Abstract
Background
We previously reported that higher levels of peripheral oxytocin are associated with lower levels of positive, general, and overall symptoms in women but not men with schizophrenia. Here we investigate the influence of sex, sex steroid hormone fluctuations, and peripheral oxytocin levels on emotional processing in men and women with schizophrenia.
Method
Twenty-two women with schizophrenia and 31 female controls completed the Penn Emotion Acuity Test (PEAT), a facial emotion recognition and perception task, during two menstrual cycle phases: 1) early follicular (Days 2–4; low estrogen/progesterone) and 2) midluteal (Days 20–22; high estrogen/progesterone). Twenty-six males with schizophrenia and 26 male controls completed testing at comparable intervals. We obtained plasma hormone assays of estrogen, progesterone, testosterone, and oxytocin.
Results
No sex differences were noted on the PEAT. Plasma oxytocin levels did not fluctuate across phases of the menstrual cycle. However, female patients and controls more accurately identified facial emotions during the early follicular versus midluteal phase (p<0.05). Higher oxytocin levels related to perceiving faces as happier in both female patients (r=−0.46, p=0.04) and controls (r=−0.40, p=0.04) but not in men.
Conclusion
Like healthy women, women with schizophrenia demonstrate menstrual-cycle dependent fluctuations in recognizing emotional cues. Like healthy women, female patients with higher levels of oxytocin perceived faces as happier. Future studies need to address whether this sex-specific relationship is associated with trust and other positive emotions, and whether exogenous oxytocin might enhance mood states and social interaction in female or all schizophrenia patients.
Keywords: oxytocin, schizophrenia, menstrual cycle, social cognition, emotion recognition, emotion perception
1. Introduction
We previously reported that higher levels of endogenous oxytocin were associated with lower symptom severity in schizophrenia (Rubin et al., 2010). Specifically, higher peripheral oxytocin levels predicted less severe positive, general, and social symptoms in women, and better prosocial behaviors in men. Additionally, clinical symptoms improved during menstrual cycle phases characterized by high levels of estrogen and progesterone (Rubin et al., 2010). Emerging literature supports our initial report, suggesting that exogenous oxytocin may be effective as an adjunctive therapy for schizophrenia (Feifel et al., 2010).
The present investigation is motivated by findings that emotion processing is impaired in schizophrenia (Kohler et al., 2000; Ziv et al., 2011), and influenced by sex (Scholten et al., 2005; Thayer and Johnsen, 2000; Williams et al., 2009), menstrual cycle phase (Derntl et al., 2008a; Derntl et al., 2008b; Guapo et al., 2009; Pearson and Lewis, 2005), and oxytocin (Goldman et al., 2011; Marsh et al., 2010). This study addresses the relationship between endogenous oxytocin and emotion processing in schizophrenia.
2. Methods
2.1. Participants
Participants included 48 patients (22 women) and 57 controls (31 women) from Rubin et al. (2010) who completed the Penn Emotion Acuity Test (PEAT) (see Table 1). All participants were age 18–40 with English as their first language. In patients, a Structured Clinical Interview for DSM confirmed diagnosis of schizophrenia or schizoaffective disorder depressed type. Female participants had regular menstrual cycles (28±5days) and were not on oral contraceptives.
Table 1.
Demographics and Other Descriptive Information.
