Abstract
Emotions are closely tied to changes in autonomic (i.e., visceral motor) function, and interoceptive sensory feedback from body to brain exerts powerful modulatory control over motivation, affect, and stress responsiveness. This manuscript reviews evidence that early life experience can shape the structure and function of central visceral circuits that underlie behavioral and physiological responses to emotive and stressful events. The review begins with a general discussion of descending autonomic and ascending visceral sensory pathways within the brain, and then summarizes what is known about the postnatal development of these central visceral circuits in rats. Evidence is then presented to support the view that early life experience, particularly maternal care, can modify the developmental assembly and structure of these circuits in a way that impacts later stress responsiveness and emotional behavior. The review concludes by presenting a working hypothesis that endogenous cholecystokinin signaling and subsequent recruitment of gastric vagal sensory inputs to the caudal brainstem may be an important mechanism by which maternal care influences visceral circuit development in rat pups. Early life experience may contribute to meaningful individual differences in emotionality and stress responsiveness by shaping the postnatal developmental trajectory of central visceral circuits.
Keywords: postnatal development, autonomic, noradrenergic, emotion, hypothalamus, limbic system, vagus
1.0 Introduction
The importance of visceral and emotional functions in health and disease is well recognized. Emotions are closely tied to changes in autonomic outflow to the viscera, and interoceptive sensory feedback from body to brain exerts powerful modulatory control over motivation, affect, and emotional learning. Indeed, central visceral and emotional neural circuits are largely coextensive [1–8]. However, only limited research has been directed towards understanding how visceral and emotional neural control circuits are shaped by developmental events that are known to profoundly impact later emotionality and stress responsiveness in humans and research animals [9–14]. This review examines how early life experience might shape the development of central visceral circuits by considering the special impact of maternal care received by rat pups during the first one to two weeks of postnatal life.
The mammalian brain exhibits a high degree of circuit plasticity during early development, and neural activity during this “sensitive period” of development can promote lifelong changes in the way that neural circuits assemble and function later in life. We have shown that central pre-autonomic circuits undergo significant synaptic assembly and functional maturation in rats during the first two weeks of postnatal life, as do the largely overlapping circuits that receive interoceptive feedback from the body [15]. This developmental timeframe represents a potential sensitive period for the synaptic assembly of visceral neural circuits, which are known to figure prominently in adult stress responsiveness, affect, and the physiological expression of emotion. Indeed, a growing body of work supports the view that early life experience impacts later emotionality and stress responsiveness in rats and other mammalian species, including humans [9–14, 16–19]. A core thesis emerging from this work is that an organism’s behavioral and physiological responses to the world are derived from interactions among its genetic heritage (including sex), early maternal care, and individual life history. In laboratory situations in which these factors can be controlled, animals raised in environments that are characterized by unusual maternal care (e.g., enhanced or disorganized) during the first one or two weeks of postnatal life will, as adults, display unusual behavioral and physiological responses to emotive and stressful stimuli. Further, laboratory models that alter the maternal care received by rat pups appear to impact their adult emotionality and stress responsiveness in a sexually dimorphic manner [20–26].
1.1 Central visceral and emotional circuits are anatomically coextensive
William James proposed that emotional feelings represent the perceptual consequences of sensory feedback from the body that occur during and after stimulus-evoked bodily responses [27]. The core of James’ theory persists today amid mounting evidence that visceral functions and interoceptive feedback about these functions are intimately associated with emotional and cognitive neurobehavioral systems [5, 28–30]. My research group subscribes to the view that emotions arise from bodily responses to real and anticipated challenges and opportunities to which the organism is exposed. The emotional responses are both innate and learned [31–33], and are supported by neural processing within brainstem, hypothalamic, limbic, and cortical circuits.
Viscerosensory signals provide continuous feedback to the organism about homeostatic balance and emotional status. Real or perceived threats to these functional states elicit a constellation of adaptive physiological and behavioral stress responses, some of which depend on noradrenergic (NA) and corticotropin releasing factor (CRF) signaling in the hypothalamus and limbic forebrain [34–40]. Recruitment of highly interacting central NA and CRF systems can occur as a result of markedly different precipitating events, including threats arising from the environment, such as the odor of a predator, or signals arising from within the body, such as visceral malaise. Stress responses can be innate or conditioned through learning [31–33], but they always include endocrine and autonomic adjustments that alter internal visceral functions. Interoceptive feedback about these altered functions is delivered via ascending NA pathways from the caudal medulla to forebrain targets that contain CRF neurons and are thought to participate in stress-related aspects of motivated behavior and affect [41]. These forebrain targets include the paraventricular nucleus of the hypothalamus (PVN), central nucleus of the amygdala (CeA), and bed nucleus of the stria terminalis (BNST). Indeed, CRF neuronal activity in these regions is closely regulated by NA inputs [cf. [41–43]] that arise primarily from viscerosensory regions of the caudal medulla, and NA/CRF interactions are strongly implicated in stress and emotional responsiveness that is sensitive to the effects of early life experience [44].
The PVN, medial preoptic area (MePO), lateral hypothalamus (LHA), CeA, nucleus accumbens (NAcc), BNST, insular cortex (IC), and medial prefrontal cortex (mPFC) serve as principal gateways for septohippocampal and cortical influences over bodily responses that include endocrine, autonomic, and somatic components [45–49] (see Figure 1). Although most interoceptive signals never reach conscious awareness [50], sensory information regarding bodily state is delivered to these diencephalic and telencephalic regions to participate in the control of physiological and behavioral outflow, thereby biasing emotional and cognitive function and guiding ongoing and future motivated behavior [1, 2, 29, 30, 51]. Thus, factors that impact the developmental assembly and functional organization of central visceral circuits should impact emotionality and stress responsiveness.
Figure 1.
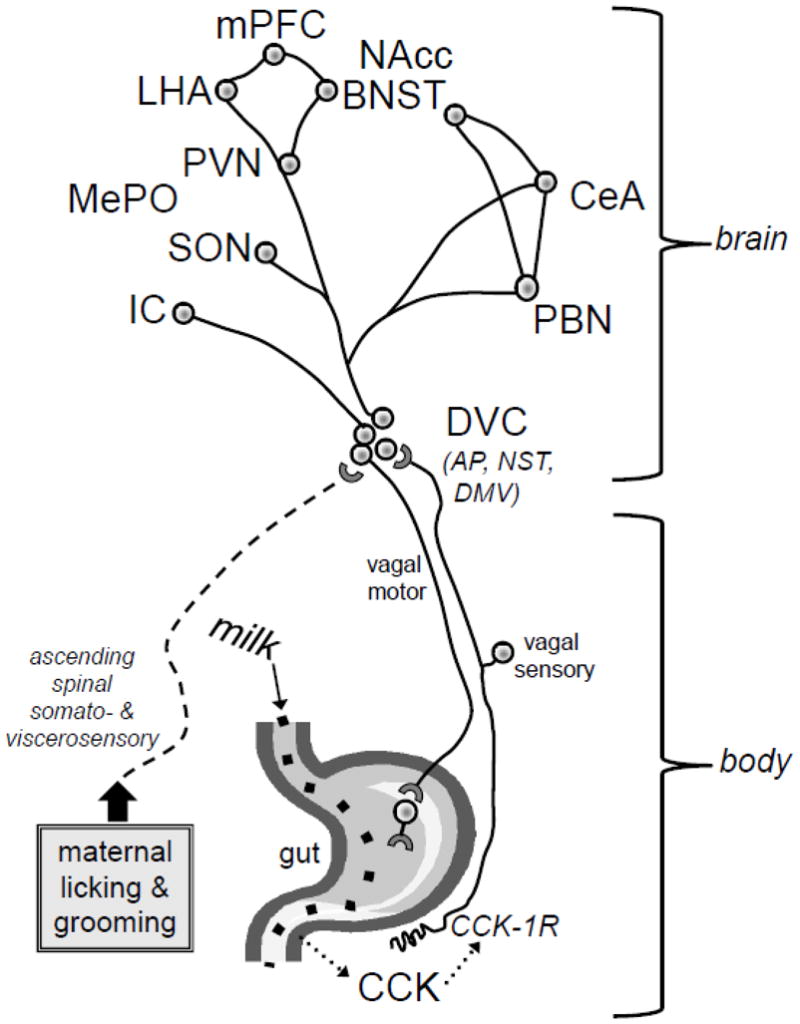
Endogenous CCK released from the gut during milk digestion in neonatal rats activates vagal sensory inputs to the caudal medulla (DVC) through a CCK-1 receptor-mediated mechanism. We hypothesize that vagal sensory signals can interact within the DVC with other somatosensory and visceral sensory signals ascending from the spinal cord via the spino-solitary tract, including signals generated by maternal licking and grooming of the rat pup. These signals may thereby modulate the functional development of ascending and reciprocated descending projections between the DVC and hypothalamic and limbic forebrain regions that modulate physiological and behavioral responses of the animal to its internal and external environments. Circles and lines are meant to represent key circuit nodes and connections among them, although the representation is not all-inclusive. Abbreviations as in the text.
