Abstract
BACKGROUND
Nodal, a TGFβ like growth factor, functions as an embryonic morphogen that maintains the pluripotency of embryonic stem cells. Nodal has been implicated in cancer progression; however, there is no information on expression and functions of Nodal in prostate cancer. In this study, we have investigated the expression of Nodal, its receptors, and its effects on proliferation and migration of human prostate cells.
METHODS
RT-PCR, qPCR, and Western blot analyses were performed to analyze expression of Nodal and Nodal receptors and its effects on phosphorylation of Smad2/3 in prostate cells. The effects on proliferation and migration were determined by 3H-Thymidine incorporation and cell migration assays in the presence or absence of Nodal receptor inhibitor (SB431542).
RESULTS
Nodal was highly expressed in WPE, DU145, LNCaP, and LNCaP-C81 cells with low expression in RWPE1 and RWPE2 cells, but not in PREC, PC3 and PC3M cells. Nodal receptors are expressed at varying levels in all prostate cells. Treatment with exogenous Nodal induced phosphorylation of Smad2/3 in WPE, DU145, and PC3 cells, which was blocked by SB431542. Nodal dose-dependently inhibited proliferation of WPE, RWPE1 and DU145 cells, but not LNCaP and PC3 cells. Nodal induced cell migration in PC3 cells, which was inhibited by SB431542; Nodal had no effect on cell migration in WPE and DU145 cells. The effects of Nodal on cell proliferation and migration are mediated via ALK4 and ActRII/ActRIIB receptors and Smad 2/3 phosphorylation.
CONCLUSIONS
Nodal may function as an autocrine regulator of proliferation and migration of prostate cancer cells.
Keywords: Nodal, ALK4/7, prostate cancer, cancer stem cells, cell proliferation, cell migration
INTRODUCTION
Transforming growth factor-β (TGF-β) superfamily is composed of nearly 30 growth factors including TGFβ proteins, bone morphogenetic proteins (BMPs), activins, inhibins, Nodal and its related proteins (1–4). These growth factors are involved in a variety of physiological processes that are essential for tissue development and homeostasis. There are two types of membrane serine/threonine kinase receptors that are required for the functions of TGFβ-like growth factors. Seven members of the type I receptors (activin receptor like kinase (ALK) 1–7) and five members of the type II receptors have been characterized in mammals (5). TGFβ superfamily ligands bind to type II receptors which then associate with specific type I receptors resulting in phosphorylation and activation of type I receptors (1–7). Consequently, the activated type I receptor phosphorylates the appropriate Smad proteins. Smad2 and Smad3 respond to Nodal, TGFβs, and activins, whereas Smad1, Smad5, and Smad8 mediate BMP signaling (6). Phosphorylated Smads interact with the co-Smad protein, Smad4, and then translocate to the nucleus to regulate expression of target genes (1–4).
TGFβ superfamily members are expressed in both embryonic and somatic stem cells (7) and several studies have implicated these ligands in the maintenance of pluripotency and self-renewal of embryonic stem (ES) cells and differentiation of various types of somatic stem cells (7,8). Nodal is a novel member of the TGFβ superfamily; it inhibits differentiation, maintains the pluripotency of human embryonic stem cells (hESCs), and promotes the self-renewing capacity of mouse ES cells (9). Nodal also plays an important role in the induction of dorsal mesoderm, anterior patterning, and formation of left-right asymmetry during early embryonic development (10–15). Gene Expression Omnibus Database shows that Nodal expression is restricted to embryonic tissues and hESCs, but re-emerges during tumorigenesis in certain types of cancers (http://www.ncbi.nlm.nih.gov/sites/entrez).
Recently, studies of neoplastic tissues have revealed evidence of self-renewing, stem-like cells within tumors called cancer stem cells (CSCs) (16), which exhibit characteristics similar to normal stem cells such as the capacity to perform self-renewal and differentiation (16–18). So far, CSCs have been identified in a variety of human cancers including leukemias (19–21), prostate (22–26), breast (27), colon (28), brain (29), ovarian (30), and pancreatic cancer (31). The first putative CSC subpopulation was identified in breast carcinoma which was isolated and characterized by high CD44 and low CD24 (CD44+/CD24−/low) expression levels (27). However, CSCs also express many of the same markers used to identify and isolate normal stem cell populations such as CD133, CD44, integrins, breast cancer resistance proteins (BCRPs), and stem cell antigen-1 (Sca1) (32). In addition, Gu et al. (33) reported direct evidence of a putative prostate CSC originating from a single cell, which differentiates into luminal and neuroendocrine epithelial cells as well as basal cells in vivo.
Since Nodal-related signaling components are upregulated in many human cancers (34–36), it has been suggested to play a role in cancer progression. Xu et al. (37) have demonstrated that Nodal induces apoptosis and inhibits proliferation via ALK7 in human ovarian epithelial cancer cells and human trophoblast cells. The zebrafish embryo model has been used to study the interaction between human melanoma cells and embryonic progenitor cells; Nodal was shown to be secreted by aggressive melanoma cells and eventually induced ectopic formation of the zebrafish embryonic axis (38). The expression of Nodal in human metastatic carcinomas (melanoma and breast) is correlated with cancer progression; inhibition of Nodal signaling decreases cell invasiveness, colony formation, and tumorigenicity (35).
Several studies have investigated the role of TGFβ superfamily members such as TGFβ (39,40), activins (41–44), and bone morphogenetic proteins (BMPs) (45–48) in prostate cancer cells; however, the expression and role of Nodal in prostate cancer development and progression have not been investigated. In this study, we have used prostate stem cells and established prostate cancer cell lines to investigate the expression of both Nodal and its cognate receptors and the biological effects of Nodal on prostate cell proliferation and migration.
MATERIALS AND METHODS
Chemicals and reagents
Recombinant human Nodal and TGFβ1 were purchased from R&D systems (Minneapolis, MN). Anti-Nodal and anti-ActRIIB antibodies were purchased from Abcam Inc. (Cambridge, MA). The antibodies against phospho-Smad2 and phospho-Smad3 were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Anti-Smad2/3, anti-ActRIB, and anti-ActRII antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-β-Actin antibody was purchased from Sigma-Aldrich (St. Louis, MO). The anti-rabbit and anti-mouse immunoglobulins coupled to horseradish peroxidase (IgG-HRP) were obtained from Promega (Madison, WI) and donkey anti-goat IgG HRP was obtained from Santa Cruz Biotechnology, Inc. SB431542 (inhibitor of activin receptor like kinase (ALK) 4/5/7) was purchased from Tocris Bioscience (Ellisville, MO). Okadaic acid potassium salt (specific inhibitor of protein phosphatases PP1and PP2A) was purchased from Sigma-Aldrich.
Cell Culture and Cell Treatments
Normal prostate epithelial cells (PREC) were obtained from Lonza (Walkersville, MD). Prostate stem cells with high expression of cytokeratin 5, 14 and MMP-2 but low expression of cytokeratin 18, androgen-independent for growth and survival cell line (WPE), immortalized prostate luminal epithelial cell line (RWPE1), k-ras transformed RWPE1 cell line (RWPE2), prostate cancer cell lines (LNCaP, DU145 and PC3) were obtained from American Type Culture Collection (ATCC, Rockville, MD). LNCaP is an androgen-dependent cell line isolated from a lymph node lesion. Androgen-independent derivative of LNCaP cells (C-81) were provided by Dr. Ming-Fong Lin (University of Nebraska). DU145 and PC3 are androgen-independent cell lines derived from brain and bone metastatic sites, respectively. PC3M cells, derived from a PC3 xenograft were obtained from Dr. Girsh Shah (University of Louisiana). PREC were cultured in prostate epithelial basal medium (Lonza Inc., Walkersville, MD). WPE, RWPE1, and RWPE2 were maintained in keratinocyte serum free medium containing 50 μg/ml gentamycin, 0.05 mg/ml bovin pituitary extract (BPE), and 5 ng/ml epidermal growth factor (EGF) (Invitrogen, Carlsbad, CA). LNCaP and C-81 cells were routinely maintained in RPMI 1640 containing 4 mM glutamine, and 50 μg/ml gentamycin. DU145, PC3, and PC3M cells were cultured in Eagle's minimum essential medium with Earle's salts with 0.1 mM of the following amino acid supplements: L-alanine, L-asparagine, L-aspartic acid, L-glutamic acid, L-proline, L-serine and L-glycine. The medium also contained 4 mM L-glutamine, 2.5 g/l NaHCO3, 1.5 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B and 50 μg/ml gentamycin. Both MEM and RPMI media (Mediatech, Herndon, VA) were supplemented with 5% fetal bovine serum (HyClone, South Logan, Utah).