| Women | Men | |||
|---|---|---|---|---|
| Patients (n = 22) |
Healthy Controls (n = 31) |
Patients (n = 26) |
Healthy Controls (n = 26) |
|
| Variables | M (SD) | M (SD) | M (SD) | M (SD) |
| Age G | 30.82 (5.80) | 27.55 (6.67) | 31.46 (6.57) | 27.58 (5.83) |
| Parental SES | 2.95 (0.95) | 3.13 (1.34) | 3.10 (1.09) | 3.38 (1.30) |
| Race | ||||
| Caucasian | 32% | 29% | 15% | 44% |
| African-American | 45% | 45% | 59% | 30% |
| Asian | 5% | 16% | 4% | 15% |
| Hispanic or Latino | 18% | 10% | 15% | 4% |
| Other/unknown | - | - | 7% | 7% |
| SCID diagnosis | ||||
| Schizophrenia | 55% | 77% | ||
| Schizoaffective (depressed type) | 45% | 23% | ||
| Duration of Illness in years† | 13.23 (5.47) | 11.58 (8.09) | ||
| Antipsychotics (on second generation) | 86% | 85% | ||
| Chlorpromazine mg equivalents S | 305 (249) | 488 (293) | ||
| Antipsychotic + antidepressant | 55% | 39% | ||
| Antipsychotic + mood stabilizer | 5% | 4% | ||
| PANSS total score | 61.47 (8.65) | 65.54 (11.50) | ||
Note. Parental SES higher scores reflect higher SES; SCID = Structured Clinical Interview for DSM; PANSS = Positive and Negative Syndrome Scale.
In years since initial treatment for psychosis.
Main effect of group was significant at p<0.05.
Main effect of sex was significant at p<0.05.
2.2. Measures
2.2.1. Penn Emotion Acuity Test
(PEAT; Erwin et al., 1992; Kohler et al., 2000). This test measures two facets of emotion processing: recognition and perception. Participants indicate on a seven-point scale how happy or sad they perceive a face to be (1=very happy, 7=very sad). Stimuli are 40 Caucasian faces (half female; 10 happy/10 sad/20 neutral), randomly presented within two blocks. Block 1 contains sad and neutral faces and Block 2 contains happy and neutral faces. Outcome measures were: (1) error rate (recognition), the percentage of incorrect responses within each valence category, (2) mean response ratings (perception) within each valence category on the 7-point scale, and (3) reaction time.
2.2.2. Serum Hormone Assays
Plasma hormone assays assessed estrogen, progesterone, testosterone, and oxytocin. For details see Rubin et al. 2010.
2.3. Procedures
Women were evaluated once during the early follicular phase (Day 2–4; low estradiol/progesterone) and once during the midluteal phase (Day 20–22; high estradiol/progesterone), where “Day 1” was the first day of menstrual bleeding. Phase was validated with estradiol and progesterone levels. All participants were tested in two separate sessions, approximately 42 days apart, to assess females across menstrual cycles, and males in parallel. Phase at first session was counterbalanced across participants, and experimenters were blinded.
2.4. Statistical Analyses
Sex differences in emotion processing were examined at session 1 in a series of ANOVAs with Emotion (“happy”, “sad”, “neutral”) as the within-subject factor and Sex (men, women) and Group (patients, controls) as the between-subject factors. Changes in emotion processing across the menstrual cycle in women were examined in a series of ANOVAs with Menstrual Phase and Emotion as the within-subjects factors and Group as the between-subjects factor. A series of parallel ANOVAs were conducted in men to examine changes in emotion processing across sessions. Pearson correlations were calculated to evaluate the relationship between cycle-related changes in hormone levels and changes in emotion processing in women. For hormone levels that did not change significantly across the cycle, correlations were calculated examining the relationship between average hormone levels (across sessions) and average PEAT performance (across sessions).
3. Results
Controls were more accurate and faster in emotion recognition compared to patients (p’s<0.05), however, there were no group differences in emotion perception. Across both groups combined, emotion recognition was best for neutral faces, followed by happy, then sad (p’s<0.001); however, reaction times were faster for happy faces, followed by neutral, then sad (p’s<0.01). There were no sex differences on PEAT performance. However, women with and without schizophrenia more accurately identified facial emotions during the follicular versus midluteal phase (p<0.05; Table 2). PEAT performance did not differ significantly across sessions in men. No other effects were significant.
Table 2.
Mean Error Rates (Emotion Recognition), Response Rating (Emotion Perception), and Reaction Time on the Perception of Emotion Acuity Test (PEAT; M and SE) as a Function of Menstrual Cycle Phase for Women and as a Function of Study Session (A, B) for Men.