1.2 Overview of central visceral circuits
The autonomic nervous system modifies visceral functions to meet the demands of immediate or anticipated changes in the organism’s internal and external environments. Autonomic outputs are strongly modulated by limbic and cortical sites that drive complex and nuanced visceral reactions to diverse threats and opportunities, including reactions based on past experience [52]. In all cases, adjustment of visceral output is highly influenced by interoceptive feedback. Indeed, visceral motor and sensory pathways are largely reciprocal, as evidenced by results from anterograde and retrograde tract-tracing studies [53–55].
The caudal medullary dorsal vagal complex (DVC) is a critical central node in both descending visceral motor and ascending interoceptive feedback pathways [56]. The DVC comprises the dorsal motor nucleus of the vagus (DMV), nucleus of the solitary tract (NST), and area postrema (AP). DMV preganglionic neurons provide parasympathetic outflow to multiple thoracic and abdominal visceral targets, and NST neurons receive direct and relayed synaptic input from vagal, glossopharyngeal, trigeminal, facial, and spinal somatosensory and viscerosensory afferents. Thus, the NST receives multidimensional sensory feedback from the entire body. The AP and a significant portion of the caudal medial NST contain fenestrated capillaries, permitting local parenchymal access by blood-borne factors (e.g., toxins, cytokines, hormones, osmolytes) that can affect local neural activity. Descending pathways from the hypothalamus and limbic forebrain to the DVC allow emotional stimuli and cognitive events to shape autonomic outflow, and reciprocal ascending pathways provide a route through which sensory feedback from the body can modulate stress hormone release (i.e., via the hypothalamic-pituitary-adrenal (HPA) axis), direct motivated behavior, shape emotional appraisals, and alter cognitive processing.
1.3 Descending visceral motor pathways
Our current view of how central pre-autonomic visceral circuits are organized owes much to the results of studies using neurotropic α-herpesviruses for retrograde transneuronal tracing of multisynaptic central circuits [4, 57–61]. Most of these studies have been conducted in adult rats, using attenuated vaccine strains of pseudorabies virus (PRV; a swine α-herpesvirus) that move selectively across synapses in the retrograde direction. For example, the Bartha strain of PRV [62] injected into the wall of the stomach [54, 63, 64] initially infects sympathetic and parasympathetic preganglionic motor neurons in the spinal cord and DVC, respectively, and subsequently is transported retrogradely and transneuronally to infect pre-autonomic neurons within circumscribed brainstem and forebrain regions. Central PRV transport from inoculated visceral targets that receive only sympathetic inputs (e.g., adrenal medulla, kidney, spleen) identifies essentially the same sets of medullary, pontine, midbrain, and diencephalic structures as are labeled by retrograde transport of classical tracers from different spinal levels of the sympathetic preganglionic column [65–68]. These structures include several hypothalamic nuclei (e.g., PVN and lateral hypothalamic area, LHA), periaqueductal gray, locus coeruleus, Barrington’s nucleus, ventromedial and ventrolateral pontine and medullary reticular formation, raphe nuclei, and the caudal medial NST. After similar post-inoculation intervals, PRV transport from viscera that receive both sympathetic and parasympathetic innervation (e.g., the wall of the stomach) identifies similar pre-autonomic regions of the brainstem and hypothalamus; however, the additional involvement of parasympathetic DVC neurons leads to additional infection of neurons within the CeA, BNST, IC, and mPFC [54, 63, 64] that are not similarly infected via sympathetic pathways. PRV tracing is uniquely suited for demonstrating, within a single animal, synaptic connections among neurons that occupy anatomically distinct nodes of multisynaptic, functionally-linked circuits [58].
1.4 Ascending visceral sensory pathways
Interoceptive information is relayed from body to brain along neural pathways that form largely reciprocal inputs to the same diencephalic and telencephalic regions that control visceral motor outflow [55, 69] (see Figure 1). Collateral projections from spinothalamic and dorsal column pathways merge with other ascending pathways that access the forebrain by relaying in the NST, reticular formation, and pontine parabrachial nucleus (PBN) [50, 70–75]. Since most spinal and cranial nerve visceral afferent pathways include a primary or collateral synaptic relay in the NST, analysis of the central projections of NST neurons effectively reveals the principle brainstem and forebrain targets of viscerosensory signaling pathways [76–78]. Neurons in the caudal visceral portion of the NST project to the ventrolateral medullary reticular formation (VLM), lateral PBN, PVN, LHA, CeA, and BNST. These projections are primarily catecholaminergic, arising from noradrenergic A2 and adrenergic C2 neurons whose cell bodies occupy the NST. For simplicity, these neurons will be referred to collectively as “noradrenergic” (NA) because they all are immunoreactive for dopamine-β-hydroxylase (DbH), the enzyme that converts dopamine to norepinephrine. Viscerosensory signals transmitted through the NST also recruit a parallel ascending pathway arising from NA neurons in the caudal VLM (A1 and C1 cell groups). Ascending NA projections participate in recruiting hypothalamic and limbic forebrain neurons during stress responses and emotional learning in adult rats [79–83]. The brain NA system is activated by stress, and modulates the activity of forebrain regions involved in behavioral and neuroendocrine output, including the PVN, CeA, and anterolateral BNST. NA inputs to these regions are implicated in behavioral and hormonal stress responses [84–86] and are sensitive to the effects of early maternal care [84]. It should be noted that hindbrain NA neurons co-express multiple neurotransmitter/neuromodulator molecules (cf.[87]), and so future work should consider the potential role of other signaling pathways that are recruited as a result of activating these neurons.
2.0 Postnatal development of central visceral circuits
Vagal sensory input to the DVC and motor output to the gastrointestinal tract are established before birth in rats [88, 89]. However, synaptic density within the DVC continues to increase over a protracted postnatal period [90–93]. Significant changes in glutamatergic and GABAergic transmission and local network properties within the DVC also occur postnatally [90, 94, 95], which contribute to the postnatal maturation of autonomic functions. For example, cardiac baroreceptor reflexes, which relay through the NST, are immature during the first postnatal week in rats [96, 97], and spinobulbospinal reflexes subserving micturition and defecation do not emerge until the third postnatal week [98]. Descending corticobulbar projection systems also become increasingly myelinated and continue to establish new synaptic connections with brainstem target neurons during the same developmental period [54, 99–101]. Collectively, these findings are consistent with our transneuronal PRV tracing results in neonatal rats, which indicate that diencephalic and telencephalic inputs to gastric DVC neurons are largely absent at birth and emerge gradually over the first two weeks postnatal [54, 102, 103]. Those studies demonstrated that descending pre-autonomic inputs from the hypothalamus, CeA, BNST, and visceral cortices reach the DVC between birth and P6, depending on their origin, but that the projections continue to mature postnatally. Indeed, many of these descending inputs do not establish synaptic connections within the rat DVC until the second postnatal week. As described below (2.1 and 2.2), the gradual maturation of central visceral circuits appears to underlie the postnatal maturation of behavioral and physiological responses to stressful events.
