Abstract
The lateral hypothalamic area (LHA) was initially described as a “feeding center” but we are now beginning to understand that the LHA contributes to other aspects of physiology as well. Indeed, the best-characterized neuronal populations of the LHA (which contain melanin-concentrating hormone (MCH) or the hypocretins/orexins (OX) are not strictly orexigenic, but also have roles in regulation of the autonomic and sympathetic nervous systems as well as in modulating motivated behavior. Leptin is an anorectic hormone that regulates energy homeostasis and the mesolimbic DA system (which transduces the wanting of food, drugs of abuse and sex) in part, via actions at the LHA. At least three populations of LHA neurons are regulated by leptin: those containing MCH, OX or the long form of the leptin receptor, LepRb. The emerging picture of leptin interaction with these LHA populations suggests that the LHA is not merely regulating feeding, but is a crucial integrator of energy balance and motivated behavior.
Keywords: Orexin, MCH, dopamine, LepRb, energy balance
1. Why Do We Need to Think About Feeding and Energy Balance?
The worldwide incidence of overweight and obesity is rapidly increasing. Obesity predisposes individuals to cardiovascular disease and type-2 diabetes, reduces life expectancy, and incurs $117 billion in annual health care costs in the U.S. alone [1, 2]. The only proven disease-modifying treatments for obesity are dietary regulation and weight loss. Bariatric surgery has been used with some success to potentiate weight loss, but this method is invasive, requires permanent lifestyle modifications and sustained weight loss is not guaranteed-some subjects regain weight in the years after surgery [3, 4]. The more conventional methods for weight loss are diet and exercise, supported by a multi-billion dollar industry and a staggering number of new supplements, diet plans and exercise regimes each year [5]. In spite of this, most dieters ultimately regain weight and these methods have poor long-term success rates. Indeed, this demonstrates that the body staunchly defends against negative changes in homeostatic set point, but is unable to defend against positive changes, resulting in progressive weight gain. Pharmacologic therapies to reduce appetite and increase energy expenditure would be useful treatments, but limited understanding of the physiologic systems regulating these processes has hindered development of truly effective therapies to treat or prevent obesity. As a result, there has been a surge of research focused on systems that impact energy homeostasis and how these might be tenable targets for therapeutic intervention.
2. The Leptin/LepRb System is a Crucial Regulator of Energy Homeostasis
2.1 Leptin
In 1950 the Jackson Laboratory identified a mouse line displaying profound hyperphagia, obesity, infertility and severe hyperglycemia resulting in diabetes. Nearly five decades later the genetic deficit underlying this phenotype was found to be a mutation on chromosome 6 in the obese (ob) gene and its gene product, leptin [6]. Leptin is a hormone produced by adipocytes and secreted into the circulation. Importantly, leptin is produced in proportion to peripheral energy reserves (i.e. fat), so leptin concentration indicates how much energy the body has on board [7]. The crucial role of leptin in energy homeostasis is revealed by the hyperphagic and obese phenotypes of leptin-deficient rodents and humans, which are normalized with leptin treatment [8, 9]. Leptin also has an important role in the regulation of glucose homeostasis, reproduction, growth, the immune response and motivated behaviors, such as intake of food and drugs of abuse and locomotor activity [10–12].
2.1 LepRb
Another obese mouse was discovered in 1965, with a genetic mutation that mapped to chromosome 4: this became known as the db/db mouse [13–15]. Coleman and colleagues characterized the differences between the obese ob/ob and db/db lines by joining the circulatory systems of these mice with normal mice or each other and observing the resulting phenotype, a technique called parabiosis (reviewed in [14].) Parabiosis of an ob/ob mouse with a normal lean mouse caused the ob/ob to lose weight, due to restoration of a missing circulatory factor provided by the lean mouse- this turned out to be leptin. By contrast, parabiosis of a db/db and normal lean mouse did not cause weight loss in the db/db, suggesting that this obese phenotype was not due to lack of circulating leptin but perhaps something necessary to transduce the leptin signal. Indeed, later experiments verified that the db gene encodes the long form of the leptin receptor (LepRb) [13, 16]. Leptin acts by binding to cells expressing LepRb, the only leptin receptor isoform containing the intracellular motifs required to convey leptin signals [15, 17–19]. Rodents lacking LepRb (i.e. db/db mice and fa/fa rats) recapitulate the phenotype of leptin-deficient models (obese, hyperphagic, infertile, diabetic), demonstrating the necessity of leptin signaling via LepRb [13, 16, 20].
The central action of leptin is crucial for energy balance and mediated via distributed populations of LepRb-expressing neurons throughout the brain [21–24]. There are several LepRb neuronal populations within the midbrain, including the dorsal raphe (DR) and periaqueductal gray (PAG), linear raphe, Edinger-Westphal nucleus and LepRb neurons in the ventral tegmental area (VTA). [24, 25]. There are also populations of LepRb neurons ranging from rostral brain areas such as the medial preoptic area (mPOA) to the caudal extent of the brain, in the hindbrain nucleus of the solitary tract (NTS). The hypothalamus, however, contains the largest density of LepRb neurons, distributed among mediobasal areas (e.g. arcuate (ARC) and ventromedial (VMH) nuclei), the ventral premammillary nucleus (PMv), the dorsomedial hypothalamus (DMH) as well as the lateral hypothalamic area (LHA).
2.3 The “Distributed Function Hypothesis” and Leptin Action
Brain function is often defined by region; for example, sensation is processed in somatosensory cortex, while motor control is regulated via separate motor cortex. The observation that LepRb-expressing neurons are broadly distributed throughout many different brain areas suggested that discrete populations of LepRb neurons may contribute to distinct aspects of central leptin action [10, 11]. This concept has been termed the “Distributed Function Hypothesis” of leptin action, and is supported by the fact that regional populations of LepRb neurons differ in their molecular and/or neurochemical expression. For example, LepRb/SF1-containing neurons of the VMH contribute to leptin control of energy homeostasis by modifying energy expenditure. Adjacent LepRb/pro-opiomelanocortin (POMC)-expressing neurons in the ARC are crucial for regulating glucose homeostasis [26–32]. By contrast, the opposing LepRb/agouti-related protein/neuropeptide Y (AgRP/NPY)-expressing neurons strongly induce feeding, suggesting that they have a more prominent role in satiety [33–35]. The role of other LepRb neuronal populations have not been studied as extensively, but have unique molecular profiles that suggest additional specificity. For example, tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine (DA) synthesis, is expressed in LepRb neurons of the VTA that project to the central amygdala [25, 36, 37]. Additionally, a population of mediobasal LepRb neurons contain neuronal nitric oxide (nNOS) and LepRb neurons in the Edinger Westphal nucleus contain urocortin-1 [38, 39]. Molecular characterization of LepRb-expressing populations has enabled site-specific examination of the role of leptin, and so we are beginning to identify the populations of LepRb neurons that contribute to various aspects of leptin action. LepRb neurons of the ARC and NTS are important for leptin-mediated satiety [26, 39–41]. Leptin contributes to glucose homeostasis via LepRb-POMC neurons of the ARC [29, 32, 42, 43], while leptin action via the PMv regulates puberty and fertility [28, 38, 40–47]. In support of the “distributed function hypothesis, ” disruption of leptin signaling via a single population of LepRb neurons does not nullify total leptin action, but rather disrupts particular facets of leptin-control [26, 28, 32, 37, 47, 48]. Thus, it will be important to characterize each population of LepRb-expressing neurons to illuminate their specified contribution to leptin action and energy homeostasis.
2.4 A Role for Leptin in the Lateral Hypothalamic Area (LHA)
Upon the identification of LepRb much attention was devoted to understanding leptin action via the dense populations of LepRb-expressing neurons in mediobasal hypothalamic nuclei, such as the ARC and VMH. By comparison, much less was known about the nearby population of LepRb-expressing neurons in the LHA. Elmquist et. al. first reported a substantial number of LepRb neurons lying within the LHA in 1998, an area historically described as a “feeding center” [22]. Over the subsequent decade, many reports described leptin effects upon various LHA neuronal populations that altered energy homeostasis, but the role of leptin action via LepRb neurons in the LHA remained unclear. We have begun to characterize LHA LepRb neurons, as well as how leptin regulates these and other LHA neuronal populations to contribute to total leptin action. The remainder of this review will examine the neuronal populations and connectivity of the LHA and how leptin acts via the LHA to contribute to energy homeostasis and behavior.