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| Patients (n = 22) |
Healthy Controls (n = 31) |
Patients (n = 26) |
Healthy Controls (n = 26) |
|||||
| Outcomes | Follicular | Midluteal | Follicular | Midluteal | Session A | Session B | Session A | Session B |
| M(SE) | M(SE) | M(SE) | M(SE) | M(SE) | M(SE) | M(SE) | M(SE) | |
| Mean Error Rates‡ | ||||||||
| Total P, G | 0.35 (0.02) | 0.39 (0.03) | 0.30 (0.02) | 0.32 (0.02) | 0.43 (0.03) | 0.39 (0.03) | 0.31 (0.03) | 0.31 (0.03) |
| Happy | 0.33 (0.03) | 0.37 (0.05) | 0.29 (0.03) | 0.28 (0.04) | 0.52 (0.04) | 0.45 (0.04) | 0.34 (0.04) | 0.32 (0.04) |
| Sad | 0.49 (0.04) | 0.57 (0.04) | 0.44 (0.03) | 0.48 (0.03) | 0.50 (0.04) | 0.47 (0.04) | 0.46 (0.04) | 0.46 (0.04) |
| Neutral | 0.21 (0.04) | 0.23 (0.05) | 0.18 (0.04) | 0.21 (0.04) | 0.28 (0.04) | 0.25 (0.04) | 0.14 (0.04) | 0.16 (0.04) |
| Mean Response Rating † | ||||||||
| Total | 3.96 (0.04) | 3.92 (0.04) | 3.88 (0.03) | 3.89 (0.04) | 3.84 (0.06) | 3.94 (0.04) | 3.93 (0.06) | 3.93 (0.04) |
| Happy | 2.32 (0.06) | 2.31 (0.07) | 2.18 (0.05) | 2.23 (0.06) | 2.29 (0.08) | 2.31 (0.08) | 2.32 (0.08) | 2.35 (0.08) |
| Sad | 5.55 (0.08) | 5.45 (0.12) | 5.49 (0.07) | 5.50 (0.10) | 5.35 (0.07) | 5.53 (0.07) | 5.52 (0.11) | 5.49 (0.07) |
| Neutral | 4.00 (0.04) | 4.00 (0.04) | 3.98 (0.03) | 3.95 (0.03) | 3.88 (0.06) | 3.99 (0.04) | 3.95 (0.06) | 3.95 (0.04) |
| Reaction Time ‖ | ||||||||
| Total G | 2679 (238) | 2944 (194) | 1982 (201) | 1809 (164) | 2715 (180) | 2804 (173) | 2101 (180) | 2109 (173) |
| Happy | 2334 (191) | 2572 (163) | 1788 (161) | 1643 (138) | 2575 (188) | 2612 (160) | 2027 (188) | 1983 (160) |
| Sad | 3195 (309) | 3275 (247) | 2173 (260) | 1983 (208) | 2907 (202) | 3043 (220) | 2228 (202) | 2317 (220) |
| Neutral | 2508 (258) | 2984 (217) | 1985 (217) | 1800 (183) | 2661 (202) | 2758 (192) | 2048 (202) | 2026 (192) |
Note.
Menstrual Phase significant at p<0.05.
Group significant for men and women at p<0.05.
Percentage of incorrect responses.
Mean response ratings on the 7-point scale where 1=very happy and 7=very sad.
Untransformed values are presented for reaction time, however, log transformed values were used for analysis since reaction time was not normally distributed.
