Abstract
The sensory processing of odorants is a dynamic process that requires plasticity at multiple levels. In the olfactory bulb (OB), inhibitory interneurons undergo lifelong replacement through a process known as adult neurogenesis. These newly born cells are incorporated in a learning-dependent fashion, a process which has led some to suggest this as a primary mechanism through which the OB retains a high degree of plasticity throughout life. A continued focus of researchers in this field has been to understand the molecular mechanisms controlling adult subventricular zone (SVZ) neurogenesis and the innate functional role of these cells. Brain-derived neurotrophic factor (BDNF) has been identified as a strong candidate molecule regulating adult OB neurogenesis. We review what is known regarding the functional role of newly-born cells, highlight the role of BDNF in this process, and describe preliminary findings from our lab implicating BDNF in the process of selecting of newly born cells for survival.
Keywords: neurotrophins, SVZ, neurogenesis, BDNF, TrkB, p75NTR, olfactory bulb
For much of the twentieth century, our understanding of the brain was shaped by the view that neurons were born during the prenatal and neonatal periods. These neurons would form a static backdrop against which subtle changes in synaptic efficacy would provide the substrate for learning and memory. If anything, the number of neurons would decrease as cell death occurred throughout the lifespan of an individual. Existing circuits sculpted by experience, however, were thought to be too complex to accommodate the addition of new neurons. This dogma was upended, when Altman and Das demonstrated that new neurons are incorporated into the adult hippocampus and olfactory bulb (Altman, 1962; Altman and Das, 1965; Altman, 1969). Since then, similar progenitor populations of cells have been identified in nearly every species of adult mammal studied, including humans, as well as in some species of songbirds. Over the last several decades, our appreciation of the extent and import of adult neurogenesis has increased tremendously. We highlight in this review the current understanding of the importance of neurogenesis to the continued plasticity of this system and how one molecule, the neurotrophin BDNF (brain derived neurotrophic factor), may contribute to the modulation of this process in a particular circuit, the rodent olfactory bulb (OB).
A Link Between Neurogenesis and Learning
Why particular brain regions incorporate a substantial number of neurons well into adulthood remains a mystery. However, a consensus is emerging that these adult-generated neurons are intricately linked with the acquisition of specific forms of memories and the maintenance of a high degree of plasticity in particular neural circuits. Beginning in the 1980s, work from Fernando Nottebohm and colleagues demonstrated a strong association between adult neurogenesis and song learning in songbirds (Goldman and Nottebohm, 1983). In the rodent hippocampus, hippocampal-dependent learning tasks increase the survival of newly-generated neurons (Gould et al., 1999) while ablating neurogenesis affects performance on these tasks (Saxe et al., 2006; Saxe et al., 2007; Imayoshi et al., 2008; Jessberger et al., 2009). Within the olfactory bulb, new neurons functionally integrate into odor-specific circuits within the bulbar network in a learning-dependent manner (Carlen et al., 2002; Belluzzi et al., 2003; Carleton et al., 2003; Magavi et al., 2005; Alonso et al., 2006; Mandairon et al., 2006). Using genetic and pharmacological means, a number of groups have shown that suppressed neurogenesis is linked with impairments in olfactory discrimination and olfactory-mediated learning while increased survival of newly born neurons is associated with enhanced olfactory short-term memory (Sultan et al.; Gheusi et al., 2000; Saghatelyan et al., 2005; Mandairon et al., 2006; Bath et al., 2008; Imayoshi et al., 2008; Breton-Provencher et al., 2009; Moreno et al., 2009; Valley et al., 2009). It should be noted that the olfactory deficits observed depend on the details of the behavioral task and the intervention used to alter neurogenesis. This likely reflects a complex process that is regulated at multiple levels by a variety of factors. Further work will be necessary to reconcile the disparate findings.
The anatomy of the olfactory bulb (OB) offers advantages in understanding the correlation between neurogenesis and systems level functioning. OB neurogenesis produces interneurons, with nearly all these new neurons becoming granule cells with a small population becoming periglomerular cells (Baker et al., 2001; Winner et al., 2002; Saino-Saito et al., 2004; Batista-Brito et al., 2008; Whitman and Greer, 2009). The activity of mitral and tufted cells, the primary projection neurons within the OB, is modulated by reciprocal interactions with the granule cells, which form inhibitory dendro-dendritic synapses onto mitral and tufted cell lateral dendrites (Shepherd et al., 2007). The dendro-dendritic interactions mediate mutual inhibition among mitral cells that appear to regulate the synchronization of mitral cell firing (Lledo and Lagier, 2006), hence shaping the temporal patterning of mitral cell odor responses (Lowe, 2003; Schoppa and Urban, 2003; Lledo and Saghatelyan, 2005). Computational models support the idea that activity-dependent survival of newborn granule cells could modulate the tuning of bulbar activity to enhance olfactory discrimination performance and potentially regulate the categorization of novel odorants following experience (Cecchi et al., 2001). Interestingly, newly born granule cells display greater synaptic plasticity than existing granule cells (Nissant et al., 2009), which may account for their unique contribution to circuit function.
Olfactory Bulb Neurogenesis
Neurogenesis comprises and is regulated at multiple steps including cell division, migration, and the subsequent differentiation of those cells into a neuronal phenotype. Newly born OB neurons arise from progenitors in the subventricular zone (SVZ) lining the lateral wall of each of the lateral ventricles. These SVZ progenitors produce neuroblasts that migrate long distances to the OB in a chain of cells known as the rostral migratory stream (RMS) (Luskin, 1993; Lois and Alvarez-Buylla, 1994). Within the SVZ, as many as 50,000 cells are born each day (Winner et al., 2002). The majority of neuroblasts that reach the OB die, with about 40% of newly born cells surviving for at least a year (Petreanu and Alvarez-Buylla, 2002; Winner et al., 2002). This “turnover” of cells, represents an approximate 6–10% replacement per month of the existing cellular population within the OB granule cell layer (Winner et al., 2002). Which neurons survive is largely influenced by the odor environment (Petreanu and Alvarez-Buylla, 2002; Rochefort et al., 2002; Saghatelyan et al., 2005; Yamaguchi and Mori, 2005). The system thus appears to function largely through an overproduction of potential neurons followed by an experience-dependent winnowing. Little is known regarding the molecular mechanism regulating the selective survival of subpopulations of these cells.