3. The Lateral Hypothalamic Area (LHA), Then and Now
3.1 Historical Perspective: The LHA is a Feeding Center
Unlike the small, circumscribed mediobasal hypothalamic nuclei (e.g. ARC, VMH, PMv), the LHA covers a broad lateral region throughout the extent of the hypothalamus. In the caudal and medial hypothalamus of rodents the LHA lies mainly above and lateral to the fornix. While not ostensibly different in cytoarchitecture, this portion of the LHA is often subdivided into the area just above and around the fornix (the perifornical area) or ventral to the fornix (the subfornical region), and these regions differ in their connectivity [49–51]. At the rostral extreme of the hypothalamus the LHA is predominantly lateral to the fornix and eventually merges into the lateral preoptic areas [52, 53]. The medial forebrain bundle (mfb) extends through the LHA to the ventral striatum, directly linking the LHA with the DA signaling system. The LHA was originally described as a “feeding center” based on findings that LHA lesions produced extreme hypophagia (to the point of starvation) while electrical stimulation of the LHA promoted food intake even in sated animals [54–59]. At the same time, the LHA was characterized as a structure important in mediating reward, largely based on studies showing that animals with electrodes placed in the LHA will self-administer electrical current [60, 61]. This paradigm of intracranial self-stimulation (ICSS) via the LHA is still used to determine how drugs of abuse and other stimuli affect DA signaling and brain reward systems [62]. While stimulation and action via the mfb (which runs through the LHA and contains mesolimbic DA fibers) might explain some of the initially observed behavior/reward effects, these reports yielded a flurry of exploration of the LHA and its role in intake and hoarding of food, salt appetite and drinking behaviors [63–65].
3.2 The Connectivity of the LHA Suggests Its Function
The initial characterization of the LHA as a center for food intake behaviors suggested that it must interact with brain regions that ultimately coordinate these behaviors (i.e. to couple LHA signals with learning/memory as well as systems required to execute feeding behavior). This line of thinking sparked an intense interest in mapping the neuronal connectivity of the LHA to better understand its functional role in neurophysiology and behavior. Interestingly, the field has since determined that neurons in the LHA project widely throughout the brain and, via polysynaptic connections, also regulate targets outside of the brain. The LHA therefore modulates the autonomic and somatomotor systems, regulating targets including (but not limited to) skeletal and cardiovascular muscle, the adrenal gland and brown adipose tissue [66–69]. This regulation likely coordinates motivation with appropriate output at the periphery (i.e. muscle movements required to seek food, altered heart rate and blood pressure needed to support motor systems, energy expenditure via brown adipose tissue, etc).
LHA neurons also directly project to diverse regions within the brain. Mapping the projections from small mediobasal hypothalamic nuclei is challenging but this task is incredibly daunting for the large LHA, which spans nearly the entire hypothalamus. LHA afferents have been described throughout the rostral-caudal axis of the brain. Prominent innervation sites in the midbrain include the dorsal raphe (DR), periaqueductal gray (PAG) parabrachial nucleus, and VTA [70–72]. The LHA also projects to, and receives dense innervation from, the amygdala [73, 74]. Rostral projection targets of the LHA include septal nuclei (such as the bed nucleus of the stria terminalis (BNST) and lateral preoptic area), the striatum (including the NAc) and pallidal regions [72]. Recent work from the laboratory of Larry Swanson has provided more detailed understanding of LHA connections by mapping the projections from sub-regions of the LHA, defined mainly by their proximity to the fornix [50, 51]. Based on their findings, Table 1 summarizes some of the major LHA afferents based on their sub-region of origin. These elegant studies suggest that certain LHA sub-regions predominantly target, and presumably regulate, a limited number of specified brain regions. Additionally, some of these sub-regions appear to preferentially target aspects of the mesolimbic, corticolimbic or pallidal systems that have been implicated in motivated behaviors, such as intake of food and drugs as well as locomotor activity. For example, the caudal and medial region of the LHA (bordering the PVH) projects more densely to the striatum than the substantia innominata (pallidum). By contrast, the portion of the LHA just above the fornix sends dense projections to the substantia innominata and the VTA but fewer projections to the dorsal and ventral striatum. Further, most projections from the LHA area below the fornix do not target the VTA but send dense projections to the basomedial amygdala. These studies, along with identification of the types of neurons within each of these sub-regions, will provide crucial understanding of how the LHA regulates physiological and neural systems.
Table 1.
| Medial | Above F | Below F, Rostral | Below F, Caudal | |
|---|---|---|---|---|
| MOTIVATED BEHAVIOR | ||||
| Basomedial Amygdala | ND | ND | ++/+++ | − |
| Dorsal Striatum | +++ | + | ND | ND |
| Ventral Striatum (NAc) | +++ | + | − | ++ |
| Substantia Innominata (SI) | − | ++++ | +/++ | ++ |
| Septal Nuclei | +++/+ | +++ | ND | ND |
| BNST | +/++ | ++/+++ | − | ++/+++ |
| VTA | − | +++ | − | − |
| Lateral habenula (LH) | +++++ | ++ | +/++ | − |
| THALAMUS | ||||
| Paraventricular nucleus (PVT) | +++ | ++++ | + | ++ |
| Nucleus reuniens | −/+ | ++++ | ++ | − |
| HYPOTHALAMUS | ||||
| Anterior Hypothalamus | +++//+ | + | ++ | ++ |
| ARC | −/+ | − | − | − |
| DMH, anterior part (DMHa) | +++ | ++ | ++ | ++/+++ |
| Posterior Hypothalamus | +++++ | +++ | ++ | ++/+++ |
| PVH | − | ++++ | − | +++ |
| VMH | ++ | + | + | − |
| Medial preoptic area | +++ | +++ | −/+ | +++ |
| AVPV | − | − | − | +++ |
| Lateral preoptic area | +/++ | +++ | ++ | +++ |
| MIDBRAIN | ||||
| DR | −/+ | +++ | ++ | ++ |
| Barrington's Nucleus | − | ++++ | − | ++/+++ |
| PAG (motor regions) | −+ | +++/+ | − | −/+ |
| PAG ventrolateral | +++ | +++++ | ++ | ++/+++ |
| Midbrain reticular nuclues | − | +++/+ | − | − |
| HINDBRAIN | ||||
| NTS, medial part, rostral zone (NTSmr) | − | +++ | ND | ND |
| Parabrachial Nucleus | − | +/+++ | ND | ND |
Medial: refers to rostral LHA area that is adjacent to the PVH and medial to the fornix. Above F: area of the LHA that is lateral to the DMH and above the fornix. Below F, Rostral: anterior area of the LHA that is below the F. Below F, Caudal: posterior area of the LHA that is below the F. ND = projections not defined in this area, F = fornix.
3.3 Current Perspectives: The LHA Regulates Motivated Behavior
The LHA is densely connected with many limbic structures (e.g. the VTA, striatum, pallidum, etc.), which are important in the regulation and execution of motivated behaviors. Motivated behavior describes the locomotor activity or work that a subject exhibits and reflects the relative value of a stimulus (i.e. drugs of abuse, alcohol, food, sex, etc.). For example, amphetamine treatment increases the locomotor activity of rodents, and quantitation of that activity is used as “read-out” of the motivational effect of the drug (as reviewed in [75]). Additionally, animals will learn to press levers or nose-poke to obtain palatable food, drugs or other wanted stimuli [76–79]. Motivated behaviors are regulated via dopamine (DA) neurons of the ventral tegmental area (VTA) that project to the nucleus accumbens (NAc) and other striatal regions; collectively these comprise the mesolimbic DA system [27, 80, 81]. The finding that the LHA projects to the VTA and NAc therefore suggests that the LHA modulates the DA system to regulate motivated behavior. In this context, we see that the historical classification of the LHA as a “feeding center” does not fully explain its neurophysiologic role. Indeed, the LHA modulates a broad spectrum of motivated behaviors, including the intake of food, as well as drugs, alcohol, water, and general locomotor activity, all via regulation of connected mesolimbic brain areas [54, 76–78, 82–86]. Thus, perhaps the LHA is better described as a “Motivation Modulation Center”, which can coordinate various stimuli with the appropriate mesolimbic regions to execute behavioral outputs.