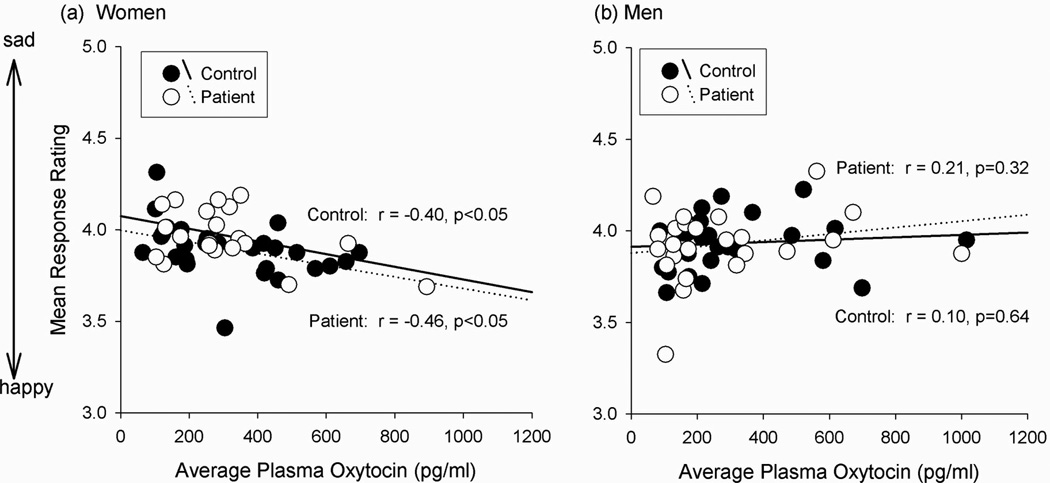
In women, despite significant cycle-related changes in emotional recognition and sex steroid hormone levels (p’s<0.001), the magnitude of change in estradiol and progesterone levels did not relate to the magnitude of change in emotion recognition. Furthermore, within each cycle phase separately, estradiol and progesterone levels did not significantly correlate with recognition accuracy. Although oxytocin did not fluctuate across phases, higher average oxytocin levels related to perceiving faces as happier (p<0.05) in both groups of women (patients r=−0.46, p=0.04; controls r=−0.40, p=0.04), but not in men (Figure 1). In women, the magnitude of the correlation between oxytocin and emotion perception did not differ between the follicular (r=−0.36, p=0.01) and luteal phase (r=−0.35, p=0.02). In patients, this pattern of effects remained significant after controlling for negative symptoms, suspiciousness/paranoia, and antipsychotic medication dose (Chlorpromazine equivalents) (Lehman et al., 2004; Woods, 2003). Testosterone levels did not relate to social cognition.
Figure 1.
Peripheral oxytocin correlates with emotion perception in (a) women but not (b) men.
Note. In women, the magnitude of correlation between oxytocin and emotion perception did not differ between the follicular (r = −0.36, p=0.01) and midluteal phase (r = −0.35, p=0.02), demonstrating that this correlation does not depend on levels of estrogen, progesterone, or both. The menstrual cycle manipulation therefore demonstrated that the relationship between oxytocin and emotion, perception did not depend on the level of circulating estrogen and progesterone.
4. Discussion
Our primary aim was to examine the relationship between endogenous oxytocin and emotion processing in patients with schizophrenia as a follow-up to our earlier demonstration that endogenous oxytocin related to improved symptom severity (Rubin et al. 2010). Higher average levels of oxytocin were associated with perceiving faces as happier in female patients and controls, but not in men. Despite similar levels of oxytocin, females in this study appeared to benefit more from endogenous oxytocin compared to men. This finding compliments our previous study, where the extent of reduced symptom severity in relation to oxytocin was broader in females (positive, general, and prosocial symptoms) than males (prosocial symptoms only). High oxytocin, especially in females, may help to produce a positive bias in emotion processing and consequently may contribute to improving trust and social interactions in schizophrenia.
Animal studies suggest that oxytocin differentially affects females and males, which may reflect interactions between oxytocin and gonadal hormones (Champagne et al., 2001; McCarthy et al.,1996; Razzoli et al., 2003). Intranasal oxytocin increases amygdala activity during emotional face processing in females (Domes et al., 2010), but reduces amygdala activity in men (Domes et al., 2007). Data suggest sex differences in the effects of oxytocin on processing of happy faces; intranasal oxytocin facilitated recognition of happy faces in both men and women in one study (Marsh et al., 2010), whereas another study in men only found no effect of oxytocin (Di Simplicio et al., 2008). The present menstrual cycle manipulation allowed us to directly examine whether the relationship between oxytocin and emotion perception depended on the level of circulating estrogen and progesterone. The magnitude of correlation between oxytocin and emotion perception did not differ between menstrual phases, demonstrating that this correlation does not depend on levels of estrogen, progesterone or both. The effects of oxytocin on emotion perception do not appear to depend on interactions with gonadal steroids in either schizophrenia patients or healthy individuals.