To determine the effects of Nodal on phospho-Smad2/3, prostate cells were cultured in 6-well plates at the density of 4 × 105 cells/well, pretreated with okadaic acid (10 μM) for 10 min or SB431542 (10 μM) for 1 h, and followed by treatment with 100 ng/ml of rhNodal for 10, 20 or 30 minutes. The cells were washed with ice-cold phosphate-buffered saline and lysed in lysis buffer (Cell Signaling Technology, Beverly, MA) containing 20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin and 1× protease inhibitor cocktail (Calbiochem, San Diego, CA). Protein concentrations were determined by the Lowry HS assay using the Bio-Rad DCProtein Assay kit (Bio-Rad, Hercules, CA) according to the instruction provided by the manufacturer.
RNA isolation, Reverse Transcription (RT), PCR and Quantitative Real Time PCR
Total RNA was isolated from prostate cells using TRIzol (Invitrogen, Carlsbad, CA) and the resulting RNA samples were quantified by optical density reading at 260 nm as described previously (49); OD260/OD280 ratio for RNA samples was between 1.8 and 2.0. Total RNA (2 μg) were reverse transcribed in a 50 μl reaction mixture containing 0.5 mM dNTP (Fisher Scientific, Pittsburgh, PA), 0.5 mM dithiotreitol (Bio-Rad, Hercules, CA), 0.5 μg of oligo dT, and 400 U of M-MLV Reverse Transcriptase (Promega, Madison, WI) at 37°C for 1.5 h. The reaction was terminated by heating the samples at 70°C for 5 min and subsequently cooled to 4°C.
The real-time PCR was performed with iCycler (Bio-Rad, Hercules, CA, USA) in 96-well plates. A SYBR-Green Master Mix (Bio-Rad) was used in a volume of 25 μl/well. The thermal profile for the real-time PCR was as follows: 1, 95°C for 3 min; 2, 95°C for 15s; 3, 60°C for 1 min; 4, repeating steps 2 and 3 for 40 times; 5, 95°C for 1min; 6, 55°C for 1 min; 7, 55°C for 10 s; 8, repeating step 7 for 100 times. Melting curves were examined for the quality of PCR amplification of each sample. Relative quantification of Nodal mRNA expression was determined using the 2−ΔΔCt method (50) with L-19 as an internal control.
Polymerase chain reaction (PCR) was performed to detect mRNA levels of Nodal, ALK4, ALK7, ActRII, ActRIIB, Smad2, Smad3, Smad4, Smad7, and L-19. The PCR reaction mixture was composed of 0.1 mM dNTPs, 0.5 U Taq DNA polymerase, 10× PCR buffer with 3 mM MgCl2 and 25 pM of the specific primers in a total volume of 15 μl. Primer information and the size of expected amplicons for individual genes are shown in Table 1. L19 (a ribosomal protein) was used as a template control. RNA samples processed without RT and PCR amplified by L-19 primers were used as negative controls. Amplification was performed at 94°C for 15s, 60°C for 15s, and 72°C for 15s for 40 cycles for Nodal; at 94°C for 20s, 60°C for 30s, 72°C for 45s for 35 cycles (for ALK4, ALK7, ActRII, ActRIIB, and L-19); at 94°C for 15s, 60°C for 15s, 72°C for 45s for 35 cycles (for Smads 2, 3, 4, and 7). For all PCRs, an initial step was at 95°C for 2 min and a final extension was at 72°C for 10 min. The PCR products were separated on 1.5–2% agarose gels or 4% super fine resolution (SFR) agarose gels (Amresco, Solon, OH) stained with ethidium bromide.
Table 1.
Primers used for the detection of target mRNAs
| Gene | Primer | Sequence (5'→3') | Product size (bp) |
|---|---|---|---|
| Forward | TGTTGGGGAGGAGTTTCATC | ||
| Nodal | Reverse | GCACAACAAGTGGAAGGGAC | 98 |
| Forward | CTGACACCATTGACATTGCC | ||
| ALK4 | Reverse | TGTGGAGAGAGGGAGCAGTT | 446 |
| Forward | GACATGAAAACATCCTTGGT | ||
| ALK7 | Reverse | ACTTCTGGTCACAAACAACC | 585 |
| Forward | ACTTGTTCCAACTCAAGACC | ||
| ActRII | Reverse | ACTTTTGATGTCCCTGTGAG | 463 |
| Forward | CTCCCTCACGGATTACCTCA | ||
| ActRIIB | Reverse | AGGGCAGCATGTACTCATCC | 428 |
| Forward | CTTTTGTTGTGTAAGCTCTCACTG | ||
| Smad2 | Reverse | GACCTTCTACCACTTTCAGAGTTG | 247 |
| Forward | GGGATACCAGCAGCAAGAGAG | ||
| Smad3 | Reverse | ATTGTGAAGACGGTTACCTGAAAG | 202 |
| Forward | TTGCGTCAGTGTCATCGACAG | ||
| Smad4 | Reverse | CCAGCCTTTCACAAAACTCATCC | 210 |
| Forward | AATATTTTCCTCCTGAGTGCTTGC | ||
| Smad7 | Reverse | ATTTCTGCTTCCCCTCTTCCTATC | 215 |
| Forward | GAAATCGCCAATGCCAACTC | ||
| L-19 | Reverse | TCTTAGACCTGCGAGCCTCA | 405 |
Western blot analysis
Western blot analyses were performed as described previously (49). Briefly, cell lysates were mixed with Laemmeli buffer (62.5 mM Tris, pH 6.8, 2% SDS, 5% β–mercaptoethanol, and 10% glycerol). Individual samples (30–35 μg protein) were subjected to SDS-PAGE in 8% –10% gels and transferred to PVDF membranes (Millipore). After blocking the membranes with 5% fat free milk in TBST (50 mM Tris, pH 7.5, containing 0.15 M NaCl, and 0.05% Tween–20) for 1h at room temperature, the membranes were incubated with appropriate dilutions of specific primary antibodies (1:1000 for anti-p-Smad2, anti-p-Smad3, anti-Smad2/3, and anti-Nodal antibodies; 1:200 for anti-ActRII antibody; 1:500 for anti-ActRIB and anti-ActRIIB antibodies; 1:20,000 dilution was used for anti-β-actin antibody) overnight at 4°C. After washing, the blots were incubated with anti-rabbit or anti-mouse IgG HRPs at a dilution range of 1:2000–1:20,000 for 1 h. The blots were developed in ECL mixture (Thermo Fisher Scientific Inc., Rockford, IL) and the density of specific protein bands were determined by QuantityOne image analysis software.