As our understanding of the LHA has evolved over time, so too has our understanding of motivated behaviors via the mesolimbic DA system. Consumption of palatable food, drugs or participation in sex were initially considered “rewarding” because they promoted DA signaling. Thus, the hypothesis was that DA itself conveyed pleasure, so DA signaling increased hedonia whereas anhedonia resulted if DA signaling decreased [87] [79]. This “anhedonia hypothesis” mainly concerned consumption/receipt of rewards, however, and did not explain the noted role for DA in the anticipatory, approach and preparatory aspects of motivated behavior [88]. Berridge and Robinson popularized an alternate hypothesis for reward, in which reward can be broken down into two separable components: liking and wanting [89–92]. “Liking” refers to the subjective appreciation of a stimulus, such as the positive facial expressions produced in infants and rodents in response to sweet tastes (lip licking, tongue protrusions) in contrast to the gapes and disliking facial expressions produced by bitter tastes [91, 93]. “Liking” is controlled via opiod, endocannabanoid and GABA-benzodiazepine neurotransmitter systems signaling to “hedonic hotspots” in the NAc shell and ventral pallidum [93–96]. In contrast, a stimulus may be “wanted” even if it is not liked; for example, individuals who take excessive amounts of drugs of abuse (i.e. induce abnormally high DA release) report higher ratings of drug wanting, but no alteration in drug liking [97]. Likewise, rodents with enhanced DA signaling exhibit increased wanting of a sweet reward without actually “liking” it any more than control mice [98]. Wanting thereby imbues a stimulus with incentive salience, meaning that the motivation to acquire, approach and consume a stimulus is increased above neutral [92, 99]. Wanting / incentive salience is regulated via mesolimbic DA neurons originating in the VTA [89, 98, 100]. Thus, the LHA (which contains neurons projecting into the NAc/pallidum and VTA) is wired to regulate both the liking and incentive salience components of reward signaling. Any consideration of LHA neurons and their actions (including those regulated by leptin) must therefore consider their role in modulating motivation, and whether they ultimately regulate the liking or incentive salience of stimuli. The remainder of this review will examine the neuronal populations and connectivity of the LHA in this context, and will ultimately address how leptin regulation of the LHA contributes to motivated behavior and energy homeostasis.
4. Getting to Know LHA Neurons: Characterization via Neuropeptide Content
As suggested by the diverse projection fields of LHA sub-regions, the neurons that make up this area are not homogeneous. The LHA contains both glutamatergic and GABAergic neurons, but expression of these classical neurotransmitters does not fully explain the role of these neurons. The discovery that sub-populations of neurons within the LHA express distinct neuropeptides has considerably advanced our understanding of the individual roles of these populations and the overall role of the LHA in homeostasis and motivated behavior. The following sections will therefore describe the neuropeptide-specific neuronal populations that lie in the LHA, their functions and how leptin regulates these populations to contribute to energy homeostasis and motivated behavior.
4.1 Melanin-Concentrating Hormone (MCH)
First discovered in the pituitary of teleost fish, melanin-concentrating hormone (MCH) was so named because it regulates skin color changes that are important for background adaption in fish and amphibians. These early studies also suggested that MCH acted via a hypothalamic circuit, since plasma concentrations of MCH varied with environmental stress [101–103]. Indeed, most MCH-expressing cell bodies in mammals are found within in the LHA, as well as minor populations in the DMH and zona incerta [104–106]. The prepro-MCH precursor yields MCH, as well as neuropeptide-E-I (NEI) and neuropeptide G-E (NGE) but the biological significance of these latter peptides remains unclear [106]. In rodents MCH acts via the G-protein coupled MCH receptor (MCHR1), and is expressed in olfaction structures, the DR, VMH, PVN, limbic areas (including the septum and amygdala) and also densely within the NAc shell [107]. Humans and primates also express a second MCH receptor (MCHR2) but its importance in MCH action is not yet known [108]. MCH neurons fall into two sub-populations, those that contain glutamate and a GABAergic population that co-expresses cocaine-and amphetamine-regulated transcript (CART) [109].
MCH has been characterized as an orexigenic neuropeptide that regulates energy homeostasis. MCH action is mediated centrally, where acute treatment promotes food intake in rodents, as well as water intake [110, 111]. Chronic treatment with either MCH or an MCHR1 agonist also increases food intake as well as body weight (including white adipose tissue), and mice over-expressing MCH are obese [112–114]. In contrast, mice lacking MCH are lean due to hypophagia and increased metabolism, and exhibit elevated heart rate suggesting increased sympathetic tone [115, 116]. MCH neurons polysynaptically project to peripheral targets (including brown adipose tissue) via the NTS, by which they can regulate sympathetic responses that contribute to energy homeostasis [67, 86]. Similarly, MCHR1 antagonists reduce food consumption and body weight, as well as improving measures of depression and anxiety. In fact, MCH may have a role in potentiating stress-regulated food intake [116, 117]. MCH is also an important regulator of the rewarding responses to drugs and alcohol [77, 115, 118] which occurs, at least in part, via regulation of the NAc [119, 120].
4.2 Hypocretins / Orexins
Two groups independently reported another LHA neural population expressing a neuropeptide that they termed as either hypocretin [121] or orexin [122], henceforth referred to as OX. There are two forms of the neuropeptide, OX-A and OX-B, which act via two G-protein coupled receptors (OXR1 and OXR2). While OX-A and OX-B themselves are very similar, they may mediate distinct actions via binding to their receptors. OxR1 is only coupled to Gq proteins and preferentially binds to OX-A. By contrast, OX2 is coupled to Gi/o and Gq proteins and binds both OX-A and -B with equal affinity [122, 123]. A few cell bodies expressing OX are found in the DMH, but most are located in the perifornical region of the LHA. In contrast to the compact localization of cell bodies, OX projections are widely dispersed throughout the rostral-caudal continuum of the brain. Particularly dense sites of OX innervation include the locus coeruleus, DR, PAG and paraventricular nucleus of the thalamus, structures important for regulation of arousal and sleep. Indeed, OX deficiency in rodents and humans results in narcolepsy, and evidence suggests that OX crucially regulates the transition between sleeping and waking [124–128]. OX neurons also send relays via the hindbrain and spinal cord to regulate the autonomic and sympathetic nervous system [66, 67, 86, 129]. Additionally, OX neurons innervate many regions involved in regulating behavior, including the amygdala, septal area, medial preoptic area, substantia innominata as well as the VTA and the NAc, the main components of the mesolimbic DA system [130, 131]. OX neurons express the neurotransmitter glutamate and increase the activity of VTA DA neurons that project to the NAc shell [132, 133]. OX neurons also express dynorphin and neuronal activity-regulated pentraxin (NARP), which is important for clustering of AMPA receptors and regulation of glutamate signaling [134, 135].
Functionally, OX is important in the regulation of sleep, energy homeostasis and motivated behavior. The OX system promotes arousal and may be a therapeutically tractable pathway for treatment of sleep disorders [136]. As such, clinical trials are investigating the efficacy of OX antagonists for treatment of insomnia [137]. The role of OX in energy homeostasis is complex, and it remains unresolved whether OX is truly an orexigenic (appetite enhancing) signal. Acute OX treatment promotes food intake in rats during the light phase (when they do not normally eat), but does not augment food intake during the dark phase (when they normally feed) [122, 138, 139]. These differences may be attributable to the role of OX in arousal-an animal must be awake to feed, so promoting arousal during the light cycle would increase the overall feeding duration and total intake. OX also regulates DA signaling, suggesting it may have a role in determining the incentive salience of rewards, such as food and drugs of abuse, that may ultimately alter food intake [76, 140–143]. Thus, OX could make food intrinsically more wanted, thus promoting increased feeding. Interestingly, the importance of the OX system in regulating motivated behavior varies according to the “reward value” of the stimulus. For example, OX-R antagonists attenuate the incentive salience of cocaine and morphine much more so than energy dense (high fat) food, but do not regulate the incentive salience of low-fat food (chow) [76]. Indeed, mice with disrupted OX signaling do not exhibit increased intake of chow, but changes in OX signaling do regulate the intake of energy dense substances, such as sucrose or high-fat chow [76, 144, 145]. The OX system also regulates locomotor activity via the mesolimbic DA system, coupling energy status/reward with behavioral response. Acute treatment with OX promotes locomotor activity and OX receptor antagonists attenuate physical activity [142, 146, 147]. Rodent models of OX deficiency exhibit decreased locomotor activity without a countermanding decrease in food intake, which contributes to the development of obesity in these animals [124, 126, 148]. OX, therefore, may collectively regulate energy homeostasis via simultaneously regulating awakeness, locomotor activity and promoting hedonic intake.
4.3 Other Neuropeptides Expressed Within the LHA
The LHA has a small population of corticotropin-releasing factor (CRF)-containing neurons, though nowhere near the number that are found in the PVH [149]. CRF has an important role in regulating stress response, and mediates some of these actions via the mesolimbic DA system [150]. Similar to the effects of acute OX treatment, CRF injection into the LHA induces grooming, eating and locomotor activity concomitant with increased heart rate [151]. Indeed, several lines of evidence suggest an interaction between the CRF and OX systems. OX neurons express receptors for CRF and mice lacking CRF receptors exhibit abnormal regulation of OX neurons in response to stress [152]. OX neurons may in turn regulate PVH CRF neurons, but OX predominantly regulates via the VTA while CRF does not. As a result, OX and CRF systems may be co-activated, but are likely mechanistically divergent [153, 154].