A second notable finding in the present study was that emotion recognition fluctuated across the menstrual cycle, and was highest during the follicular phase. Although the inclusion of women with schizoaffective disorders may have contributed to the finding of cycle-related changes in emotion recognition, this finding expands upon previous studies in healthy women in which the effect was similar (Derntl et al., 2008a,b). This also complements our previous finding that clinical symptoms fluctuate across the menstrual cycle in women with schizophrenia, and are worst during the follicular phase. However, cycle-related changes in estrogen and progesterone did not explain changes in emotion recognition. Thus, we did not replicate previous reports that estrogen (Guapo et al., 2009) or progesterone (Derntl et al., 2008a,b) relates to the accuracy of emotion recognition across the menstrual cycle in healthy women. The absence of effects of sex steroid hormones (particularly estrogen) on emotion recognition observed in this study may be related to the characteristics of the experimental paradigm employed, which evaluated only two emotions (happiness and sadness) and on a seven-point scale.
Cycle-related changes in emotion processing may reflect ovarian hormone effects on the amygdala; amygdala activation during performance of an emotional recognition task is more extensive in the follicular compared to the luteal phase (Derntl et al., 2008a,b). It is interesting that clinical symptoms were worst during the follicular phase when emotion recognition was best. Previous studies suggest that the amygdala is hyperactive during emotion processing in schizophrenia, which relates to worse symptoms (Rauch et al., 2010). Future studies might explore whether heightened activation of the amygdala during the follicular phase relates to both improved emotion recognition and higher levels of symptom severity. A broad psychological hypothesis is that higher levels of emotional reactivity may contribute to symptom severity and perhaps risk for relapse. Regardless of mechanism of action, oxytocin may have beneficial effects on emotional function, and potentially adverse effects on psychiatric symptomatology. This latter possibility might be considered in future clinical trials of oxytocin.
Conclusions
We report the novel finding that higher levels of endogenous oxytocin are associated with perceiving emotional faces as happier in both healthy women and women with schizophrenia. This extends our previous finding that higher levels of endogenous oxytocin are associated with improved clinical symptoms in women with schizophrenia. In light of the emerging literature supporting exogenous oxytocin as a potential adjunctive therapy for schizophrenia (Feifel et al., 2010), further study of the relationships among oxytocin, symptoms, and emotion perception is warranted.
Acknowledgements
This publication was made possible by Grant Number F31MH082480 from the National Institute of Mental Health, Grant Number K12HD055892 from the National Institute of Child Health and Human Development (NICHD), and the National Institutes of Health Office of Research on Women's Health (ORWH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health. Other support for this work was by a Psi Chi Graduate Research Grant, by the Alice J. Dan Dissertation Award from the UIC Center for Research on Women and Gender (CRWG), and by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS), Award Number UL1RR029879 from the National Center for Research Resources, and by the Core Lab of the GCRC/CNRU at the University of Alabama, which is supported by NIH grants M01-RR-00032, P30-DK56336). Validation of the oxytocin assay was supported by MH 072935 (CSC). Dr. Sweeney is supported by a Humboldt Research Award for Senior Research Scientists. We would also like to thank Erin Eatough, Antonia Savarese, Mary Winters, Pamela Perschler, Stephanie Klenotich, Jessica Jandak, and Melissa Arcabos for their assistance with this study. Special thanks goes to Ellen Herbener, Jim Pellegrino, Julie Dumas, Cherise Rosen, Sheila Dowd, Sandra Wilkniss and the Thresholds Psychiatric Rehabilitation Center, and the Center for Cognitive Medicine at the University of Illinois at Chicago.