Abstract
Bovine pulmonary artery endothelial cells (BPAEC) are extremely sensitive to oxygen, mediated by superoxide production. Ionizing radiation is known to generate superoxide in oxygenated aqueous media; however, at systemic oxygen levels (3%), no oxygen enhancement is observed after irradiation. A number of markers (cell growth, alamarBlue, mitochondrial membrane polarization) for metabolic activity indicate that BPAEC maintained under 20% oxygen grow and metabolize more slowly than cells maintained under 3% oxygen. BPAEC cultured in 20% oxygen grow better when they are transiently transfected with either manganese superoxide dismutase (MnSOD) or copper zinc superoxide dismutase (CuZnSOD) and exhibit improved survival after irradiation (0.5–10 Gy). Furthermore, X irradiation of BPAEC grown in 20% oxygen results in very diffuse colony formation, which is completely ameliorated by either growth in 3% oxygen or overexpression of MnSOD. However, MnSOD overexpression in BPAEC grown in 3% oxygen provides no further radioprotection, as judged by clonogenic survival curves. Radiation does not increase apoptosis in BPAEC but inhibits cell growth and up-regulates p53 and p21 at either 3% or 20% oxygen.
INTRODUCTION
The acute effects of ionizing radiation on humans, leading to death within a few days to a few weeks after irradiation, are usually thought of in terms of three to four principal syndromes that are dependent upon the dose received and the organ systems involved: (1) cerebrovascular syndrome, after >10 Gy total-body irradiation (TBI); (2) gastrointestinal syndrome, after 8–10 Gy TBI; (3) hematopoietic syndrome, after 5.5–7.5 Gy TBI; and (4) cutaneous syndrome after external exposure to β-particle emitters. However, other organs are also sensitive, including kidney, liver and lung. In situations where there has been an airborne release of nuclear material, lung injury can contribute significantly to mortality (1–3). Such studies of radiation-induced lung injury have often focused on end points days to weeks after irradiation (4–8), but there is a paucity of information concerning the early mechanistic processes that lead to radiation-induced lung injury.
Even lethal doses of ionizing radiation may generate relatively small bursts of free radicals in tissue, but the initial insult appears to become amplified in mammalian cells over time. That is, the net flux of damaging oxidants and free radicals produced by cell signaling processes associated with the radiobiological response can be greater than that experienced during the irradiation (9–12). Much effort has been directed toward investigation of cell lines that undergo radiation-induced apoptosis in the case of both directly irradiated cells and those exhibiting a bystander response. The overexpression of manganese superoxide dismutase (MnSOD) has been shown to be radioprotective in cells and in vivo, establishing the involvement of mitochondrially generated superoxide in radiation-induced apoptosis (13–15). The possible role of nitric oxide, superoxide and their derivatives in other mechanisms of radiation-induced cell death is an important issue. However, the significant fluxes of free radicals associated with apoptosis in many cell lines represent a serious confounding interference in such studies.
Here we show that bovine pulmonary artery endothelial cells (BPAEC) undergo cell growth arrest mediated by the up-regulation of p53 and p21 after irradiation at intermediate doses (0.5–10 Gy) while exhibiting negligible apoptosis. The apparent lack of any role for oxygen-derived species in this radiobiological response at systemic oxygen levels is described.
MATERIALS AND METHODS
Cells and Cell Culture
Bovine pulmonary artery endothelial cells (BPAEC) were purchased from Lonza and used at passages 4–8. Cells were grown in Opti-Mem medium supplemented with 10% fetal bovine serum, 5 mM glutamate, 100 U/ml penicillin and 100 μg/ml streptomycin under 5% CO2 and oxygen levels of 3%, 12% or 20% (95% air). Cells were grown under defined conditions (e.g. 20%, 12% or 3% oxygen) for at least 48 h prior to experiments. During irradiation, cells grown under non-atmospheric oxygen levels were placed in gas-impermeable containers; otherwise, the cells were minimally handled (~10 min) under normally oxygenated conditions. Unless stated to the contrary, culture medium was purchased from Invitrogen, and all reagents, ACS grade or better, were obtained from Sigma-Aldrich.
Overexpression of MnSOD, CuZnSOD and iNOS
MnSOD plasmids containing a 22-amino acid mitochondrial targeting sequence and CuZnSOD plasmids were a kind gift from Michael W. Epperly (16). Plasmids containing iNOS were prepared as follows. Briefly, a 3.7 kb XbaI/AflII human hepatocyte iNOS restriction fragment from pBSskhiNOS (obtained from Dr. Tim Billiar, UPMC, Pittsburgh, PA) was cloned into pCA14 adenoviral vector from Microbix System (Toronto, Canada). Two days after transfection, supernatants of BPAEC transfected with 1.3 μg (per 10,000 cells) pCA14hiNOS yielded an increase of 43 ± 3 μM in (by Greiss) over control. Cells were transfected with empty vector, iNOS, CuZnSOD or MnSOD plasmids using LipofectaminePLUS (Invitrogen) according to the manufacturer’s instructions. Cells were used for clonogenic survival and other assays 24 to 48 h after transfections. Up-regulation of MnSOD and CuZnSOD was confirmed by Western blotting and activity assays (see below).
Assays
Cell growth was determined by seeding known numbers of cells, administering experimental treatments at the times given in the figure legends, then recounting. Viability was determined by detaching the plated cells using trypsin, diluting 1:1 with 0.4% trypan blue, and then counting the stained and total cells in a hemocytometer (17). Only live cells (unstained) were included for cell growth determinations, but at no time were there more than 3% trypan blue-positive cells. Mitochondrial membrane polarization (Δψ) was determined by measuring JC-1 (Invitrogen) fluorescence changes (excitation 485 nm, emission 535 and 590 nm) using CCCP (carbonyl cyanide m-chlorophenylhydrazone) to establish complete depolarization (Invitrogen). Cells (1 × 106) were incubated with 2 μM JC-1 at 37°C for 10 min, washed with PBS, then resuspended in 2 ml PBS for fluorescence measurements (18). Caspase 3/7 activities were determined using a kit from Invitrogen (EnzChek), and results were normalized to protein concentrations measured by the BCA assay (Pierce). Annexin V assays were performed using a kit from Enzo Life Sciences according to the manufacturer’s instructions. Cells were incubated in the binding buffer supplied along with annexin V cy3-labeled antibody (excitation 543 nm, emission 570 nm). Cells undergoing apoptosis emitted a red fluorescence and were subsequently counted along with the total number of cells. Calcium fluxes were detected using the calcium-specific indicator Rhod-2 AM (Invitrogen, excitation 552 nm, emission 581 nm) according to the manufacturer’s recommendations. Cells were incubated with hexagon-shaped beads (Nunc, 2D MicroHex™) to which they became attached after several hours. The cells were then allowed to grow on beads for 2 days prior to loading with the calcium fluorescent dye. After loading, the beads were placed in a stirred cell holder/thermostat and allowed to equilibrate at 37°C for 60 s before fluorescence data were collected by taking measurements every 0.1 s. Dihydrohodamine 123 (Invitrogen) was used to detect mitochondrial oxidant production and/or membrane depolarization. The dye was incubated with irradiated or control cells at a concentration of 10 μM for 1 h at 37°C prior to fluorescence detection (excitation 500 nm, emission 535 nm). DAF-2 FM diacetate (4,5-diaminofluorescein diacetate) employed for NO detection was added to cells 30 min prior to irradiation and cells were harvested immediately for fluorescence measurements (excitation 485 nm, emission 538 nm). To assess metabolic activity, alamarBlue (10% solution) was added to plated BPAEC, and the cells were incubated at 37°C for 2 h and then assayed by measuring changes in the fluorescence intensity (excitation 535 nm, emission 590 nm). BPAEC were fixed and stained for senescence-activated β-galactosidase (SA-β-gal) activity as described previously (19). Briefly, cells were fixed for 10 min in 2% formaldehyde, 0.2% glutaraldehyde in PBS and incubated overnight in β-gal staining solution (1 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactopyranose, 5 mM potassium ferrocyanide, 5 mM ferricyanide and 2 mM MgCl2, pH 6.0) at 37°C without CO2. The cells were counterstained with 0.2 mg/ml DAPI (4′6-diaminophenylindole) in 10 mM NaCl to count the total number of cells. The assays for MnSOD and CuZnSOD were performed with or without sodium cyanide, which inhibits CuZnSOD but not MnSOD, on lysed cells and mitochondrial extracts using a kit from Cayman Chemical Co. as described previously (18).
