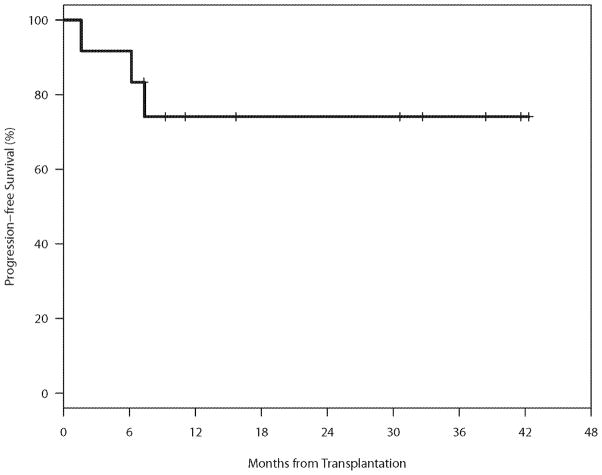
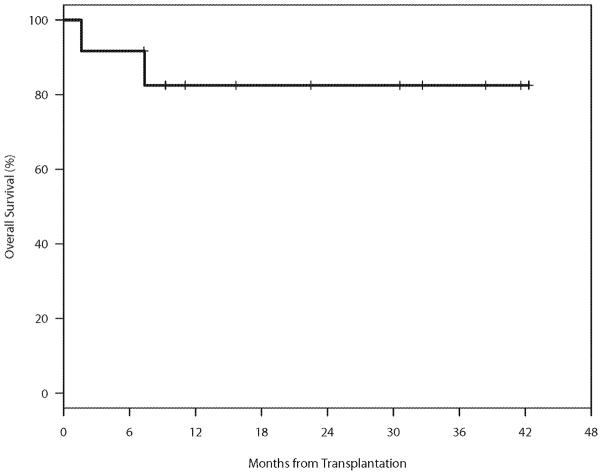
The median follow-up for the 9 patients alive without relapse was 31 months (range 7–42). Outcomes are presented in table 2, Figures 3 and 4. Eight (67%) patients achieved CR after allogeneic HSCT. One patient relapsed 8 months after allogeneic HSCT. Treatment with bendamustine and rituximab and rapid taper of immunosuppression resulted in a GVHD flare followed by a CR. For the entire cohort, the 2-year PFS (SE) and OS (SE) were 74% (11%), and 83% (11%) respectively.
Figure 3.
Progression-free Survival (PFS)
Figure 4.
Overall Survival (OS)
DISCUSSION
In the present study, the addition of the radioimmunoconjugate prior to allogeneic HSCT was associated with excellent OS and PFS with no apparent increase in NRM or GVHD compared with regimens that do not include RIT. The relapse rate appears to be low, although a precise estimate will depend on analysis of a larger cohort.
Conventional RIC allogeneic HSCT has the benefit of less immediate TRM than high dose conditioning regimens at the cost of a higher relapse rate. A CIBMTR report compared 88 RIC to 120 myeloablative matched sibling allogeneic HSCT in relapsed FL. Relapse rates with RIC were twice as high compared with myeloablative allogeneic HSCT (17% vs 8%) [7]. This result suggested that we could take advantage of the inherently radiosensitive nature of FL by using RIT to avoid the toxicities associated with the use of myeloablative regimens.