Cell proliferation assay
The effects of Nodal on cell proliferation were determined using 3H-thymidine incorporation assay as described previously (49). Briefly, prostate cells were plated in 24-well plates at the density of 4 × 104 cells/well and maintained with 5% FBS. Cell cycle synchronization was performed by serum starvation for 24h. The cells were treated at various concentrations (10, 100, and 500 ng/ml) of rhNodal in the presence of 5% FBS for 18h. The media were then removed and fresh media containing 1 μci/ml 3H-Thymidine (GE-Amersham, Piscataway, NJ) were added and the cells were incubated for 4 h prior to determining the incorporation of 3H-thymidine into DNA. The radiolabelled medium was replaced with cold distilled water and the cells were lysed by ultrasonication. The cell lysates were allowed to pass through DE81 ion-exchange filter membrane using Millipore vacuum module and washed twice with distilled water. The filters were transferred to scintillation vials containing scintillation cocktail (Fisher Scientific, Pittsburgh, PA) and the radioactivity was determined by a scintillation counter (Beckman Instruments). Each treatment group was assayed in replicates of four and each experiment was repeated at least three times.
Cell migration assay
In vitro cell migration assay was performed using 24-well transwell inserts (8 μm) (51). Briefly, cells were washed once with MEM and harvested from cell culture dishes by EDTA-trypsin into 50 ml conical tubes. The cells were centrifuged at 500 g for 10 min at room temperature; the pellets were resuspended in MEM supplemented with 0.2% bovine serum albumin (BSA) at a cell density of 3 × 105 cells/ml. The outside of the transwell insert membrane was coated with 50 μl rat tail collagen (50 μg/ml) overnight at 4°C. The next day, aliquots of rat tail collagen (50 μl) were added into the transwell inserts to coat the inside of the membranes. The inserts were left for 1.5 hr at room temperature before being washed thoroughly with 3 ml MEM. Chemoattractant solutions were made by diluting rhNodal (10, 100, 500 ng/mL) or EGF (3 ng/ml) into MEM for DU145 and PC3 cells or KSFM for WPE-stem cells supplemented with 0.2% BSA. MEM or KSFM containing 0.2% BSA served as a negative control. EGF was used as a positive control (52). 400 μl of control and chemoattractant solutions were added into different wells of a 24-well plate. Aliquots of 100 μl cell suspension were loaded into transwell inserts that were subsequently placed into the 24-well plate. The transwell insert-loaded plate was placed in a cell culture incubator for 5 h. At the end of the incubation, transwell inserts were removed from the plate individually; the cells inside transwell inserts were removed with cotton swabs. The cleaned inserts were fixed in 300 μl of 4% paraformaldehye (pH 7.5) for 20 minutes at room temperature. Cells on the outside of the transwell insert membrane were stained using HEMA 3 staining kit (Fisher Scientific Inc, TX). The stained cells were counted in four non-overlapping low power fields of a light microscope, and the average number of cells reflected the cell migration status in each transwell insert. To avoid experimental bias, a systematic random sampling technique was applied in the selection of representative fields, which was executed by the comparison of representative fields selected and counted by different persons.
The results were expressed as migration index defined as: the average number of cells per field for test substance/the average number of cells per field for the medium control. The experiments were conducted at least three times using independent cell preparations.
Statistical analysis
All experiments were repeated at least three times using a different cell preparation. The results are presented as means ± SEM of three independent experiments and images from a single representative experiment are presented. ANOVA and Duncan's modified multiple range test were employed to assess the significance of differences between treatment groups.
RESULTS
Expression of Nodal and its signaling components in prostate cell lines
Nodal mRNA and protein
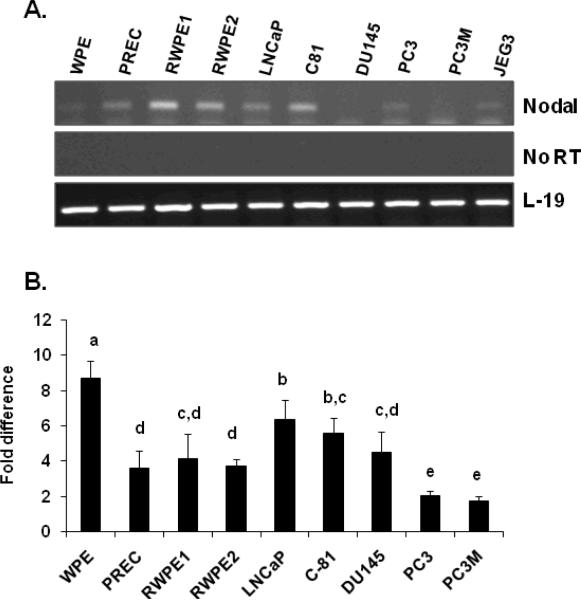
Total RNAs and proteins were extracted from prostate stem cells (WPE), normal prostate epithelial cells (PREC), immortalized prostate luminal epithelial cell line (RWPE1), k-ras transformed RWPE1 (RWPE2) cells, and prostate cancer cell lines (LNCaP, LNCaP-C81, DU145, PC3 and PC3M). The mRNA levels of Nodal were determined by RT-PCR, real time PCR and the proteins levels were determined by Western blot analysis. As shown in Fig. 1A, RT-PCR detected Nodal mRNA in all cell lines; however, the expression levels differed in specific cell lines. The identity of the RT-PCR product with Nodal was confirmed by sequencing. To determine the quantitative differences in the expression of Nodal, the levels of mRNA in the cell lines was determined using quantitative real-time PCR. As shown in Fig. 1B, the basal levels of Nodal mRNA were detected in PC3 and PC3M cells. In contrast, Nodal mRNA levels in WPE cells were 6.84 fold higher than those observed in PC3 and PC3M cells. Intermediate Nodal mRNA levels were observed in LNCaP (4.48 fold), LNCaP-C81 (3.7 fold), and DU145 (2.6 fold) cells. Nodal mRNA levels in PREC, RWPE1, and RWPE2 cells were 1.74, 2.27, and 1.83 fold higher than those detected in PC3 and PC3M cells.
FIGURE 1.
Steady-state levels of Nodal mRNA in prostate cell lines. A. Total RNAs were isolated and semi-quantitative RT-PCR was performed to determine the mRNA expression of Nodal in prostate cell lines. JEG3 is the human choriocarcinoma cell lines, which was used as a positive control. L-19 was used as an internal control. A panel of No RT samples derived from the same RNAs is also included. B. Quantitative real-time PCR was performed to quantify the expression level of Nodal. The relative concentration of each PCR product was determined using the 2−ΔΔCt method. L-19 was used as an internal control. Data are expressed as Mean ± SEM (n=3), and were analyzed by ANOVA and Duncan's modified range test. Statistically significant differences between groups in a given category (P < 0.05) are designated with different lowercase letters.
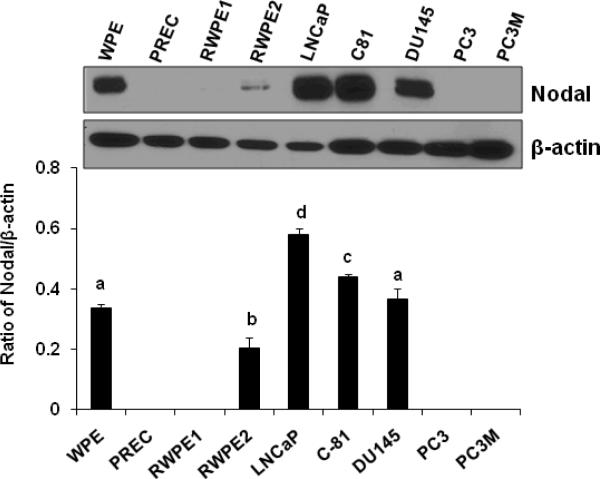
To examine the presence of Nodal protein in these prostate cell lines, the total cell lysate proteins were separated on 10% SDS-PAGE and transferred onto PVDF membrane for Western blot analysis with specific anti-Nodal antibody. The Western blot analysis detected pro-Nodal protein in WPE, RWPE2, LNCaP, LNCaP-C81, and DU145 cells, but not in PREC, RWPE1, PC3, and PC3M cells (Fig. 2). Quantitative analysis showed that Pro-Nodal protein levels were significantly (P<0.05) higher in WPE, LNCaP, LNCaP-C81, and DU145 cells. Low levels of Nodal protein were detected in RWPE2 cells; however, it was significantly (P<0.05) lower than that observed in other cell lines. Nodal protein was not detected in PREC, RWPE1, PC3, and PC3M cells.