2.1 Postnatal maturation of dehydration anorexia
Dehydration anorexia provides an interesting example of how postnatal maturation of central visceral pathways corresponds with maturation of behavioral responses to an interoceptive challenge. Centrally-mediated responses to plasma hyperosmolality in adult rats include compensatory drinking [104], neurohypophyseal secretion of oxytocin and vasopressin [105], inhibition of vagally-mediated gastric motility and emptying [106, 107], and inhibition of food intake [106]. The first two responses also occur in neonatal rats [108–110], whereas the latter response, termed “dehydration anorexia”, does not emerge until after the first 2 weeks of postnatal development [109, 111]. The mechanisms that underlie the postnatal emergence of dehydration anorexia in rats may be related to delayed maturation of osmotic influences on neural signaling within the hindbrain DVC. This idea is supported by observations that only a subset of hindbrain regions that are activated to express Fos in adult rats after acute osmotic dehydration treatment are similarly activated in 2-day-old rats, including a lack of dehydration-induced Fos within the neonatal NST [112]. Conversely, prominent NST Fos expression occurs in adult rats after osmotic dehydration and other treatments that inhibit both feeding and gastric motility [112, 113]. The lack of NST Fos expression in dehydrated 2-day-old rats suggests that plasma hyperosmolality does not activate NST neurons in neonates, which led to the prediction that acute dehydration would not inhibit gastric emptying in neonatal rats, as it does in adult rats. This prediction was later found to be correct: an inhibitory effect of dehydration on gastric emptying emerges in rats between postnatal days 11 and 19, the same developmental period during which osmotic dehydration begins to inhibit rather than stimulate independent ingestion in rat pups [114].
These results point to an overlap between the functional maturation of central circuits that mediate dehydration-induced inhibition of gastric motility and emptying, and those that mediate inhibition of food intake. It is possible that these circuits share common structural features. For example, the ontogeny of dehydration-induced inhibition of both feeding and gastric emptying might be related to the postnatal maturation of central oxytocin (OT)-containing neural pathways. Magnocellular and parvocellular OT neurons in the paraventricular nucleus of the hypothalamus are activated by osmotic dehydration in adult and neonatal rats [112], and central OT signaling is implicated in dehydration anorexia in adult rats [115]. A subset of hypothalamic OT neurons projects to the DVC in adult rats [116, 117], and these projections play a physiologically important role in hypothalamic inhibition of vagally-mediated gastric motility [118]. Considered together, these findings suggest that central OT pathways contribute to both the hypophagic and gastric inhibitory effects of osmotic dehydration in adult rats. Conversely, central OT-containing pathways (including OT inputs to the DVC) are quite immature in neonatal rats, becoming gradually adult-like between 2 and 3 weeks postnatal [103]. Thus, postnatal maturation of central OT pathways may at least partially support the postnatal onset of dehydration-induced hypophagia and inhibition of gastric emptying.
2.2 The postnatal “stress hyporesponsive period”
The first 2–3 postnatal weeks of life correspond to a so-called “stress hyporesponsive period” that is characterized by reduced or absent HPA axis responses to many stimuli that robustly activate the HPA axis in adult rats. NA inputs to the medial parvocellular PVN provide important control over the activity of CRF-containing neurons at the apex of the HPA axis [37, 39, 119–122], and so immaturity of ascending NA viscerosensory pathways likely contributes to the documented hyporesponsiveness of CRF neurons to interoceptive stimuli in neonatal rats [123–127]. DbH fiber immunolabeling increases progressively in the rat PVN after postnatal day (P)1, with adult-like levels achieved by the end of the third postnatal week [127]. Functional immaturity of ascending viscerosensory pathways also has been demonstrated by analyzing neural Fos expression patterns in 2-day-old rats after systemic administration of cholecystokinin octapeptide (CCK) [128]. In striking contrast to results in adult rats, CCK treatment in 2-day-old rats does not activate Fos expression in the hypothalamus or other forebrain regions, and does not stimulate pituitary hormone release [128], consistent with other evidence for delayed postnatal maturation of ascending viscerosensory projections from the NST and VLM [54] to the hypothalamus and limbic forebrain [126, 129–133]. Later work in our laboratory also revealed significant postnatal maturation of central Fos responses to another interoceptive challenge, systemically administered lithium chloride (LiCl) [126].
The findings summarized above support the view that central visceral circuits are structurally and functionally immature in neonatal rats. Thus, these circuits may exhibit plasticity as they assemble during their developmentally-defined sensitive period. Highly specialized mechanisms are crucial for the initial establishment of postsynaptic specializations during synaptogenesis, and for activity-related changes in synaptic strength that underlie experience-dependent plasticity [134]. By analogy with other CNS systems, evoked neural activity within visceral circuits should shape ongoing synapse formation during the first two weeks of postnatal life.
3.0 Early experience modifies emotionality and stress responsiveness
Several model systems have been used to study the effects of early life experience on adult emotionality and stress responsiveness, including repeated daily episodes of brief (e.g., 15 min; MS15) or more extended (e.g., 180 min; MS180) periods of maternal separation in order to manipulate maternal care received by rat pups during the first two postnatal weeks [135]. Maternal interactions with rat pups generate interoceptive signals that include olfactory, thermal, metabolic, hydrational, gastrointestinal, tactile, and hormonal components [136, 137]. Perhaps paradoxically, the mild nest disruption inherent to the brief daily maternal separation paradigm (MS15) serves to significantly increase the amount of time that dams spend each day in active contact with their pups, including more time spent licking, grooming, and actively nursing them [14, 138, 139] compared to the behavior of dams that are not separated from their pups. Adult rats with a developmental history of daily MS15 during the first two weeks of postnatal life are less anxious in temperament and less behaviorally and hormonally responsive to laboratory stress paradigms compared to rats that were not separated from their dams during postnatal development [21, 135, 140–144]. However, and in sharp contrast to the increased maternal care elicited by MS15, rat pups exposed to daily MS180 during the first two weeks postnatal receive cumulatively less “high quality”, active maternal care each day compared to pups in either MS15 or non-separated control litters [14, 138, 139, 143, 144]. Mature rats with a developmental history of MS180 generally are more anxious and hyper-responsive to stress as adults compared to rats with a developmental history of MS15 or no separation [17, 135, 145].
The long-term effects of MS15 and MS180 appear to originate from the differential antecedent effects of these manipulations on tactile aspects of maternal behavior. In non-separated control litters, these forms of maternal behavior, such as licking and grooming, are normally distributed across dams and litters [138, 139]. Natural variations in maternal care received by pups in non-manipulated litters are correlated with differences in adult stress responsiveness [16, 146, 147], suggesting that relatively subtle alterations in early experience can significantly impact neural circuit development. As a result, non-separated litters represent a group that receives more variable maternal care compared to MS15 or MS180 litters; therefore, data from non-separated control litters tend to be more variable [139], and the most consistent experimental differences are often between rats raised in MS15 and MS180 litters [142]. Macrí and Würbel and colleagues [142–144] have effectively argued that active maternal care received by pups cannot entirely account for differences in their later endocrine and behavioral responsiveness to stressful/fearful events. Instead, maternal care received by pups appears to interact with maternal separation to exert relatively independent and opposing effects on the offspring, such that increased maternal care may act to buffer the adverse consequences of long separations [143].
4.0 Early experience modifies visceral circuit assembly
It has always been assumed that the effects of early experience on life-long emotionality and stress responsiveness involve epigenetic modification of CNS systems [17, 148–151]. The long-term consequences of MS15 and MS180 on behavioral and physiological responses to stress are at least partially mediated by early experience-dependent alterations of central CRF signaling pathways and glucocorticoid receptors involved in negative feedback control over the HPA axis [12, 17, 140, 152–158]. Most studies have focused on the neuroendocrine effects of MS15 and MS180, particularly alterations in the HPA axis that shape hormonal responses to acute and chronic stress. Far less attention has been paid to the potential impact of early life experience on adult visceral motor responses to stress, or on the neural pathways that relay sensory feedback about visceral responses to the brain. For example, adult rats with a developmental history of MS180 exhibit visceral hypersensitivity and are more prone to stress-induced intestinal mucosal dysfunction [155, 159–162]. Sex-specific alterations in adult baseline mean arterial blood pressure and hypoxic ventilatory responses in MS180 rats are at least partly due to enhanced responsiveness of the phrenic and carotid sinus nerves [163–166].
