Abstract
The association between smoking and HIV disease progression has been examined in several studies; however, findings have been inconsistent. We examined the effect of recent cigarette smoking on CD4+ T cell count/µL (CD4 count) and HIV RNA concentration (HIV viral load [VL]) among two HIV-infected cohorts with alcohol problems in Massachusetts in the periods 1997–2001 and 2001–2006 using a prospective cohort design and linear mixed models. Smoking groups were defined as: minimal or non-smokers, light smokers, moderate smokers and heavy smokers. Age, alcohol use, injection drug use, depressive symptoms, gender, annual income, and antiretroviral therapy (ART) adherence were considered as potential confounders. Among 462 subjects, no significant differences in CD4 count or viral load were found between smoking groups. Using minimal or non-smokers as the reference group, the adjusted mean differences in CD4 count were: 8.2 (95% confidence interval (CI): −17.4, 33.8) for heavy smokers; −0.1 (95% CI: −25.4, 5.1) for moderate smokers; and −2.6 (95% CI: −28.3, 3.0) for light smokers. For log10 VL, the adjusted differences were: 0.03 (95% CI: −0.12, 0.17) for heavy smokers; −0.06 (95% CI: −0.20, 0.08) for moderate smokers; and 0.14 (95% CI −0.01, 0.28) for light smokers. This study did not find an association between smoking cigarettes and HIV disease progression as measured by CD4 cell count and VL.
Keywords: Cigarette Smoking, CD4+ T cells, Viral Load, HIV
Introduction
With the major prognostic advance of highly active antiretroviral therapy (HAART) (Palella et al., 1998; Detels et al., 1998; Crum et al., 2006), impetus to understand other potential avenues to prevent disease progression among Human Immunodeficiency Virus (HIV)-infected persons has been sought (Jia et al., 2007; Baum et al., 1995; Fawzi et al., 2004; Cheng et al., 2007; Cook et al., 2008). Since smoking is common among HIV-infected persons (Webb, Vanable, Carey, & Blair, 2007) (Niaura et al., 2005) its effect on HIV disease progression has merited study. Smoking can suppress the maturation of dendritic cells in the lymph nodes thereby weakening the function of CD4+ T cells (Robbins, Franco, Mouded, Cernadas, & Shapiro, 2008; Robbins et al., 2004). It can also affect the efficiency of peripheral blood mononuclear cells to secrete cytokines (Ouyang et al., 2000). Smoking has also been reported to up regulate the expression of Fas (cell surface molecules mediating apoptotic cell death) on peripheral blood lymphocytes, rendering them susceptible to apoptosis (Bijl et al., 2001). Other reported possible mechanisms explaining the adverse effects of smoking on immunological function have been described (Sopori & Kozak,1998; Kalra, Singh, Savage, Finch, & Sopori, 2000), (Petersen, Steimel, & Callaghan, 1983; Silverman, Potvin, Alexander Jr, & Chretien, 1975), (Tollerud et al.,1989; Sopori, Gairola, DeLucia, Bryant, & Cherian, 1985), (Carrillo, Castro, Cuevas, Diaz, & Cabrera, 1991), (Kuniak et al., 1995), (Abbud, Finegan, Guay, & Rich, 1995).
Several epidemiologic studies have investigated the relation between cigarette smoking and the course of HIV infection yielding mixed results. Two cohort studies (Crothers et al., 2005; Conley et al., 1996) and two cross-sectional studies (Slavinsky III et al., 2002; Palacio, Hilton, Canchola, & Greenspan, 1997) found an association between smoking and development of opportunistic infections (OIs). In contrast, eight cohort studies (Webber, Schoenbaum, Gourevitch, Buono, & Klein, 1999; Stephenson et al., 1999; Coates et al., 1990; Burns et al., 1996; Eskild & Petersen, 1994; Galai et al., 1997; Craib et al., 1992; Nieman, Fleming, Coker, William, & Mitchell, 1993) and two cross sectional studies (Webb, Vanable, Carey, & Blair, 2007; Gritz, Vidrine, Lavez, Amick, & Arduino, 2004) reported a null association. One study (Royce & Winkelstein, 1990) linked smoking with an increase in CD4+ T cells among males although the increase was less pronounced in HIV-infected individuals.
Inconsistent results regarding the effect of smoking on HIV disease progression may in part be attributed to the differing characteristics of the study populations. Adjustment for potential confounders (e.g. alcohol use) did not consistently occur. As most previous epidemiologic studies used the incidence of OIs as a primary endpoint, we considered the other useful biological markers that might complement the observations of clinical outcomes in the examination of whether smoking accelerates HIV disease progression.
We therefore analyzed data from a prospectively assessed two cohorts of HIV-infected patients with alcohol problems to examine the association of cigarette smoking with CD4+ T cell count (CD4 count) and HIV viral load (VL), We hypothesized that smoking would be associated with a lower CD4 count and a higher VL.
Design and Methods
Study population
Study participants were from two longitudinal cohorts of HIV-infected persons with alcohol problems: HIV Alcohol Longitudinal Cohort (HIV-ALC) study only (1997–2001) (n=78); HIV Longitudinal Interrelationships of Viruses and Ethanol (HIV-LIVE) study only (2001–2006) (n=230); and both HIV-ALC and HIV-LIVE studies (n=154). Eligibility criteria and recruitment methods for HIV-ALC (Samet, Horton, Traphagen, Lyon, & Freedberg, 2003) and HIV-LIVE (Samet et al., 2007) were the same with follow-up visits planned every six months. Inclusion criteria included a documented HIV antibody test, a history of alcohol problems as measured by the CAGE questionnaire or a clinical investigator’s assessment (Samet, Phillips, & Horton, 2004), age 18 years or older, ability to speak English or Spanish, and at least one contact person to assist with the follow-up. Exclusion criteria included score<21 on the 30-item Mini-Mental State Examination (M.F. Folstein, S.E. Folstein, & McHugh, 1975; Smith, Horton, Saitz, & Samet, 2006), inability to provide informed consent or answer the interview questions, and plans to move from the Boston area in the subsequent 12 months. For the current analysis, we also excluded those who did not have at least one follow-up visit. Laboratory measurements were obtained at each interview. Additional details on this population have been provided elsewhere (Samet et al., 2007; Samet et al., 1995). The study was approved by the Boston Medical Center and Beth Israel Deaconess Medical Center Institutional Review Boards.