Radioimmunoconjugates have been used for over a decade in hematological malignancies and are potent drugs for the treatment of NHL. 90Y-Ibritumomab tiuxetan was approved by the FDA in 2002 for the treatment of patients with relapsed or refractory low-grade, follicular, or transformed B-cell NHL, including patients with rituximab refractory FL. In addition to antibody and complement-dependent cytotoxicity, radioimmunoconjugates exert a large part of their clinical activity through the radioisotope [10]. Because 90Y does not emit gamma rays compared to 131I used with tositumomab, radiation exposure to household members of patients treated with 90Y-Ibritumomab tiuxetan is minimal, rendering outpatient administration feasible. Furthermore, 90Y has a 5mm radiation path length compared to 0.8 mm for 131I making 90Y-Ibritumomab tiuxetan potentially better suited for bulky tumor load reduction. Through their unique mechanism of action, radioimmunoconjugates offer high response rates even in patients who are refractory to combination chemotherapy and rituximab [22, 12] with a limited toxicity profile. An integrated analysis of 250 patients with relapsed/refractory or transformed indolent NHL treated with 131I-tositumomab revealed overall response (ORR) and complete response (CR) rates ranging from 47–68% and 20–38 % respectively. Although in the majority of patients responses were not durable, among the 32% of patients who stayed in remission for ≥ 1 year, the median duration of response was an impressive 45.8 months [23]. Similar findings have also been reported with 90Y-ibritumomab tiuxetan [9].
These promising results have lead to the introduction of standard dose and high dose RIT as part of the conditioning regimen for ASCT in NHL with encouraging results [24–25]. Few studies have been published to date on RIT combined with RIC allogeneic HSCT in NHL. A recent German phase II prospective study evaluated the use of 90Y-ibritumomab tiuxetan followed by RIC allogeneic HSCT using fludarabine and 2 Gy TBI as conditioning in 40 patients with relapse indolent NHL. The outcomes of 17 patients with FL were considerably superior to other histologies resulting in 2-year OS and PFS of 67% and 57% respectively compared to 51% and 43% respectively for the entire cohort [26]. Similarly, Shimoni and colleagues reported the outcomes of 12 patients with extensively treated advanced NHL of various histologies with active disease who underwent RIT combined with RIC HSCT. The conditioning regimen consisted of 90Y-Ibritumomab tiuxetan and fludarabine combined with busulfan or melphalan followed by allogeneic HSCT. An ORR of 83% was observed along with a 2 year PFS of 33%. Only three patients had FL but all three were reported as long term survivors [17]. Our reported 2-year PFS and OS rates of 74% and 83% respectively are encouraging in this high risk, heavily pretreated FL patient population. These results compare favorably to previous reports of RIC allogeneic HSCT in FL with and without the use of radioimmunoconjugates [7, 18, 27]. Direct comparisons between these studies are limited by variations in patient and disease characteristics as well as conditioning regimens. Taken together, these results suggest that FL appears to be particularly amenable to the use of RIT followed by RIC allogeneic HSCT compared to other indolent NHL histologies, although the reasons for this remain uncertain.
The use of RIT prior to RIC allogeneic HSCT in our study did not result in increased toxicity with a cumulative incidence of NRM at 2 years of 18%, similar to previous reports of RIC allogeneic HSCT in FL [7, 27]. Furthermore, engraftment was not delayed with a time to neutrophil and platelet recovery of 14 and 20 days respectively, similar to RIC allogeneic HSCTs our institution performed without RIT [18]. Moreover, no graft failures have been observed. GVHD remains a major cause of morbidity and mortality in RIC allogeneic HSCT. Previous reports of RIT followed by RIC allogeneic HSCT have highlighted a significant NRM attributable to such high rates of GVHD. Until now, published studies on RIT followed by RIC allogeneic HSCT in NHL reported high rates of aGVHD ranging from 43% to 67% leading to a 2-year NRM of 42% to 45% [17, 26]. The authors attributed this high rate of aGVHD to a combination of heavily pretreated patient population, unrelated mismatched donors, rapid achievement of complete chimerism and early withdrawal of immunosuppression. We observed a considerably lower cumulative incidence of Grade II–IV aGVHD (25%), which is comparable to previous reports of RIC allogeneic HSCT in indolent NHL [7, 18, 27]. Based on these findings, RIT prior to RIC allogeneic HSCT does not appear to be an important contributor to the development of aGVHD. Furthermore, a recent CIBMTR analysis revealed that exposure to anti-CD20 antibodies in the peri-transplant period may actually diminish the risk of acute GVHD [28]. Lastly, half of our patients received sirolimus plus tacrolimus, a combination which appears to result in a lower risk of aGVHD compared to a calcineurin inhibitor plus methotrexate [29].