4.1 Postnatal plasticity of visceral motor circuits
Based on anatomical and functional findings summarized in preceding sections, we hypothesized that experimental manipulation of early maternal care (via MS15 and/or MS180) would alter the ongoing developmental assembly of central visceral circuits in rat pups, thereby providing a potential structural correlate for early experience-dependent effects on later physiological and behavioral responsiveness to emotionally evocative stimuli. To test this hypothesis, we traced central visceral circuit development in neonatal rats exposed to MS15 or MS180, using synapse-dependent retrograde transneuronal transport of PRV from the stomach wall [167]. Previous PRV tracing work in non-maternally separated rat pups had demonstrated that the first ten days of postnatal life represent a potential sensitive period of development, characterized by progressive synaptic assembly of central pre-autonomic circuits [54, 102]. To study the effects of early life experience on ongoing circuit development and synaptic assembly, rat pups from each MS group were injected with PRV on P8 and perfused on P10, during the course of daily MS15 or MS180 [167]. Quantitative analysis of central PRV transneuronal transport in pups from non-separated control litters confirmed our previous observations of age-dependent assembly of hypothalamic, limbic, and cortical inputs to autonomic motor neurons [54, 102]. Strikingly, however, circuit assembly was significantly altered in MS15 and MS180 pups, in which fewer neurons in the LHA, certain PVN subregions, CeA, BNST, and visceral cortices (mPFC, IC) were transneuronally labeled compared to labeling in age-matched controls [167]. Rather surprisingly, reductions in hypothalamic and limbic forebrain transneuronal infection were similar in MS15 and MS180 pups, and no sex differences were apparent. It is important to note, however, that these PRV tracing analyses were limited to a single early developmental time point (i.e., P8/P10) within ongoing circuit assembly. The results suggest that daily MS15 and MS180 manipulations exert a shared ability to delay the ongoing assembly of central visceral circuits during early postnatal development in both male and female rat pups; potentially, the daily handling/nest disruption intrinsic to both MS15 and MS180 could underlie this shared effect. Alternatively, or in addition, the surprisingly similar results that we obtained in 10-day old MS15 and MS180 pups [167] could have been due to the lack of pup isolation from littermates during daily MS180 treatment (e.g., see [144]). We subsequently have discovered that isolation is necessary for the ability of MS180 to increase anxiety-like behavior in the EPMZ in our experimental environment (unpublished observations), perhaps because isolation further reduces tactile and other sensory stimulation received by pups during the daily MS period. Thus, the MS180 group of pups used in our neonatal virus tracing study might not have grown up to exhibit the expected behavioral and endocrine phenotype.
It is of considerable interest to determine how early alterations in central pre-autonomic control circuits are manifested later in life, after synaptic assembly is complete and the circuits presumably are less plastic. Our recent findings in post-weaning juvenile rats (~P30) indicate that rats exposed to MS15 during the first 2 weeks of life display significantly enhanced transneuronal PRV labeling within pre-autonomic regions of the PVN compared to labeling in rats raised in non-separated control litters [168]. These results suggest that the reduction seen in neonatal MS15 rats reflects a delay in the developmental assembly of preautonomic circuits, rather than a permanent reduction in circuit strength. In ongoing research we are working to determine whether our new model for MS180 (i.e., incorporating pup isolation from littermates during each daily separation period) also promotes long-lasting effects on the structural organization of central visceral circuits.
4.2 Postnatal plasticity of visceral sensory pathways
The early MS paradigm has been used to show that maternal care has a significant impact on stressor-evoked norepinephrine release in the adult rat PVN [84]. Further, this effect is associated with altered NA α2 autoreceptor binding in the caudal medullary DVC, suggesting altered negative feedback control over stress-responsive NA neurons [84] that are heavily involved in local coordination of vagal motor outflow [169–172]} and other behavioral functions, such as inhibition of food intake [53, 81, 173, 174]. NA neurons within the DVC also are critically involved in relaying visceral sensory feedback to the PVN and BNST to initiate and modulate HPA axis responses to a variety of stressors [81, 83, 175, 176]. This ascending NA projection pathway undergoes significant structural and functional maturation during the first two weeks postnatal [12, 127, 177], the same period during which visceral motor circuits are developing (discussed above). We also have demonstrated that DbH-positive NA axon terminals form appositions and synapses with pre-autonomic PVN neurons that were transneuronally and retrogradely labeled with PRV from the stomach wall in adult rats [178]. Thus, alteration of ascending NA pathways could contribute to experience-dependent differences in the effect of stressful and emotive stimuli on both endocrine (i.e., HPA axis) and pre-autonomic PVN functions, and thus, to differences in central neural, physiological, behavioral responsiveness to stress.
To test this idea, we examined whether MS15 and/or MS180 might alter the later ability of an interoceptive challenge to recruit central neural circuits that receive visceral sensory signals and generate stress responses [179]. We previously had demonstrated that central neural Fos responses to systemic LiCl matured during postnatal development [126], suggesting that the circuits underlying these responses might be susceptible to alteration by manipulating pups’ early life experience during that period of maturation. Similar to previous reports in adult rats, adolescent rats (P35-45) with a developmental history of MS15 displayed less anxiety-like behavior on the elevated plus maze compared to control and MS180 rats [179]. MS15 rats tended to display fewer LiCl-activated neurons in most brain regions compared with rats in the other two rearing groups. The ability of MS15 to reduce central neural activation in rats after LiCl treatment may reflect, in a more general way, how early life experience can modulate later physiological and behavioral responses to homeostatic challenge. More recent work in our laboratory has extended these findings by demonstrating that MS15 also attenuates the ability of restraint stress to activate NA neurons within the DVC in juvenile rats [180].
5.0 How might maternal care shape central visceral circuit assembly?
Although the physiological effects of MS15 and/or MS180 on rat pups have not been rigorously examined, the available evidence suggests that some effects occur rapidly [181]. Conversely, plasma corticosterone levels do not begin to increase until pups have been isolated for several hours [182]. Thus, daily MS180 is unlikely to activate the HPA axis of developing pups, although this possibility has not been rigorously examined. Plasma osmolality and glucose levels also do not change in pups during daily MS180, although hypovolemia emerges within 2–3 hrs of maternal separation in the youngest pups [183]. Most laboratories utilizing the MS180 paradigm use an incubator to maintain pup body temperature during maternal separation, so hypothermia is unlikely to play a significant role.
In premature incubator-isolated human infants, supplementing tactile stimulation promotes marked gains in body weight and behavioral development, improved habituation and motor control, and significantly enhanced sympatho-adrenal maturation [184]. Clinical studies have demonstrated that providing premature human infants with active and passive touch, including skin-to-skin “kangaroo care” [10, 185, 186], alleviates many of the adverse effects of sensory neglect on behavior and physiology. Perhaps these interventions are effective because they alter the development of visceral neural circuits. Factors related to the presence of milk in the gut also are likely to affect the postnatal assembly and functional organization of central visceral circuits, and these factors may interact with tactile stimulation in a way that is altered by MS15 and/or MS180. For example, the upper gastrointestinal tract contains significantly less milk at the end of a 3 hr maternal separation period compared to the beginning [187]. Hofer has demonstrated that the presence of milk in the gastrointestinal tract potently modulates the activity of cardiac visceral sensory and motor pathways in neonatal rats, supporting his view that milk is a physiological regulator of early autonomic activity and balance [97]. Suckling also provides significant somatosensory stimulation and exerts profound behavioral effects on newborn rat pups and human infants [188]. Maternally derived stimuli initiate simple somato-visceral and viscero-visceral reflexes in neonatal rat pups, including urination and defecation induced by anogenital licking by the dam.
Interestingly, intragastric infusion of milk (or other liquid nutrients) and patterned ano-genital stimulation of rat pups can partially ameliorate the effects of prolonged maternal separation [189]. In this regard, it is particularly interesting that rat dams lick the perineal region of their male pups significantly more than that of their female pups [147], a factor which will alter the sensory stimulation received by pups and which may contribute to sex differences in how their central neural circuits assemble during the first two weeks postnatal. Thus, maternal nursing and licking of rat pups promotes rhythms of activity within central visceral circuits during a sensitive period of development in which the nature and frequency of stimulation may affect the establishment and strength of synaptic connections.