Outcome assessment
The primary outcomes for this analysis were CD4+ T cell count/µl (CD4 count) and log10 plasma HIV RNA/ ml (VL). CD4 count was determined by flow cytometry at the hospital laboratories. VL was measured using a branched-chain assay (lowest detection threshold= 75 copies/ ml) or a polymerase chain reaction (lowest detection threshold= 50 copies/ ml for ultrasensitive assay, and 500 copies/ ml for standard assay) (Pachl et al., 1995).
Exposure assessment
Information on smoking was collected at each study visit. Smoking status was categorized per Okuyemi et al. (2002) and Okuyemi et al. (2004) as follows: Minimal or non-smoker (smoked less than one cigarette per day); light smoker (smoked one to less than 10 cigarettes per day); moderate smoker (smoked 10 to less than 20 cigarettes per day); or heavy smoker (smoked 20 or more cigarettes per day). We used non-parametric local linear polynomial curves (loess) (Cleveland, Grosse, & Shyu, 1992) to verify that the cutoffs were reasonable for our data. We also performed a secondary, confirmatory analysis including smoking categorized based on quintiles of the distribution.
We defined a smoker as someone who answered affirmatively to the question “Do you currently smoke cigarettes?” As a secondary analysis, we defined smoking based on the subject’s reported smoking status at two successive visits. That is, the outcome at each time point was modeled as a function of smoking from the current and previous study visit, thus accounting for whether the subjects changed or maintained their recent smoking behavior. In the case of missed visits, smoking status from the last available visit was used. We categorized smoking as follows: consistent smokers (smoked in two consecutive visits); consistent minimal or non-smokers (smoked <1 cigarette per day in two consecutive visits); recent quitters (smoked in the last visit but stopped smoking in the current visit); and new/relapsed smokers (not smoked in the last visit but started or resumed smoking in the current visit). The categorization was based on data from previous studies which suggest that the effect of smoking on the immune system is acute (Tollerud et al.,1989,Hersey, Prendergast, & Edwards, 1983; Sunyer et al., 1996), with induction period of about five weeks (Thomas, Holt, & Keast, 1975) to 10 weeks (Chalmer, Holt, & Keast, 1975), and lasts for about six to 35 weeks since quitting (Thomas, Holt, & Keast, 1975; Miller, Goldstein, Murphy, & Ginns, 1982; Radloff, 1977). Since the time elapsed between the last HIV-ALC visit to the first HIV-LIVE visit was too long (range one month to 66 months) for some of the subjects enrolled in both cohorts, this secondary analysis was restricted to subjects who participated in HIV-LIVE.
Statistical analysis
We performed descriptive analyses to characterize the study population, overall and by baseline smoking status.
We applied linear mixed effects models to account for correlated measures within subjects. The models included subject-specific random intercepts and slopes, and adjusted for the value of the outcome (i.e. CD4 count and VL) at the previous study visit. We fitted a separate model for each outcome. Graphs illustrating trajectories of HIV disease progression over time were also plotted using outcome estimates from linear mixed effects models.
Age (modeled as a continuous variable), alcohol use (≤ 2 drinks per day, >2-≤4 drinks per day, and > 4 drinks per day), current injection drug use (user vs. non-user), depressive symptoms (CESD score >23 vs. ≤23), gender, annual income (>median ($7,500) vs ≤ median), and antiretroviral therapy (ART) adherence (not on medication, on medication and adherent, and on medication but not adherent) were considered as potential confounders during analysis. The categories for alcohol use were made narrower than those suggested by Cook et al. (2009) so as to account for residual confounding. The cutoff point for depressive symptoms was determined with Radloff et al. (1977) criteria. ART adherence was measured using the AIDS Clinical Trials Group criteria (Chesney et al., 2000) and defined as a self-report of 100% adherent in the past three days (Samet, Horton, Meli, Freedberg, & Palepu, 2004). Preliminary models were fitted separately for each potential confounder. Confounders were then added sequentially in the models according to the magnitude of their effect on the association between cigarette smoking and outcome. Any potential confounder that changed the point estimate of cigarette smoking by more than 10% was included in the final model. Smoking status and all covariates with the exception of gender and age were analyzed as time-varying variables and updated at each time point. An interaction between smoking and time was included in the models and evaluated for its statistical significance. To assess whether ART modifies the effect of smoking, subgroup analyses were repeated separately for subjects not on ART and for subjects on ART and adherent to medication. Subjects who changed their ART status contributed observations to each stratum depending on the ART status of the observation.
For the primary analysis, undetectable VL was imputed as half the value of the lowest threshold of assay sensitivity. Secondary analyses evaluated the potential bias due to imputing assay values using half the limit of detection (Greenland & Lash, 2008) as follows: The undetectable assay measurements were imputed with plausible values from a logit-logistic distribution (Lesaffre, Rizopoulos, & Tsonaka, 2007) with a scale parameter of 0.8, accounting for the fact that VL values are bounded by 0. The distribution also took into account that 0 VL is unlikely as no current treatment can completely eliminate HIV. This process was repeated in 10,000 simulation runs and 95% simulation intervals (SI) were obtained. Statistical analysis was done using SAS version 9.1 (SAS Institute, Inc., Cary, North Carolina).