Western Blotting
Cell lysates were normalized to protein prior to Western blotting and/or probing for β-actin according to published methods (20). Mitochondria from cell lysates were isolated by differential centrifugation (21). Cell lysates or isolated mitochondria were mixed with Laemmli sample buffer (1:1) and boiled for 5 min. Ten micrograms of each sample was subjected to 4–20% SDS-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes, and blocked with 3% nonfat milk. Blots were incubated with primary antibodies (1: 2,000) for at least 2 h prior to rinsing and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10,000) for 1 h at room temperature. MnSOD, CuZnSOD, p53, p16, p21 and HRP-conjugated antibodies were purchased from Santa Cruz Biotechnology and detected using chemiluminescence according to the manufacturer’s instructions (Western Lighting, Perkin Elmer).
Radiation Survival Curves
BPAEC were irradiated at ~80% confluence with 0–5 Gy. Cells were then diluted and placed in six-well plates. Cells were cultured, fixed (glutaraldehyde) and stained with crystal violet 7 days later. Colonies of more than 50 cells were counted, and all experiments were performed at least in triplicate. The survival curves were normalized to the starting number of cells adjusted by the plating efficiency (SF = colonies/[cells seeded × %PE]) for each condition. Data points were fitted to curves using the single-hit, multitarget and linear-quadratic models as described previously (18).
Instrumentation
Irradiation of cells was carried out using a Precision X-ray Inc. X-RAD 160 X-ray system. Doses of 0.5 to 10 Gy were administered at a dose rate of 4.4 Gy/min. Fluorescence measurements were carried out using either a Shimadzu RF-5301PC spectrofluorophotometer or a BMG Labtechnology Fluostar Galaxy plate reader.
Statistics
Values are means ± standard errors from at least three independent experiments. Statistical analysis of the data was performed using ANOVA with Tukey’s post hoc test for comparison of two groups and ANOVA with a Dunnett post hoc test for multiple comparisons. P < 0.05 was considered significant. Statistical analyses were performed with Kaleidagraph® (Synergy Software).
RESULTS
Radiation-Independent Cytotoxicity of Oxygen in BPAEC
BPAEC have previously been shown to be extremely sensitive to oxidative stress, and these cells grow slowly in 20% oxygen (essentially hyperoxic) compared to cells grown in 3% oxygen (approximating the systemic levels in vivo). Overexpession of MnSOD (mitochondrial) or CuZnSOD (cytosolic) by transient transfections clearly protects BPAEC against oxidative stress according to various indicators (Fig. 1). The results of cell growth experiments showed that cells transfected with either MnSOD or CuZnSOD and grown in 20% oxygen maintained cell numbers and metabolic rates similar to those of untransfected cells grown in 3% oxygen (Fig. 1A and B). In addition, cells transfected with MnSOD or CuZnSOD and cultured in 20% oxygen had mitochondria that were more highly polarized (greater Δψ) compared to control BPAEC in 20% oxygen (Fig. 1C). Thus the unirradiated cells are clearly oxygen-sensitive in a manner suggesting that the toxicity is mediated by superoxide.
FIG. 1.
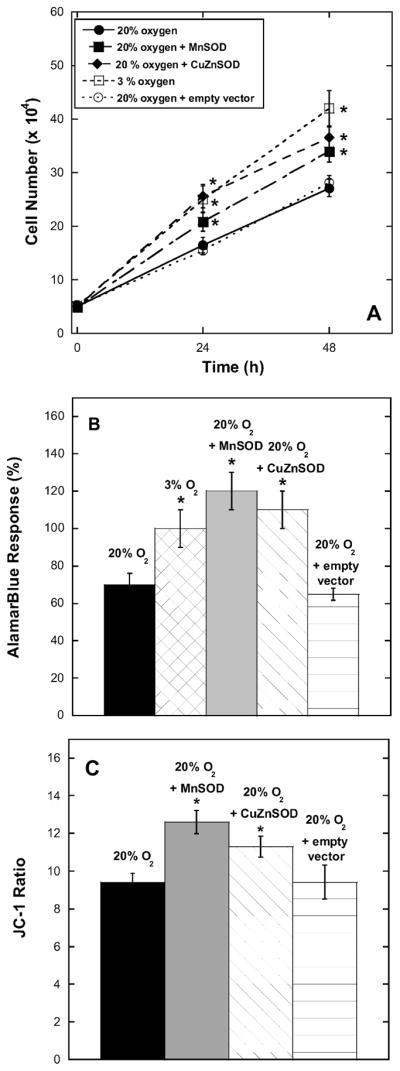
Oxidative stress in BPAEC at 20% oxygen is ameliorated by overexpression of MnSOD and CuZnSOD or lowering the oxygen level (3%). Panel A: BPAEC were seeded at 5 × 104 cells per well, grown for 24 h or 48 h, and counted. Panel B: BPAEC were seeded at 5 × 104 cells per well, grown for 24 h, and then assayed for metabolic activity using 10% alamarBlue. Values were normalized (arbitrarily set at 100%) to the 3% oxygen condition. Panel C: BPAEC were seeded at 5 × 104 cells per well, grown for 24 h, and then assayed for mitochondrial function using JC-1. *Significant difference at P < 0.05 compared to the 20% oxygen group using ANOVA with Dunnett’s post hoc test. Results are expressed as means ± SE from three to six experiments.