This study has inherent limitations due to its retrospective nature. First, selection bias may limit the applicability of these results. However, our patient population consisted of high-risk heavily pretreated FL patients. Nearly half of them had either transformed to a more aggressive histology or become refractory to therapy. This accurately represents patients with relapsed/refractory or transformed FL undergoing allogeneic HSCT and whose expected outcomes are very poor [30]. Nevertheless, the outcomes of the 12 patients in this study were not different from those of 29 FL patients who underwent allogeneic HSCT at DFCI during the same period with less advanced and more chemosensitive disease (not shown). Only one of thirteen patients considered for this therapy failed to have an adequate response to 90Y ibritumomab tiuxetan and hence did not proceed to RIC allogeneic HSCT. The use of RIT therefore allowed us to achieve adequate disease control in the vast majority of patients who had previously difficult to control FL. The outcome of this patient was not included in our KM curves as our intent was to describe the outcomes of patients successfully salvaged by RIT who were then consolidated by RIC allogeneic HSCT. Second, the uncontrolled nature of this study prevents us from drawing firm conclusions on the magnitude of the effect of adding RIT to a RIC allogeneic HSCT regimen in this patient population, as our results can only be compared to historical controls. As such, the potential impact of RIT on OS, PFS, rates of GVHD and NRM have to be interpreted with caution. Despite these limitations, combining RIT with RIC allogeneic HSCT for relapsed, refractory or transformed FL is well-tolerated and the outcomes are encouraging.
Although radioimmunoconjugates have well documented clinical efficacy and safety, they continue to be underutilized in the management of NHL. There is now growing evidence that these agents can play a role both as frontline and in relapse NHL. Furthermore, safety and efficacy data is becoming available to support their use in HSCT where they offer effective cytoreduction with low associated toxicity. In this study, we conclude that 90Y ibritumomab tiuxetan followed by RIC allogeneic HSCT is associated with favorable outcomes including acceptable rates of GVHD and relapse in this high-risk relapsed, refractory or transformed FL patient population. Further validation through well designed prospective studies is warranted.
Acknowledgments
Supported in part by NIH grant CA142106 and the Jock and Bunny Adams Research and Teaching Endowment. ASF is supported in part by NIH/NCI P01 CA92625.
Footnotes
All authors of this manuscript have no relevant financial disclosures or conflict of interest to declare.
References
- 1.Czuczman MS. Controversies in follicular lymphoma: “who, what, when, where, and why?. ” (not necessarily in that order!) Hematology Am Soc Hematol Educ Program. 2006;1:303–310. doi: 10.1182/asheducation-2006.1.303. [DOI] [PubMed] [Google Scholar]
- 2.Giné E, Montoto S, Bosch F, Arenillas L, Mercadal S, Villamor N, et al. The Follicular Lymphoma International Prognostic Index (FLIPI) and the histological subtype are the most important factors to predict transformation in follicular lymphoma. Ann Oncol. 2006;17(10):1539–45. doi: 10.1093/annonc/mdl162. [DOI] [PubMed] [Google Scholar]