Results
The cohort (n=462) is described in Table 1 with the following demographic characteristics: black (43%); male (77%); median age 42 years (range 21–71 years); and median annual income of $7,500 or less. In the combined cohort, 77% (358/462) of subjects reported having smoked cigarettes within the last month before enrollment; 23% were minimal or non-smokers, 25% were light, 22% were moderate and 31% were heavy smokers. The median baseline CD4 count and viral load were 380 cells/ µl and 1175 copies/ ml respectively. The median follow-up time was 18 months for those enrolled in only HIV-LIVE, 14 months for those in only HIV-ALC, and 40 months for those in both studies. In the overall cohort, the median number of visits was seven (range: 2–14 visits). Subjects who completed the majority of study of visits had higher mean baseline CD4 count (difference=117.3; p<0.0001) and lower mean baseline log10 VL (difference=0.35; p=0.03) than those who did not complete the majority of study visits. There was no significant association between baseline smoking status and whether the subject completed the majority of study visits in this cohort (chi-square=0.41; p=0.52). Subjects contributed 3141 observations across all follow-up visits: 827 (26%) classified as minimal or non-smokers, 696 (22%) light smokers, 772 (25%) moderate smokers and 846 (27%) as heavy smokers.
Table 1.
Sociodemographic and clinical characteristics of HIV-infected persons with a history of alcohol problems in two prospective cohorts stratified by baseline smoking status (n=462)
| n (%) |
||||||
|---|---|---|---|---|---|---|
| Covariates |
Total n (%) n=462 |
Minimal or non-smokers n=104 |
Light smokers n=115 |
Mod. smokers n=100 |
Heavy smokers n=143 |
Mean no. cigarretes1 smoked per day (SD) n=358 |
| Sociodemographic variables | ||||||
| Age | ||||||
| ≤30 | 22 (5) | 5 (5) | 6 (5) | 3 (3) | 8 (6) | 14.18 (8.79) |
| 31–40 | 174 (38) | 34 (33) | 47 (41) | 43 (43) | 50 (35) | 14.11 (10.14) |
| >40 | 266 (58) | 65 (63) | 62 (54) | 54 (54) | 85 (59) | 13.79 (9.00) |
| Gender | ||||||
| Females | 108 (23) | 81 (78) | 86 (75) | 73 (73) | 114 (80) | 14.30 (9.59) |
| Males | 354 (77) | 23 (22) | 29 (25) | 27 (27) | 29 (20) | 12.72 (8.87) |
| Race | ||||||
| Black | 198 (43) | 43 (41) | 64 (56) | 57 (57) | 34 (24) | 10.92 (7.08) |
| White | 154 (33) | 38 (37) | 15 (13) | 21 (21) | 80 (56) | 19.28 (10.85) |
| Hispanic | 87 (19) | 18 (17) | 29 (25) | 17 (17) | 23 (16) | 12.18 (8.43) |
| Other | 23 (5) | 5 (5) | 7 (6) | 5 (5) | 6 (4) | 14.36 (7.15) |
| Income | ||||||
| >Median ($7,500) | 215 (47) | 55 (53) | 43 (37) | 48 (48) | 69 (49) | 15.60 (10.02) |
| <=Median ($7,500) | 245 (53) | 49 (47) | 72 (63) | 51 (52) | 73 (51) | 12.63 (8.79) |
| Average no. drinks/day | ||||||
| 0–2 | 381 (83) | 91 (88) | 93 (81) | 80 (81) | 117 (82) | 13.87 (9.42) |
| >2–4 | 30 (7) | 8 (8) | 11 (10) | 6 (6) | 5 (4) | 12.86 (8.16) |
| >4 | 50 (11) | 5 (5) | 11 (10) | 13 (13) | 21 (15) | 14.96 (10.13) |
| Injecting drug use | ||||||
| User | 79 (17) | 7 (7) | 14 (12) | 25 (25) | 33 (23) | 15.96 (9.43) |
| Non user | 383 (83) | 97 (93) | 101 (88) | 75 (75) | 110 (77) | 13.60 (9.41) |
| ART status | ||||||
| Not on meds | 173 (38) | 35 (34) | 34 (30) | 41 (41) | 63 (44) | 13.98 (9.04) |
| On meds, not adherent | 84 (18) | 14 (13) | 31 (27) | 13 (13) | 26 (18) | 15.35 (11.61) |
| On meds, adherent | 204 (44) | 55 (53) | 50 (43) | 45 (45) | 54 (38) | 13.42 (8.90) |
| Depressive symptoms | ||||||
| Depressed | 217 (47) | 35 (34) | 55 (47) | 48 (48) | 79 (55) | 15.08 (9.95) |
| Not Depressed | 245 (53) | 69 (66) | 60 (52) | 52 (52) | 64 (45) | 12.92 (8.86) |
| Cohort | ||||||
| ALC-only | 78 (17) | 17 (16) | 15 (13) | 18 (18) | 28 (20) | 16.82 (13.30) |
| LIVE-only | 230 (50) | 58 (56) | 52 (45) | 52 (52) | 68 (48) | 13.91 (8.16) |
| Combined | 154 (33) | 29 (28) | 48 (42) | 30 (30) | 47 (33) | 13.46 (9.64) |
| Median baseline CD4 count | 390 | 383 | 410 | 324 | 422 | |
| Median baseline viral load | 1188 | 388 | 1723 | 1382 | 1251 | |
Minimal or non-smokers are excluded
The plots in Figure 1 show the unadjusted mean CD4 count or VL over time across smoking categories. The plots suggest potential variation in mean differences in CD4 count or VL over time between smoking categories. However, the smoking-time interaction term was not statistically significant in any of the regression models and was therefore excluded from subsequent analyses.
Figure 1.