FIGURE 2.
Western blot analysis of Nodal protein levels in prostate cell lines (Upper panel). Total cellular proteins were separated by SDS polyacrylamine gel electrophoresis and blotted using an anti-Nodal antibody. Anti-β-actin blots were used as loading controls. Quantitative analysis of Nodal protein in prostate cell lines was carried out after normalization to the signal obtained with β-actin (Lower panel). Each bar represents Mean ± SEM (n=3). Statistically significant differences between groups in a given category (P < 0.05) are designated with different lowercase letters.
Nodal Receptors
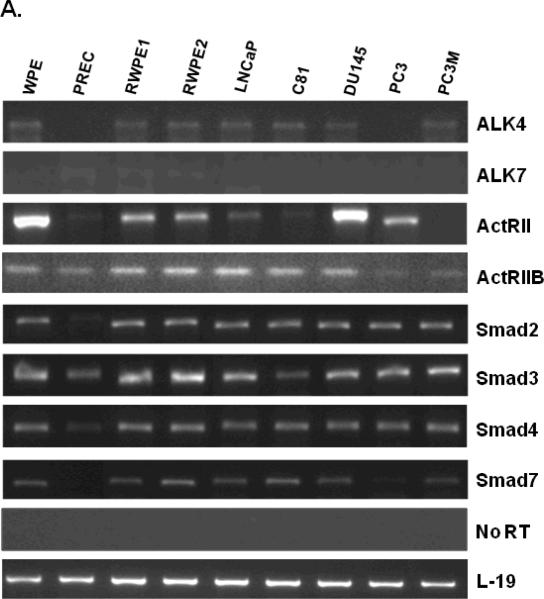
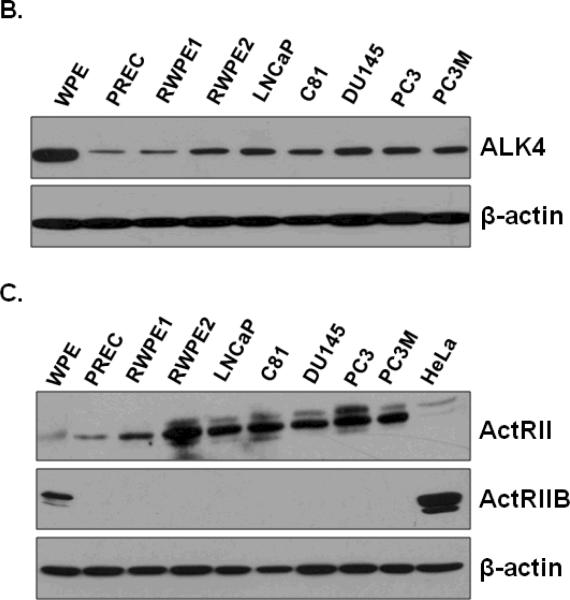
Nodal signals by binding to heterodimeric complexes between type I (ALK4 and ALK7) and type II (ActRII and ActRIIB) receptors (53). This results in activation of the Smad2/3/4 complex, which translocates to the nucleus where it regulates gene expression (53). To determine whether Nodal signaling receptors are expressed in prostate cells, we determined the levels of receptor mRNA and proteins in all cell lines used above. As shown in Fig. 3, Nodal receptors (ALK4, ALK7, ActRII, and ActRIIB) and Smads 2, 3, 4, and 7 were expressed at varying levels in prostate cell lines. The expression of ALK4 mRNA was detected in WPE, PREC, RWPE1, RWPE2, LNCaP, LNCaP-C81, DU145, PC3 and PC3M cells. ALK7 mRNA was undetectable in all prostate cell lines. ActRII mRNA was expressed in all cell lines at different levels. Interestingly, ActRII mRNA levels were highest in WPE and DU145 cells. ActRIIB mRNA was also detected in all prostate cell lines. Additionally, Smads 2, 3, 4, and 7 are intracellular signal components of Nodal receptors, which were expressed ubiquitously in all prostate cell lines (Fig. 3A). The Western blot analysis showed that ALK4 and ActRII proteins were present in all prostate cell lines; however, both receptors were highly expressed in all prostate cancer cell lines compared to the normal cells (PREC, RWPE1) (Fig. 3B). On the other hand, ActRIIB protein was only detected in the WPE cells and HeLa cells (used as positive controls) (Fig. 3B).
FIGURE 3.
A. Steady-state mRNA levels of Nodal receptors (ALK4, ALK7, ActRII, and ActRIIB) and Smads 2, 3, 4, and 7, in prostate cell lines. Total RNAs were extracted and semi-quantitative RT-PCR analyses were performed. L-19 was used as an internal control. A panel of No RT samples derived from the same RNAs is also included. B and C. Western blot analysis of type I (ALK4) and type II (ActRII and ActRIIB) receptors protein levels in prostate cell lines. Total cellular proteins were separated by SDS polyacrylamine gel electrophoresis and blotted using anti-ALK4, anti-ActRII, and anti-ActRIIB antibodies. HeLa cells were used a positive control. Anti-β-actin blots were used as loading controls.
Table 2 summarizes the data on the expression of Nodal and its receptors in 9 prostate cell lines. On the basis of the expression patterns of Nodal and Nodal receptors, we chose WPE, RWPE1, DU145 and PC3 cells for functional studies. While all four cell lines express Nodal receptors, WPE and DU145 cells express Nodal protein but RWPE1 and PC3 cells lack the expression of Nodal.
Table 2.
Expression of Nodal and Nodal Receptors in Prostate Cell Lines
| Cell line | Ligand | Nodal Receptors | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nodal | ALK4 | ALK7 | ActRII | ActRIIB | ||||||
| mRNA | Protein | mRNA | Protein | mRNA | Protein | mRNA | Protein | mRNA | Protein | |
| WPE | + | + | + | + | − | − | + | + | + | + |
| PREC | + | − | + | + | − | − | + | + | + | − |
| RWPE1 | + | − | + | + | − | − | + | + | + | − |
| RWPE2 | + | + | + | + | − | − | + | + | + | − |
| LNCaP | + | + | + | + | − | − | + | + | + | − |
| LNCaP-C81 | + | + | + | + | − | − | + | + | + | − |
| DU145 | + | + | + | + | − | − | + | + | + | − |
| PC3 | + | − | + | + | − | − | + | + | + | − |
| PC3M | − | − | + | + | − | − | + | + | + | − |
Activation of Nodal signaling in prostate cell lines
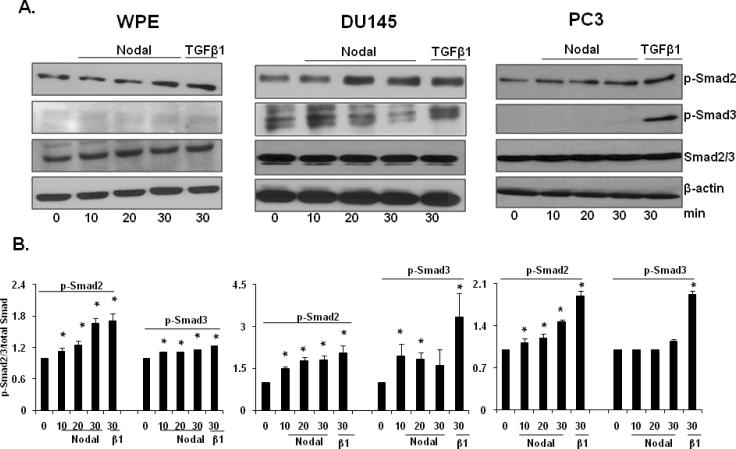
Type I and Type II receptors of Nodal and related Smads are expressed in stem cells and prostate cancer cells; therefore, we investigated whether functional Nodal receptors are present in prostate cell lines and whether they are coupled to Smad signaling pathway. To achieve this, we studied the effects of exogenous rhNodal on Smad 2/3 phosphorylation in WPE, DU145, and PC3 cells. These cells were treated with rhNodal and TGFβ1 (as a positive control) for different time periods. Western blot analysis showed that Smad2 was phosphorylated in a time-dependent manner in WPE (1.67 fold), DU145 (1.81 fold), and PC3 (1.47 fold) cells in response to Nodal and TGFβ1 at 30 min after treatment (Fig. 4A, B). Phosphorylation of Smad3 was much weaker than that of phosphorylation of Smad2.