Does the Role of Newly Born Neurons Change as Animals Age?
A key question is whether adult neurogenesis is merely an extension of developmental neurogenesis, numerically smaller but functionally equivalent. Several lines of evidence indicate that this is not the case. From a purely anatomical view, the RMS itself does not exist in its adult form in the neonatal animal. Instead, this structure arises over the first postnatal month (Law et al., 1999). The alterations in the structure that produces the adult born neurons implies the existence of discrete cues regulating their production, migration, and differentiation from those seen in the juvenile. Furthermore, adult neurogenesis produces granule cells that integrate into different OB circuits than those produced by neonatal neurogenesis. As mentioned above, granule cells form reciprocal synapses with two anatomically and functionally distinct classes of projection neurons—the mitral and tufted cells. These connections are formed by two classes of granule cells, with superficial granule cells interacting with tufted cells and deep granule cells interacting with the mitral cells. Interestingly, the granule cells generated during early postnatal development occupy the superficial granule cell layer while those generated in adulthood occupy the deep granule cell layer (Lemasson et al., 2005; Mandairon et al., 2006). Adult-generated granule cells thus contribute preferentially to different circuits than neonatally-generated cells. These findings highlight that neurogenesis persisting in the adult, while similar in many ways to developmental neurogenesis, differs in the anatomical pathway that neuronal precursors traverse, the OB circuits to which they contribute, and the molecules that regulate these processes. A primary focus of this field has been to identify key molecules and signaling pathways that may be unique in controlling the proliferation, migration, and survival of these newly born neurons in adulthood. BDNF and its associated receptors (Figure 1) have been identified as strong candidates for mediating these processes. Furthermore, the developmental onset and peak expression of BDNF correlates well with the transition from embryonic to adult-like neurogenesis in the OB (Figure 2).
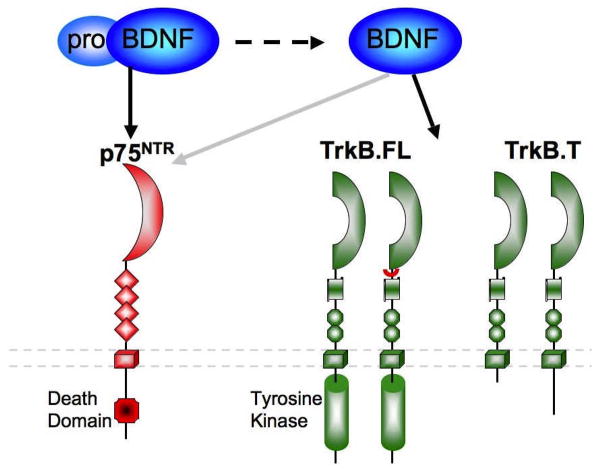
Figure 1. BDNF and its receptors.
Brain derived neurotrophic factor (BDNF) is a member of the neurotrophin family of polypeptide growth factors. BDNF is synthesized from a 32 kDa precursor protein known as ProBDNF, which preferentially binds to the p75NTR neurotrophin receptor (Lee et al., 2001; Teng et al., 2005). ProBDNF binding to p75NTR and ensuing signaling has been linked with the induction of cell death and suppression of cell cycling in a variety of cellular populations. ProBDNF is cleaved to produce a mature 14 kDa form of BDNF which binds preferentially to the tropomysin related kinase receptor, TrkB (Chao, 2003). Mature BDNF signaling depends upon which of the 4 distinct isoforms of TrkB it binds to. There are two full-length forms of TrkB (TrkB.FL) which differ in a short amino acid insert on the extracellular domain, but both contain an active kinase domain with multiple phosphorylation sites. Activation of the full-length form of TrkB is thought to promote cellular differentiation, survival, neurite outgrowth, and synaptic plasticity (Reichardt, 2006). In addition, two truncated forms of TrkB have been identified, TrkB.T1 and TrkB.T2 which both lack the intracellular kinase domain (Stoilov et al., 2002; Reichardt, 2006). The truncated forms of TrkB are largely thought to function as endogenous dominant negatives (Eide et al., 1996; Haapasalo et al., 2001). However, recent work by Mattson and colleagues have identified a potential role for truncated TrkB in signaling via trans-activation of a G-protein coupled receptor (Cheng et al., 2007). In this model, truncated TrkB is though to function in the process of gliogenesis.
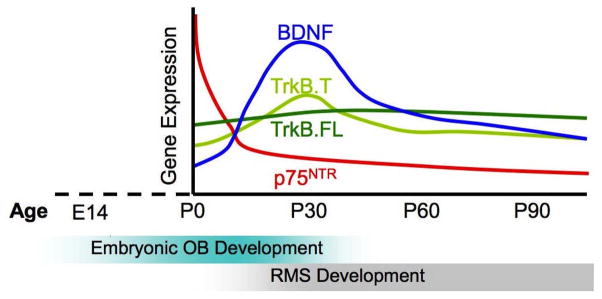
Figure 2. Developmental Expression of Neurotrophins.
The expression of BDNF is dynamically regulated across development. In the OB of the mouse, BDNF levels are low during prenatal development and increase over the first weeks of life, peaking during adolescence and then declining across the remainder of life. Expression of p75NTR also changes dramatically over the lifespan. p75NTR is highly expressed prenatally and is then rapidly suppressed in most brain regions postnatally. The full-length form of the TrkB receptor appears be stably expressed across the lifespan, while TrkB.T1 expression appears to mirror that of BDNF, peaking during adolescence (unpublished data). The developmental peak in BDNF expression, occurs following the embryonic and early postnatal organization of the OB. This peak appears to correlate well with development of a proper RMS and the onset of adult-like neurogenesis.