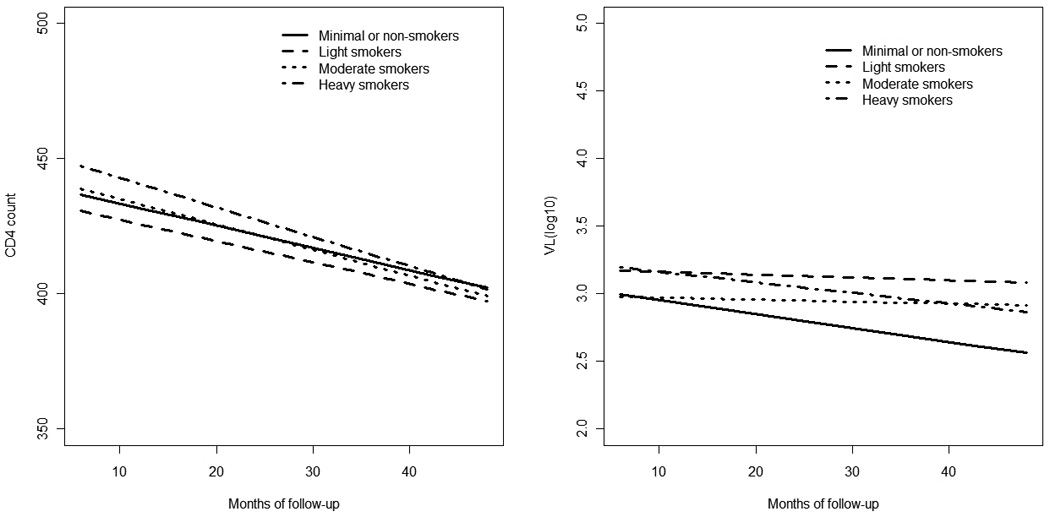
Unadjusted mean CD4 count and VL over time
We did not find any substantial differences in CD4 count or VL across categories of smoking (Table 2). Using minimal or non-smokers as the reference group, the adjusted mean differences in CD4 count were: 8.2 (95% confidence interval (CI): −17.4, 33.8; p=0.44) for heavy smokers; −0.1 (95% CI: −25.4, 5.1; p=0.48) for moderate smokers; and −2.6 (95% CI: −28.3, 3.0; p=0.90) for light smokers. For log10 VL, the adjusted differences were: 0.03 (95% CI: −0.12, 0.17; p=0.39) for heavy smokers; −0.06 (95% CI: −0.20, 0.08; p=0.83) for moderate smokers; and 0.14 (95% CI: −0.01, 0.28; p=0.06) for light smokers.
Table 2.
The association between smoking status and markers of HIV disease progression
| Smoking status | Mean differences in CD4 count1 | Mean differences in HVL2 | ||
|---|---|---|---|---|
| All subjects combined (n=462)3 | ||||
| Crude [95% CI] | Adjusted [95% CI] | Crude [95% CI] | Adjusted [95% CI] | |
| Heavy smokers | −3.2 [−27.7, 21.2] | 8.2 [−17.4, 33.8] | 0.18 [0.03, 0.32] | 0.03 [−0.12, 0.17] |
| Moderate smokers | −11.7 [−36.0, 12.6] | −0.1 [−25.4, 25.1] | 0.11 [−0.04, 0.25] | −0.06 [−0.20, 0.08] |
| Light smokers | −13.0 [−37.8, 11.9] | −2.6 [−28.3, 23.0] | 0.27 [0.12, 0.42] | 0.14 [−0.01, 0.28] |
| Minimal or non smokers |
Reference | Reference | Reference | Reference |
| Not on ART4,5 | ||||
| Heavy smokers | 24.0 [−22.4, 70.3] | 27.4 [−19.7, 74.4] | −0.11 [−0.36, 0.14] | −0.09 [−0.35, 0.17] |
| Moderate smokers | 27.7 [−18.0, 73.4] | 30.1 [−16.0, 76.1] | −0.22 [−0.47, 0.03] | −0.20 [−0.56, 0.06] |
| Light smokers | 13.8 [−30.7, 58.4] | 16.0 [−28.9, 60.9] | −0.15 [−0.39, 0.09] | −0.14 [−0.38, 0.11] |
| Minimal or non smokers |
Reference | Reference | Reference | Reference |
| On ART and adhered to medication4,6 | ||||
| Heavy smokers | −8.4 [−34.3, 17.5] | −6.4 [−32.4, 19.6] | 0.05 [−0.14, 0.24] | 0.03 [−0.16, 0.21] |
| Moderate smokers | −14.5 [−40.4, 11.3] | −14.2 [−40.0, 11.6] | 0.05 [−0.14, 0.23] | 0.01 [−0.18, 0.19] |
| Light smokers | −11.2 [−38.2, 15.8] | −11.4 [−38.3, 15.6] | 0.25 [0.06, 0.45]* | 0.24 [0.04, 0.44]* |
| Minimal or non smokers |
Reference | Reference | Reference | Reference |
Adjusted analyses controlled for previous CD4+ cell count, ART status, time, and depressive symptoms
Adjusted analyses controlled for previous log10 HIV RNA, ART status, income, depressive symptoms, injection drug use, age, alcohol, time, and gender
No. observations was 3141
ART was removed in the model because we stratified on it
No. subjects was 291. No. observations was 1026. Included only observations when subject was on ART.
No. subjects was 363. No. observations was 1586. Included only observations when subject was on ART and adhered to medication.
Results are statistically significant
When analysis was stratified by ART status, no clear evidence of an association between smoking with CD4 count or HIV VL was found (Table 2). We observed a small but statistically significant increase in VL for light smokers compared with minimal or non-smokers among subjects who adhered to ART (the adjusted mean difference was 0.24; 95% CI: 0.04, 0.44; p=0.01). However, similar associations were not observed for categories of heavier smoking.