Recall that the DVC receives direct sensory inputs from the vagus and other cranial nerves, and spinal sensory inputs via the spino-solitary tract. Within the DVC, signals related to gastrointestinal milk may interact with signals related to the amount and frequency of maternal licking and grooming received (Figure 1). Visceral and somatosensory signals derived from maternal contact also may associate with increased or decreased levels of circulating factors such as growth hormone and CCK, both of which fall in pups during maternal separation and increase during and after feeding and/or tactile stimulation [190, 191]. Such factors might participate in the interactive effects of milk and maternal touch to regulate visceral circuit development during early postnatal life [192–195]. CCK is released from intestinal mucosal secretory cells when nutrients, including those present in milk [196], are transferred from stomach to small intestine [197]. CCK subsequently binds to CCK-1 receptors expressed in the gastrointestinal tract and along vagal afferent fibers [198, 199] to increase the activity of vagal sensory inputs to the medullary dorsal vagal complex [198]. CCK-1 receptors are especially abundant and widely distributed in the upper gastrointestinal tract in neonatal rats [200, 201], in which intragastric milk and exogenously administered CCK have calming effects [202–204]. Further, functional antagonism of CCK-1 receptors with systemically administered devazepide counteracts the calming effects of milk infusion or normal suckling in rat pups [203, 204].
We performed a study to examine whether the ability of MS15 to reduce later anxiety-like behavior in rats is at least partly due to increased gastrointestinal CCK release and signaling at CCK-1 receptors during the early postnatal period [205]. We predicted that rats with a developmental history of MS15 would display reduced anxiety-like behavior, as previously reported, and that this anxiolytic effect would be attenuated or reversed in rats in which daily MS15 was accompanied by systemic administration of devazepide to antagonize endogenous CCK-1 receptors. We reasoned that upon maternal reunion, increased somatosensory-related stimulation of pups by their dam promotes increased gastric emptying and delivery of existing milk from the pup’s stomach into the duodenum (i.e., similar to maternal licking-induced micturition and defecation reflexes), contributing to an increase in plasma CCK levels that would increase gastrointestinal vagal sensory activation over the course of early postnatal development. As predicted, rats with a developmental history of MS15 displayed reduced anxiety-like behavior, and this behavioral phenotype was reversed in devazepide-treated rats [205]. It should be noted that devazepide does cross the blood-brain barrier [206], and so its pharmacological actions in our study cannot conclusively be ascribed only to reduced CCK-1 receptor signaling within gastrointestinal tissue and vagal sensory afferents. Nonetheless, our results support the view that endogenous CCK-1 receptor signaling in infants is a potential pathway through which maternal-pup interactions regulate the development and functional organization of emotional circuits that control anxiety-like behavior in the offspring.
6.0 Conclusion
Early postnatal life events can strongly influence the functional development of central neural systems that mediate the expression of behavioral, emotional, autonomic, and endocrine responses to stress. Although central visceral and emotional neural circuits are largely coextensive, relatively little research effort has been directed towards understanding how these circuits are shaped by developmental events that profoundly impact later emotionality and stress responsiveness. Experimental outcomes reviewed in this manuscript provide insight into the biological impact of early life experience on central circuits that respond to interoceptive and exteroceptive events, including events that elicit behavioral and physiological stress responses. Early life experience can shape the developmental trajectory of these neural circuits, leading to altered responses to internal and external sensory cues that may contribute to individual differences in emotionality and stress responsiveness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- 1.Cameron OG. Interoception: The inside story -- A model for psychosomatic processes. Psychosomatic Medicine. 2001;63:697–710. doi: 10.1097/00006842-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews - Neuroscience. 2002;2:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 3.Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neuroscience. 2008;156:1093–102. doi: 10.1016/j.neuroscience.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annual Review of Neuroscience. 2002;25:433–69. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 5.Thayer JF, Lane RD. A model of neurovisceral integration in emotional regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–16. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 6.King AB, Menon RS, Hachinski V, Cechetto DF. Human forebrain activation by visceral stimuli. The Journal of Comparative Neurology. 1999;413:572–82. [PubMed] [Google Scholar]
- 7.Price JL. Prefrontal cortical networks related to visceral function and mood. Annals of the New York Academy of Sciences. 1999;877:383–96. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- 8.Berntson GG, Sarter M, Cacioppo JT. Ascending visceral regulation of cortical affective information processing. European Journal of Neuroscience. 2003;18:2103–9. doi: 10.1046/j.1460-9568.2003.02967.x. [DOI] [PubMed] [Google Scholar]
- 9.Feldman R, Eidelman AI. Skin-to-skin contact (Kangaroo Care) accelerates autonomic and behavioural maturation in preterm infants. Dev Med Child Neurology. 2003;45:274–81. doi: 10.1017/s0012162203000525. [DOI] [PubMed] [Google Scholar]
- 10.Feldman R, Weller A, Sirota L, Eidelman AI. Skin-to-skin contact (kangaroo care) promotes self-regulation in premature infants: sleep-wake cyclicity, arousal modulation, and sustained exploration. Developmental Psychology. 2002;38:194–207. doi: 10.1037//0012-1649.38.2.194. [DOI] [PubMed] [Google Scholar]
- 11.Field T. Maternal depression effects on infants and early interventions. Preventive Medicine. 1998;27:200–3. doi: 10.1006/pmed.1998.0293. [DOI] [PubMed] [Google Scholar]
- 12.Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor-norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biological Psychiatry. 1999;46:1153–66. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- 13.Gunnar MB. Quality of early care and buffering of neuroendocrine stress reactions: potential effects on the developing human brain. Preventive Medicine. 1998;27:208–11. doi: 10.1006/pmed.1998.0276. [DOI] [PubMed] [Google Scholar]
- 14.Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, et al. Epigenetic programming of stress responses through variations in maternal care. Annals of the New York Academy of Sciences. 2004;1036:167–80. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- 15.Rinaman L, Koehnle TJ. Development of Central Visceral Circuits. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 298–321. [Google Scholar]
- 16.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–92. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 17.Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- 18.Maunder RG, Hunter JJ. Attachment and psychosomatic medicine: developmental contributions to stress. Psychosomatic Medicine. 2001;63:556–67. doi: 10.1097/00006842-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Champagne FA, Curley JP. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neuroscience and Biobehavioral Reviews. 2009;33:593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Faraday MM. Rat sex and strain differences in responses to stress. Physiology and Behavior. 2002;75:507–22. doi: 10.1016/s0031-9384(02)00645-5. [DOI] [PubMed] [Google Scholar]
- 21.Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiology and Behavior. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Reviews in the Neurosciences. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann J, Pryce CR, Bettschen D, Feldon J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacology Biochemistry and Behavior. 1999;64:705–15. doi: 10.1016/s0091-3057(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 24.Slotten HA, Kalinichev M, Hagan JJ, Marsden CA, Fone KC. Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: gender-dependent effects. Brain Research. 2006;1097:123–32. doi: 10.1016/j.brainres.2006.04.066. [DOI] [PubMed] [Google Scholar]
- 25.Champagne FA, Curley JP. Maternal regulation of estrogen receptor a methylation. Current Opinion in Pharmacology. 2008;8:735–9. doi: 10.1016/j.coph.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Champagne DL, Bagot RC, Hasselt FV, Ramakers G, Meaney MJ, deKloet ER, et al. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. The Journal of Neuroscience. 2008;28:6037–45. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James W. What is an emotion? Mind. 1884:9. [Google Scholar]