Neurotensin has been described in the LHA but also in neurons distributed throughout the forebrain, midbrain and in various regions in the hypothalamus, including the ARC, DMH and PMV [155, 156]. Central neurotensin is implicated in the sensitivity to pain and temperature, osmotic control, reward signaling, and food intake, though these actions have not been attributed to any particular populations of neurotensin-containing neurons [157–159]. Some LHA neurons containing neurotensin project to the parabrachial region and DR, though the functional significance of these connections remains unclear [160, 161]
The LHA also contains a population of neurons that express galanin, and some of these also co-express vasopressin [162]. Galanin injections into the LHA increase food intake in fasted and sated rats through a yet-unclear mechanism. [162]. The related Galanin-like peptide (GALP) also stimulates feeding behavior and may regulate MCH and OX-containing neurons [163, 164].
5. The Role of Leptin Action via the LHA
The discovery and characterization of leptin, an anorectic hormone, suggested that it might diametrically regulate the LHA, which had initially been considered as a “feeding center.” Fasting (which decreases circulating leptin) and diet-induced obesity (in which neurons no longer respond normally to leptin) increase neuronal activation in the LHA- these data suggested that leptin normally acts to suppress the LHA “feeding center” [165, 166]. The evolving understanding of the LHA as a general “motivation modulation area, ” however, has broadened our scope for leptin action via the LHA beyond feeding alone. For example, intracranial self-stimulation in the LHA (a measure of general reward, not necessarily reward induced by consumption of food or drugs) is also attenuated by leptin treatment [167]. Thus, in order to understand leptin action at the LHA we must consider not only the classical studies concerning food intake effects, but also how motivated behaviors for non-food stimuli may be affected.
5.1 The LHA is an Intersection of the Energy Balance and Mesolimbic DA Systems: A Role for Leptin Regulation
It is becoming clear that energy status modulates intake of “rewards”, such as drugs of abuse or alcohol, suggesting that there must be a link between systems regulating homeostasis and DA-mediated reward signaling. For example, caloric restriction increases the motivated intake of food and drugs, relapse to drug taking, and the amount of work an animal will do to obtain drugs [80, 168]. DA is crucial for feeding and locomotor behavior, including the hyperphagia observed in obese leptin-deficient mice [169]. Further, obesity deranges DA-regulated motivated behaviors: diet-induced obese, but not lean rodents, exhibit compulsive-like feeding behaviors that are not altered by aversive stimuli [170]. These effects may even be translated via the maternal environment to offspring- the motivated intake of high-fat food is increased in progeny from obese dams compared to offspring from lean dams [171]. One possible interpretation of these data is that the incentive salience of “reward” stimuli is altered in models of disrupted energy state, thereby promoting increased intake. As such, signals communicating energy status might play a regulatory role in this process, as has been documented for insulin and ghrelin (the appetite-increasing hormone), which regulate the motivated intake of food, drugs and locomotor activity [172–174].
Leptin also regulates DA signaling circuits, and thus could regulate the incentive salience of food and non-food stimuli. For example, leptin treatment of leptin-deficient children diminishes their ratings for food “rewards, ” and leptin status modifies reward responding in rodents [36, 37, 167, 175–177]. While some leptin-responsive mediobasal neurons innervate the LHA [178] these neurons do not account for leptin regulation of incentivized intake and motivated behavior, indicating that there must be another population of LepRb neurons mediating these effects. Neurons within the LHA, however, directly regulate mesolimbic centers and leptin regulates several LHA neuronal populations, including LepRb neurons within the LHA [133, 146, 150, 179]. Thus, the LHA represents an intersection between the homeostatic and mesolimbic DA systems and is a likely site by which leptin modulates DA signaling. While the physiologic relevance of leptin-regulated DA changes are yet unclear, the DA system is a crucial regulator of the incentive salience of stimuli. This suggests that leptin could regulate the “wanting” component of reward via regulation of the mesolimbic DA system, though more work is required to definitively explore this hypothesis. Regardless of how this DA signal is ultimately interpreted (wanting, learning, etc), the LHA is poised to integrate the leptin and mesolimbic DA systems, and so the remainder of this review will therefore address leptin action via known LHA neuronal populations, both in terms of their roles in energy balance and DA signaling.
5.2 Leptin Regulation of MCH Neurons
The presence of orexigenic MCH neurons within the LHA suggested that these might be targets of anorectic leptin signals. Acute leptin treatment decreases MCH expression [180], and conversely mice deficient in leptin have increased MCH and MCHR1 expression that is reduced by leptin treatment [181–183]. Mice lacking MCH are hypophagic and lean, suggesting that MCH and leptin signals are antagonistic [115]. Indeed, mice double null for MCH and leptin weigh less than mice only null for leptin (ob/ob). While both lines of mice are similarly hyperphagic, the MCH/leptin null animals have increased energy expenditure and locomotor activity that potentiates weight loss [184]. Thus, MCH may not be as important as an orexigenic factor as it is a suppressor of activity. This is consistent with the finding that MCH reduces neuronal firing in the NAc, where DA acts to promote locomotor activity [120]. Further, MCH neurons project to OX neurons and inhibit their activity [185, 186]; since OX regulates mesolimbic DA to promote locomotor activity, this circuitry may further suppress locomotor responses. The increased ambulatory activity in mice lacking MCH may therefore be due, in part, to lifted suppression of OX neurons and increased DA signaling.
5.3 Leptin Regulation of OX Neurons
Almost immediately after its discovery OX was investigated as a likely regulation point of the anorectic leptin system. Indeed, initial results suggested that leptin acts to suppress OX signaling. Depleting leptin via acute fasting increases OX and OXR1 expression and increases the excitatory synaptic inputs onto OX neurons. By contrast, leptin treatment inhibits OX expression and neuronal activity in normal fed animals, in part by reducing the excitatory inputs onto OX neurons [187–190]. Disruption of appropriate leptin signaling, however, disregulates the OX system: obese rodents with genetically ablated leptin signaling (ob/ob and db/db mice, fa/fa rats) have reduced OX expression that is restored to normal levels with leptin treatment [191–193]. OX levels in obese humans are also increased by weight loss and restoration of leptin sensitivity [194]. Thus, OX expression itself is not an indicator of appetite; in fact OX over-expression protects animals from diet-induced obesity and the associated decrease in locomotor activity, including reducing food intake. Increased OX expression also potentiates weight loss with leptin treatment [144]. The presence of leptin, however, is crucial for OX action; OX over-expression does not protect leptin-deficient mice from diet-induced obesity [144]. Overall these data suggest that leptin control of OX neurons is complex but required for appropriate energy balance. Less clear is how this leptin regulation of OX neurons affects the mesolimbic DA system, a main output of OX signaling. Given that leptin normalizes both OX expression and the DA system of leptin-deficient animals, it seems likely that leptin might regulate these processes via an integrated system.
5.4 Leptin Action via LepRb neurons in the LHA
In 1998 a large population of LepRb neurons were reported in the LHA [22] but the role of these LHA LepRb neurons is just beginning to be appreciated. LHA LepRb neurons do not co-express MCH or OX and represent a unique population in the LHA. The LHA LepRb neurons are primarily co-distributed amongst the OX neuronal population within the perifornical area, and both populations are surrounded by the MCH-containing neurons (Figure 1). All LHA LepRb neurons express the inhibitory neurotransmitter GABA, consistent with the hypothesis that anorectic leptin would suppress LHA actions in feeding and behavior. Indeed, leptin administered selectively into the LHA inhibits food intake and promotes weight loss in rats. In addition to regulation of energy homeostasis, LHA LepRb neurons project to and regulate the mesolimbic DA system. Restoring normal leptin levels only in the LHA of leptin-deficient ob/ob mice increases TH expression in the VTA (which is abnormally blunted) and increases DA content in the NAc. These data suggest that leptin action via the LHA is crucial for normal regulation of the DA system and energy homeostasis [179]
Figure 1.