Similarly, no substantial outcome differences were observed for subjects who switched or maintained their smoking behavior (Table 3).
Table 3.
The association between smoking status at two successive visits and markers of HIV disease progression (n=383)1
| Mean difference in CD4 count [95% CI] | Mean difference in HVL[95% CI] | |||
|---|---|---|---|---|
| Crude | Adjusted2 | Crude | Adjusted3 | |
| Smoking Status | ||||
| New/relapsed smokers | 16.87 [−6.60,60.35] | 22.73 [−0.28,65.74] | −0.11 [−.41,0.19] | −0.11 [−.39,0.17] |
| Consistent smokers | −12.14 [−30.00,5.69] | −2.25 [−20.18,15.68] | 0.18 [0.05,0.30] | 0.11 [−0.05,0.26] |
| Recent quitters | −32.39 [−71.00,6.23] | −33.20 [−71.39,4.98] | −0.07 [−.34,0.20] | −0.04 [−0.22,0.29] |
| Consistent minimal or | Reference | Reference | Reference | Reference |
| non-smokers | ||||
Analysis restricted to HIV LIVE cohort. No. observations was 1974. All p values>0.05
Adjusted for CD4 count at previous visit, ART status, age, time, and alcohol use
Adjusted for HVL at previous visit, ART status, time and depressive symptoms
Results (not shown) remained similar in analyses examining the potential bias due to the imputed assay measurements, suggesting that the unobserved values in the lower range did not have a large impact on results. Similarly, the analysis with smoking categorized based on quintiles did not alter the study findings.
Discussion
This study does not provide evidence that cigarette smoking is associated with a decrease in CD4 count or an increase in VL among HIV- infected patients. Specifically, we observed no substantial differences in CD4 count or VL between minimal or non-smokers, light smokers, moderate smokers and heavy smokers. Moreover, we found no substantial differences in CD4 count or VL when smoking status changed in two consecutive visits.
Our findings are in accordance with previous cohort studies which did not detect an association between cigarette smoking and HIV disease progression (Webber, Schoenbaum, Gourevitch, Buono, & Klein, 1999; Stephenson et al., 1999; Coates et al., 1990; Burns et al., 1996; Eskild & Petersen, 1994; Galai et al., 1997; Craib et al., 1992) when using the onset of an AIDS defining condition as a primary outcome. Our design, using CD4 count and VL as markers for HIV disease progression, is well suited for HIV cohorts with less advanced disease, fewer anticipated OIs.
Results from this study are in contrast with findings from two cohort studies which reported that smoking enhances HIV disease progression using OIs as outcomes (Crothers et al., 2005; Conley, Bush, Buchbinder, & Penley, 1996). In one of these studies CD4 counts were also examined (Conley, Bush, Buchbinder, & Penley, 1996), but not associated with smoking. As smoking may selectively affect target organs (e.g. lungs), the latter study underscores the need to investigate biological markers of immunological dysfunction in addition to OIs in order to assess its impact on the immune system.
Although we found no evidence of a relation between smoking and HIV disease progression, we could not rule out the potential effect of smoking on CD4 cells or HIV which are not in peripheral blood. As high concentrations of smoke can get trapped in the lungs, it would not be surprising if most of the affected cells are those surrounding the lungs. For example, one study (Wewers et al., 1998) found that, compared to minimal or non-smokers, HIV-infected smokers had a significant depletion in CD4 cells in their bronchoalveolar lavage fluid. Analyzing samples of immunological markers derived from sites other than the peripheral blood may shed some light on the issue.
Our study was conducted using a cohort of patients with current or past alcohol problems. As alcohol drinkers tend to smoke more than non-alcohol drinkers (Collins & Marks, 1995), the choice of this cohort ensured availability of ample smokers for analysis. The selection of this cohort came with a caution in that the generalizability of its findings may be limited to a population with alcohol problems. However, given the prevalence of past alcohol problems among HIV-infected individuals, up to 40% (Samet, Phillips, & Horton, 2004), these findings would still be of importance even if not applicable to a non-alcohol affected HIV population. An interaction term between smoking and current alcohol drinking (categorized by dose) was not statistically significant in regression models.
The main strength of this study was the inclusion of assessments of changes in immunological biological markers over time using repeated measures on the same individuals. We identified the use of such methodology in only one other study (Sunyer et al., 1996) among HIV seronegative subjects in which a positive association existed between smoking and an increase in white blood cells. We performed post-hoc power calculations to assess the differences in CD4 count our study could detect with reasonably high power. While we utilized longitudinal regression methods in the analyses, for the purposes of power calculations we considered a simpler setting utilizing a single time point. Thus our estimates are conservative as the longitudinal analyses are expected to increase the study power. Assuming a standard deviation of 42 (based on our observed data at baseline), the minimum detectable difference in mean CD4 count between any two smoking groups that our sample size could detect with 80% power was 16 cells/µl. hence this study was adequately powered to detect effect sizes observed previously (Sunyer et al. 1996), a difference in CD4 count of 74 cells/µl between heavy smokers and never smokers. Other strengths included the ability to analyze short term effects of smoking initiation and cessation, information on smoking dosage and the availability of many important potential confounders.
We note Our study had limitations. First, information on cigarette smoking was self reported. Second, the number of cigarettes smoked may not necessarily reflect the amount of smoke inhaled, underestimating the actual smoking dosage.
In summary we did not find significant associations between cigarette smoking and CD4 count or VL among HIV-infected patients with alcohol problems. Future epidemiologic studies concerning the impact of smoking on HIV disease progression may provide more insight if focused on this substance’s effect on specific tissues and particular immunological systems in addition to immunological markers (i.e., CD4 count and VL) in the peripheral blood.