- 3.Brown JR, Feng Y, Gribben JG, Neuberg D, Fisher DC, Mauch P, et al. Long-Term Survival after Autologous Bone Marrow Transplantation for Follicular Lymphoma in First Remission. Biol Blood Marrow Transplant. 2007;13(9):1057–1065. doi: 10.1016/j.bbmt.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabloff M, Atkins HL, Bence-Bruckler I, Bredeson C, Fergusson D, Genest P, et al. A 15-Year Analysis of Early and Late Autologous Hematopoietic Stem Cell Transplant in Relapsed, Aggressive, Transformed, and Nontransformed Follicular Lymphoma. Biol Blood Marrow Transplant. 2007;13:956–964. doi: 10.1016/j.bbmt.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Schouten HC, Qian W, Kvaloy S, Porcellini A, Hagberg H, Johnson HE, et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin’s lymphoma: results from the randomized European CUP trial. J Clin Oncol. 2003;21(21):3918–27. doi: 10.1200/JCO.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Khouri IF, Saliba RM, Giralt SA, Lee MS, Okoroji GJ, Hagemeister FB, et al. Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: Low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortality. Blood. 2001;98:3595–3599. doi: 10.1182/blood.v98.13.3595. [DOI] [PubMed] [Google Scholar]
- 7.Hari P, Carreras J, Zhang MJ, Gale RP, Bolwell BJ, Bredeson CN, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant. 2008;14:236–245. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters MV, Busch RS, Brown TC, Reid J. The place of radiotherapy in the control on non-Hodgkin’s lymphomata. Br J Cancer. 1975;2:386–401. [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon LI, Molina A, Witzig T, Emmanouilides C, Raubtischek A, Darif M, et al. Durable responses after ibritumomab tiuxetan radioimmunotherapy for CD20+ B-cell lymphoma: Long-term follow-up of a phase 1/2 study. Blood. 2004;103:4429–4431. doi: 10.1182/blood-2003-11-3883. [DOI] [PubMed] [Google Scholar]
- 10.Davis TA, Kaminski MS, Leonard JP, Hsu FJ, Wilkinson M, Zelenetz A, et al. The radioisotope contributes significantly to the activity of radioimmunotherapy. Clin Cancer Res. 2004;10(23):7792–8. doi: 10.1158/1078-0432.CCR-04-0756. [DOI] [PubMed] [Google Scholar]
- 11.Wiseman GA, Gordon LI, Multani PS, Witzig TE, Spies S, Bartlett NL, et al. Ibritumomab tiuxetan radioimmunotherapy for patients with relapsed or refractory non-Hodgkin lymphoma and mild thrombocytopenia: a phase II multicenter trial. Blood. 2002;99(12):4336–42. doi: 10.1182/blood.v99.12.4336. [DOI] [PubMed] [Google Scholar]
- 12.Witzig TE, Flinn IW, Gordon LI, Emmanouilides C, Czuczman MS, Saleh MN, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20 (15):3262–69. doi: 10.1200/JCO.2002.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Witzig TE, Molina A, Gordon LI, Emmanouilides C, Schilder RJ, Flinn IW, et al. Long-term responses in patients with recurring or refractory B-cell non-Hodgkin lymphoma treated with yttrium 90 ibritumomab tiuxetan. Cancer. 2007;109(9):1804–10. doi: 10.1002/cncr.22617. [DOI] [PubMed] [Google Scholar]
- 14.Emmanouilides C, Witzig TE, Wiseman GA, Gordon LI, Wang H, Schilder R, et al. Safety and efficacy of yttrium-90 ibritumomab tiuxetan in older patients with non-Hodgkin’s lymphoma. Cancer Biother Radiopharm. 2007;22(5):684–91. doi: 10.1089/cbr.2007.359. [DOI] [PubMed] [Google Scholar]
- 15.Pagel JM, Gooley TA, Rajendran J, Fisher DR, Wilson WA, Sandmaier BM, et al. Allogeneic hematopoietic-cell transplantation after conditioning with 131I-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood. 2009;114(27):5444–53. doi: 10.1182/blood-2009-03-213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fietz T, Uharek L, Gentilini C, Muessig A, Rieger K, Marinets O, et al. Allogeneic hematopoietic cell transplantation following conditioning with 90Y-ibritumomab-tiuxetan. Leuk Lymphoma. 2006;47:59–63. doi: 10.1080/10428190500260478. [DOI] [PubMed] [Google Scholar]
- 17.Shimoni A, Zwas ST, Oksman Y, Hardan I, Shem-Tov N, Rand A, et al. Ibritumomab tiuxetan (Zevalin) combined with reduced-intensity conditioning and allogeneic stem-cell transplantation (SCT) in patients with chemorefractory non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2008;41:355–361. doi: 10.1038/sj.bmt.1705919. [DOI] [PubMed] [Google Scholar]