Impact of exogenous BDNF on SVZ neurogenesis
Since its discovery, BDNF has been known to function as a potent mediator of neuronal survival and differentiation throughout the nervous system (Barde, 1994). A role for BDNF in the control of SVZ neurogenesis was initially identified by Goldman and colleagues in the mid 1990’s. They found that BDNF administered to rat SVZ derived neuroblasts in vitro, promoted the long-term survival of those cells (Kirschenbaum and Goldman, 1995). In the following years, a number of labs found that infusion or viral overexpression of BDNF in the lateral ventricles of adult rats led to a near doubling of newly born neurons in the OB (Zigova et al., 1998; Benraiss et al., 2001; Chmielnicki et al., 2004; Henry et al., 2007). However, recent work has challenged the theory that BDNF infusion into the lateral ventricles promotes neurogenesis. In that report, BDNF infusion had no impact on cell survival in mouse and led to a decrease in cell survival in the OB of rats (Galvao et al., 2008). These contradictory results could be due to differences in the reagents being used by the various labs. It is known that production of BDNF in some recombinant systems leads to misfolding of the protein and possibly changes in its efficacy to stimulate target receptors. In addition, differences in the abundance of the pre-processed pro form of BDNF relative to the mature form may also contribute to the differences in observed results. With advances in techniques for the induction of BDNF expression in this region, increased quality of reagents, and interest in the potential of these cells as a therapeutic tool, the impact of exogenous BDNF on neurogenesis will undoubtedly receive additional attention.
Impact of endogenous BDNF on SVZ neurogenesis
While it is generally accepted that cells of the SVZ are sensitive to the application of exogenous BDNF, additional studies were needed to clarify whether these populations of cells use endogenous sources of neurotrophins as signaling molecules to regulate postnatal neurogenesis. To address this question, researchers took advantage of newly developed reagents to localize BDNF and its receptors in the SVZ and RMS of adult animals and used genetic techniques to manipulate BDNF expression and signaling in rodent model systems. In the following sections, we review work implicating BDNF and its various receptors in each step of the process of neurogenesis, from stem cell to differentiated neuron. We will detail what is known regarding gene expression and cell-specific localization as well as the impact of genetic manipulations on the survival, proliferation, and migration of newly born cells.
Before reviewing this literature, it should be noted that a common concern when working with genetically engineered lines of mice is that any effects of genetic manipulation observed in adulthood may be the remnant of early organizational effects on the system. Indeed, previous reports from Miller and colleagues have demonstrated that disrupting BDNF signaling during embryonic development leads to impairments in cortical development and a subsequent thinning of the subventricular zone (Bartkowska et al., 2007). Despite the thinning of the SVZ, loss of BDNF does not appear to impact the pools of cells contributing to the embryonic development of the OB. Parada and colleagues have shown that the complete loss of BDNF or any neurotrophin receptor, does not alter the prenatal development of the OB (Nef et al., 2001). Furthermore, in BDNF null mice, alterations in SVZ neurogenesis cannot be detected until at least 2 weeks after birth (Linnarsson et al., 2000). Thus, we interpret many of the subsequent findings to be unique to the process of “adult” neurogenesis and not remnants of early organizational effects of disruptions in the BDNF signaling pathway.
Survival
Some of the earliest work investigating the role of endogenous BDNF on neurogenesis found that mice in which the bdnf gene was genetically knocked out had an increase in cell death in the SVZ (Linnarsson et al., 2000). Using this same mouse line, we found that the loss of a single bdnf allele led to a ~30% decrease in the eventual survival of newly born cells in the adult OB. Using pharmacological and cell specific deletion of BDNF, Snapyan and colleagues found a similar decrease in the eventual survival of new neurons in the OB of adult mice (Snapyan et al., 2009). A significant question that has arisen in this field regards the receptor mediating these effects, as well as the specific process that BDNF may regulate (e.g. proliferation, migration, or differentiation).
To investigate the contribution of the BDNF receptors, TrkB and p75NTR, to adult neurogenesis, researchers again used genetically engineered mouse lines. Mice in which a single copy of the trkb gene was genetically ablated, were found to have a significant reduction in the survival of newly born cells in the OB, an effect that mirrored the effects observed in BDNF heterozygous mice (Bath et al., 2008). These results were consistent with subsequent work by (Galvao et al., 2008), who found that the same TrkB heterozygous mice showed a 14% reduction in cell survival in the OB. However due to a small n, the effect observed by Galvao did not reach statistical significance (p < 0.07). Mice in which the p75NTR gene was completely ablated were found to have no change in the density of newly born cells in the OB relative to wild type control mice (Bath et al., 2008). However, in a parallel series of studies by Young and colleagues, they found a significant reduction in OB volume, and biomarkers indicative of a decrease in the number of proliferating and migrating neuroblasts in p75NTR null animals (Young et al., 2007). Work in the dentate gyrus (DG), the other brain region that undergoes high rates of postnatal neurogenesis, has shown that loss of either the TrkB or p75NTR expression decreases neurogenesis (von Bohlen und Halbach et al., 2003; Bath et al., 2009) (Bernabeu and Longo, 2010). These findings, taken together, provide compelling evidence to implicate BDNF signaling as critical in the control of neurogenesis and implicate both BDNF receptors as potential mediators of some step of this process. A number of labs have carried out additional studies to attempt to define the specific step in neurogenesis that might be supported by BDNF and each of its receptors.