FIGURE 4.
Activation of Nodal signaling in prostate cell lines. A. Western blot analyses of phosphorylated Smad2 and 3, Smad2/3, and β-actin in WPE, DU145, PC3 cells at different time periods after treatment with rhNodal (200 ng/ml). TGFβ (10 ng/ml) was used as a positive control. Western blot using anti-Smad2/3 and anti-β-actin antibodies were used as internal controls. B. Quantitative analysis of p-Smad2 and p-Smad3 in WPE, DU145, PC3 cells treated with rhNodal were relative to that of the untreated control (designated as one) after normalization to the signal obtained with Smad2/3. Each bar represents Mean ± SEM (n=3). *Significant differences (P < 0.05) compared to untreated controls.
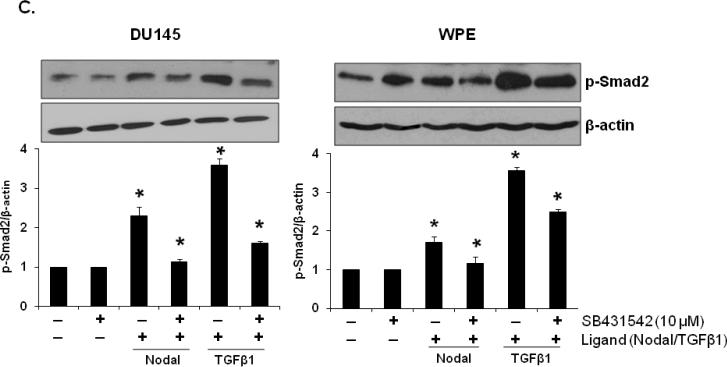
C. Western blot analyses of phosphorylated Smad2 and β-actin in WPE and DU145 cells after pre-treatment with ALK4/5/7 inhibitor (SB431542, 10 µM). Quantitative analysis p-Smad2 in WPE and DU145 cells treated with rhNodal, after pretreatment with SB431542. For further details, see legend to Fig. 4B. Each bar represents Mean ± SEM (n=3). *Significant differences (P < 0.05) compared to untreated controls.
A specific inhibitor of ALK4/5/7 (SB431542), which has previously been shown to abrogate the effects of Nodal in target cells [10], blocked phosphorylation of Smad2 in WPE and DU145 cells in response to rhNodal (Fig. 4C).
The effects of Nodal on cell proliferation in prostate cell lines
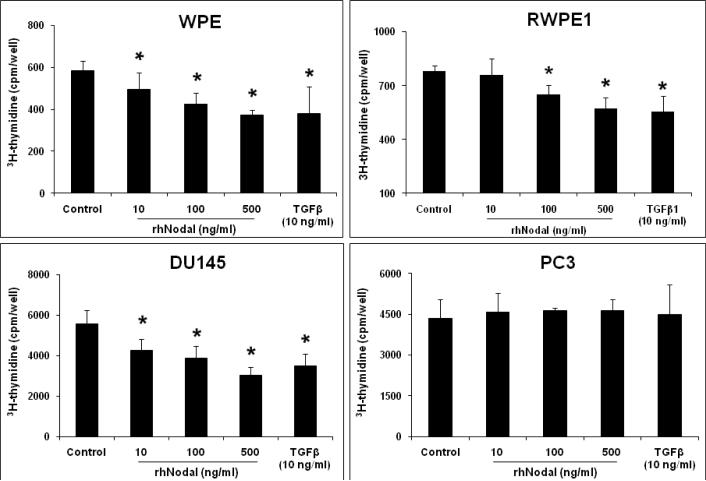
Nodal and TGFβ growth factors have been shown to regulate cell growth in different types of cells (34–36). We investigated the effects of different concentrations of rhNodal on cellular proliferation in WPE, RWPE1, LNCaP, DU145, and PC3 cells under normal growth conditions. 3H-thymidine incorporation assay showed that treatment with rhNodal dose-dependently decreased DNA synthesis in WPE, RWPE1, and DU145 cells (Fig. 5). On the other hand, treatment with rhNodal had no effect on proliferation of LNCaP (data not shown) and PC3 cells (Fig. 5).
FIGURE 5.
Effects of Nodal on DNA synthesis in WPE, RWPE1, DU145, and PC3 cells as determined by 3H-thymidine incorporation assay. The cells were serum-starved for 24 h and treated with different concentrations of rhNodal for 18 h in the presence of 5% FBS. The cells were then pulse-labeled for 4 h with 1μci/ml 3H-Thymidine and radioactivity was determined by liquid scintillation counting. Each bar represents Mean ± SEM (n=3). *Significant differences (P < 0.05) compared to untreated controls.
The effects of Nodal on cell migration in prostate cell lines
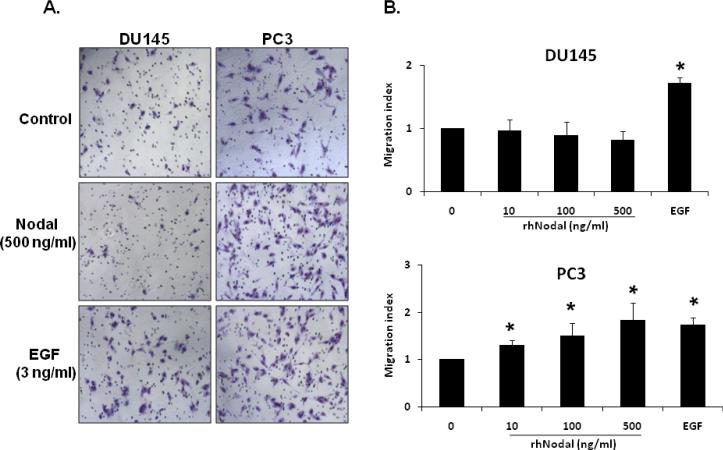
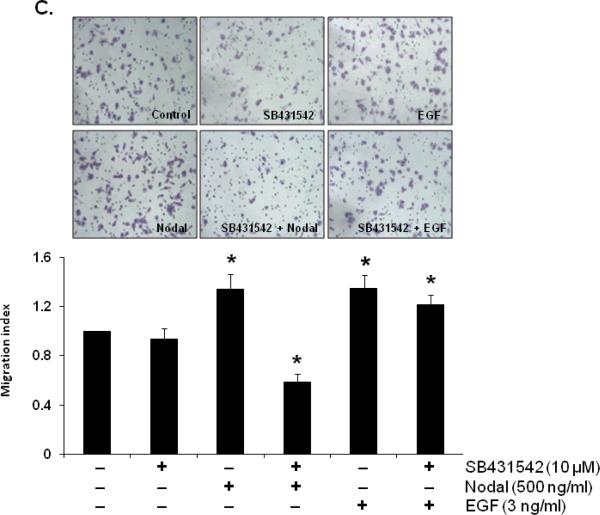
Previous studies have shown that Nodal expression was considerably higher in invasive and metastatic human cancer cells (54,55) and it has been suggested that Nodal may be involved in the invasive and metastatic behavior of the cancer cells. To test this possibility, we investigated the biological effects of Nodal on cell migration in WPE, DU145 and PC3 cells. Our results showed that treatment with rhNodal stimulated cell migration in PC3 cells (1.82 fold) but not in WPE (data not shown) or DU145 cells (Fig. 6A, B). On the other hand, EGF used as a positive control induced cell migration in both DU145 (1.71 fold) and PC3 (1.73 fold) cells (Fig. 6A, B). As shown in Fig. 6C, pretreatment with a specific inhibitor of ALK4/5/7 (SB431542) completely blocked the stimulatory effects of Nodal on migration of PC3 cells indicating the involvement of ALK4 in Nodal effects. The inhibitor did not influence the EGF (3 ng/ml) induced migration of PC3 cells.