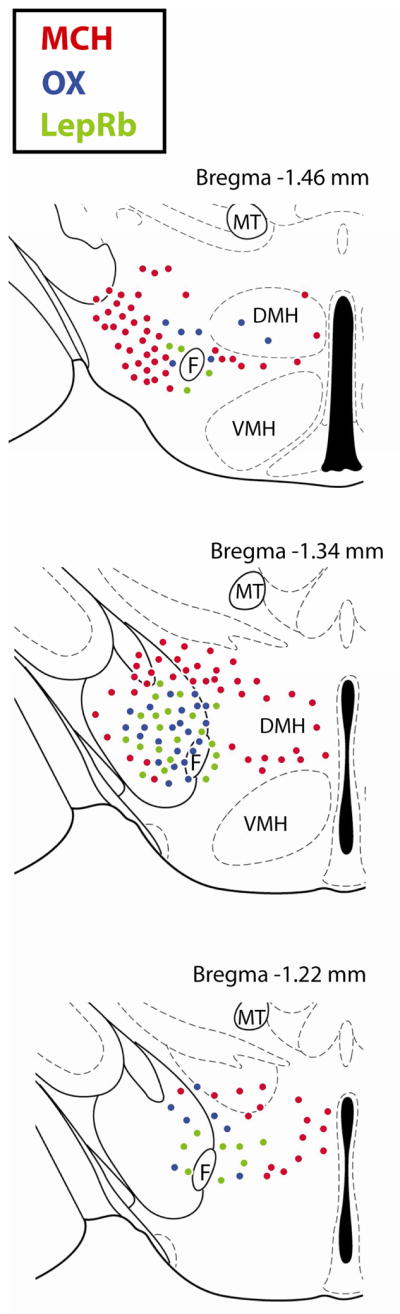
Distribution of MCH, OX and LepRb Neurons in the LHA of the Mouse. Levels of the mouse brain according to the stereotaxic atlas of Paxinos and Watson [52]. Only the left hemisphere is shown for each brain level. Red circles = MCH neurons, blue circles = OX neurons, green circles = LepRb neurons. F = Fornix, VMH = ventromedial hypothalamus, DMH = dorsomedial hypothalamus, MT = mammillothalamic tract.
LHA LepRb neurons also regulate neurons within the LHA, and may thus indirectly regulate energy balance and the mesolimbic DA system. In addition to the VTA, LHA LepRb neurons also project onto OX neurons. Leptin treatment increases OX expression in ob/ob mice through this connection [193]. Intriguingly, some LepRb neurons also project to MCH neurons, but LHA LepRb neurons do not. The population of LepRb neurons that regulate MCH neurons remains to be determined; identification of this circuitry will be an important step in understanding how leptin regulates MCH action.
6. Conclusions
The connectivity of the LHA with autonomic, sympathetic and mesolimbic systems indicates that is more than a “feeding center”, as it was historically described, but rather regulates the range of physiological responses to stimuli (food, drugs, stress, etc.) Despite the increasing evidence that there is more to the LHA than feeding alone, it is still most often described as containing two populations of orexigenic neurons- MCH and OX. Indeed, further understanding of these neuronal populations suggest that their roles are more than merely appetitive. Similarly, the role of leptin via the LHA has, thus far, mainly been considered in terms of effects on food intake and weight. While leptin does regulate energy balance via the LHA, the functional mechanism(s) are not as straightforward as first imagined—i.e., leptin does not just inhibit LHA neurons to reduce feeding. Regulation of OX and the leptin system are directly linked, and both are required for functional energy homeostasis. Collectively, these data suggest that we expand our view of the LHA and not only assess its role in appetite, but also in other mechanisms (such as locomotor activity, incentive salience, energy expenditure, etc.) that contribute to energy homeostasis.
Highlights.
The Lateral Hypothalamic Area (LHA) is not just a “feeding center” but a “motivation modulation center”
Leptin is an anorectic hormone that modulates energy homeostasis and motivated behavior
MCH and Orexin neurons of the LHA regulate the mesolimbic dopamine system and motivated behavior
Leptin regulates LHA neurons to integrate energy balance and DA signaling.
Acknowledgments
Special thanks to Martin G. Myers, Jr. for helpful discussions and comments. This work is supported by a grant from the NIH (NIDDK).
Footnotes
The author declares that they have no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farag YM, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrol Dial Transplant. 2011;26:28–35. doi: 10.1093/ndt/gfq576. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Dayyeh BK, Lautz DB, Thompson CC. Gastrojejunal Stoma Diameter Predicts Weight Regain After Roux-en-Y Gastric Bypass. Clin Gastroenterol Hepatol. 2010 doi: 10.1016/j.cgh.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meguid MM, Glade MJ, Middleton FA. Weight regain after Roux-en-Y: a significant 20% complication related to PYY. Nutrition. 2008;24:832–42. doi: 10.1016/j.nut.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Olson E. Diet Companies Promote New Ways to Reduce. New York Times; New York: 2011. p. B2. New York Edition ed. [Google Scholar]
- 6.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 7.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 8.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–84. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 9.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–6. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 10.Leinninger GM. Location, location, location: the CNS sites of leptin action dictate its regulation of homeostatic and hedonic pathways. Int J Obes (Lond) 2009;33 (Suppl 2):S14–7. doi: 10.1038/ijo.2009.66. [DOI] [PubMed] [Google Scholar]
- 11.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–23. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson SA, Leinninger GM, Myers MG., Jr Molecular and neural mediators of leptin action. Physiology behavior. 2008;94:637–42. doi: 10.1016/j.physbeh.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–6. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 14.Coleman DL. A historical perspective on leptin. Nat Med. 2010;16:1097–9. doi: 10.1038/nm1010-1097. [DOI] [PubMed] [Google Scholar]
- 15.White DW, Kuropatwinski KK, Devos R, Baumann H, Tartaglia LA. Leptin receptor (OB-R) signaling. Cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization. J Biol Chem. 1997;272:4065–71. doi: 10.1074/jbc.272.7.4065. [DOI] [PubMed] [Google Scholar]
- 16.Bahary N, Leibel RL, Joseph L, Friedman JM. Molecular mapping of the mouse db mutation. Proc Natl Acad Sci U S A. 1990;87:8642–6. doi: 10.1073/pnas.87.21.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kloek C, Haq AK, Dunn SL, Lavery HJ, Banks AS, Myers MG., Jr Regulation of Jak kinases by intracellular leptin receptor sequences. J Biol Chem. 2002;277:41547–55. doi: 10.1074/jbc.M205148200. [DOI] [PubMed] [Google Scholar]
- 18.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–7. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 19.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 20.Truett GE, Bahary N, Friedman JM, Leibel RL. Rat obesity gene fatty (fa) maps to chromosome 5: evidence for homology with the mouse gene diabetes (db) Proc Natl Acad Sci U S A. 1991;88:7806–9. doi: 10.1073/pnas.88.17.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–21. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–47. [PubMed] [Google Scholar]
- 23.Leshan RL, Bjornholm M, Munzberg H, Myers MG., Jr Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring) 2006;14 (Suppl 5):208S–12S. doi: 10.1038/oby.2006.310. [DOI] [PubMed] [Google Scholar]
- 24.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–32. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, et al. Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci. 2010;30:5713–23. doi: 10.1523/JNEUROSCI.1001-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–91. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Berthoud HR. Interactions between the "cognitive" and "metabolic" brain in the control of food intake. Physiology & behavior. 2007;91:486–98. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 29.Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 30.Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci. 2007;30:367–98. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- 31.Morton GJ, Niswender KD, Rhodes CJ, Myers MG, Jr, Blevins JE, Baskin DG, et al. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fa(k)/fa(k)) rats. Endocrinology. 2003;144:2016–24. doi: 10.1210/en.2002-0115. [DOI] [PubMed] [Google Scholar]
- 32.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–85. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2010 doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Q, Palmiter RD. GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism. Eur J Pharmacol. 2011 doi: 10.1016/j.ejphar.2010.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–22. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 38.Donato J, Jr, Frazao R, Fukuda M, Vianna CR, Elias CF. Leptin induces phosphorylation of neuronal nitric oxide synthase in defined hypothalamic neurons. Endocrinology. 2010;151:5415–27. doi: 10.1210/en.2010-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu L, Scheenen WJ, Leshan RL, Patterson CM, Elias CF, Bouwhuis S, et al. Leptin Signaling Modulates the Activity of Urocortin 1 Neurons in the Mouse Nonpreganglionic Edinger-Westphal Nucleus. Endocrinology. 2011 doi: 10.1210/en.2010-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11:77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng H, Patterson LM, Rhodes CJ, Louis GW, Skibicka KP, Grill HJ, et al. A potential role for hypothalamomedullary POMC projections in leptin-induced suppression of food intake. Am J Physiol Regul Integr Comp Physiol. 2010;298:R720–8. doi: 10.1152/ajpregu.00619.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, et al. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–97. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donato J, Jr, Cravo RM, Frazao R, Elias CF. Hypothalamic Sites of Leptin Action Linking Metabolism and Reproduction. Neuroendocrinology. 2010 doi: 10.1159/000322472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donato J, Jr, Silva RJ, Sita LV, Lee S, Lee C, Lacchini S, et al. The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J Neurosci. 2009;29:5240–50. doi: 10.1523/JNEUROSCI.0405-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leshan RL, Louis GW, Jo YH, Rhodes CJ, Munzberg H, Myers MG., Jr Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci. 2009;29:3138–47. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donato J, Jr, Cravo RM, Frazao R, Gautron L, Scott MM, Lachey J, et al. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355–68. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, et al. Leptin Regulates Energy Balance and Motivation Through Action at Distinct Neural Circuits. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leibowitz SF, Rossakis C. Mapping study of brain dopamine- and epinephrine-sensitive sites which cause feeding suppression in the rat. Brain Res. 1979;172:101–13. doi: 10.1016/0006-8993(79)90898-9. [DOI] [PubMed] [Google Scholar]