Overexpression of SOD Activity
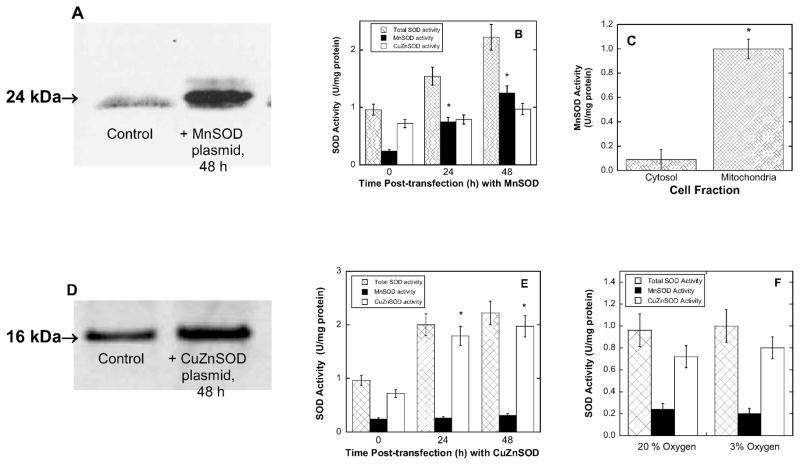
BPAEC transfected with MnSOD plasmid showed up-regulation by Western blot analysis (Fig. 2A) and exhibited MnSOD activity levels up to fivefold greater than controls by enzyme assays (Fig. 2B). In addition, the MnSOD activity in transfected cells was found to be localized to the mitochondria (Fig. 2C). In separate experiments, CuZnSOD was up-regulated in BPAEC by transfection as shown by Western blot analysis (Fig. 2D) and activity assays (Fig. 2E), with the latter indicating a twofold increase in CuZnSOD activity that was essentially confined to the cytosol (data not shown). The level of SOD activity (either MnSOD or CuZnSOD) was not measurably changed upon incubation in 3% oxygen as opposed to 20% oxygen (Fig. 2F), indicating that SOD activity in BPAEC apparently is not subject to oxygen-dependent regulation in this range.
FIG. 2.
SOD overexpression and accompanying increases in enzyme activity after transient transfections of BPAEC with either MnSOD or CuZnSOD. The results of activity assays have been normalized to total protein concentration. Panel A: Western blot analysis of whole cell lysates transfected with MnSOD plasmids 24 h post-transfection. Panel B: Cell lysates transfected with MnSOD and assayed for MnSOD and CuZnSOD activity 24 and 48 h post-transfection (see the Materials and Methods for details). Panel C: Mitochondria in which MnSOD was overexpressed were separated from whole cell lysates by differential centrifugation, and both samples were assayed for MnSOD activity. Panel D: Western blot analysis of whole cell lysates 24 h after transfection with CuZnSOD. Panel E: Cell lysates were assayed for MnSOD and CuZnSOD activity. Panel F: Cell lysates were assayed for total SOD, MnSOD and CuZnSOD activities after BPAEC were incubated in either 3% or 20% oxygen for at least 48 h.
Lack of Radioprotection by MnSOD at Systemic Oxygen Levels
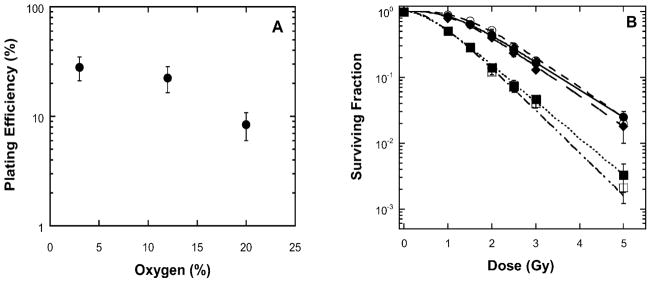
Overexpression of MnSOD (but not CuZnSOD) in several cell lines exhibiting significant apoptosis has previously been shown to be radioprotective, indicating superoxide production in the mitochondria as an integral radiation response (16, 22). The radiation response for BPAEC transfected with MnSOD was compared to controls in the presence of 20% or 3% oxygen. BPAEC grown in 20% oxygen did not form well-defined colonies compared to those grown at 3% oxygen, but transfection with MnSOD (or CuZnSOD) increased colony formation (data not shown). The plating efficiency under three different oxygen concentrations (20, 12 and 3%) is shown in Fig. 3A. The change in the plating efficiency of BPAEC reflected the oxygen toxicity (Fig. 1). A marked improvement in clonogenic survivability was observed (Fig. 3B and Table 1) when cells were grown, irradiated and maintained in 3% oxygen compared to those handled throughout under 20% oxygen. The survival curves for BPAEC cultured in 20% oxygen and transfected with MnSOD prior to irradiation were very similar to those for the untransfected cells handled in 3% oxygen. Cells cultured in 3% oxygen and transfected with MnSOD prior to irradiation did not appreciably increase their survival over that of untransfected cells grown in 3% oxygen. None of the observed protective effects appeared to be due to any general stimulation of the pathways involved in the transfection of cells, because iNOS overexpression had no effect on the survival curve obtained under 20% oxygen.
FIG. 3.
Clonogenic survival curves for BPAEC. Panel A: Plating efficiency at 3, 12 and 20% oxygen. Panel B: Radiation survival curves of BPAEC generated in 20% oxygen (■, dotted line), 20% oxygen with iNOS overexpressed (□, dashed-dotted line), 3% oxygen (○, solid line), 20% oxygen with MnSOD overexpressed (◆, long dashed line), and 3% oxygen with MnSOD overexpressed (●, short dashed line).
TABLE 1.
Parameters of Single-Hit Multitarget Survival Curves Indicating that Overexpression of MnSOD or Low Oxygen (3%) Increases Survival of BPAEC
| Treatment | D0 | n |
|---|---|---|
| 20% oxygen | 1.47 (0.05) | 2.7 (0.2) |
| 20% oxygen + MnSOD | 1.10 (0.06) | 4.3 (0.5)* |
| 20% oxygen + iNOS | 1.50 (0.06) | 2.8 (0.2) |
| 3% oxygen | 1.10 (0.06) | 4.2 (0.5)* |
| 3% oxygen + MnSOD | 1.20 (0.1) | 4.8 (0.8)* |
Note. Results are the means with standard errors in parentheses.
Significant difference compared to 20% oxygen (P < 0.05) using ANOVA with Dunnett’s test.
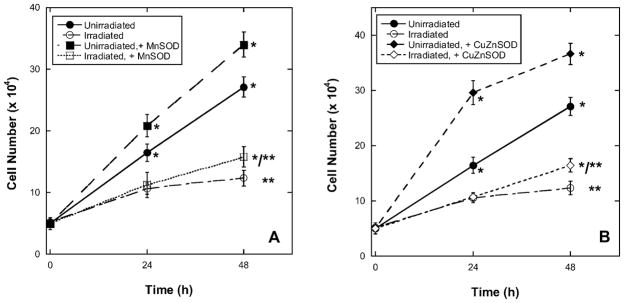
Using a trypan blue exclusion assay to monitor cell death, at 20% oxygen and over 24–48 h, BPAEC growth was slowed in irradiated cells compared to unirradiated controls (Fig. 4). When unirradiated cells had previously been transfected with MnSOD (Fig. 4A) or CuZnSOD (Fig. 4B), there was a significant increase in the number of viable cells at 24 or 48 compared to untransfected controls. At 24 h after irradiation, growth of both transfected (MnSOD or CuZnSOD) and untransfected cells was similarly inhibited. However, at 48 h, there was a small but significant increase in the growth of the cells in which SOD activity had been overexpressed compared to untransfected cells.