FIGURE 6.
Differential effects of Nodal on migration of prostate cell lines. A. Representative images from different treatments of DU145 and PC3 cell lines. Cells were visualized under 10× objectives. EGF (3ng/ml) used as a positive control, induced migration in both cell lines. B. Nodal dose-dependently induced migration in PC3 cells but not in DU145 cells in a transwell migration assay. Each bar represents Mean ± SEM (n=3). *Significant differences (P < 0.05) compared to untreated controls. C. Pretreatment with SB431542 (10 μM) for 30 min blocked the migration of PC3 cells induced by Nodal (500 ng/ml) but not in EGF (3 ng/ml). The data were presented as Mean ± SEM (n=3). *Significant differences (P < 0.05) compared to untreated controls.
DISCUSSION
The results presented in this study demonstrate for the first time that Nodal and its receptors are expressed in prostate epithelial stem cells (WPE) and prostate cancer cells (LNCaP, LNCaP-C81, and DU145). All prostate cells express ALK4 and ActRII receptors and exogenous Nodal induced a time dependent phosphorylation of Smad2 indicating the presence of functional receptors. Nodal exerted differential effects on prostate cancer cells; it inhibited proliferation in WPE, RWPE1, and DU145 cells while it had no effect on the proliferation of LNCaP and PC3 cells. On the other hand, it induced migration in PC3 cells but not in WPE or DU145 cells.
It has been suggested by several studies that cancer cells reacquire characteristics of stem cells as indicated by the expression of genes associated with ES cells (16). Nodal plays an important role in the maintenance of pluripotency of human ES cells and its expression is lost in differentiated cells; however, several cancer cells reacquire the expression of Nodal and its receptors. Previous studies have shown that Nodal was expressed in human breast (56), melamona (54), and ovarian (37,57) cancer cell lines, but is weakly expressed in normal epithelial cells. Our results confirm the presence of Nodal in WPE, LNCaP, LNCaP-C81, and DU145 cells, but its expression was low or absent in PrEC, RWPE1, RWPE2 cells. Our results indicated significant discrepancies in the levels of Nodal mRNA and Nodal protein in various cell types. While Nodal mRNA was detected in most cell lines by RT-PCR and real-time PCR, Nodal protein was present only in some cell lines. Both Nodal mRNA and Nodal protein were undetectable in PC3 and its derivative PC3M cell line. These results indicate the involvement of post-transcriptional and post-translational mechanisms in the regulation of Nodal expression in different cell types. Our results also indicate that the data on mRNA levels determined by gene expression analysis without simultaneous protein measurements need to be interpreted with caution.
Our results also confirm the similarity of WPE-stem cells to ES cells. WPE-stem cells express high levels of Nodal in addition to other ES markers such as cytokeratins 5 and 14 and MMP-2 but low expression of cytokeratin 18 (58). They are also androgen-independent for growth and survival (58). Our data confirms the previous findings in several other cell types and shows that Nodal is also expressed in prostate stem cells and its expression is low or absent in differentiated prostate epithelial cells, and that Nodal expression is reacquired by the prostate cancer cells.
Recently several studies have reported that cancers may arise from cancer stem cells (CSCs) which exhibit characteristics similar to normal stem cells (16). CSCs have been identified in a variety of human cancers including leukemias (19–21) and solid tumors such as prostate (22–26), breast (27), colon (28), brain (29), ovarian (30), and pancreatic cancers (31). In prostate, Kasper has suggested that during tumorigenesis, different types of cells in the prostate cell lineage may acquire epigenetic modifications and genetic mutations(32,59). The resulting tumors exhibit differentiated or undifferentiated characteristics depending upon the stem/progenitor cell-of-origin (32,59,60). Mutations in early tissue progenitor cells would promote development of poorly differentiated cancer and solid tumors. Gu et al. (33) presented the first direct evidence for the existence of a putative prostate cancer stem cell. A specific population of prostate cancer stem cells was identified with the capability of developing into basal, luminal, and neuroendrocrine epithelial cell types in vivo, indicating that they arise from a common stem/progenitor cell (33). We observed significant differences in the expression of Nodal in different prostate cancer cell lines. Nodal mRNA and protein expression was relatively high in LNCaP and DU145 cells but it was completely absent in PC3 and PC3M cells. These differences in the expression of Nodal support the notion that different cancer cells may originiate from cancer stem cells at different levels of differentiation (59).
Nodal signaling is initiated by binding of the ligand to type II receptors (ActRII and ActRIIB) which form heterodimers with type I activin-like kinase receptors (ALK 4 or ALK7) and leading to the phosphorylation of Smad2 and Smad3 proteins (53). Phosphorylated Smad2/3 then associate with Smad4 and translocate to the nucleus to regulate gene expression. We observed that mRNA for Nodal receptors, especially type I receptor (ALK4) and type II receptors (ActRII and ActRIIB) were expressed ubiquitously in all prostate cells used in this study. The transcript of ALK7 was very low or undetectable in all prostate cell lines, indicating that ALK7 may not be involved in the possible effects of Nodal in prostate cancer cells. In addition, protein expression of Nodal type I (ALK4) and type II (ActRII) receptors were detected in all prostate cells; however, ActRIIB was expressed only in WPE and HeLa cells. Based on these results, it is logical to assume that Nodal may signal via heterodimeric complexes composed of type II (ActRII) and type I (ALK4) receptors in prostate cancer cell lines while WPE stem cells may also be able to utilize ActRIIB in Nodal effects on these cells.
The expression of functional Nodal receptors was confirmed by the ability of exogenous Nodal to induce phosphorylation of Smad2. We treated WPE, DU145, and PC3 cells with rhNodal and TGFβ1 (as a positive control) at various time points. We observed that Nodal and TGFβ1 stimulation led to Smad2 and Smad3 phosphorylation at Serine 423/425 and 465/467. However, the effects of Nodal on Smad3 phosphorylation were not as significant as those on Smad2 phosphorylation in WPE, DU145, and PC3 cells. These results indicate that Nodal primarily employs Smad2 for intracellular signaling in prostate cell lines. Since ALK7 expression was not detectable in prostate cell lines, it appears that Nodal activity in prostate cells is mediated by ALK4 via a Smad2 mechanism. This finding was further confirmed by the use of a specific ALK4/5/7 inhibitor (SB431542); Nodal effects on Smad2 phosphorylation were blocked by pretreatment with SB431542 in both WPE and DU145 cells.
The expression of both Nodal and its cognate receptors in some prostate cell lines indicates paracrine and autocrine roles of Nodal in these cells. It is interesting to note that some cancer cell lines (LNCaP, DU145 cells) express both Nodal ligands and the signaling receptors, while others (PC3 and PC3M) do not express the ligand but express the Nodal receptors. These results indicate that development and progression of prostate cancer may involve both autocrine and paracrine effects of Nodal. It is tempting to suggest that Nodal secreted by putative cancer stem cells (33) may exert paracrine effects on cancer cells until they acquire autocrine mechanisms. Alternatively, prostate cancer cells differing in the expression of Nodal ligand may represent their origin from stem cells with varying degrees of differentiation (32,59).