- 50.Goto M, Canteras NS, Burns G, Swanson LW. Projections from the subfornical region of the lateral hypothalamic area. J Comp Neurol. 2005;493:412–38. doi: 10.1002/cne.20764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hahn JD, Swanson LW. Distinct patterns of neuronal inputs and outputs of the juxtaparaventricular and suprafornical regions of the lateral hypothalamic area in the male rat. Brain Res Rev. 2010;64:14–103. doi: 10.1016/j.brainresrev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paxinos G, Franklin B. The Mouse Brain in Stereotaxic Coordinates. 2. San Diego, CA: Academic Press; 2001. [Google Scholar]
- 53.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. San Diego, CA: Academic Press; 2004. [Google Scholar]
- 54.Delgado JM, Anand BK. Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am J Physiol. 1953;172:162–8. doi: 10.1152/ajplegacy.1952.172.1.162. [DOI] [PubMed] [Google Scholar]
- 55.Morrison SD, Barrnett RJ, Mayer J. Localization of lesions in the lateral hypothalamus of rats with induced adipsia and aphagia. Am J Physiol. 1958;193:230–4. doi: 10.1152/ajplegacy.1958.193.1.230. [DOI] [PubMed] [Google Scholar]
- 56.Wyrwicka W, Dobrzecka C. Relationship between feeding and satiation centers of the hypothalamus. Science. 1960;132:805–6. doi: 10.1126/science.132.3430.805. [DOI] [PubMed] [Google Scholar]
- 57.Morgane PJ. Distinct "feeding" and "hunger motivating" systems in the lateral hypothalamus of the rat. Science. 1961;133:887–8. doi: 10.1126/science.133.3456.887. [DOI] [PubMed] [Google Scholar]
- 58.Morgane PJ. Evidence of a 'hunger motivational' system in the lateral hypothalamus of the rat. Nature. 1961;191:672–4. doi: 10.1038/191672a0. [DOI] [PubMed] [Google Scholar]
- 59.Teitelbaum P, Stellar E. Recovery from the failure to eat produced by hypothalamic lesions. Science. 1954;120:894–5. doi: 10.1126/science.120.3126.894. [DOI] [PubMed] [Google Scholar]
- 60.Olds J. Satiation effects in self-stimulation of the brain. J Comp Physiol Psychol. 1958;51:675–8. doi: 10.1037/h0039616. [DOI] [PubMed] [Google Scholar]
- 61.Olds J. Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science. 1958;127:315–24. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]
- 62.Kenny PJ. Brain reward systems and compulsive drug use. Trends Pharmacol Sci. 2007;28:135–41. doi: 10.1016/j.tips.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 63.Herberg LJ, Blundell JE. Lateral hypothalamus: hoarding behavior elicited by electrical stimulation. Science. 1967;155:349–50. doi: 10.1126/science.155.3760.349. [DOI] [PubMed] [Google Scholar]
- 64.Mendelson J. Lateral hypothalamic stimulation in satiated rats: the rewarding effects of self-induced drinking. Science. 1967;157:1077–9. doi: 10.1126/science.157.3792.1077. [DOI] [PubMed] [Google Scholar]
- 65.Wayner MJ. The lateral hypothalamus and adjunctive drinking. Prog Brain Res. 1974;41:371–94. doi: 10.1016/S0079-6123(08)61919-6. [DOI] [PubMed] [Google Scholar]
- 66.Kerman IA, Akil H, Watson SJ. Rostral elements of sympatho-motor circuitry: a virally mediated transsynaptic tracing study. J Neurosci. 2006;26:3423–33. doi: 10.1523/JNEUROSCI.5283-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kerman IA, Bernard R, Rosenthal D, Beals J, Akil H, Watson SJ. Distinct populations of presympathetic-premotor neurons express orexin or melanin-concentrating hormone in the rat lateral hypothalamus. J Comp Neurol. 2007;505:586–601. doi: 10.1002/cne.21511. [DOI] [PubMed] [Google Scholar]
- 68.Cerri M, Morrison SF. Activation of lateral hypothalamic neurons stimulates brown adipose tissue thermogenesis. Neuroscience. 2005;135:627–38. doi: 10.1016/j.neuroscience.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 69.Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110:515–26. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- 70.Pelosi GG, Tavares RF, Correa FM. Rostrocaudal somatotopy in the neural connections between the lateral hypothalamus and the dorsal periaqueductal gray of the rat brain. Cell Mol Neurobiol. 2006;26:635–43. doi: 10.1007/s10571-006-9015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–43. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- 72.Saper CB, Swanson LW, Cowan WM. An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J Comp Neurol. 1979;183:689–706. doi: 10.1002/cne.901830402. [DOI] [PubMed] [Google Scholar]
- 73.Petrovich GD, Risold PY, Swanson LW. Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1996;374:387–420. doi: 10.1002/(SICI)1096-9861(19961021)374:3<387::AID-CNE6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 74.Petrovich GD, Ross CA, Mody P, Holland PC, Gallagher M. Central, but not basolateral, amygdala is critical for control of feeding by aversive learned cues. J Neurosci. 2009;29:15205–12. doi: 10.1523/JNEUROSCI.3656-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–77. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- 76.Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–25. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A, et al. The melanin-concentrating hormone system modulates cocaine reward. Proc Natl Acad Sci U S A. 2009;106:6772–7. doi: 10.1073/pnas.0811331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56 (Suppl 1):112–21. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–82. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 80.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–11. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–7. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watts AG, Sanchez-Watts G. Rapid and preferential activation of Fos protein in hypocretin/orexin neurons following the reversal of dehydration-anorexia. J Comp Neurol. 2007;502:768–82. doi: 10.1002/cne.21316. [DOI] [PubMed] [Google Scholar]
- 83.DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–68. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- 84.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, et al. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–11. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nixon JP, Smale L. Individual differences in wheel-running rhythms are related to temporal and spatial patterns of activation of orexin A and B cells in a diurnal rodent (Arvicanthis niloticus) Neuroscience. 2004;127:25–34. doi: 10.1016/j.neuroscience.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 86.Oldfield BJ, Allen AM, Davern P, Giles ME, Owens NC. Lateral hypothalamic 'command neurons' with axonal projections to regions involved in both feeding and thermogenesis. Eur J Neurosci. 2007;25:2404–12. doi: 10.1111/j.1460-9568.2007.05429.x. [DOI] [PubMed] [Google Scholar]
- 87.Wise RA, Spindler J, deWit H, Gerberg GJ. Neuroleptic-induced "anhedonia" in rats: pimozide blocks reward quality of food. Science. 1978;201:262–4. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- 88.Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–83. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 90.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–13. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 91.Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: 'liking', 'wanting', and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic 'liking' for food: map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- 94.Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances 'liking' of a sweet reward. Neuropsychopharmacology. 2007;32:2267–78. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- 95.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose "liking" and food intake. J Neurosci. 2005;25:8637–49. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste "liking"/"disliking" reactions, place preference/avoidance, and fear. J Neurosci. 2002;22:7308–20. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Evans AH, Pavese N, Lawrence AD, Tai YF, Appel S, Doder M, et al. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol. 2006;59:852–8. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- 98.Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards. J Neurosci. 2003;23:9395–402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berridge KC. 'Liking' and 'wanting' food rewards: brain substrates and roles in eating disorders. Physiology & behavior. 2009;97:537–50. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ostlund SB, Wassum KM, Murphy NP, Balleine BW, Maidment NT. Extracellular Dopamine Levels in Striatal Subregions Track Shifts in Motivation and Response Cost during Instrumental Conditioning. J Neurosci. 2011;31:200–7. doi: 10.1523/JNEUROSCI.4759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Green JA, Baker BI. The influence of repeated stress on the release of melanin-concentrating hormone in the rainbow trout. J Endocrinol. 1991;128:261–6. doi: 10.1677/joe.0.1280261. [DOI] [PubMed] [Google Scholar]