Proliferation
Studies attempting to define a potential role for BDNF in regulating the proliferation of cells of the SVZ have led to complicated and sometimes contradictory findings. As an initial study to investigate whether the loss of either BDNF or its receptors (p75NTR or TrkB) alters proliferation of cell of the SVZ in vivo, we used BDNF heterozygous, TrkB heterozygous, and p75NTR null mice. We found no effect of gene deletion on the density of newly dividing cells in the SVZ of these mice compared with wild type controls (Bath et al., 2008). Based upon these data, we hypothesized that BDNF and its receptors are not involved in the endogenous control of cell division. However, subsequent studies using alternative approaches have questioned our findings and provided evidence that BDNF signaling may in fact impact cell proliferation within the SVZ.
Using cell culture and immunohistochemical studies, the majority of groups (including our own) have identified p75NTR on a small subpopulation of cells in the SVZ (Gascon et al., 2005; Young et al., 2007; Bath et al., 2008; Snapyan et al., 2009). Using cell sorting via flow cytometry, Bartlett and colleagues demonstrated that approximately 0.3% of cells within the SVZ are p75NTR-positive (Young et al., 2007). The localization of p75NTR to such a small subset of cells could make sense in light of data from other systems implicating p75NTR as a suppressor of cell cycling (Khwaja and Djakiew, 2003; Khwaja et al., 2006; Vilar et al., 2006). Using an in vitro neurosphere assay, Young and colleagues concluded that p75NTR positive precursors were alone neurogenic. Using whole mount immunostaining they further showed a significant reduction in PSA-NCAM labeling in p75NTR-null mice suggesting a general decrease in postmitotic migrating neuroblasts (Young et al., 2007). A role for p75NTR as a controller of cell proliferation is consistent with recent work in the DG by Longo. They found that the loss of p75NTR led to a significant reduction in proliferating cells in the DG compared with wildtype controls (Bernabeu and Longo, 2010). Certainly more work will be beneficial in clarifying a possible role for p75NTR in the process of cell proliferation within the SVZ.
A subset of groups have failed to localize p75NTR to proliferating cells, and instead found the full length and/or truncated forms of the TrkB receptors to be highly expressed in this population of cells (Tervonen et al., 2006; Galvao et al., 2008). Galvao and colleagues found that TrkB knockout mice had a decrease in cell proliferation and increased cell death in the SVZ at birth and a general reduction in cell number in the OB. They further showed that the truncated TrkB receptor alone was enriched in the SVZ relative to adjacent neural structures. Despite such findings, they argue that many of the effects of TrkB are not selective to the process of postnatal neurogenesis, and may instead reflect early organizational effects. Tervonen and colleagues using cell cultures of SVZ-derived progenitor cells from mice in which the truncated TrkB.T1 was overexpressed, found a significant reduction in the number of neurosphere-forming progenitors compared with cells derived from wild type mice. Interestingly, these same cells showed an increased rate of cell proliferation relative to wild type-derived cells (Tervonen et al., 2006). These data suggest a potential role for TrkB.T1 in regulating SVZ neurogenesis by impacting proliferative potential as well as rates of cell division. With the recent development of a line of mouse in which the truncated TrkB receptor has been selectively genetically deleted (Carim-Todd et al., 2009), we are interested to see how loss of this receptor might also impact the proliferation and survival of cells in vivo.
Migration
Work aimed at testing the role of BDNF in the migration of SVZ-derived cells has been an area of higher consensus than work on proliferation. In BDNF heterozygous mice as well as a line of mice in which a single nucleotide polymorphism (SNP) in the BDNF gene has been knocked-in (BDNF Val66Met mice (Chen et al., 2006)), we found a decrease in the survival but not proliferation of cells of the SVZ (Bath et al., 2008). Using mice that carry the BDNF Val66Met SNP, we found following a single injection of BrdU and a survival time of 6 days, that BrdU-positive neuroblasts in BDNF SNP carrying mice were primarily localized to the caudal portion of the RMS, while neuroblasts in wild type mice were primary found in the anterior RMS (Bath et al., 2008). These data implicate BDNF in either the rate or ability of neuroblasts to migrate through the RMS. These data are supported by work in slice and cell culture preparations, where the application of BDNF increases the motility of cells in the RMS (Chiaramello et al., 2007; Bagley and Belluscio, 2010). In addition, work in which BDNF is sequestered using TrkBFc or when BDNF is genetically ablated from vascular endothelial cells, neuroblasts show decreased migration in vitro and in vivo (Snapyan et al. 2009).
The primary source of conflict regarding the potential contribution of BDNF to the process of neuroblast migration has been questions about the receptor thought to mediate this process. We and others have found TrkB or the activated form of TrkB (pTrkB) to be selectively localized to migrating neuroblasts (Chiaramello et al., 2007; Bath et al., 2008). Consistent with these results, a similar selective expression of TrkB on postmitotic immature neuroblasts has been identified within the SGZ of the hippocampus (Donovan et al., 2008; Li et al., 2008). Through work in vitro, TrkB signaling via PI3-K and ERK was shown to be critical for the appropriate migration of neuroblasts (Chiaramello et al., 2007). In further studies, BDNF stimulation of TrkB was shown to be capable of rescuing the inactivation or loss of the polysialated head (PSA) of PSA-NCAM, a molecule critical for cell adhesion during the migratory process (Vutskits et al., 2001). Based upon these data, BDNF signaling via TrkB may be involved in the stabilization or destabilization of contacts between migrating neuroblasts. However more work will be needed to strengthen such a hypothesis.