FIG. 4.
Radiation slowed growth of BPAEC, but no radioprotection occurred with overexpression of MnSOD or CuZnSOD in 20% oxygen at less than 48 h. *Significant difference at P < 0.05 compared with unirradiated cells, ANOVA with Dunnett’s post hoc test. **Significant increase in numbers observed for irradiated cells transfected with either MnSOD (panel A) or CuZnSOD (panel B) compared to nontransfected irradiated cells; ANOVA with Tukey’s post hoc test.
BPAEC do not Undergo Significant Apoptosis in Response to Radiation
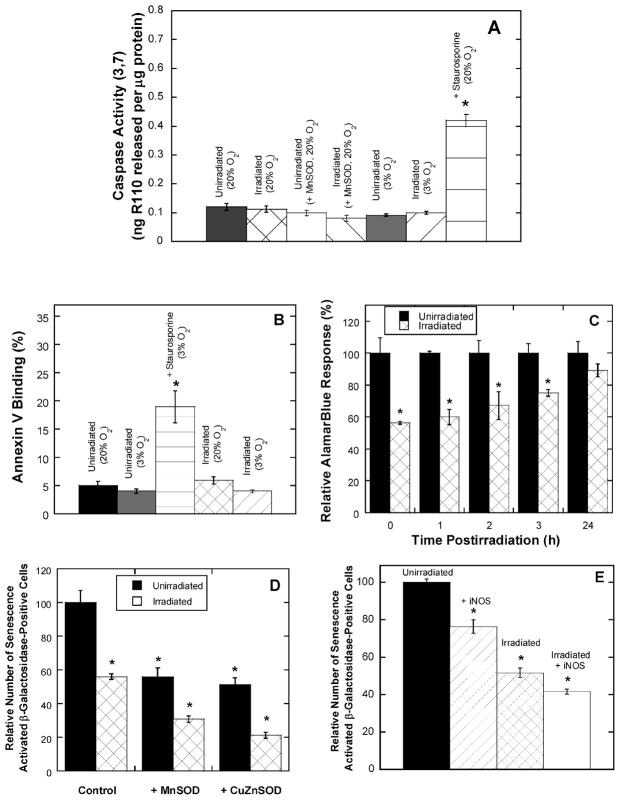
Apoptosis is difficult to induce by some commonly employed experimental stimuli in BPAEC (23, 24). Irradiation of BPAEC did not increase caspase activity or annexin V binding (Fig. 5A and B), and while we observed distinct changes in cell morphology at 24 h (not shown), none were consistent with apoptosis. In contrast, using staurosporine as a positive control to initiate apoptosis, a readily detectable increase in caspase activity and annexin V binding was obtained (Fig. 5A and B). In addition, while there was an immediate change in metabolic activity, as observed by the alamarBlue assay (Fig. 5C), this was reversed after 24 h. We also examined whether the inhibition of growth of BPAEC was due to senescence by determining senescence-activated β-galactosidase activity (SA-β-Gal). However, SA-β-Gal activity staining of normal compared to irradiated cells (up to 20 Gy) indicated that senescence was actually decreased after irradiation (Fig. 5D). MnSOD or CuZnSOD overexpression similarly decreased SA-β-Gal expression in both unirradiated and irradiated cells (Fig. 5D). Because both protective and damaging roles for nitric oxide are sometimes suggested for situations where there may be oxidative stress, we also looked at the effect of iNOS overexpression. iNOS did not protect BPAEC against ionizing radiation, but it did significantly reduce SA-β-Gal activity either alone or in conjunction with radiation (Fig. 5E).
FIG. 5.
Irradiated BPAEC do not exhibit apoptosis and senescence is ameliorated. Panel A: BPAEC cultured in 20% oxygen or 3% oxygen were exposed to 10 Gy iradiation, and whole cell lysates were harvested and assayed for caspase 3/7 activity 24 h postirradiation. When required, transfections with MnSOD were carried out 24 h before irradiation. As a positive control, BPAEC were treated with 1 μM staurosporine for 3 h prior to conducting the caspase activity assay. Panel B: Annexin V activity in unirradiated cells (20% and 3% oxygen), irradiated cells (10 Gy, 3% and 20% oxygen), and staurosporine-treated cells (1 μM for 3 h in 20% oxygen). Panel C: BPAEC were irradiated (10 Gy, 20% oxygen) and incubated along with control cells in 10% alamarBlue, starting at the times indicated, for 2 h before determining changes in fluorescence. Panel D: BPAEC cultured in 20% oxygen were exposed to 10 Gy and then analyzed for senescence-activated β-galactosidase activity 24 h postirradiation. When required, transfections with MnSOD or CuZnSOD were carried out 24 h before irradiation. Panel E: BPAEC cultured in 20% oxygen were exposed to 10 Gy and then analyzed for β-galactosidase activity 24 h postirradiation. When required, transfections with iNOS were carried out 24 h before irradiation.
Western blot analysis indicated that irradiation led to up-regulation of p53 (Fig. 6, upper two panels), a key protein involved in a number of antiproliferative cell activities and p21 (Fig. 6, middle two panels), consistent with inhibition of the cell cycle leading to reproductive death. The deactivation of p16, a protein involved in senescence, was also confirmed (Fig. 6, lower two panels). These changes all became apparent within 3 h after irradiation (10 Gy) and persisted for at least 24 h. The blots shown were obtained with cells cultured under 20% oxygen. At 3% oxygen (data not shown), we detected little p16 both before and after irradiation, indicating fewer senescent cells in the cultures; however, p53 and p21 were up-regulated in a manner analogous to the examples shown in Fig. 6. While these data suggest that there had been a perturbation of the cell cycle, more data are required to determine whether and at what point the putative cell cycle arrest occurs. Consequently, irrespective of the prevailing oxygen level (in the range 3–20%), the overwhelmingly dominant radiobiological response of BPAEC is apparently to undergo diminished growth. It is important to note that these experiments were performed using subconfluent (80%) cultures. The same changes in p53, p21 and p16 levels can also occur in overly confluent (but unirradiated) endothelial cells, presumably in association with contact inhibition.
FIG. 6.