Acknowledgements
This study was supported by funding from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) RO1-AA11785, RO1-AA13216, K24-AA015674 and Fogarty International Center D43-TW006808. The authors thank Emily Quinn for her data management support. We also appreciate the collaboration of Dr. Howard Libman at Beth Israel Deaconess Medical Center who led the recruitment of participants at that institution.
References
- Abbud RA, Finegan CK, Guay LA, Rich EA. Enhanced production of human immunodeficiency virus type 1 by in vitro-infected alveolar macrophages from otherwise healthy cigarette smokers. The Journal of Infectious Diseases. 1995;172:859–863. doi: 10.1093/infdis/172.3.859. [DOI] [PubMed] [Google Scholar]
- Baum MK, Shor-Posner G, Lu Y, Rosner B, Sauberlich HE, Fletcher MN, Hennekens CH. Micronutrients and HIV-1 disease progression. AIDS. 1995;9:1051–1056. doi: 10.1097/00002030-199509000-00010. [DOI] [PubMed] [Google Scholar]
- Bijl M, Horst G, Limburg PC, Kallenberg CG. Effects of smoking on activation markers, Fas expression and apoptosis of peripheral blood lymphocytes. European Journal of Clinical Investigation. 2001;31:550–553. doi: 10.1046/j.1365-2362.2001.00842.x. [DOI] [PubMed] [Google Scholar]
- Burns DN, Hillman D, Neaton JD, Sherer R, Mitchell T, Capps L, Michael D. Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. Journal of Acquired Immune Deficiency Syndrome. 1996;13:374–383. doi: 10.1097/00042560-199612010-00012. [DOI] [PubMed] [Google Scholar]
- Carrillo T, Castro FR, Cuevas M, Diaz F, Cabrera P. Effect of cigarette smoking on the humoral immune response in pigeon fanciers. Allergy. 1991;46:241–244. doi: 10.1111/j.1398-9995.1991.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Chalmer J, Holt P, Keast D. Cell-mediated responses to transplanted tumors in mice chronically exposed to cigarette smoke. Journal of the National Cancer Institute. 1975;55:1129–1134. doi: 10.1093/jnci/55.5.1129. [DOI] [PubMed] [Google Scholar]
- Cheng DM, Nunes D, Libman H, Vidaver J, Alperen JK, Saitz R, Samet J. Impact of hepatitis C on HIV Progression in adults with alcohol problems. Alcoholism: Clinical and Experimental Research. 2007;31:829–836. doi: 10.1111/j.1530-0277.2007.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Patient Care Committee and Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG Adherence Instrument. AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Cleveland WS, Grosse E, Shyu WM. Local regression models. In: Chambers JM, Hastie TJ, editors. Statistical models in S. Chapman & Hall/CRC; 1992. pp. 309–373. [Google Scholar]
- Coates RA, Farewell VT, Raboud J, Read SE, Macfadden DK, Calzavara LM, Fanning MM. Cofactors of progression to acquired immunodeficiency syndrome in a cohort of male sexual contacts of men with human immunodeficiency virus disease. American Journal of Epidemiology. 1990;132:717–722. doi: 10.1093/oxfordjournals.aje.a115713. [DOI] [PubMed] [Google Scholar]
- Collins AC, Marks MJ. Animal models of alcohol-nicotine interactions. In: Fertig JB, Allen JP, editors. Alcohol and Tobacco: From Basic Science to Clinical Practice. NIAAA Research Monograph. No. 30. NIH Pub; 1995. pp. 129–143. 95–3931. [Google Scholar]
- Conley LJ, Bush TJ, Buchbinder SP, Penley KA. The association between cigarette smoking and selected HIV-related medical conditions. AIDS. 1996;10:1121–1126. [PubMed] [Google Scholar]
- Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, Grey DD. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22:355–1363. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RL, Zhu F, Belnap BH, Weber K, Cook JA, Vlahov D, Cohen MH. Longitudinal trends in hazardous alcohol consumption among women with human immunodeficiency virus infection, 1995–2006. American Journal of Epidemiology. 2009;169:1025–1032. doi: 10.1093/aje/kwp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craib KJ, Schechter MT, Montaner JSG, Le TN, Sestak P, Willoughby B, O'Shaughnessy MV. The effect of cigarette smoking on lymphocyte subsets and progression to AIDS in a cohort of homosexual men. Clinical and Investigative Medicine. 1992;15:301–308. [PubMed] [Google Scholar]
- Crothers K, Griffith TA, McGinnis KA, Rodriguez-Barradas MC, Leaf DA, Weissman S, Justice AC. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. Journal of General Internal Medicine. 2005;20:1142–1145. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, Mark R. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (Highly Active Antiretroviral Therapy) eras. Journal of Acquired Immune Deficiency Syndromes. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- Detels R, Munoz A, McFarlane G, Kingsley LA, Margolick JB, Giorgi J, Phair JP. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study. The Journal of the American Medical Association. 1998;280:1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- Eskild A, Petersen G. Cigarette smoking and drinking alcohol are not associated with rapid progression to acquired immunodeficiency syndrome among homosexual men in Norway. Scandinavian Journal of Social Medicine. 1994;3:209–212. doi: 10.1177/140349489402200309. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga GI, Spiegelman D, Wei R, Kapiga S, Villamor E, Hunter DJ. A randomized trial of multivitamin supplements and HIV disease progression and mortality. The New England Journal of Medicine. 2004;351:23–32. doi: 10.1056/NEJMoa040541. [DOI] [PubMed] [Google Scholar]