A separate line of work has suggested that instead of TrkB, p75NTR is selectively localized to immature and migrating neuroblasts (Gascon et al., 2005; Gascon et al., 2007; Galvao et al., 2008; Snapyan et al., 2009). Snapyan and colleagues propose that p75NTR is localized to migrating neuroblasts and TrkB is instead localized to astrocytes within the RMS. They argue that TrkB is indirectly involved in migration by sequestering or presenting BDNF to p75NTR-expressing migrating neuroblasts, an effect that can be modulated by GABAergic internalization of TrkB from the cell surface of astrocytes. These findings are supported by findings demonstrating that an antibody capable of inactivating p75NTR diminishes the migration of cells in culture and decreases the number of newly born cells in the OB in vivo. Despite such findings, the direct mechanisms by which p75NTR might alter cell motility or cell guidance remain to be clarified. During embryonic development p75NTR interacts with non-neurotrophin signaling partners to regulate axonal guidance. It is possible that in the RMS, p75NTR may function in a similar capacity to either guide the migration of cells, or more likely to promote the death of cells that fail to reach appropriate targets
Given the differences in the characterization of which receptors are expressed on the various subpopulations of cells in the SVZ and RMS, more work will be required to fully understand the functional role of these receptors in this complex process. Despite these disparities, both pathways stand as reasonable candidates to control either cell migration or the survival of cells during this complex process.
Source(s) of BDNF regulating neurogenesis
A major question that remains to be addressed is to localize the source of BDNF regulating SVZ neurogenesis in adulthood. Goldman and colleagues proposed endothelial cells as the primary endogenous source of BDNF in vivo (Leventhal et al., 1999). This hypothesis was based upon the observations that endothelial cells produce a significant amount of BDNF and, when co-cultured, lead to increased survival of SVZ derived cells—an effect that could be blocked by TrkB-Fc. This theory has since been bolstered by an elegant series of studies from Saghatelyan & colleagues (Snapyan et al., 2009). They used targeted KO mice and pharmacological means to demonstrate that loss of BDNF from the vasculature and endothelial cells leads to a significant decrement in the number of cells that reach the OB and survive. In a recent series of pilot experiments, we demonstrated a similar effect in a small number of mice that lacked BDNF in nestin-positive cells (which include vascular endothelial cells), and observed a decrease in the survival of newly born cells in the OB (Figure 3a; see supplemental methods).
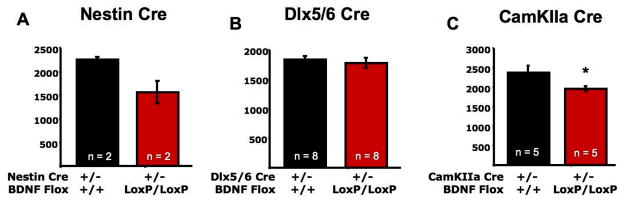
Figure 3. Cell-specific deletion of BDNF impacts rates of OB neurogenesis.
Histograms depicting the effects of selective genetic deletion of BDNF from A) Nestin-positive, B) Dlx5/6-positive, or C) CamKIIa-positive cells on rates of cell survival within the adult mouse OB. All groups were compared with Cre-positive mice that did not contain LoxP sites flanking the bdnf gene. Nestin-Cre BDNF LoxP groups were small (n=2) due to high postnatal mortality of this line. * p < 0.05.
In our recent report investigating the role of the BDNF Val66Met SNP on SVZ neurogenesis, we raised the possibility of an alternate source of BDNF, beyond that of endothelial cells that could function to regulate adult neurogenesis. This hypothesis was based upon a combination of findings. First, we previously showed that the BDNF Val66Met SNP leads to no changes in the total amount of BDNF protein produced in the brain (Chen et al., 2006). Second, this SNP leads to a significant reduction in regulated but not constitutive secretion of BDNF (Chen et al., 2005; Chen et al., 2006). Third, endothelial cells generally lack a regulated release mechanism, and are thus unlikely to account for the observed decrease in survival of newly born neurons in BDNF Val66Met mice. Furthermore, BDNF has been identified as a possible molecule of interest in regulating the activity-dependent survival of newly born cells and their selective integration into circuits. While in the RMS, neuroblasts use endothelial cells as a substrate for migration and as a likely source of BDNF (Snapyan et al., 2009; Whitman et al., 2009). When neuroblasts reach the bulb, they migrate tangentially to integrate into the existing circuit (Luskin, 1993; Lois and Alvarez-Buylla, 1994). During this final process, these cells may rely upon an alternate source of BDNF to support their continued survival, migration, and/or eventual differentiation. In a recent series of studies, Whitman and Greer demonstrated that newly born cells receive initial inputs from centrifugal afferents prior to their development of apical dendrites and integration with mitral cells (Whitman and Greer, 2007). Enhanced activation of centrifugal afferents following odorant detection, in addition to aiding in the tuning of outputs from the OB, may also provide signals that support the selective survival of newly born cells in regions of high activity. Based upon these various lines of evidence, we hypothesize that BDNF release from centrifugal afferents may influence neurogenesis, and specifically the eventual survival of newly born cells.
As a very preliminary study to address this question, we used cell specific Cre mediated recombination. This methodology relies utilizes genetically engineered mice in which LoxP sites flank a gene of interest. These mice are then crossed with mice that express Cre under the control of a cell specific promoter. When Cre is expressed, it cuts the LoxP sites surrounding the gene of interest and in effect eliminates the ability of Cre positive cells to express that gene. We used mice in which the bdnf gene is flanked by LoxP sites (Rios et al., 2001) and crossed those mice with either Dlx5/6 Cre mice (provided by lab of Stewart A Anderson) to eliminate BDNF from interneurons, or with CamKIIa Cre mice (Minichiello et al., 1999) to eliminate BDNF from projection neurons (including centrifugal afferents to the OB (Zou et al., 2002)). We found that the selective loss of BDNF from projection neurons but not interneurons led to a decrease in the survival of newly born cells in the OB (Figure 3b,c; see supplemental material for description of procedures). These results, while extremely preliminary, suggest that in fact BDNF from centrifugal afferents may function to control the survival of a subpopulation of cells in the OB. This work is consistent with findings from several other labs demonstrating that suppression of cholinergic and noradrenergic inputs to the OB significantly impact discrimination ability (Mandairon et al., 2006; Mandairon et al., 2008) as well as impact the survival and regional distribution of newly born cells (Moreno et al., under review). Such studies provide a tantalizing glimpse into a potential additional mechanism by which BDNF may function to regulate the selective survival of newly born cells, through activity dependent release from projection neurons. Through this process, feedback from cortical and subcortical circuits could function to promote the selection of subsets of cells to enhance local inhibitory tone, and thus tuning of the system to newly or repeatedly encountered stimuli. Such a process could significantly contribute to continued plasticity of this circuit and aid in circuit adaptation to the nearly limitless possible odorants and odorant mixtures that may be encountered in an animals environment. Far more work will be needed to more thoroughly test this hypothesis, but we hope that these results will prompt more work on this important question.