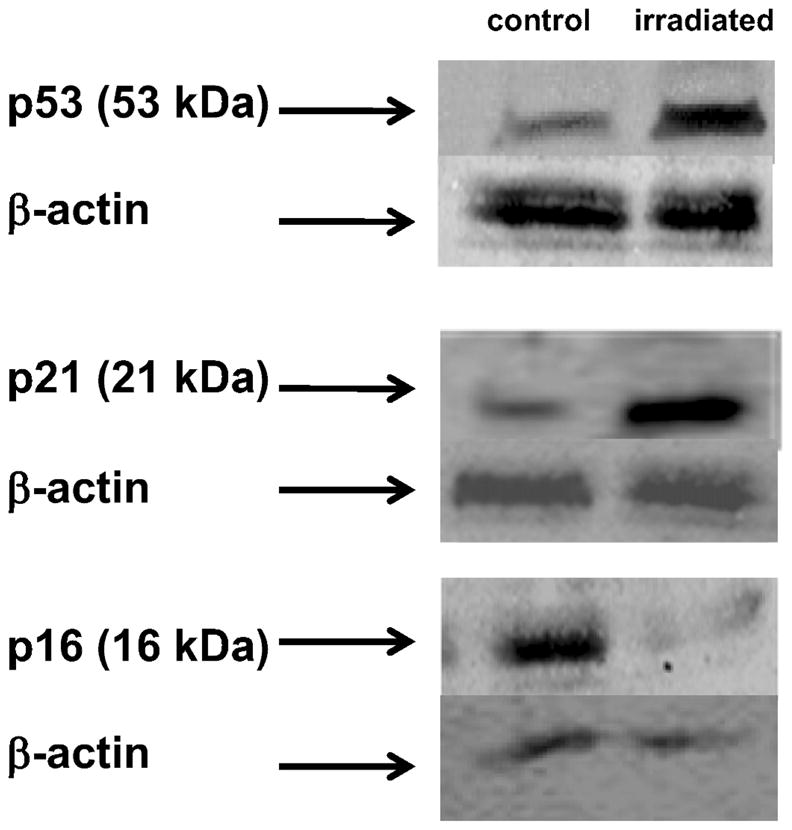
Irradiation of BPAEC up-regulates p53 and p21 within 3 h and leads to lower p16 expression.
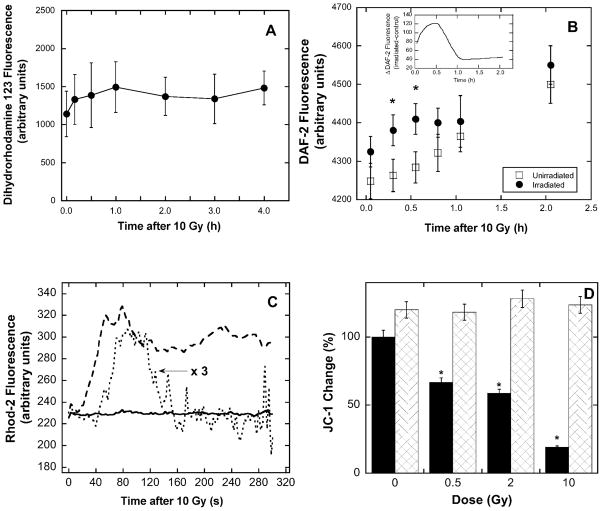
Free Radical Production and Mitochondrial Response in Irradiated BPAEC
Irradiation of aqueous solutions generates predominantly solvated electrons, hydroxyl radicals, hydrogen atoms and, in the presence of oxygen, superoxide ions (25, 26). Subsequently, irradiated cells/tissue produce further reactive species, all of which can act to oxidize or reduce biological compounds. Dihydrorhodamine 123 accumulates predominantly in the mitochondria and has been used previously to detect ROS/RNS production in mitochondria (27). BPAEC that were irradiated with up to 10 Gy in 20% oxygen and then exposed to dihydrorhodamine 123 showed no significant increase in fluorescence (Fig. 7A), indicating very little reactive mitochondrial oxidant production compared to unirradiated control cells for at least 4 h. This is in contrast to some other cells, such as hematopoietic progenitor cells, that produce a small oxidant burst within 30 min (L. L. Pearce and J. Peterson, unpublished observations). Under the same conditions, no changes in either ATP or GSH levels were detected in BPAEC over several hours after the dose (data not shown).
FIG. 7.
A rapid mitochondrial response with no detectable oxidant burst is observed in irradiated BPAEC. Panel A: BPAEC cultured in 20% oxygen, preincubated with dihydrorhodamine 123 and assayed after irradiation showed no oxidant burst. Panel B: Incubation of irradiated BPAEC (10 Gy, ■) with DAF-2 resulted in a small NO increase shortly after irradiation compared to control (●); P < 0.05 (*). Inset: Differences in DAF-2 fluorescence between the results for irradiated and control cells were plotted as a cubic spline calculated to pass through the data. Panel C: Calcium fluxes after irradiation of BPAEC. Within 1 min, irradiation of cells loaded with the Ca2+ indicator dye Rhod-2 AM (2 μM) exhibited an increase in fluorescence indicating an increase in cytosolic calcium levels (dashed line) compared to control (solid line). After preloading of the cells with Rhod-2 for 24 h and subsequent irradiation, a weaker and transient calcium flux was detected (dotted line). Panel D: Changes in mitochondrial membrane polarization as measured by JC-1 30 min after irradiation (solid bars). Cells that overexpress MnSOD (crosshatched bars) are protected against membrane depolarization.
Irradiation of BPAEC resulted in a small elevation in the rate of NO production as detected by increased DAF-2 fluorescence (Fig. 7B). The increase in DAF-2 fluorescence of unirradiated cells was essentially linear with respect to time, indicating a constant rate of NO production during the course of the experiment. However, irradiated cells exhibited small but significantly increased levels of NO at 20 and 30 min. By taking the difference in DAF-2 fluorescence between for irradiated and control cells and plotting this as a cubic spline calculated to pass through the data (Fig. 7B, inset) it is apparent that there was a transient elevation of NO production that peaked at about 25 min postirradiation. Other cell lines have shown similar “bursts” of NO (9, 10), and some authors have suggested that increased NO production can be protective when cells are irradiated (28). Thus we reasoned that increased NO production by the overexpression of iNOS might be protective in BPAEC, but upon examination of these transiently transfected with iNOS, we found no change in survival (Fig. 3B and Table 1).
Leach et al. observed a transient calcium flux in Chinese hamster ovary cells immediately after irradiation coincident with NO production (9). After 10 Gy, a rise in cellular Ca2+ was detected within ~1 min postirradiation in BPAEC (Fig. 7C). When cells are incubated with the Ca2+-sensitive dye Rhod-2 for 24 h prior to irradiation, the indicator accumulates in mitochondria (29). BPAEC prepared in this fashion also showed an increase in mitochondrial Ca2+ after 10 Gy, but unlike the predominantly cytoplasmic response, the elevated mitochondrial flux persisted for only ~2 min (Fig. 7C).