Earlier studies in human trophoblast cells and human epithelial ovarian cancer cells have shown that Nodal induces apoptosis and inhibits proliferation via ALK7 and Smad2/3 (37,57,61,62). In the present study, we observed the inhibitory effects of Nodal on cellular proliferation of WPE, RWPE1, and DU145 cells, but had no effect on the proliferation of LNCaP and PC3 cells. These results suggest that some prostate cell lines develop resistance to exogenous rhNodal, while other cell lines such as WPE, RWPE1, and DU145 cells are sensitive to the growth inhibitory effects of rhNodal on their proliferation. Nodal inhibits proliferation in more differentiated cells (WPE) while more metastatic and undifferentiated cells (PC3) are not affected. However, Nodal induces migration of PC3 cells. Previous studies also have shown that Nodal expression was considerably higher in invasive and metastatic cell lines such as human ovarian, melanoma and breast cancer cells (54,55,63); therefore, we also studied the effects of Nodal on cell migration. Interestingly, Nodal had significant effects on migration of PC3 cells but had no effect on migration of WPE and DU145 cells. Pretreatment with a specific inhibitor of ALK4/5/7 (SB431542) completely blocked Nodal induced cell migration of PC3 cells. While SB431542 did not influence EGF (3 ng/ml) induced cell migration of WPE, DU145, and PC3 cells.
Nodal exerts different biological effects on cell proliferation and migration which are specific to different cell lines indicating that Nodal may have different effects in prostate cancer cells depending upon the stage in cancer progression and the cell microenvironment. Similar differential effects during different stages of cancer progression have been demonstrated in several studies of the TGFβ superfamily members. For example, in earlier stages of cancer development, TGFβ inhibits proliferation of prostate epithelial cells and prostate cancer cells; however, in the later stages, TGFβ does not inhibit proliferation but is involved in invasive and metastatic behavior of these cells (39,40). The effects of Nodal and TGFβ on cell proliferation were comparable in prostate cell lines used in the present study. TGFβ isoforms signal through ALK5 (Type I receptor) and our studies have not ruled out an involvement of ALK5 in Nodal effects. Further studies are required to investigate whether there is a cross-talk between TGFβ and Nodal signaling in prostate cancer cells. However, our recent results on the comparative effects of TGFβ1 and Nodal in LNCaP cells indicate that the two cytokines use distinct cell signaling mechanisms (Vo et al., unpublished data).
In conclusion, we demonstrate for the first time that Nodal is expressed in prostate epithelial stem cells and that its expression is lost in differentiated epithelial cells. However, Nodal mRNA and protein (and its receptors) are expressed in some prostate cancer cells and exogenous Nodal inhibits proliferation and induces migration depending upon the cellular context and microenvironment. These studies suggest autocrine and paracrine roles of Nodal during different stages of prostate cancer development and progression.
ACKNOWLEDGMENTS
We would like to thank Dr. Natalya Kueva, Dr. Miao Zhong, Dr. Paulette Dillard, Ana Cecilia Millena and Mojgan Zavareh for their help in this study. These studies were supported by the NIH/NCRR/RCMI grant #2G12RR003062, NIH P20 grant #5P20MD002285-02, DOD grant # W8I-08-1-0077 and NIGMS/NIH grant #5R25GM0604.
REFERENCES
- 1.Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23(6):787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- 2.Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14(6):627–644. [PubMed] [Google Scholar]
- 3.Zimmerman CM, Padgett RW. Transforming growth factor beta signaling mediators and modulators. Gene. 2000;249(1–2):17–30. doi: 10.1016/s0378-1119(00)00162-1. [DOI] [PubMed] [Google Scholar]
- 4.Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370(6488):341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 5.Peng C. The TGF-beta superfamily and its roles in the human ovary and placenta. J Obstet Gynaecol Can. 2003;25(10):834–844. doi: 10.1016/s1701-2163(16)30674-0. [DOI] [PubMed] [Google Scholar]
- 6.Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells. 2002;7(12):1191–1204. doi: 10.1046/j.1365-2443.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- 7.Watabe T, Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009;19(1):103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 8.Mishra L, Derynck R, Mishra B. Transforming growth factor-beta signaling in stem cells and cancer. Science. 2005;310(5745):68–71. doi: 10.1126/science.1118389. [DOI] [PubMed] [Google Scholar]
- 9.Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275(2):403–421. doi: 10.1016/j.ydbio.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 10.Brennan J, Norris DP, Robertson EJ. Nodal activity in the node governs left-right asymmetry. Genes Dev. 2002;16(18):2339–2344. doi: 10.1101/gad.1016202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eimon PM, Harland RM. Effects of heterodimerization and proteolytic processing on Derriere and Nodal activity: implications for mesoderm induction in Xenopus. Development. 2002;129(13):3089–3103. doi: 10.1242/dev.129.13.3089. [DOI] [PubMed] [Google Scholar]
- 12.Nonaka S, Shiratori H, Saijoh Y, Hamada H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature. 2002;418(6893):96–99. doi: 10.1038/nature00849. [DOI] [PubMed] [Google Scholar]
- 13.Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403(6768):385–389. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- 14.Smith WC, McKendry R, Ribisi S, Jr., Harland RM. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell. 1995;82(1):37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 15.Kimelman D. Mesoderm induction: from caps to chips. Nat Rev Genet. 2006;7(5):360–372. doi: 10.1038/nrg1837. [DOI] [PubMed] [Google Scholar]
- 16.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 17.Sell S. Cellular origin of cancer: dedifferentiation or stem cell maturation arrest? Environ Health Perspect. 1993;101(Suppl 5):15–26. doi: 10.1289/ehp.93101s515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sell S, Pierce GB. Maturation arrest of stem cell differentiation is a common pathway for the cellular origin of teratocarcinomas and epithelial cancers. Lab Invest. 1994;70(1):6–22. [PubMed] [Google Scholar]
- 19.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 20.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 21.Holyoake TL, Jiang X, Drummond MW, Eaves AC, Eaves CJ. Elucidating critical mechanisms of deregulated stem cell turnover in the chronic phase of chronic myeloid leukemia. Leukemia. 2002;16(4):549–558. doi: 10.1038/sj.leu.2402444. [DOI] [PubMed] [Google Scholar]
- 22.Rizzo S, Attard G, Hudson DL. Prostate epithelial stem cells. Cell Prolif. 2005;38(6):363–374. doi: 10.1111/j.1365-2184.2005.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 24.De Marzo AM, Meeker AK, Epstein JI, Coffey DS. Prostate stem cell compartments: expression of the cell cycle inhibitor p27Kip1 in normal, hyperplastic, and neoplastic cells. Am J Pathol. 1998;153(3):911–919. doi: 10.1016/S0002-9440(10)65632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hudson DL. Epithelial stem cells in human prostate growth and disease. Prostate Cancer Prostatic Dis. 2004;7(3):188–194. doi: 10.1038/sj.pcan.4500745. [DOI] [PubMed] [Google Scholar]
- 26.Lukacs RU, Lawson DA, Xin L, Zong Y, Garraway I, Goldstein AS, Memarzadeh S, Witte ON. Epithelial stem cells of the prostate and their role in cancer progression. Cold Spring Harb Symp Quant Biol. 2008;73:491–502. doi: 10.1101/sqb.2008.73.012. [DOI] [PubMed] [Google Scholar]