- Feikin DR, Feldmanb C, Schuchata A, Janoff DN. Global strategies to prevent bacterial pneumonia in adults with HIV disease. The Lancet Infectious Diseases. 2004;4:445–455. doi: 10.1016/S1473-3099(04)01060-6. [DOI] [PubMed] [Google Scholar]
- Feldman JG, Minkoff H, Schneider MF, Gange SJ, Cohen M, Watts DH, Anastos K. Association of cigarette smoking with HIV prognosis among women in the HAART era: A report from the women’s interagency HIV study. The American Journal of Public Health. 2006;96:1060–1065. doi: 10.2105/AJPH.2005.062745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Galai N, Park LP, Wesch J, Visscher B, Riddler S, Margolick JB. Effect of smoking on the clinical progression of HIV-1 infection. Journal of Acquired Immune Deficiency Syndromes. 1997;14:451–458. doi: 10.1097/00042560-199704150-00009. [DOI] [PubMed] [Google Scholar]
- Greenland S, Lash TL. Bias Analysis. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 345–380. [Google Scholar]
- Gritz ER, Vidrine DJ, Lavez AB, Amick BC, Arduino RC. Smoking behavior in a low-income multiethnic HIV/AIDS population. Nicotine & Tobacco Research. 2004;6:71–77. doi: 10.1080/14622200310001656885. [DOI] [PubMed] [Google Scholar]
- Hersey P, Prendergast D, Edwards A. Effects of cigarette smoking on the immune system. Follow-up studies in normal subjects after cessation of smoking. The Medical Journal of Australia. 1983;2:425–429. [PubMed] [Google Scholar]
- Hughes DA, Haslam PI, Townsend PJ, Turner-Warwick M. Numerical and functional alterations in circulatory lymphocytes in cigarette smokers. Clinical & Experimental Immunology. 1985;61:459–466. [PMC free article] [PubMed] [Google Scholar]
- Jia H, Uphold CR, Zheng Y, Wu S, Chen GJ, Findley K, Duncan PW. A further investigation of health-related quality of life over time among men with HIV infection in the HAART era. Quality of Life Research. 2007;16:961–968. doi: 10.1007/s11136-007-9214-4. [DOI] [PubMed] [Google Scholar]
- Kalra R, Singh SP, Savage SM, Finch GL, Sopori ML. Effects of cigarette smoke on immune response: chronic exposure to cigarette smoke impairs antigen-mediated signaling in T cells and depletes IP3- sensitive Ca2+ stores. The Journal of Pharmacology and Experimantal Therapeutics. 2000;293:166–171. [PubMed] [Google Scholar]
- Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, Lindegren ML, Jones JL. Epidemiology of human immunodeficiency virus–associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clinical Infectious Diseases. 2000;30:S5–S14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- Kuniak MP, Tsunoda M, Weiss ST, Satoh T, Guevarra L, Tollerud DJ. Cigarette smoking and serum cytokine levels. American Journal of Respiratory and Critical Care Medicine. 1995;15:A565. [Google Scholar]
- Lesaffre E, Rizopoulos D, Tsonaka R. The logistic transform for bounded outcome scores. Biostatistics. 2007;8:72–85. doi: 10.1093/biostatistics/kxj034. [DOI] [PubMed] [Google Scholar]
- Miller LG, Goldstein G, Murphy M, Ginns LC. Reversible alterations in immunoregulatory T cells in smoking. Chest. 1982;5:527–529. doi: 10.1378/chest.82.5.526. [DOI] [PubMed] [Google Scholar]
- Morris A, Lundgren JD, Masur H, Walzer PD, Hanson DL, Frederick T, Kaplan JE. Current epidemiology of pneumocystis pneumonia. Emerging Infectious Diseases. 2004;10 doi: 10.3201/eid1010.030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clinical Infectious Diseases. 2000;31:808–812. doi: 10.1086/314048. [DOI] [PubMed] [Google Scholar]
- Nieman RB, Fleming J, Coker RJ, William HJR, Mitchell DM. The effect of cigarette smoking on the development of AIDS in HIV-1-seropositive individuals. AIDS. 1993;7:705–710. doi: 10.1097/00002030-199305000-00015. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Ahluwalia JS, Banks R, Harris KJ, Mosier MC, Nazir N, Powell J. Differences in smoking and quitting experiences by levels of smoking among African Americans. Ethnicity & Disease. 2004;14:127–133. [PubMed] [Google Scholar]
- Okuyemi KS, Harris KJ, Scheibmeir M, Choi WS, Powell J, Ahluwalia JS. Light smokers: Issues and recommendations. Nicotine & Tobacco Research. 2002;4:S103–S112. doi: 10.1080/1462220021000032726. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Virasch N, Hao P, Aubrey MT, Mukerjee N, Bierer BE, Freed BM. Suppression of human IL-1β, IL-2, IFN-γ, and TNF-α production by cigarette smoke extracts. Journal of Allergy and Clinical Immununology. 2000;106:280–287. doi: 10.1067/mai.2000.107751. [DOI] [PubMed] [Google Scholar]
- Pachl C, Todd JA, Kern DG, Sheridan PJ, Fong S, Stempien M, Urdea MS. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. Journal of Acquired Immune Deficiency Syndromes. 1995;8:446–454. doi: 10.1097/00042560-199504120-00003. [DOI] [PubMed] [Google Scholar]
- Palacio H, Hilton JF, Canchola AJ, Greenspan D. Effect of cigarette smoking on HIV-related oral lesions. Journal of Acquired Immune Deficiency Syndromes. 1997;14:338–342. doi: 10.1097/00042560-199704010-00005. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. New England Journal of Medicine. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Petersen B, Steimel L, Callaghan J. Suppression of mitogen-induced Lymphocyte transformation in cigarette smokers. Clinical Immunology and Immunopathology. 1983;27:35–140. doi: 10.1016/0090-1229(83)90062-4. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Robbins CS, Dawe DE, Goncharova SI, Pouladi MA, Drannik AG, Swirski FK, Stampfli MR. Cigarette smoke decreases pulmonary dendritic cells and impacts antiviral immune responsiveness. American Journal of Respiratory Cell and Molecular Biology. 2004;30:202–211. doi: 10.1165/rcmb.2003-0259OC. [DOI] [PubMed] [Google Scholar]