- 102.Green JA, Baker BI, Kawauchi H. The effect of rearing rainbow trout on black or white backgrounds on their secretion of melanin-concentrating hormone and their sensitivity to stress. J Endocrinol. 1991;128:267–74. doi: 10.1677/joe.0.1280267. [DOI] [PubMed] [Google Scholar]
- 103.Rance T, Baker BI. The teleost melanin-concentrating hormone -- a pituitary hormone of hypothalamic origin. Gen Comp Endocrinol. 1979;37:64–73. doi: 10.1016/0016-6480(79)90047-9. [DOI] [PubMed] [Google Scholar]
- 104.Naito N, Kawazoe I, Nakai Y, Kawauchi H. Melanin-concentrating hormone-like immunoreactive material in the rat hypothalamus; characterization and subcellular localization. Cell Tissue Res. 1988;253:291–5. doi: 10.1007/BF00222284. [DOI] [PubMed] [Google Scholar]
- 105.Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, et al. The melanin-concentrating hormone system of the rat brain: an immuno-and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–45. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 106.Nahon JL, Presse F, Bittencourt JC, Sawchenko PE, Vale W. The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology. 1989;125:2056–65. doi: 10.1210/endo-125-4-2056. [DOI] [PubMed] [Google Scholar]
- 107.Chung S, Saito Y, Civelli O. MCH receptors/gene structure-in vivo expression. Peptides. 2009;30:1985–9. doi: 10.1016/j.peptides.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bednarek MA, Tan C, Hreniuk DL, Palyha OC, MacNeil DJ, Van Der Ploeg LH, et al. Synthesis and biological evaluation in vitro of a selective, high potency peptide agonist of human melanin-concentrating hormone action at human melanin-concentrating hormone receptor 1. J Biol Chem. 2002;277:13821–6. doi: 10.1074/jbc.M200563200. [DOI] [PubMed] [Google Scholar]
- 109.Elias CF, Lee CE, Kelly JF, Ahima RS, Kuhar M, Saper CB, et al. Characterization of CART neurons in the rat and human hypothalamus. J Comp Neurol. 2001;432:1–19. doi: 10.1002/cne.1085. [DOI] [PubMed] [Google Scholar]
- 110.Clegg DJ, Air EL, Benoit SC, Sakai RS, Seeley RJ, Woods SC. Intraventricular melanin-concentrating hormone stimulates water intake independent of food intake. Am J Physiol Regul Integr Comp Physiol. 2003;284:R494–9. doi: 10.1152/ajpregu.00399.2002. [DOI] [PubMed] [Google Scholar]
- 111.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–7. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 112.Shearman LP, Camacho RE, Sloan Stribling D, Zhou D, Bednarek MA, Hreniuk DL, et al. Chronic MCH-1 receptor modulation alters appetite, body weight and adiposity in rats. Eur J Pharmacol. 2003;475:37–47. doi: 10.1016/s0014-2999(03)02146-0. [DOI] [PubMed] [Google Scholar]
- 113.Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, et al. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–86. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Glick M, Segal-Lieberman G, Cohen R, Kronfeld-Schor N. Chronic MCH infusion causes a decrease in energy expenditure and body temperature, and an increase in serum IGF-1 levels in mice. Endocrine. 2009;36:479–85. doi: 10.1007/s12020-009-9252-5. [DOI] [PubMed] [Google Scholar]
- 115.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–4. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 116.Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, et al. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–30. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- 117.Pankevich DE, Teegarden SL, Hedin AD, Jensen CL, Bale TL. Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. J Neurosci. 2010;30:16399–407. doi: 10.1523/JNEUROSCI.1955-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duncan EA, Sorrell JE, Adamantidis A, Rider T, Jandacek RJ, Seeley RJ, et al. Alcohol drinking in MCH receptor-1-deficient mice. Alcohol Clin Exp Res. 2007;31:1325–37. doi: 10.1111/j.1530-0277.2007.00427.x. [DOI] [PubMed] [Google Scholar]
- 119.Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–40. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sears RM, Liu RJ, Narayanan NS, Sharf R, Yeckel MF, Laubach M, et al. Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin-concentrating hormone. J Neurosci. 2010;30:8263–73. doi: 10.1523/JNEUROSCI.5858-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92 doi: 10.1016/s0092-8674(00)80949-6. 1 page following 696. [DOI] [PubMed] [Google Scholar]
- 123.Zhu Y, Miwa Y, Yamanaka A, Yada T, Shibahara M, Abe Y, et al. Orexin receptor type-1 couples exclusively to pertussis toxin-insensitive G-proteins, while orexin receptor type-2 couples to both pertussis toxin-sensitive and -insensitive G-proteins. J Pharmacol Sci. 2003;92:259–66. doi: 10.1254/jphs.92.259. [DOI] [PubMed] [Google Scholar]
- 124.Anaclet C, Parmentier R, Ouk K, Guidon G, Buda C, Sastre JP, et al. Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J Neurosci. 2009;29:14423–38. doi: 10.1523/JNEUROSCI.2604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Blouin AM, Thannickal TC, Worley PF, Baraban JM, Reti IM, Siegel JM. Narp immunostaining of human hypocretin (orexin) neurons: loss in narcolepsy. Neurology. 2005;65:1189–92. doi: 10.1212/01.wnl.0000175219.01544.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 127.Huang H, Ghosh P, van den Pol AN. Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J Neurophysiol. 2006;95:1656–68. doi: 10.1152/jn.00927.2005. [DOI] [PubMed] [Google Scholar]
- 128.Kirouac GJ, Parsons MP, Li S. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2005;1059:179–88. doi: 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 129.Shiuchi T, Haque MS, Okamoto S, Inoue T, Kageyama H, Lee S, et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009;10:466–80. doi: 10.1016/j.cmet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 130.Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–37. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- 131.Espana RA, Reis KM, Valentino RJ, Berridge CW. Organization of hypocretin/orexin efferents to locus coeruleus and basal forebrain arousal-related structures. J Comp Neurol. 2005;481:160–78. doi: 10.1002/cne.20369. [DOI] [PubMed] [Google Scholar]
- 132.Vittoz NM, Schmeichel B, Berridge CW. Hypocretin /orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur J Neurosci. 2008;28:1629–40. doi: 10.1111/j.1460-9568.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, et al. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reti IM, Reddy R, Worley PF, Baraban JM. Selective expression of Narp, a secreted neuronal pentraxin, in orexin neurons. J Neurochem. 2002;82:1561–5. doi: 10.1046/j.1471-4159.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- 136.Diniz Behn CG, Klerman EB, Mochizuki T, Lin SC, Scammell TE. Abnormal sleep/wake dynamics in orexin knockout mice. Sleep. 2010;33:297–306. doi: 10.1093/sleep/33.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–66. doi: 10.1146/annurev-pharmtox-010510-100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Espana RA, Plahn S, Berridge CW. Circadian-dependent and circadian-independent behavioral actions of hypocretin/orexin. Brain Res. 2002;943:224–36. doi: 10.1016/s0006-8993(02)02653-7. [DOI] [PubMed] [Google Scholar]
- 139.Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol. 1999;160:R7–12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- 140.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–73. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 142.Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sharf R, Sarhan M, Dileone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry. 2008;64:175–83. doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9:64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matsuo E, Mochizuki A, Nakayama K, Nakamura S, Yamamoto T, Shioda S, et al. Decreased Intake of Sucrose Solutions in Orexin Knockout Mice. J Mol Neurosci. 2010 doi: 10.1007/s12031-010-9475-1. [DOI] [PubMed] [Google Scholar]
- 146.Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, et al. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–7. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- 147.Zheng H, Patterson LM, Berthoud HR. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol. 2005;485:127–42. doi: 10.1002/cne.20515. [DOI] [PubMed] [Google Scholar]
- 148.Espana RA, McCormack SL, Mochizuki T, Scammell TE. Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep. 2007;30:1417–25. doi: 10.1093/sleep/30.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kawano H, Daikoku S, Shibasaki T. CRF-containing neuron systems in the rat hypothalamus: retrograde tracing and immunohistochemical studies. J Comp Neurol. 1988;272:260–8. doi: 10.1002/cne.902720208. [DOI] [PubMed] [Google Scholar]
- 150.Borgland SL, Ungless MA, Bonci A. Convergent actions of orexin/hypocretin and CRF on dopamine neurons: Emerging players in addiction. Brain Res. 2010;1314:139–44. doi: 10.1016/j.brainres.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 151.Diamant M, Kashtanov SI, Fodor M, de Wied D. Corticotropin-releasing factor induces differential behavioral and cardiovascular effects after intracerebroventricular and lateral hypothalamic/perifornical injections in rats. Neuroendocrinology. 1992;56:750–60. doi: 10.1159/000126303. [DOI] [PubMed] [Google Scholar]