- 28.Damasio A. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- 29.Prinz JJ. Gut Reactions: A Perceptual Theory of Emotion. New York: Oxford University Press; 2004. [Google Scholar]
- 30.Mayer EA, Saper CB. Progress in Brain Research. Amsterdam: Elsevier; 2000. The Biological Basis for Mind Body Interactions. [Google Scholar]
- 31.Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. Heterogeneous neurochemical responses to different stressors: a test of Selye’s doctrine of nonspecificity. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 1998;275:R1247–R55. doi: 10.1152/ajpregu.1998.275.4.R1247. [DOI] [PubMed] [Google Scholar]
- 32.Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocrine Reviews. 2001;22:502–48. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- 33.Palkovits M. Interconnections between the neuroendocrine hypothalamus and the central autonomic system. Frontiers in Neuroendocrinology. 1999;20:270–95. doi: 10.1006/frne.1999.0186. [DOI] [PubMed] [Google Scholar]
- 34.Kitazawa S, Shioda S, Nakai Y. Catecholaminergic innervation of neurons containing corticotropin-releasing factor in the paraventricular nucleus of the rat hypothalamus. Acta Anatomica (Basel) 1987;129:337. [PubMed] [Google Scholar]
- 35.Phelix CF, Liposits Z, Paull WK. Catecholamine-CRF synaptic interaction in a septal bed nucleus: afferents of neurons in the bed nucleus of the stria terminalis. Brain Research Bulletin. 1994;33:109–19. doi: 10.1016/0361-9230(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 36.Day T, Ferguson AV, Renaud L. Noradrenergic afferents facilitate the activity of tuberoinfundibular neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology. 1985;41:17. doi: 10.1159/000124148. [DOI] [PubMed] [Google Scholar]
- 37.Alonso G, Szafarczyk A, Balmefrezol M, Assenmacher I. Immunocytochemical evidence of stimulatory control by the ventral noradrenergic bundle of parvicellular neurons of the paraventricular nucleus secreting corticotropin-releasing hormone and vasopressin in rats. Brain Research. 1986;397:297–307. doi: 10.1016/0006-8993(86)90631-1. [DOI] [PubMed] [Google Scholar]
- 38.Asan E, Yilmazer-Hanke DM, Eliava M, Hantsch M, Lesch KP, Schmitt A. The corticotropin-releasing factor (CRF)-system and monoaminergic afferents in the central amygdala: investigations in different mouse strains and comparison with the rat. Neuroscience. 2005;131:953–67. doi: 10.1016/j.neuroscience.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 39.Liposits Z, Phelix C, Paull WK. Adrenergic innervation of corticotropin releasing factor (CRF) - synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. Histochemistry. 1986;84:201–5. doi: 10.1007/BF00495783. [DOI] [PubMed] [Google Scholar]
- 40.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biological Psychiatry. 1999;46:1167–80. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 41.Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Annals of the New York Academy of Sciences. 2004;1018:25–34. doi: 10.1196/annals.1296.003. [DOI] [PubMed] [Google Scholar]
- 42.Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. The Journal of Comparative Neurology. 2001;436:430–55. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 43.Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. The Journal of Neuroscience. 2004;24:8198–204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahuque LL, Kullberg EF, Mcgeehan AJ, Kinder JR, Hicks MP, Blanton MG, et al. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology. 2006;186:122–32. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein MB. Neurobiological perspectives on social phobia: from affiliation to zoology. Biological Psychiatry. 1998;44:1277–85. doi: 10.1016/s0006-3223(98)00265-0. [DOI] [PubMed] [Google Scholar]
- 46.Pratt JA. The neuroanatomical basis of anxiety. Pharmacology and Therapeutics. 1992;55:149–81. doi: 10.1016/0163-7258(92)90014-q. [DOI] [PubMed] [Google Scholar]
- 47.Vouimba RM, Garcia R, Jaffard R. Opposite effects of lateral septal LTP and lateral septal lesions on contextual fear conditioning in mice. Behavioral Neuroscience. 1998;112:875–84. doi: 10.1037//0735-7044.112.4.875. [DOI] [PubMed] [Google Scholar]
- 48.Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nature Neuroscience. 1999;2:833–9. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- 49.Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learning and Memory. 1994;1:34–44. [PubMed] [Google Scholar]
- 50.Bielefeldt K, Christianson JA, Davis BM. Basic and clinical aspects of visceral sensation: transmission in the CNS. Neurogastroenterology and Motility. 2005;17:488–99. doi: 10.1111/j.1365-2982.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- 51.Bechara A, Naqvi N. Listening to your heart: interoceptive awareness as a gateway to feeling. Nature Neuroscience. 2004;7:102–3. doi: 10.1038/nn0204-102. [DOI] [PubMed] [Google Scholar]
- 52.Price JL. Free will versus survival: brain systems that underlie intrinsic constraints on behavior. The Journal of Comparative Neurology. 2005;493:132–9. doi: 10.1002/cne.20750. [DOI] [PubMed] [Google Scholar]
- 53.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Research. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rinaman L, Levitt P, Card JP. Progressive postnatal assembly of limbic-autonomic circuits revealed by central transneuronal transport of pseudorabies virus. The Journal of Neuroscience. 2000;20:2731–41. doi: 10.1523/JNEUROSCI.20-07-02731.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rinaman L, Schwartz GJ. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. The Journal of Neuroscience. 2004;24:2782–6. doi: 10.1523/JNEUROSCI.5329-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rinaman L. Postnatal development of hypothalamic inputs to the dorsal vagal complex in rats. Physiology and Behavior. 2003;79:65–70. doi: 10.1016/s0031-9384(03)00105-7. [DOI] [PubMed] [Google Scholar]
- 57.Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Research. 1989;491:156–62. doi: 10.1016/0006-8993(89)90098-x. [DOI] [PubMed] [Google Scholar]
- 58.Card JP. Exploring brain circuitry with neurotropic viruses: new horizons in neuroanatomy. The Anatomical Record (New Anat) 1998;253:176–85. doi: 10.1002/(SICI)1097-0185(199812)253:6<176::AID-AR6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 59.Strack AM, Loewy AD. Pseudorabies virus: a highly specific transneuronal cell body marker in the sympathetic nervous system. Journal of Neuroscience. 1990;10:2139–47. doi: 10.1523/JNEUROSCI.10-07-02139.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loewy AD. Pseudorabies virus: a transneuronal tracer for neuroanatomical studies. In: Loewy AD, Kaplitt MG, editors. Viral Vectors. Gene therapy and neuroscience applications. San Diego: Academic Press; 1995. pp. 349–66. [Google Scholar]
- 61.Saper CB. Central Autonomic System. In: Paxinos G, editor. The Rat Nervous System. 3. San Diego: Elsevier Academic Press; 2004. pp. 761–96. [Google Scholar]
- 62.Bartha A. Experimental reduction of virulence of Aujeszky’s disease virus. Magy Allotorv Lapja. 1961;16:42–5. [Google Scholar]
- 63.Card JP, Rinaman L, Lynn RB, Lee BH, Meade RP, Miselis RR, et al. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J Neurosci. 1993;13:2515–39. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang M, Card JP, Tirabassi RS, Miselis RR, Enquist LW. Retrograde, transneuronal spread of pseudorabies virus in defined neuronal circuitry of the rat brain is facilitated by gE mutations that reduce virulence. Journal of Virology. 1999;73:4350–9. doi: 10.1128/jvi.73.5.4350-4359.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tucker DC, Saper CB. Specificity of spinal projections from hypothalamic and brainstem areas which innervate sympathetic preganglionic neurons. Brain Research. 1985;360:159–64. doi: 10.1016/0006-8993(85)91231-4. [DOI] [PubMed] [Google Scholar]
- 66.Cano G, Card JP, Rinaman L, Sved AF. Connections of Barrington’s nucleus to the sympathetic nervous system in rats. Journal of the Autonomic Nervous System. 2000;79:117–28. doi: 10.1016/s0165-1838(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 67.Cano G, Sved AF, Rinaman L, Rabin BS, Card JP. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. The Journal of Comparative Neurology. 2001;439:1–18. doi: 10.1002/cne.1331. [DOI] [PubMed] [Google Scholar]
- 68.Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Research. 1989;491:274–96. doi: 10.1016/0006-8993(89)90063-2. [DOI] [PubMed] [Google Scholar]
- 69.Loewy AD. Central autonomic pathways. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. 1. New York: Oxford University Press; 1990. pp. 88–103. [Google Scholar]
- 70.Potts JT, Lee SM, Anguelov PI. Tracing of projection neurons from the cervical dorsal horn to the medulla with the anterograde tracer biotinylated dextran amine. Autonomic Neuroscience: Basic and Clinical. 2002;98:64–9. doi: 10.1016/s1566-0702(02)00034-6. [DOI] [PubMed] [Google Scholar]