Mitochondrial membrane polarization (Δψ) changes transiently in hematopoietic progenitor cells after irradiation. MnSOD (and not CuZnSOD) overexpression in 32D cells was shown to be radioprotective and ameliorated a transient change in Δψ observed at 15–30 min that returned to control levels by 2 h (18). In BPAEC, Δψ was transiently decreased within minutes of irradiation, as measured using JC-1, and overexpression of MnSOD prevented the transient decrease in Δψ (Fig. 7D). It is possible that the membrane potential dropped more quickly in cells overexpressing MnSOD; such changes would be difficult to detect if they were completed less than ~5 min postirradiation. We did not observe any later changes in Δψ up to 8 h after irradiation.
Similar mitochondrial responses (but including oxidant production and more persistent membrane depolarization) have been reported previously in other cell lines, including those undergoing a bystander response; however, in those cases apoptosis was the end point (30–31). It follows that the transient nature of the mitochondrial response we observed in BPAEC may be linked to the avoidance of apoptosis, but it is unclear whether this is coincidental or whether there is a direct cause-and-effect relationship.
DISCUSSION
BPAEC exhibit limited growth under hyperoxic conditions (>80% oxygen), but they appear to thrive under near systemic (~3%) oxygen levels (Bruce R. Pitt, personal communication). This is broadly in keeping with the realization that many cell types (hematopoietic, fibroblast, etc.) grow and proliferate better at lower oxygen tensions, e.g. cultured fibroblasts in 7% oxygen (32). Pitt et al. (24) showed that up-regulation of metallothionein, predominantly in the cytosol of sheep pulmonary artery endothelial cells (SPAEC), also attenuated oxidative stress, probably through reduction of oxidants by the many cysteine thiols present in this protein. We have clearly demonstrated that BPAEC grown under 20% oxygen exhibit a number of markers of oxidative stress that are attenuated after over-expression of SOD activity (Fig. 1). Both CuZnSOD (cytosolic) and MnSOD (mitochondrial) significantly ameliorate radiation-independent oxidative stress in this cell line. Recent work demonstrated that Nox4 (33–34) and Nox2 (34) (NADPH oxidases) have a physiological role in hyperoxia-induced production of reactive oxidants and are probably localized to a number of membrane structures within the cell. It follows that NADPH oxidases may be key producers of superoxide in BPAEC in addition to mitochondrial sources, but further work is required to identify the center(s) in question.
The dependence of colony formation in BPAEC on oxidative stress is striking; elevated (20%) oxygen largely prevents proper colony formation, while either over-expression of SODs or reducing the oxygen to systemic (3%) levels results in the growth of easily identifiable, well-formed colonies. It has been reported that VEGF (vascular endothelial growth factor) promotes colony formation (35, 36) and stimulates up-regulation of MnSOD (37), in agreement with our results showing that alleviation of oxidative stress promotes cell growth and colony formation.
While the behavior of BPAEC in relation to superoxide-mediated oxygen toxicity is of great interest, our primary goal was to ascertain the mechanism(s) by which ionizing radiation injures these cells. A number of relevant studies on other endothelial cells have been carried out with a wide variety of results (38, 39). Endothelial cells from the GI tract, capillary endothelium of lung, liver and the central nervous system all appear to undergo substantial apoptosis, while some other endothelial types do not. For example, Li et al. found no apoptosis in bone marrow endothelial cells isolated from irradiated mice, although DNA damage and repair were initiated within 3 h after lethal irradiation (38). In the present work, we have shown that BPAEC essentially do not undergo apoptosis but instead appear to undergo reproductive death exclusively after irradiation, although the mechanistic details have not been established. However, within 3 h postirradiation, we observed that p53 and p21 were up-regulated and p16 was down-regulated (Fig. 6). Normally p53 up-regulation is associated with apoptosis but not in the case of BPAEC (Fig. 5A). In keeping with these findings, the up-regulation of p21, which may or may not be a consequence of p53 up-regulation, has been reported to be important in suppressing apoptosis (40).
Leach et al. proposed (10) that ionizing radiation initiates an oxidative event that results in calcium release. Elevated mitochondrial calcium may then cause a depolarization of the mitochondria membrane, and this could lead to elevated mitochondrial ROS/RNS generation. In addition, these authors suggested that rather small initial signals from difficult-to-detect ROS/RNS may then be propagated from mitochondria to mitochondria. While we did not detect any significant mitochondrial ROS/RNS after irradiation, we did observe a small increase in NO. Mitochondrial nitric oxide synthase (mtNOS) appears to be a form of neuronal nitric oxide synthase (nNOS), and both are activated by calcium (21). Thus the calcium flux may lead to the observed increase in NO production. It is possible that some ROS/RNS may result (e.g. peroxynitrite from the combination of nitric oxide and superoxide) from the mitochondria that is below our detection limit. However, it is also likely that there may be non-mitochondrial sources of ROS/RNS, for example, NADPH oxidase and/or eNOS.
Because we had confirmed the suspected sensitivity of BPAEC to superoxide-mediated oxygen toxicity, the apparent lack of any radiosensitizing effect attributable to superoxide (and hence oxygen) at systemic (~3% O2) levels was unexpected. There is generally only a marginal change in the cellular response to radiation between 3% and 100% oxygen (26). Conversely, there is usually an exponential decrease in survival observed when the oxygen level at which cell cultures are irradiated is increased from 0.5% to 3% (26). In the present study, overexpression of MnSOD in cells irradiated at 3% oxygen had no impact on the survival curve, and we observed a significant decrease in colony survival when the irradiations were performed at 20% compared to 3% oxygen (Fig. 3B). That is, the oxygen dependence we observed does not occur in the correct concentration range for it to be a classic radiosensitizing effect involving oxygen-derived reactive species generated at the time of irradiation. The current findings strongly suggest that the superoxide-mediated oxygen dependence apparent in BPAEC grown in 20% oxygen (Fig. 3B) is an exacerbating effect associated with postirradiation cell signaling that may not be specific to radiation. Although previous studies with hematopoietic progenitor cells and other lines showing that overexpression of MnSOD or SOD mimetics increased postirradiation survival were carried out at 20% oxygen (11, 18, 41–43), radioprotection was also demonstrated at systemic oxygen levels in vivo (42, 44). However, unlike BPAEC, the cell lines used in the other investigations exhibit substantial radiation-induced apoptosis.
It has been well documented that many cells are more sensitive to radiation at various points in their cell cycles. Balasubramaniam et al. showed that asynchronous PAEC (ovine) had a higher number (almost double) of cells in S phase at 3% oxygen than at 20% oxygen after 48 h growth (45). Because S-phase cells are generally much less sensitive to radiation, this could at least partially explain our survival curve results. For example, Quiet et al. compared two human squamous cell lines and found twice the number of S-phase cells in one line with a difference of ~1 Gy in their survival curve D0 parameters (46). Although we saw a smaller difference in the D0s (i.e. ~0.3 Gy) in our survival curves, the cell cycle parameters of BPAEC will be examined with respect to oxygen and cell cycle radiation sensitivity in future studies. In addition, while we did not see any changes in cell survival with increasing NO production (Fig. 3B), others found that decreasing NO levels can perturb cell cycle progression (47). We also note that while MnSOD has not been observed to alter cell cycle distribution in other cell lines, this may not be the case for BPAEC (48).