- 152.Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, et al. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–48. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci. 2010;30:2300–10. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 2009;65:857–62. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kahn D, Abrams GM, Zimmerman EA, Carraway R, Leeman SE. Neurotensin neurons in the rat hypothalamus: an immunocytochemical study. Endocrinology. 1980;107:47–54. doi: 10.1210/endo-107-1-47. [DOI] [PubMed] [Google Scholar]
- 156.Jennes L, Stumpf WE, Kalivas PW. Neurotensin: topographical distribution in rat brain by immunohistochemistry. J Comp Neurol. 1982;210:211–24. doi: 10.1002/cne.902100302. [DOI] [PubMed] [Google Scholar]
- 157.Caceda R, Kinkead B, Nemeroff CB. Neurotensin: role in psychiatric and neurological diseases. Peptides. 2006;27:2385–404. doi: 10.1016/j.peptides.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 158.Geisler S, Berod A, Zahm DS, Rostene W. Brain neurotensin, psychostimulants, and stress--emphasis on neuroanatomical substrates. Peptides. 2006;27:2364–84. doi: 10.1016/j.peptides.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 159.Watts AG, Sanchez-Watts G, Kelly AB. Distinct patterns of neuropeptide gene expression in the lateral hypothalamic area and arcuate nucleus are associated with dehydration-induced anorexia. J Neurosci. 1999;19:6111–21. doi: 10.1523/JNEUROSCI.19-14-06111.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moga MM, Saper CB, Gray TS. Neuropeptide organization of the hypothalamic projection to the parabrachial nucleus in the rat. J Comp Neurol. 1990;295:662–82. doi: 10.1002/cne.902950409. [DOI] [PubMed] [Google Scholar]
- 161.Behbehani MM, Park MR, Clement ME. Interactions between the lateral hypothalamus and the periaqueductal gray. J Neurosci. 1988;8:2780–7. doi: 10.1523/JNEUROSCI.08-08-02780.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Skofitsch G, Jacobowitz DM. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985;6:509–46. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- 163.Kageyama H, Takenoya F, Kita T, Hori T, Guan JL, Shioda S. Galanin-like peptide in the brain: effects on feeding, energy metabolism and reproduction. Regul Pept. 2005;126:21–6. doi: 10.1016/j.regpep.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 164.Takenoya F, Hirayama M, Kageyama H, Funahashi H, Kita T, Matsumoto H, et al. Neuronal interactions between galanin-like-peptide- and orexin- or melanin-concentrating hormone-containing neurons. Regul Pept. 2005;126:79–83. doi: 10.1016/j.regpep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 165.Diano S, Horvath B, Urbanski HF, Sotonyi P, Horvath TL. Fasting activates the nonhuman primate hypocretin (orexin) system and its postsynaptic targets. Endocrinology. 2003;144:3774–8. doi: 10.1210/en.2003-0274. [DOI] [PubMed] [Google Scholar]
- 166.Xi Y, Ryan J, Noble S, Yu M, Yilbas AE, Ekker M. Impaired dopaminergic neuron development and locomotor function in zebrafish with loss of pink1 function. Eur J Neurosci. 2010;31:623–33. doi: 10.1111/j.1460-9568.2010.07091.x. [DOI] [PubMed] [Google Scholar]
- 167.Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–8. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- 168.Hao J, Cabeza de Vaca S, Pan Y, Carr KD. Effects of central leptin infusion on the reward-potentiating effect of D-amphetamine. Brain Res. 2006;1087:123–33. doi: 10.1016/j.brainres.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 169.Szczypka MS, Rainey MA, Palmiter RD. Dopamine is required for hyperphagia in Lep(ob/ob) mice. Nat Genet. 2000;25:102–4. doi: 10.1038/75484. [DOI] [PubMed] [Google Scholar]
- 170.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–41. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Verhagen LA, Luijendijk MC, Adan RA. Leptin reduces hyperactivity in an animal model for anorexia nervosa via the ventral tegmental area. Eur Neuropsychopharmacol. 2010 doi: 10.1016/j.euroneuro.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 172.Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci. 2004;118:479–87. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- 173.Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol. 2008;13:358–63. doi: 10.1111/j.1369-1600.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- 174.Clifford S, Zeckler RA, Buckman S, Thompson J, Hart N, Wellman PJ, et al. Impact of food restriction and cocaine on locomotion in ghrelin- and ghrelin-receptor knockout mice. Addict Biol. 2010 doi: 10.1111/j.1369-1600.2010.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fulton S, Richard D, Woodside B, Shizgal P. Food restriction and leptin impact brain reward circuitry in lean and obese Zucker rats. Behav Brain Res. 2004;155:319–29. doi: 10.1016/j.bbr.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 177.Figlewicz DP, Higgins MS, Ng-Evans SB, Havel PJ. Leptin reverses sucrose-conditioned place preference in food-restricted rats. Physiology & behavior. 2001;73:229–34. doi: 10.1016/s0031-9384(01)00486-3. [DOI] [PubMed] [Google Scholar]
- 178.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–86. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 179.Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sahu A. Leptin decreases food intake induced by melanin-concentrating hormone (MCH), galanin (GAL) and neuropeptide Y (NPY) in the rat. Endocrinology. 1998;139:4739–42. doi: 10.1210/endo.139.11.6432. [DOI] [PubMed] [Google Scholar]
- 181.Tritos NA, Elmquist JK, Mastaitis JW, Flier JS, Maratos-Flier E. Characterization of expression of hypothalamic appetite-regulating peptides in obese hyperleptinemic brown adipose tissue-deficient (uncoupling protein-promoter-driven diphtheria toxin A) mice. Endocrinology. 1998;139:4634–41. doi: 10.1210/endo.139.11.6308. [DOI] [PubMed] [Google Scholar]
- 182.Huang Q, Viale A, Picard F, Nahon J, Richard D. Effects of leptin on melanin-concentrating hormone expression in the brain of lean and obese Lep(ob)/Lep(ob) mice. Neuroendocrinology. 1999;69:145–53. doi: 10.1159/000054413. [DOI] [PubMed] [Google Scholar]
- 183.Kokkotou EG, Tritos NA, Mastaitis JW, Slieker L, Maratos-Flier E. Melanin-concentrating hormone receptor is a target of leptin action in the mouse brain. Endocrinology. 2001;142:680–6. doi: 10.1210/endo.142.2.7981. [DOI] [PubMed] [Google Scholar]
- 184.Segal-Lieberman G, Bradley RL, Kokkotou E, Carlson M, Trombly DJ, Wang X, et al. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci U S A. 2003;100:10085–90. doi: 10.1073/pnas.1633636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rao Y, Lu M, Ge F, Marsh DJ, Qian S, Wang AH, et al. Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. J Neurosci. 2008;28:9101–10. doi: 10.1523/JNEUROSCI.1766-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gao XB, van den Pol AN. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J Physiol. 2001;533:237–52. doi: 10.1111/j.1469-7793.2001.0237b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, Arch JR, et al. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes. 1999;48:2132–7. doi: 10.2337/diabetes.48.11.2132. [DOI] [PubMed] [Google Scholar]
- 188.Lopez M, Seoane L, Garcia MC, Lago F, Casanueva FF, Senaris R, et al. Leptin regulation of prepro-orexin and orexin receptor mRNA levels in the hypothalamus. Biochem Biophys Res Commun. 2000;269:41–5. doi: 10.1006/bbrc.2000.2245. [DOI] [PubMed] [Google Scholar]
- 189.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–13. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 190.Horvath TL, Gao XB. Input organization and plasticity of hypocretin neurons: possible clues to obesity's association with insomnia. Cell Metab. 2005;1:279–86. doi: 10.1016/j.cmet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 191.Yamamoto Y, Ueta Y, Serino R, Nomura M, Shibuya I, Yamashita H. Effects of food restriction on the hypothalamic prepro-orexin gene expression in genetically obese mice. Brain Res Bull. 2000;51:515–21. doi: 10.1016/s0361-9230(99)00271-3. [DOI] [PubMed] [Google Scholar]
- 192.Cai XJ, Lister CA, Buckingham RE, Pickavance L, Wilding J, Arch JR, et al. Down-regulation of orexin gene expression by severe obesity in the rats: studies in Zucker fatty and zucker diabetic fatty rats and effects of rosiglitazone. Brain Res Mol Brain Res. 2000;77:131–7. doi: 10.1016/s0169-328x(00)00041-3. [DOI] [PubMed] [Google Scholar]
- 193.Louis GW, Leinninger GM, Rhodes CJ, Myers MG., Jr Direct innervation and modulation of orexin neurons by lateral hypothalamic LepRb neurons. J Neurosci. 2010;30:11278–87. doi: 10.1523/JNEUROSCI.1340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bronsky J, Nedvidkova J, Zamrazilova H, Pechova M, Chada M, Kotaska K, et al. Dynamic changes of orexin A and leptin in obese children during body weight reduction. Physiol Res. 2007;56:89–96. doi: 10.33549/physiolres.930860. [DOI] [PubMed] [Google Scholar]