Conclusions
Throughout this review, we have highlighted the potential role of adult neurogenesis in promoting the ongoing plasticity of the OB, and identified a key molecule controlling the various steps of this process (BDNF). While this review focused on neurogenesis in the OB, in recent years, a number of groups have attempted to use overexpression of BDNF in typically non-neurogenic regions, such as the striatum, to increase the survival of grafted progenitor cells as a therapeutic strategy for stroke and other neurodegenerative conditions with some success (Schabitz et al., 2004; Chen et al., 2005; Chen et al., 2007; Cho et al., 2007; Schabitz et al., 2007). In a similar line of work, the induction of BDNF expression through the use of pharmacological means has also been shown to be capable of reintroducing plasticity into systems that were long thought to have crystallized. For example, work by Maffei and colleagues have shown that augmentation of BDNF through treatment with the SSRI fluoxetine may be an effective treatment for amblyopia, a condition in which the visual input of one eye is highly favored resulting in poor development of acuity in the unfavored eye (Maya Vetencourt et al., 2008, also see Morishita & Hensch in this issue). Based upon the results from the OB and other system, BDNF is emerging as a strong candidate in regulating the continued plasticity of multiple brain systems long beyond the early organization that occurs during embryonic and perinatal period.
Supplementary Material
Acknowledgments
Stewart A. Anderson and Qing Xu for the Dlx5/6 Cre mice, This work was supported by the Sackler Institute (K.G.B.), DeWitt-Wallace Fund of the New York Community Trust (F.S.L), Irma T. Hirschl/Monique Weill-Caulier Trust (F.S.L.), International Mental Health Research Organization (F.S.L.), Burroughs Wellcome Foundation (FSL), Pritzker Consortiun (F.S.L), NARSAD (F.S.L., K.G.B.) and National Institutes of Health grants MH060478 (K.G.B.), NS052819 (F.S.L.), MH088814 (F.S.L.) and MH090237 (M.R.A.).
References
- Alonso M, Viollet C, Gabellec MM, Meas-Yedid V, Olivo-Marin JC, Lledo PM. Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J Neurosci. 2006;26:10508–10513. doi: 10.1523/JNEUROSCI.2633-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. JComp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Bagley JA, Belluscio L. Dynamic imaging reveals that brain-derived neurotrophic factor can independently regulate motility and direction of neuroblasts within the rostral migratory stream. Neuroscience. 2010;169:1449–1461. doi: 10.1016/j.neuroscience.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Liu N, Chun HS, Saino S, Berlin R, Volpe B, Son JH. Phenotypic differentiation during migration of dopaminergic progenitor cells to the olfactory bulb. J Neurosci. 2001;21:8505–8513. doi: 10.1523/JNEUROSCI.21-21-08505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res. 1994;390:45–56. [PubMed] [Google Scholar]
- Bartkowska K, Paquin A, Gauthier AS, Kaplan DR, Miller FD. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development. 2007;134:4369–4380. doi: 10.1242/dev.008227. [DOI] [PubMed] [Google Scholar]
- Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen ZY, Khan T, Proenca CC, Kraemer R, Cleland TA, Hempstead BL, Chao MV, Lee FS. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28:2383–2393. doi: 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Voss HU, Jing D, Anderson S, Hempstead B, Lee FS, Dyke JP, Ballon DJ. Quantitative intact specimen magnetic resonance microscopy at 3.0 T. Magn Reson Imaging. 2009;27:672–680. doi: 10.1016/j.mri.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Close J, Machold R, Fishell G. The distinct temporal origins of olfactory bulb interneuron subtypes. J Neurosci. 2008;28:3966–3975. doi: 10.1523/JNEUROSCI.5625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O, Benedusi M, Ackman J, LoTurco JJ. Electrophysiological differentiation of new neurons in the olfactory bulb. J Neurosci. 2003;23:10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabeu RO, Longo FM. The p75 neurotrophin receptor is expressed by adult mouse dentate progenitor cells and regulates neuronal and non-neuronal cell genesis. BMC Neurosci. 2010;11:136. doi: 10.1186/1471-2202-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton-Provencher V, Lemasson M, Peralta MR, 3rd, Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J Neurosci. 2009;29:15245–15257. doi: 10.1523/JNEUROSCI.3606-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carim-Todd L, Bath KG, Fulgenzi G, Yanpallewar S, Jing D, Barrick CA, Becker J, Buckley H, Dorsey SG, Lee FS, Tessarollo L. Endogenous truncated TrkB.T1 receptor regulates neuronal complexity and TrkB kinase receptor function in vivo. J Neurosci. 2009;29:678–685. doi: 10.1523/JNEUROSCI.5060-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, Cassidy RM, Brismar H, Smith GA, Enquist LW, Frisen J. Functional integration of adult-born neurons. Curr Biol. 2002;12:606–608. doi: 10.1016/s0960-9822(02)00771-6. [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Cecchi GA, Petreanu LT, Alvarez-Buylla A, Magnasco MO. Unsupervised learning and adaptation in a model of adult neurogenesis. J Comput Neurosci. 2001;11:175–182. doi: 10.1023/a:1012849801892. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Henry RA, Hughes SM, Connor B. Creating a neurogenic environment: the role of BDNF and FGF2. Mol Cell Neurosci. 2007;36:108–120. doi: 10.1016/j.mcn.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25:6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Coksaygan T, Tang H, Khatri R, Balice-Gordon RJ, Rao MS, Mattson MP. Truncated tyrosine kinase B brain-derived neurotrophic factor receptor directs cortical neural stem cells to a glial cell fate by a novel signaling mechanism. J Neurochem. 2007;100:1515–1530. doi: 10.1111/j.1471-4159.2006.04337.x. [DOI] [PubMed] [Google Scholar]