It is tempting to speculate that cells undergoing postirradiation apoptosis may benefit much more from protection against oxidative stress than cells like BPAEC that exhibit a different radiological response. Applying this consideration to whole tissues, where oxidative stress due to secondary inflammation can be an additional problem, it follows that antioxidant therapy (including MnSOD gene therapy) can be expected to affect some radiobiological outcomes, such as amelioration of pulmonary pneumonitis (42, 49), but have no impact on others. The difficulty of assessing the potential role of oxidative stress in late effects like organ failure (50–52) may be implicit here.
Acknowledgments
This research was supported by NIH grant no. U19-AI068021 (Project 3 to JP and LLP).
References
- 1.Vanmarcke H. Radon: a special case in radiation protection. Radiat Prot Dosimetry. 2008;130:76–80. doi: 10.1093/rpd/ncn106. [DOI] [PubMed] [Google Scholar]
- 2.Belyaeva ZD, Osovets SV, Scott BR, Zhuntova GV, Grigoryeva ES. Modeling of respiratory system dysfunction among nuclear workers: a preliminary study. Dose Response. 2008;6:319–332. doi: 10.2203/dose-response.06-117.Belyaeva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott BR, Peterson VL. Risk estimates for deterministic health effects of inhaled weapons grade plutonium. Health Phys. 2003;85:280–293. doi: 10.1097/00004032-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Breuer R, Tochner Z, Conner MW, Nimrod A, Gorecki M, Or R, Slavin S. Superoxide dismutase inhibits radiation-induced lung injury in hamsters. Lung. 1992;170:19–29. doi: 10.1007/BF00164752. [DOI] [PubMed] [Google Scholar]
- 5.Kang SK, Rabbani ZN, Folz RJ, Golson ML, Huang H, Yu D, Samulski TS, Dewhirst MW, Anscher MS, Vujaskovic Z. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2003;57:1056–1066. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- 6.Law MP, Ahier RG, Coultas PG. The role of vascular injury in the radiation response of mouse lung. Br J Cancer Suppl. 1986;7:327–329. [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson LM, Evans ML, Graham MM, Eary JF, Dahlen DD. Vascular response to radiation injury in the rat lung. Radiat Res. 1992;129:139–148. [PubMed] [Google Scholar]
- 8.Serin M, Gulbas H, Gurses I, Erkal HS, Yucel N. The histopathological evaluation of the effectiveness of melatonin as a protectant against acute lung injury induced by radiation therapy in a rat model. Int J Radiat Biol. 2007;83:187–193. doi: 10.1080/09553000601129093. [DOI] [PubMed] [Google Scholar]
- 9.Leach JK, Black SM, Schmidt-Ullrich RK, Mikkelsen RB. Activation of constitutive nitric-oxide synthase activity is an early signaling event induced by ionizing radiation. J Biol Chem. 2002;277:15400–15406. doi: 10.1074/jbc.M110309200. [DOI] [PubMed] [Google Scholar]
- 10.Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001;61:3894–3901. [PubMed] [Google Scholar]
- 11.Epperly MW, Sikora CA, DeFilippi SJ, Gretton JA, Zhan Q, Kufe DW, Greenberger JS. Manganese superoxide dismutase (SOD2) inhibits radiation-induced apoptosis by stabilization of the mitochondrial membrane. Radiat Res. 2002;157:568–577. doi: 10.1667/0033-7587(2002)157[0568:msdsir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Isoir M, Buard V, Gasser P, Voisin P, Lati E, Benderitter M. Human keratinocyte radiosensitivity is linked to redox modulation. J Dermatol Sci. 2006;41:55–65. doi: 10.1016/j.jdermsci.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Greenberger JS, Epperly MW, Gretton J, Jefferson M, Nie S, Bernarding M, Kagan V, Guo HL. Radio-protective gene therapy. Curr Gene Ther. 2003;3:183–195. doi: 10.2174/1566523034578384. [DOI] [PubMed] [Google Scholar]
- 14.Guo H, Epperly MW, Bernarding M, Nie S, Gretton J, Jefferson M, Greenberger JS. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) intratracheal gene therapy reduction of irradiation-induced inflammatory cytokines does not protect orthotopic Lewis lung carcinomas. In Vivo. 2003;17:13–21. [PubMed] [Google Scholar]
- 15.Zhang X, Epperly MW, Kay MA, Chen ZY, Dixon T, Franicola D, Greenberger BA, Komanduri P, Greenberger JS. Radioprotection in vitro and in vivo by minicircle plasmid carrying the human manganese superoxide dismutase transgene. Hum Gene Ther. 2008;19:820–826. doi: 10.1089/hum.2007.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epperly MW, Gretton JE, Sikora CA, Jefferson M, Bernarding M, Nie S, Greenberger JS. Mitochondrial localization of superoxide dismutase is required for decreasing radiation-induced cellular damage. Radiat Res. 2003;160:568–578. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 17.Tennant JR. Evaluation of the trypan blue technique for determination of cell viability. Transplantation. 1964;2:685–694. doi: 10.1097/00007890-196411000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Epperly MW, Melendez JA, Zhang X, Nie S, Pearce L, Peterson J, Franicola D, Dixon T, Greenberger BA, Greenberger JS. Mitochondrial targeting of a catalase transgene product by plasmid liposomes increases radioresistance in vitro and in vivo. Radiat Res. 2009;171:588–595. doi: 10.1667/RR1424.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epperly MW, Gretton JA, DeFilippi SJ, Greenberger JS, Sikora CA, Liggitt D, Koe G. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD2-PL) gene therapy. Radiat Res. 2001;155:2–14. doi: 10.1667/0033-7587(2001)155[0002:morice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Kanai AJ, Pearce LL, Clemens PR, Birder LA, VanBibber MM, Choi SY, de Groat WC, Peterson J. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci USA. 2001;98:14126–14131. doi: 10.1073/pnas.241380298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong GH. Protective roles of cytokines against radiation: induction of mitochondrial MnSOD. Biochim Biophys Acta. 1995;1271:205–209. doi: 10.1016/0925-4439(95)00029-4. [DOI] [PubMed] [Google Scholar]
- 23.Tang ZL, Wasserloos KJ, Liu X, Stitt MS, Reynolds IJ, Pitt BR, St Croix CM. Nitric oxide decreases the sensitivity of pulmonary endothelial cells to LPS-induced apoptosis in a zinc-dependent fashion. Mol Cell Biochem 234–. 2002;235:211–217. [PubMed] [Google Scholar]
- 24.Pitt BR, Schwarz M, Woo ES, Yee E, Wasserloos K, Tran S, Weng W, Mannix RJ, Watkins SA, Lazo JS. Overexpression of metallothionein decreases sensitivity of pulmonary endothelial cells to oxidant injury. Am J Physiol. 1997;273:L856–865. doi: 10.1152/ajplung.1997.273.4.L856. [DOI] [PubMed] [Google Scholar]