- 71.Hylden JL, Anton F, Nahin RL. Spinal lamina I projection neurons in the rat: collateral innervation of parabrachial area and thalamus. Neuroscience. 1989;28:27–37. doi: 10.1016/0306-4522(89)90229-7. [DOI] [PubMed] [Google Scholar]
- 72.Menétrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. The Journal of Comparative Neurology. 1987;255:439–50. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- 73.Green T, Dockray GJ. Calcitonin gene-related peptide and substance P in afferents to the upper gastrointestinal tract in the rat. Neuroscience Letters. 1987;76:151–6. doi: 10.1016/0304-3940(87)90707-5. [DOI] [PubMed] [Google Scholar]
- 74.Wang CC, Willis WD, Westlund KN. Ascending projections from the area around the spinal cord central canal: a Phaseolus vulgaris leucoagglutinin study in rats. The Journal of Comparative Neurology. 1999;415:341–67. doi: 10.1002/(sici)1096-9861(19991220)415:3<341::aid-cne3>3.0.co;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Helmeke C, Ovtscharoff WJ, Poeggel G, Braun K. Juvenile emotional experience alters synaptic inputs on pyramidal neurons in the anterior cingulate cortex. Cerebral Cortex. 2001;11:717–27. doi: 10.1093/cercor/11.8.717. [DOI] [PubMed] [Google Scholar]
- 76.Ter Horst GJ, Streefland C. Ascending projections of the solitary tract nucleus. In: Barraco IRA, editor. Nucleus of the Solitary Tract. Boca Raton: CRC Press; 1994. pp. 93–103. [Google Scholar]
- 77.Ter Horst GJ, Boer PD, Luiten PGM, Willigen JDV. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31:785–97. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- 78.Bailey TW, Hermes SM, Andresen MC, Aicher AA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. The Journal of Neuroscience. 2006;26:11893–902. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis: a target site for noradrenergic actions in opiate withdrawal. Annals of the New York Academy of Sciences. 1999;877:486–98. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- 80.Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–4. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- 81.Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. The Journal of Neuroscience. 2003;23:10084–92. doi: 10.1523/JNEUROSCI.23-31-10084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. American Journal of Physiology. 1999;277:R582–R90. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- 83.Li HY, Ericsson A, Sawchenko PE. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proceedings of the National Academy of Sciences, USA. 1996;93:2359–64. doi: 10.1073/pnas.93.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinephrine release in the hypothalamic paraventricular nucleus. Journal of Neuroendocrinology. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- 85.Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine, acting on alpha1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002;43:1139–47. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 86.Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 87.Rinaman L. Hindbrain Noradrenergic A2 Neurons: Diverse Roles in Autonomic, Endocrine, Cognitive, and Behavioral Functions. Am J Physiol Regul Integr Comp Physiol. 2010 doi: 10.1152/ajpregu.00556.2010. ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rinaman L, Levitt P. Establishment of vagal sensory-motor circuits during fetal development in rats. Journal of Neurobiology. 1993;24:641–59. doi: 10.1002/neu.480240509. [DOI] [PubMed] [Google Scholar]
- 89.Zhang LL, Ashwell KWS. The development of cranial nerve and visceral afferents to the nucleus of the solitary tract in the rat. Anatomy and Embryology. 2001;204:135–51. doi: 10.1007/s004290100185. [DOI] [PubMed] [Google Scholar]
- 90.Rao H, Jean A, Kessler JP. Postnatal ontogeny of glutamate receptors in the rat nucleus tractus solitarii and ventrolateral medulla. Journal of the Autonomic Nervous System. 1997;65:25–32. doi: 10.1016/s0165-1838(97)00031-3. [DOI] [PubMed] [Google Scholar]
- 91.Miller AJ, McKoon M, Pinneau M, Silverstein R. Postnatal synaptic development of the nucleus tractus solitarius (NTS) of the rat. Developmental Brain Research. 1983;8:205–13. doi: 10.1016/0165-3806(83)90005-6. [DOI] [PubMed] [Google Scholar]
- 92.Lachamp P, Tell F, Kessler JP. Successive episodes of synapse production in the developing rat nucleus tractus solitarii. Journal of Neurobiology. 2002;52:336–42. doi: 10.1002/neu.10091. [DOI] [PubMed] [Google Scholar]
- 93.Vincent A, Tell F. Postnatal development of rat nucleus tractus solitarius neurons: morphological and electrophysiological evidence. Neuroscience. 1999;93:293–305. doi: 10.1016/s0306-4522(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 94.Kawai Y, Senba E. Postnatal differentiation of local networks in the nucleus of the tractus solitarius. Neuroscience. 2000;100:109–14. doi: 10.1016/s0306-4522(00)00257-8. [DOI] [PubMed] [Google Scholar]
- 95.Dufour A, Tell F, Baude A. Perinatal development of inhibitory synapses in the nucleus tractus solitarii of the rat. European Journal of Neuroscience. 2010;32:538–49. doi: 10.1111/j.1460-9568.2010.07309.x. [DOI] [PubMed] [Google Scholar]
- 96.Quigley KS, Myers MM, Shair HN. Development of the baroreflex in the young rat. Autonomic Neuroscience: Basic and Clinical. 2005;121:26–32. doi: 10.1016/j.autneu.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 97.Hofer MA. Early stages in the organization of cardiovascular control. Proceedings of the Society for Experimental Biology and Medicine. 1984;175:147–57. doi: 10.3181/00379727-175-41780. [DOI] [PubMed] [Google Scholar]
- 98.Araki I, Groat WCd. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. The Journal of Neuroscience. 1997;17:8402–7. doi: 10.1523/JNEUROSCI.17-21-08402.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sarnat HB. Do the corticospinal and corticobulbar tracts mediate functions in the human newborn? Canadian Journal of Neurological Sciences. 1989;16:157–60. doi: 10.1017/s0317167100028821. [DOI] [PubMed] [Google Scholar]
- 100.Iriki A, Nozaki S, Nakamura Y. Feeding behavior in mammals: corticobulbar projection is reorganized during conversion from sucking to chewing. Brain Research. 1988;44:189–96. doi: 10.1016/0165-3806(88)90217-9. [DOI] [PubMed] [Google Scholar]
- 101.Martin GF, Cabana T, Culberson JL, Curry JJ, Tschismadia I. The early development of corticobulbar and corticospinal systems. Studies using the North American opossum. Anatomy and Embryology. 1980;161:197–213. doi: 10.1007/BF00305344. [DOI] [PubMed] [Google Scholar]
- 102.Rinaman L, Roesch MR, Card JP. Retrograde transynaptic pseudorabies virus infection of central autonomic circuits in neonatal rats. Developmental Brain Research. 1999;114:207–16. doi: 10.1016/s0165-3806(99)00039-5. [DOI] [PubMed] [Google Scholar]
- 103.Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. The Journal of Comparative Neurology. 1998;399:101–9. doi: 10.1002/(sici)1096-9861(19980914)399:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 104.Fitzsimons JT. The effects of slow infusions of hypertonic solutions on drinking and drinking thresholds in rats. Journal of Physiology. 1963;167:344–54. doi: 10.1113/jphysiol.1963.sp007154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stricker EM, Verbalis JG. Interaction of osmotic and volume stimuli in regulation of neurohypophyseal secretion in rats. American Journal of Physiology. 1986;250:R267–R75. doi: 10.1152/ajpregu.1986.250.2.R267. [DOI] [PubMed] [Google Scholar]
- 106.Flanagan LM, Blackburn RE, Verbalis JG, Stricker EM. Hypertonic NaCl inhibits gastric motility and food intake in rats with lesions in the rostral AV3V region. American Journal of Physiology. 1992;263:R9–R14. doi: 10.1152/ajpregu.1992.263.1.R9. [DOI] [PubMed] [Google Scholar]
- 107.Flanagan LM, Verbalis JG, Stricker EM. Effects of anorexigenic treatments on gastric motility in rats. American Journal of Physiology. 1989;256:R955–R61. doi: 10.1152/ajpregu.1989.256.4.R955. [DOI] [PubMed] [Google Scholar]
- 108.Almli CR. The ontogeny of the onset of drinking and plasma osmotic pressure regulation. Developmental Psychobiology. 1973;6:147–58. doi: 10.1002/dev.420060209. [DOI] [PubMed] [Google Scholar]
- 109.Bruno JP. Development of drinking behavior in preweanling rats. Journal of Comparative and Physiological Psychology. 1981;95:1016–27. [Google Scholar]
- 110.Sinding C, Robinson AG, Seif SM. Levels of neurohypophyseal peptides in the rat during the first month of life. II. Response to physiological stimuli. Endocrinology. 1980;107:755–60. doi: 10.1210/endo-107-3-755. [DOI] [PubMed] [Google Scholar]
- 111.Bruno JP, Hall WG. Olfactory contributions to dehydration-induced anorexia in weanling rats. Developmental Psychobiology. 1982;15:493–505. doi: 10.1002/dev.420150602. [DOI] [PubMed] [Google Scholar]
- 112.Rinaman L, Stricker EM, Hoffman GE, Verbalis JG. Central c-fos expression in neonatal and adult rats after subcutaneous injection of hypertonic saline. Neuroscience. 1997;79:1165–75. doi: 10.1016/s0306-4522(97)00022-5. [DOI] [PubMed] [Google Scholar]