- 27.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat Rev Cancer. 2008;8(6):415–424. doi: 10.1038/nrc2392. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand J, Begaud-Grimaud G, Bessette B, Verdier M, Battu S, Jauberteau MO. Cancer stem cells from human glioma cell line are resistant to Fas-induced apoptosis. Int J Oncol. 2009;34(3):717–727. doi: 10.3892/ijo_00000198. [DOI] [PubMed] [Google Scholar]
- 30.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65(8):3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 31.LaMarca HL, Rosen JM. Minireview: hormones and mammary cell fate--what will I become when I grow up? Endocrinology. 2008;149(9):4317–4321. doi: 10.1210/en.2008-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasper S. Identification, characterization, and biological relevance of prostate cancer stem cells from clinical specimens. Urol Oncol. 2009;27(3):301–303. doi: 10.1016/j.urolonc.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007;67(10):4807–4815. doi: 10.1158/0008-5472.CAN-06-4608. [DOI] [PubMed] [Google Scholar]
- 34.Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol. 2005;23(9):2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 35.Glasgow E, Mishra L. Transforming growth factor-beta signaling and ubiquitinators in cancer. Endocr Relat Cancer. 2008;15(1):59–72. doi: 10.1677/ERC-07-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jakowlew SB. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev. 2006;25(3):435–457. doi: 10.1007/s10555-006-9006-2. [DOI] [PubMed] [Google Scholar]
- 37.Xu G, Zhong Y, Munir S, Yang BB, Tsang BK, Peng C. Nodal induces apoptosis and inhibits proliferation in human epithelial ovarian cancer cells via activin receptor-like kinase 7. J Clin Endocrinol Metab. 2004;89(11):5523–5534. doi: 10.1210/jc.2004-0893. [DOI] [PubMed] [Google Scholar]
- 38.Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12(8):925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 39.Robson CN, Gnanapragasam V, Byrne RL, Collins AT, Neal DE. Transforming growth factor-beta1 up-regulates p15, p21 and p27 and blocks cell cycling in G1 in human prostate epithelium. J Endocrinol. 1999;160(2):257–266. doi: 10.1677/joe.0.1600257. [DOI] [PubMed] [Google Scholar]
- 40.Wu KJ, Zhu GF, Zhang D, Zeng J, Wang XY, Xue Y, Zhang LL, He DL. Identification of TGF-beta/Smads pathway in human prostate cancer cell lines and its significance. Zhonghua Nan Ke Xue. 2009;15(10):920–924. [PubMed] [Google Scholar]
- 41.Simon DP, Vadakkadath Meethal S, Wilson AC, Gallego MJ, Weinecke SL, Bruce E, Lyons PF, Haasl RJ, Bowen RL, Atwood CS. Activin receptor signaling regulates prostatic epithelial cell adhesion and viability. Neoplasia. 2009;11(4):365–376. doi: 10.1593/neo.81544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang HY, Huang HY, Hsieh CY, Li CF, Shyr CR, Tsai MY, Chang C, Chuang YC, Huang KE. Activin A enhances prostate cancer cell migration through activation of androgen receptor and is overexpressed in metastatic prostate cancer. J Bone Miner Res. 2009;24(7):1180–1193. doi: 10.1359/jbmr.090219. [DOI] [PubMed] [Google Scholar]
- 43.Fujii Y, Kawakami S, Okada Y, Kageyama Y, Kihara K. Regulation of prostate-specific antigen by activin A in prostate cancer LNCaP cells. Am J Physiol Endocrinol Metab. 2004;286(6):E927–931. doi: 10.1152/ajpendo.00443.2003. [DOI] [PubMed] [Google Scholar]
- 44.Dowling CR, Risbridger GP. The role of inhibins and activins in prostate cancer pathogenesis. Endocr Relat Cancer. 2000;7(4):243–256. doi: 10.1677/erc.0.0070243. [DOI] [PubMed] [Google Scholar]
- 45.Morrissey C, Brown LG, Pitts TE, Vessella RL, Corey E. Bone morphogenetic protein 7 is expressed in prostate cancer metastases and its effects on prostate tumor cells depend on cell phenotype and the tumor microenvironment. Neoplasia. 12(2):192–205. doi: 10.1593/neo.91836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye L, Kynaston H, Jiang WG. Bone morphogenetic protein-10 suppresses the growth and aggressiveness of prostate cancer cells through a Smad independent pathway. J Urol. 2009;181(6):2749–2759. doi: 10.1016/j.juro.2009.01.098. [DOI] [PubMed] [Google Scholar]
- 47.Ye L, Kynaston H, Jiang WG. Bone morphogenetic protein-9 induces apoptosis in prostate cancer cells, the role of prostate apoptosis response-4. Mol Cancer Res. 2008;6(10):1594–1606. doi: 10.1158/1541-7786.MCR-08-0171. [DOI] [PubMed] [Google Scholar]
- 48.Yuen HF, Chan YP, Cheung WL, Wong YC, Wang X, Chan KW. The prognostic significance of BMP-6 signaling in prostate cancer. Mod Pathol. 2008;21(12):1436–1443. doi: 10.1038/modpathol.2008.94. [DOI] [PubMed] [Google Scholar]
- 49.Millena AC, Reddy SC, Bowling GH, Khan SA. Autocrine regulation of steroidogenic function of Leydig cells by transforming growth factor-alpha. Mol Cell Endocrinol. 2004;224(1–2):29–39. doi: 10.1016/j.mce.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zigmond SH, Foxman EF, Segall JE. Chemotaxis assays for eukaryotic cells. Curr Protoc Cell Biol. 2001;Chapter 12(Unit 12):11. doi: 10.1002/0471143030.cb1201s00. [DOI] [PubMed] [Google Scholar]
- 52.Kharait S, Dhir R, Lauffenburger D, Wells A. Protein kinase Cdelta signaling downstream of the EGF receptor mediates migration and invasiveness of prostate cancer cells. Biochem Biophys Res Commun. 2006;343(3):848–856. doi: 10.1016/j.bbrc.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 53.Yeo C, Whitman M. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol Cell. 2001;7(5):949–957. doi: 10.1016/s1097-2765(01)00249-0. [DOI] [PubMed] [Google Scholar]
- 54.Postovit LM, Margaryan NV, Seftor EA, Hendrix MJ. Role of nodal signaling and the microenvironment underlying melanoma plasticity. Pigment Cell Melanoma Res. 2008;21(3):348–357. doi: 10.1111/j.1755-148X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 55.Strizzi L, Postovit LM, Margaryan NV, Lipavsky A, Gadiot J, Blank C, Seftor RE, Seftor EA, Hendrix MJ. Nodal as a biomarker for melanoma progression and a new therapeutic target for clinical intervention. Expert Rev Dermatol. 2009;4(1):67–78. doi: 10.1586/17469872.4.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, Abbott DE, Seftor RE, Hendrix MJ. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci U S A. 2008;105(11):4329–4334. doi: 10.1073/pnas.0800467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu G, Zhou H, Wang Q, Auersperg N, Peng C. Activin receptor-like kinase 7 induces apoptosis through up-regulation of Bax and down-regulation of Xiap in normal and malignant ovarian epithelial cell lines. Mol Cancer Res. 2006;4(4):235–246. doi: 10.1158/1541-7786.MCR-05-0174. [DOI] [PubMed] [Google Scholar]
- 58.Tokar EJ, Ancrile BB, Cunha GR, Webber MM. Stem/progenitor and intermediate cell types and the origin of human prostate cancer. Differentiation. 2005;73(9–10):463–473. doi: 10.1111/j.1432-0436.2005.00047.x. [DOI] [PubMed] [Google Scholar]
- 59.Kasper S. Exploring the origins of the normal prostate and prostate cancer stem cell. Stem Cell Rev. 2008;4(3):193–201. doi: 10.1007/s12015-008-9033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasper S. Stem cells: The root of prostate cancer? J Cell Physiol. 2008;216(2):332–336. doi: 10.1002/jcp.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Munir S, Xu G, Wu Y, Yang B, Lala PK, Peng C. Nodal and ALK7 inhibit proliferation and induce apoptosis in human trophoblast cells. J Biol Chem. 2004;279(30):31277–31286. doi: 10.1074/jbc.M400641200. [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Tsang BK. Nodal signalling and apoptosis. Reproduction. 2007;133(5):847–853. doi: 10.1530/REP-07-0053. [DOI] [PubMed] [Google Scholar]
- 63.Strizzi L, Abbott DE, Salomon DS, Hendrix MJ. Potential for cripto-1 in defining stem cell-like characteristics in human malignant melanoma. Cell Cycle. 2008;7(13):1931–1935. doi: 10.4161/cc.7.13.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]