- Robbins CS, Franco F, Mouded M, Cernadas M, Shapiro SD. Cigarette smoke exposure impairs dendritic cell maturation and T cell proliferation in thoracic lymph nodes of mice. The Journal of Immunology. 2008;180:6623–6628. doi: 10.4049/jimmunol.180.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce RA, Winkelstein W., Jr HIV infection, cigarette smoking and CD4+ T-lymphocyte counts: preliminary results from the San Francisco Men's Health Study. AIDS. 1990;4:327–334. [PubMed] [Google Scholar]
- Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. Journal of Acquired Immune Deficiency Syndromes. 2007;46:194–199. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcoholism: Clinical and Experimental Research. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Traphagen ET, Lyon SM, Freedberg KA. Alcohol consumption and HIV disease progression: are they related? Alcoholism: Clinical and Experimental Research. 2003;27:862–867. doi: 10.1097/01.ALC.0000065438.80967.56. [DOI] [PubMed] [Google Scholar]
- Samet JH, Libman H, LaBelle C, Steger K, Lewis R, Craven DE, Freedberg KA. A model clinic for the initial evaluation and establishment of primary care for persons infected with human immunodeficiency virus. Archives of Internal Medicine. 1995;155:1629–1633. [PubMed] [Google Scholar]
- Samet JH, Phillips SJ, Horton NJ. Detecting alcohol problems in HIV-infected patients. Use of the CAGE questionnaire. AIDS Research and Human Retroviruses. 2004;20:151–155. doi: 10.1089/088922204773004860. [DOI] [PubMed] [Google Scholar]
- Silverman N, Potvin C, Alexander JC, Jr, Chretien PB. In vitro lymphocyte reactivity and T Cell levels in chronic cigarette smokers. Clinical & Experimental Immunology. 1975;22:285–292. [PMC free article] [PubMed] [Google Scholar]
- Slavinsky J, III, Myers T, Swoboda RK, Leigh JE, Hager S, Fidel PL., Jr Th1/Th2 cytokine profiles in saliva of HIV-positive smokers with oropharyngeal candidiasis. Oral Microbioogy andl Immunology. 2002;17:38–43. doi: 10.1046/j.0902-0055.2001.00080.x. [DOI] [PubMed] [Google Scholar]
- Smith KL, Horton NJ, Saitz R, Samet JH. The use of the mini-mental state examination in recruitment for substance abuse research studies. Drug and Alcohol Dependency. 2006;82:231–237. doi: 10.1016/j.drugalcdep.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Sopori MH, Gairola CC, DeLucia AJ, Bryant LR, Cherian S. Immune responsiveness of Monkeys exposed chronically to cigarette smoke. Clinical Immunology and Immunopathology. 1985;36:338–344. doi: 10.1016/0090-1229(85)90054-6. [DOI] [PubMed] [Google Scholar]
- Sopori ML, Kozak W. Immunomodulatory effects of cigarette smoke. Journal of Neuroimmunology. 1998;83:148–156. doi: 10.1016/s0165-5728(97)00231-2. [DOI] [PubMed] [Google Scholar]
- Stephenson JM, Griffioen A, Woronowski H, Phillips AN, Petruckevitch A, Keenlyside R, Doyle C. Survival and progression of HIV disease in women attending GUM/HIV clinics in Britain and Ireland. Sexually Transmitted Infections. 1999;75:247–252. doi: 10.1136/sti.75.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer J, Munoz A, Peng Y, Margolick J, Chmiel JS, Oishi J, Samet JM. Longitudinal relation between smoking and white blood cells. American Journal of Epidemiology. 1996;144:734–741. doi: 10.1093/oxfordjournals.aje.a008997. [DOI] [PubMed] [Google Scholar]
- Thomas W, Holt P, Keast D. Humoral immune response of mice with long-term exposure to cigarette smoke. Archives of Environmental Health. 1975;30:78–80. doi: 10.1080/00039896.1975.10666647. [DOI] [PubMed] [Google Scholar]
- Tollerud DJ, Clark JW, Brown LM, Neuland CY, Mann DL, Pankiw-Trost LK, Hoover RN. The effects of cigarette smoking on T cell subsets. A population-based survey of healthy Caucasians. American Journal of Respiratory and Critical Care Medicine. 1989;139:1446–1451. doi: 10.1164/ajrccm/139.6.1446. [DOI] [PubMed] [Google Scholar]
- Tollerud DJ, Clark JW, Brown LM, Neuland CY, Mann DL, Pankiw-Trost LK, Hoover RN. Association of cigarette smoking with decreased numbers of circulating natural killer cells. The American Review of Respiratory Disease. 1989;139:194–196. doi: 10.1164/ajrccm/139.1.194. [DOI] [PubMed] [Google Scholar]
- Webb MS, Vanable PA, Carey MP, Blair DC. Cigarette smoking among HIV+ men and women: examining health, substance use, and psychosocial correlates across the smoking spectrum. Journal of Behavioral Medicine. 2007;30:371–383. doi: 10.1007/s10865-007-9112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber PM, Schoenbaum EE, Gourevitch MN, Buono D, Klein RS. A prospective study of HIV disease progression in female and male drug users. AIDS. 1999;13:257–262. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- Wewers MD, Diaz PT, Wewers ME, Lowe MP, Nagaraja HN, Clanton TL. Cigarette smoking in HIV infection induces a suppressive inflammatory environment in the lung. American Journal of Respiratory and Critical Care Medicine. 1998;158:1543–1549. doi: 10.1164/ajrccm.158.5.9802035. [DOI] [PubMed] [Google Scholar]