- 25.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 26.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 6. Lippincott Williams & Wilkins; Philadelphia, PA: 2005. [Google Scholar]
- 27.Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chazotte-Aubert L, Pluquet O, Hainaut P, Ohshima H. Nitric oxide prevents gamma-radiation-induced cell cycle arrest by impairing p53 function in MCF-7 cells. Biochem Biophys Res Commun. 2001;281:766–771. doi: 10.1006/bbrc.2001.4423. [DOI] [PubMed] [Google Scholar]
- 29.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 30.Hei TK, Zhou H, Ivanov VN, Hong M, Lieberman HB, Brenner DJ, Amundson SA, Geard CR. Mechanism of radiation-induced bystander effects: a unifying model. J Pharm Pharmacol. 2008;60:943–950. doi: 10.1211/jpp.60.8.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nugent SM, Mothersill CE, Seymour C, McClean B, Lyng FM, Murphy JE. Increased mitochondrial mass in cells with functionally compromised mitochondria after exposure to both direct gamma radiation and bystander factors. Radiat Res. 2007;168:134–142. doi: 10.1667/RR0769.1. [DOI] [PubMed] [Google Scholar]
- 32.Bradley TR, Hodgson GS, Rosendaal M. The effect of oxygen tension on haemopoietic and fibroblast cell proliferation in vitro. J Cell Physiol. 1978;97:517–522. doi: 10.1002/jcp.1040970327. [DOI] [PubMed] [Google Scholar]
- 33.Collins-Underwood JR, Zhao W, Sharpe JG, Robbins ME. NADPH oxidase mediates radiation-induced oxidative stress in rat brain microvascular endothelial cells. Free Radic Biol Med. 2008;45:929–938. doi: 10.1016/j.freeradbiomed.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffith B, Pendyala S, Hecker L, Lee PJ, Natarajan V, Thannickal VJ. NOX enzymes and pulmonary disease. Antioxid Redox Signal. 2009;11:2505–2516. doi: 10.1089/ars.2009.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciulla MM, Giorgetti A, Silvestris I, Cortiana M, Montelatici E, Paliotti R, Annoni GA, Fiore AV, Giordano R, Lazzari L. Endothelial colony forming capacity is related to C-reactive protein levels in healthy subjects. Curr Neurovasc Res. 2006;3:99–106. doi: 10.2174/156720206776875876. [DOI] [PubMed] [Google Scholar]
- 36.Pierre M, Yoshimoto M, Huang L, Richardson M, Yoder MC. VEGF and IHH rescue definitive hematopoiesis in Gata-4 and Gata-6-deficient murine embryoid bodies. Exp Hematol. 2009;37:1038–1053. doi: 10.1016/j.exphem.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abid MR, Tsai JC, Spokes KC, Deshpande SS, Irani K, Aird WC. Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. FASEB J. 2001;15:2548–2550. doi: 10.1096/fj.01-0338fje. [DOI] [PubMed] [Google Scholar]
- 38.Li XM, Hu Z, Jorgenson ML, Wingard JR, Slayton WB. Bone marrow sinusoidal endothelial cells undergo nonapoptotic cell death and are replaced by proliferating sinusoidal cells in situ to maintain the vascular niche following lethal irradiation. Exp Hematol. 2008;36:1143–1156. doi: 10.1016/j.exphem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 39.Fu P, Birukova AA, Xing J, Sammani S, Murley JS, Garcia JG, Grdina DJ, Birukov KG. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur Respir J. 2009;33:612–624. doi: 10.1183/09031936.00014808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez R, Meuth M. Chk1 and p21 cooperate to prevent apoptosis during DNA replication fork stress. Mol Biol Cell. 2006;17:402–412. doi: 10.1091/mbc.E05-07-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epperly MW, Rugo R, Cao S, Wang H, Franicola D, Goff JP, Shen H, Zhang X, Wiktor-Brown D, Greenberger JS. Investigation of the effects of aging on homologous recombination in long-term bone marrow cultures. In Vivo. 2009;23:669–677. [PMC free article] [PubMed] [Google Scholar]
- 42.Epperly M, Bray J, Kraeger S, Zwacka R, Engelhardt J, Travis E, Greenberger J. Prevention of late effects of irradiation lung damage by manganese superoxide dismutase gene therapy. Gene Ther. 1998;5:196–208. doi: 10.1038/sj.gt.3300580. [DOI] [PubMed] [Google Scholar]
- 43.Vorotnikova E, Rosenthal RA, Tries M, Doctrow SR, Braunhut SJ. Novel synthetic SOD/catalase mimetics can mitigate capillary endothelial cell apoptosis caused by ionizing radiation. Radiat Res. 2010;173:748–759. doi: 10.1667/RR1948.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epperly MW, Sikora CA, DeFilippi SJ, Gretton JE, Bar-Sagi D, Archer H, Carlos T, Guo H, Greenberger JS. Pulmonary irradiation-induced expression of VCAM-I and ICAM-I is decreased by manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) gene therapy. Biol Blood Marrow Transplant. 2002;8:175–187. doi: 10.1053/bbmt.2002.v8.pm12014807. [DOI] [PubMed] [Google Scholar]
- 45.Balasubramaniam V, Maxey AM, Fouty BW, Abman SH. Nitric oxide augments fetal pulmonary artery endothelial cell angiogenesis in vitro. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1111–1116. doi: 10.1152/ajplung.00431.2005. [DOI] [PubMed] [Google Scholar]
- 46.Quiet CA, Weichselbaum RR, Grdina DJ. Variation in radiation sensitivity during the cell cycle of two human squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 1991;20:733–738. doi: 10.1016/0360-3016(91)90016-w. [DOI] [PubMed] [Google Scholar]
- 47.Janssen YM, Soultanakis R, Steece K, Heerdt E, Singh RJ, Joseph J, Kalyanaraman B. Depletion of nitric oxide causes cell cycle alterations, apoptosis, and oxidative stress in pulmonary cells. Am J Physiol. 1998;275:L1100–1109. doi: 10.1152/ajplung.1998.275.6.L1100. [DOI] [PubMed] [Google Scholar]
- 48.Epperly MW, Bray JA, Esocobar P, Bigbee WL, Watkins S, Greenberger JS. Overexpression of the human manganese superoxide dismutase (MnSOD) transgene in subclones of murine hematopoietic progenitor cell line 32D cl 3 decreases irradiation-induced apoptosis but does not alter G2/M or G1/S phase cell cycle arrest. Radiat Oncol Investig. 1999;7:331–342. doi: 10.1002/(SICI)1520-6823(1999)7:6<331::AID-ROI3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 49.Epperly MW, Defilippi S, Sikora C, Gretton J, Greenberger JS. Radioprotection of lung and esophagus by overexpression of the human manganese superoxide dismutase transgene. Mil Med. 2002;167:71–73. [PubMed] [Google Scholar]
- 50.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80:251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 51.Robbins ME, Zhao W, Davis CS, Toyokuni S, Bonsib SM. Radiation-induced kidney injury: a role for chronic oxidative stress? Micron. 2002;33:133–141. doi: 10.1016/s0968-4328(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 52.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16:130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]