- Chiaramello S, Dalmasso G, Bezin L, Marcel D, Jourdan F, Peretto P, Fasolo A, De Marchis S. BDNF/TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAP-K signalling pathways. Eur J Neurosci. 2007;26:1780–1790. doi: 10.1111/j.1460-9568.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- Chmielnicki E, Benraiss A, Economides AN, Goldman SA. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J Neurosci. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SR, Benraiss A, Chmielnicki E, Samdani A, Economides A, Goldman SA. Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. J Clin Invest. 2007;117:2889–2902. doi: 10.1172/JCI31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan MH, Yamaguchi M, Eisch AJ. Dynamic expression of TrkB receptor protein on proliferating and maturing cells in the adult mouse dentate gyrus. Hippocampus. 2008;18:435–439. doi: 10.1002/hipo.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide FF, Vining ER, Eide BL, Zang K, Wang XY, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao RP, Garcia-Verdugo JM, Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J Neurosci. 2008;28:13368–13383. doi: 10.1523/JNEUROSCI.2918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon E, Vutskits L, Jenny B, Durbec P, Kiss JZ. PSA-NCAM in postnatally generated immature neurons of the olfactory bulb: a crucial role in regulating p75 expression and cell survival. Development. 2007;134:1181–1190. doi: 10.1242/dev.02808. [DOI] [PubMed] [Google Scholar]
- Gascon E, Vutskits L, Zhang H, Barral-Moran MJ, Kiss PJ, Mas C, Kiss JZ. Sequential activation of p75 and TrkB is involved in dendritic development of subventricular zone-derived neuronal progenitors in vitro. Eur J Neurosci. 2005;21:69–80. doi: 10.1111/j.1460-9568.2004.03849.x. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci U S A. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Haapasalo A, Koponen E, Hoppe E, Wong G, Castren E. Truncated trkB.T1 is dominant negative inhibitor of trkB.TK+-mediated cell survival. Biochem Biophys Res Commun. 2001;280:1352–1358. doi: 10.1006/bbrc.2001.4296. [DOI] [PubMed] [Google Scholar]
- Henry RA, Hughes SM, Connor B. AAV-mediated delivery of BDNF augments neurogenesis in the normal and quinolinic acid-lesioned adult rat brain. Eur J Neurosci. 2007;25:3513–3525. doi: 10.1111/j.1460-9568.2007.05625.x. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja F, Djakiew D. Inhibition of cell-cycle effectors of proliferation in bladder tumor epithelial cells by the p75NTR tumor suppressor. Mol Carcinog. 2003;36:153–160. doi: 10.1002/mc.10106. [DOI] [PubMed] [Google Scholar]
- Khwaja F, Tabassum A, Allen J, Djakiew D. The p75(NTR) tumor suppressor induces cell cycle arrest facilitating caspase mediated apoptosis in prostate tumor cells. Biochem Biophys Res Commun. 2006;341:1184–1192. doi: 10.1016/j.bbrc.2006.01.073. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum B, Goldman SA. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc Natl Acad Sci U S A. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AK, Pencea V, Buck CR, Luskin MB. Neurogenesis and neuronal migration in the neonatal rat forebrain anterior subventricular zone do not require GFAP-positive astrocytes. Dev Biol. 1999;216:622–634. doi: 10.1006/dbio.1999.9498. [DOI] [PubMed] [Google Scholar]
- Lee FS, Kim AH, Khursigara G, Chao MV. The uniqueness of being a neurotrophin receptor. Curr Opin Neurobiol. 2001;11:281–286. doi: 10.1016/s0959-4388(00)00209-9. [DOI] [PubMed] [Google Scholar]
- Lemasson M, Saghatelyan A, Olivo-Marin JC, Lledo PM. Neonatal and adult neurogenesis provide two distinct populations of newborn neurons to the mouse olfactory bulb. J Neurosci. 2005;25:6816–6825. doi: 10.1523/JNEUROSCI.1114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnarsson S, Willson CA, Ernfors P. Cell death in regenerating populations of neurons in BDNF mutant mice. Brain Res Mol Brain Res. 2000;75:61–69. doi: 10.1016/s0169-328x(99)00295-8. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Lagier S. Adjusting neurophysiological computations in the adult olfactory bulb. Semin Cell Dev Biol. 2006;17:443–453. doi: 10.1016/j.semcdb.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lowe G. Electrical signaling in the olfactory bulb. Curr Opin Neurobiol. 2003;13:476–481. doi: 10.1016/s0959-4388(03)00092-8. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Mitchell BD, Szentirmai O, Carter BS, Macklis JD. Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo. J Neurosci. 2005;25:10729–10739. doi: 10.1523/JNEUROSCI.2250-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci. 2006;24:3234–3244. doi: 10.1111/j.1460-9568.2006.05212.x. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Peace S, Karnow A, Kim J, Ennis M, Linster C. Noradrenergic modulation in the olfactory bulb influences spontaneous and reward-motivated discrimination, but not the formation of habituation memory. Eur J Neurosci. 2008;27:1210–1219. doi: 10.1111/j.1460-9568.2008.06101.x. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Sacquet J, Garcia S, Ravel N, Jourdan F, Didier A. Neurogenic correlates of an olfactory discrimination task in the adult olfactory bulb. Eur J Neurosci. 2006;24:3578–3588. doi: 10.1111/j.1460-9568.2006.05235.x. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, Castren E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Moreno MM, Linster C, Escanilla O, Sacquet J, Didier A, Mandairon N. Olfactory perceptual learning requires adult neurogenesis. Proc Natl Acad Sci U S A. 2009;106:17980–17985. doi: 10.1073/pnas.0907063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nef S, Lush ME, Shipman TE, Parada LF. Neurotrophins are not required for normal embryonic development of olfactory neurons. Dev Biol. 2001;234:80–92. doi: 10.1006/dbio.2001.0240. [DOI] [PubMed] [Google Scholar]