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Kryspin-Exner I, Fernbach E, Moser E, Habel U. Emotion recognition accuracy in healthy young females is associated with cycle phase. Horm Behav. 2008a;53:90–95. doi: 10.1016/j.yhbeh.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, Lamplmayr E, Kryspin-Exner I, Gur RC, Moser E, Habel U. Facial emotion recognition and amygdala activation are associated with menstrual cycle phase. Psychoneuroendocrinology. 2008b;33:1031–1040. doi: 10.1016/j.psyneuen.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simplicio M, Massey-Chase R, Cowen P, Harmer C. Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. J Psychopharmacol. 2008 doi: 10.1177/0269881108095705. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Gur RC, Gur RE, Skolnick B, Mawhinney-Hee M, Smailis J. Facial Emotion Discrimination: I. Task Construction Behavioral Findings in Normal Subjects. Psychiatry Research. 1992;42:231–240. doi: 10.1016/0165-1781(92)90115-j. [DOI] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A. Adjunctive Intranasal Oxytocin Reduces Symptoms in Schizophrenia Patients. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Gomes AM, Carter CS, Lee R. Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2193-8. [DOI] [PubMed] [Google Scholar]
- Guapo VG, Graeff FG, Zani AC, Labate CM, dos Reis RM, Del-Ben CM. Effects of sex hormonal levels and phases of the menstrual cycle in the processing of emotional faces. Psychoneuroendocrinology. 2009;34:1087–1094. doi: 10.1016/j.psyneuen.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Bilker W, Hagendoorn M, Gur RE, Gur RC. Emotion recognition deficit in schizophrenia: association with symptomatology and cognition. Biol Psychiatry. 2000;48:127–136. doi: 10.1016/s0006-3223(00)00847-7. [DOI] [PubMed] [Google Scholar]
- Lehman AF, Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB, Goldberg R, Green-Paden LD, Tenhula WN, Boerescu D, Tek C, Sandson N, Steinwachs DM. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003. Schizophr Bull. 2004;30:193–217. doi: 10.1093/oxfordjournals.schbul.a007071. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Blair RJ. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology (Berl) 2010;209:225–232. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- Pearson R, Lewis MB. Fear recognition across the menstrual cycle. Horm Behav. 2005;47:267–271. doi: 10.1016/j.yhbeh.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Rauch AV, Reker M, Ohrmann P, Pedersen A, Bauer J, Dannlowski U, Harding L, Koelkebeck K, Konrad C, Kugel H, Arolt V, Heindel W, Suslow T. Increased amygdala activation during automatic processing of facial emotion in schizophrenia. Psychiatry Res. 2010;182:200–206. doi: 10.1016/j.pscychresns.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Razzoli M, Cushing BS, Carter CS, Valsecchi P. Hormonal regulation of agonistic and affiliative behavior in female mongolian gerbils (Meriones unguiculatus) Horm Behav. 2003;43:549–553. doi: 10.1016/s0018-506x(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophr Res. 2010;124:13–21. doi: 10.1016/j.schres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten MR, Aleman A, Montagne B, Kahn RS. Schizophrenia and processing of facial emotions: sex matters. Schizophr Res. 2005;78:61–67. doi: 10.1016/j.schres.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Johnsen BH. Sex differences in judgement of facial affect: a multivariate analysis of recognition errors. Scand J Psychol. 2000;41:243–246. doi: 10.1111/1467-9450.00193. [DOI] [PubMed] [Google Scholar]
- Williams LM, Mathersul D, Palmer DM, Gur RC, Gur RE, Gordon E. Explicit identification and implicit recognition of facial emotions: I. Age effects in males and females across 10 decades. J Clin Exp Neuropsychol. 2009;31:257–277. doi: 10.1080/13803390802255635. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Ziv I, Leiser D, Levine J. Social cognition in schizophrenia: cognitive and affective factors. Cogn Neuropsychiatry. 2011;16:71–91. doi: 10.1080/13546805.2010.492693. [DOI] [PubMed] [Google Scholar]