- 18.Armand P, Kim HT, Ho VT, Cutler CS, Koreth J, Antin JH, et al. Allogeneic transplantation with reduced-intensity conditioning for Hodgkin and non-Hodgkin lymphoma: importance of histology for outcome. Biol Blood Marrow Transplant. 2008;14(4):418–25. doi: 10.1016/j.bbmt.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alyea EP, Li S, Kim HT, Cutler C, Ho V, Soiffer RJ, et al. Sirolimus, tacrolimus, and low-dose methotrexate as graft-versus-host disease prophylaxis in related and unrelated donor reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2008;14(8):920–6. doi: 10.1016/j.bbmt.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho VT, Aldridge J, Kim HT, Cutler C, Koreth J, Armand P, et al. Comparison of Tacrolimus and Sirolimus (Tac/Sir) versus Tacrolimus, Sirolimus, and mini-methotrexate (Tac/Sir/MTX) as acute graft-versus-host disease prophylaxis after reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(7):844–50. doi: 10.1016/j.bbmt.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 22.Morschhauser F, Radford J, Van Hoof A, Vitolo U, Soubeyran P, Tilly H, et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol. 2008;26:5156–5164. doi: 10.1200/JCO.2008.17.2015. [DOI] [PubMed] [Google Scholar]
- 23.Fisher RI, Kaminski MS, Wahl RL, Knox SJ, Zelenetz AD, Vose JM, et al. Tositumomab and iodine-131 tositumomab produces durable complete remissions in a subset of heavily pretreated patients with low-grade and transformed non-Hodgkin’s lymphomas. J Clin Oncol. 2005;23:7565–7573. doi: 10.1200/JCO.2004.00.9217. [DOI] [PubMed] [Google Scholar]
- 24.Shimoni A, Zwas ST, Oksman Y, Hardan I, Shem-Tov N, Yerushalmi R, et al. Yttrium-90-ibritumomab tiuxetan (Zevalin) combined with high-dose BEAM chemotherapy and autologous stem cell transplantation for chemo-refractory aggressive non-Hodgkin’s lymphoma. Exp Hematol. 2007;35:534–540. doi: 10.1016/j.exphem.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 25.Vose JM, Bierman PJ, Enke C, Hankins J, Bociek G, Lynch JC, et al. Phase I trial of iodine-131 tositumomab with high-dose chemotherapy and autologous stem-cell transplantation for relapsed non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:461–467. doi: 10.1200/JCO.2005.05.117. [DOI] [PubMed] [Google Scholar]
- 26.Bethge WA, Lange T, Meisner C, von Harsdorf S, Bornhaeuser M, Federmann B, et al. Radioimmunotherapy with yttrium-90-ibritumomab tiuxetan as part of a reduced intensity conditioning regimen for allogeneic hematopoietic cell transplantation in patients with advanced non-Hodgkin lymphoma: results of a phase II study. Blood. 2010;116(10):1795–802. doi: 10.1182/blood-2010-02-270538. [DOI] [PubMed] [Google Scholar]
- 27.Rezvani AR, Storer B, Maris M, Sorror ML, Agura E, Maziarz RT, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(2):211–7. doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- 28.Ratanatharathorn V, Logan B, Wang D, Horowitz M, Uberti JP, Ringden O, et al. Prior rituximab correlates with less acute graft-versus-host disease and better survival in B-cell lymphoma patients who received allogeneic peripheral blood stem cell transplantation. Br J Haematol. 2009;145(6):816–24. doi: 10.1111/j.1365-2141.2009.07674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armand P, Gannamaneni S, Kim HT, Cutler CS, Ho VT, Koreth J, et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol. 2008;26(35):5767–74. doi: 10.1200/JCO.2008.17.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson SP, Goldstone AH, Mackinnon S, Carella A, Russell N, de Elvira CR, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reducedintensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100:4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]