- Nissant A, Bardy C, Katagiri H, Murray K, Lledo PM. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nat Neurosci. 2009;12:728–730. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghatelyan A, Roux P, Migliore M, Rochefort C, Desmaisons D, Charneau P, Shepherd GM, Lledo PM. Activity-dependent adjustments of the inhibitory network in the olfactory bulb following early postnatal deprivation. Neuron. 2005;46:103–116. doi: 10.1016/j.neuron.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Saino-Saito S, Sasaki H, Volpe BT, Kobayashi K, Berlin R, Baker H. Differentiation of the dopaminergic phenotype in the olfactory system of neonatal and adult mice. J Comp Neurol. 2004;479:389–398. doi: 10.1002/cne.20320. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci U S A. 2007;104:4642–4646. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabitz WR, Berger C, Kollmar R, Seitz M, Tanay E, Kiessling M, Schwab S, Sommer C. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35:992–997. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, Kuhn HG. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38:2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci. 2003;26:501–506. doi: 10.1016/S0166-2236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Chen WR, Willhite D, Migliore M, Greer CA. The olfactory granule cell: from classical enigma to central role in olfactory processing. Brain Res Rev. 2007;55:373–382. doi: 10.1016/j.brainresrev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, Parent A, Saghatelyan A. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoilov P, Castren E, Stamm S. Analysis of the human TrkB gene genomic organization reveals novel TrkB isoforms, unusual gene length, and splicing mechanism. Biochem Biophys Res Commun. 2002;290:1054–1065. doi: 10.1006/bbrc.2001.6301. [DOI] [PubMed] [Google Scholar]
- Sultan S, Mandairon N, Kermen F, Garcia S, Sacquet J, Didier A. Learning-dependent neurogenesis in the olfactory bulb determines long-term olfactory memory. FASEB J. 24:2355–2363. doi: 10.1096/fj.09-151456. [DOI] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervonen TA, Ajamian F, De Wit J, Verhaagen J, Castren E, Castren M. Overexpression of a truncated TrkB isoform increases the proliferation of neural progenitors. Eur J Neurosci. 2006;24:1277–1285. doi: 10.1111/j.1460-9568.2006.05010.x. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Valley MT, Mullen TR, Schultz LC, Sagdullaev BT, Firestein S. Ablation of mouse adult neurogenesis alters olfactory bulb structure and olfactory fear conditioning. Front Neurosci. 2009;3:51. doi: 10.3389/neuro.22.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar M, Murillo-Carretero M, Mira H, Magnusson K, Besset V, Ibanez CF. Bex1, a novel interactor of the p75 neurotrophin receptor, links neurotrophin signaling to the cell cycle. EMBO J. 2006;25:1219–1230. doi: 10.1038/sj.emboj.7601017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Minichiello L, Unsicker K. Haploinsufficiency in trkB and/or trkC neurotrophin receptors causes structural alterations in the aged hippocampus and amygdala. Eur J Neurosci. 2003;18:2319–2325. doi: 10.1046/j.1460-9568.2003.02953.x. [DOI] [PubMed] [Google Scholar]
- Vutskits L, Djebbara-Hannas Z, Zhang H, Paccaud JP, Durbec P, Rougon G, Muller D, Kiss JZ. PSA-NCAM modulates BDNF-dependent survival and differentiation of cortical neurons. Eur J Neurosci. 2001;13:1391–1402. doi: 10.1046/j.0953-816x.2001.01516.x. [DOI] [PubMed] [Google Scholar]
- Whitman MC, Fan W, Rela L, Rodriguez-Gil DJ, Greer CA. Blood vessels form a migratory scaffold in the rostral migratory stream. J Comp Neurol. 2009;516:94–104. doi: 10.1002/cne.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman MC, Greer CA. Synaptic integration of adult-generated olfactory bulb granule cells: basal axodendritic centrifugal input precedes apical dendrodendritic local circuits. J Neurosci. 2007;27:9951–9961. doi: 10.1523/JNEUROSCI.1633-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman MC, Greer CA. Adult neurogenesis and the olfactory system. Prog Neurobiol. 2009;89:162–175. doi: 10.1016/j.pneurobio.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Cooper-Kuhn CM, Aigner R, Winkler J, Kuhn HG. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur J Neurosci. 2002;16:1681–1689. doi: 10.1046/j.1460-9568.2002.02238.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Mori K. Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc Natl Acad Sci U S A. 2005;102:9697–9702. doi: 10.1073/pnas.0406082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, Mark W, Tessarollo L, Lee FS, Lu B, Hempstead BL. Neuronal release of proBDNF. Nat Neurosci. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Merson TD, Sotthibundhu A, Coulson EJ, Bartlett PF. p75 neurotrophin receptor expression defines a population of BDNF-responsive neurogenic precursor cells. J Neurosci. 2007;27:5146–5155. doi: 10.1523/JNEUROSCI.0654-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigova T, Pencea V, Wiegand SJ, Luskin MB. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci. 1998;11:234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]
- Zou DJ, Greer CA, Firestein S. Expression pattern of alpha CaMKII in the mouse main olfactory bulb. J Comp Neurol. 2002;443:226–236. doi: 10.1002/cne.10125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.