- 113.Olson BR, Freilino M, Hoffman GE, Stricker EM, Sved AF, Verbalis JG. c-Fos expression in rat brain and brainstem nuclei in reponse to treatments that alter food intake and gastric motility. Molecular and Cellular Neuroscience. 1993;4:93–106. doi: 10.1006/mcne.1993.1011. [DOI] [PubMed] [Google Scholar]
- 114.Callahan JB, Rinaman L. The postnatal emergence of dehydration anorexia in rats is temporally associated with the emergence of dehydration-induced inhibition of gastric emptying. Physiology and Behavior. 1998;64:683–7. doi: 10.1016/s0031-9384(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 115.Olson BR, Drutarosky MC, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology. 1991;129:785–91. doi: 10.1210/endo-129-2-785. [DOI] [PubMed] [Google Scholar]
- 116.Swanson LW, Kuypers HGJM. The paraventricular nucleus of the hypothalamus: Cytoarchitectonic subdivisions and the organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. Journal of Comparative Neurology. 1980;194:555–70. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 117.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2004;287:R87–R96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 118.Rogers RC, Hermann GE. Central regulation of brainstem gastric vago-vagal control circuits. In: Ritter S, Ritter RC, Barnes CD, editors. Neuroanatomy and Physiology of Abdominal Vagal Afferents. Boca Raton: CRC Press; 1992. pp. 99–134. [Google Scholar]
- 119.Sarkar S, Fekete C, Legradi G, Lechan RM. Glucagon like peptide-1 (7–36) amide (GLP-1) nerve terminals densely innervate corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Research. 2003;985:163–8. doi: 10.1016/s0006-8993(03)03117-2. [DOI] [PubMed] [Google Scholar]
- 120.Gaillet S, Lachuer J, Malaval F, Assenmacher I, Szafarczyk A. The involvement of noradrenergic ascending pathways in the stress-induced activation of ACTH and corticosterone secretions is dependent on the nature of the stressors. Experimental Brain Research. 1991;87:173–80. doi: 10.1007/BF00228518. [DOI] [PubMed] [Google Scholar]
- 121.Al-Damluji S. Adrenergic mechanisms in the control of corticotropin secretion. Journal of Endocrinology. 1988;119:5–14. doi: 10.1677/joe.0.1190005. [DOI] [PubMed] [Google Scholar]
- 122.Kiss A, Aguilera G. Participation of alpha1-adrenergic receptors in the secretion of hypothalamic corticotropin-releasing hormone during stress. Neuroendocrinology. 1992;56:153–60. doi: 10.1159/000126223. [DOI] [PubMed] [Google Scholar]
- 123.Pihoker C, Owens MJ, Kuhn CM, Schanberg SM, Nemeroff CB. Maternal separation in neonatal rats elicits activation of the hypothalamic-pituitary-adrenocortical axis: a putative role for corticotropin-releasing factor. Psychoneuroendocrinology. 1993;18:485–93. doi: 10.1016/0306-4530(93)90042-j. [DOI] [PubMed] [Google Scholar]
- 124.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews. 1986;4:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 125.Walker CD, Scribner VA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time dependent and stress-specific fashion. Endocrinology. 1991;128:1385–96. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- 126.Koehnle T, Rinaman L. Progressive postnatal increases in Fos immunoreactivity in the forebrain and brainstem of rats after viscerosensory stimulation with lithium chloride. American Journal of Physiology Regulatory Integrative Comparative Physiology. 2007;292:R1212–R23. doi: 10.1152/ajpregu.00666.2006. [DOI] [PubMed] [Google Scholar]
- 127.Rinaman L. Postnatal development of catecholamine inputs to the paraventricular nucleus of the hypothalamus in rats. The Journal of Comparative Neurology. 2001;438:411–22. doi: 10.1002/cne.1324. [DOI] [PubMed] [Google Scholar]
- 128.Rinaman L, Hoffman GE, Stricker EM, Verbalis JG. Exogenous cholecystokinin activates cFos expression in medullary but not hypothalamic neurons in neonatal rats. Developmental Brain Research. 1994;77:140–5. doi: 10.1016/0165-3806(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 129.Rinaman L, Hoffman GE, Dohanics J, Le WW, Stricker EM, Verbalis JG. Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus in rats. The Journal of Comparative Neurology. 1995;360:246–56. doi: 10.1002/cne.903600204. [DOI] [PubMed] [Google Scholar]
- 130.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Research Reviews. 1982;4:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- 131.Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214:685–7. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- 132.Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. The Journal of Neuroscience. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oladehin A, Blatteis CM. Lipopolysaccharide-induced fos expression in hypothalamic nuclei of neonatal rats. Neuroimmunomodulation. 1995;2:282–9. doi: 10.1159/000097207. [DOI] [PubMed] [Google Scholar]
- 134.Pérez-Otaño I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends In Neurosciences. 2005;28:229–38. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 135.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Molecular Brain Research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 136.Levine S. Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in the rat. Physiology and Behavior. 2001;73:255–60. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- 137.Van Oers HJJ, deKloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. Journal of Neuroscience. 1998;18:10171–9. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu D, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 139.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiology and Behavior. 2003;79:359–71. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 140.Ladd CO, Huot RL, Thriikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. In: Mayer EA, Saper CB, editors. Progress in Brain Research. Amsterdam: Elsevier; 2000. pp. 81–103. [DOI] [PubMed] [Google Scholar]
- 141.Huot RL, Gonzalez ME, Ladd CO, Thrivikraman KV, Plotsky PM. Foster litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinology. 2004;29:279–89. doi: 10.1016/s0306-4530(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 142.Macrì S, Würbel H. Developmental plasticity of HPA and fear responses in rats: A critical review of the maternal mediation hypothesis. Hormones and Behavior. 2006;50:667–80. doi: 10.1016/j.yhbeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 143.Macrì S, Chiarotti F, Würbel H. Maternal separation and maternal care act independently on the development of HPA responses in male rats. Behavioural Brain Research. 2008;191:227–34. doi: 10.1016/j.bbr.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 144.Macrì S, Mason GJ, Wurbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring’s HPA and fear responses in rats. European Journal of Neuroscience. 2004;20:1017–24. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- 145.Daniels WMU, Pietersen CU, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metabolic Brain Disease. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- 146.Francis DD, Champagne FC, Meaney MJ. Variations in maternal behavior are associated with differences in oxytocin receptor levels in the rat. Journal of Neuroendorinology. 2000;12:1145–8. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 147.Moore C, Power K. Variation in maternal care and individual differences in play, exploration, and grooming of juvenile Norway rat offspring. Developmental Psychobiology. 1992;25:165–82. doi: 10.1002/dev.420250303. [DOI] [PubMed] [Google Scholar]
- 148.Vicentic A, Francis D, Moffett M, Lakatos A, Rogge G, Hubert GW, et al. Maternal separation alters serotonergic transporter densities and serotonergic 1A receptors in rat brain. Neuroscience. 2006;140:355–65. doi: 10.1016/j.neuroscience.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 149.Levine S. Infantile experience and resistance to stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- 150.Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1967;156:258–60. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- 151.Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–29. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 152.Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Developmental Neuroscience. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 153.Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–46. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 154.Bhatnagar S, Shanks N, Plotsky PM, Meaney MJ. Hypothalamic-pituitary-adrenal responses in neonatally handled and nonhandled rats: differences in facilitatory and inhibitory neural pathways. In: McCarthy R, Agulara G, Sabba E, Kvetnansky R, editors. Stress: Molecular and Neurobiological Advances. New York: Gordon & Breach; 1996. pp. 1–24. [Google Scholar]
- 155.Schwetz I, McRoberts JA, Coutinho SV, Bradesi S, Gale G, Fanselow M, et al. Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long-Evans rats. American Journal of Physiology - Regulatory, Integrative, and Comparative Physiology. 2005;289:G704–G12. doi: 10.1152/ajpgi.00498.2004. [DOI] [PubMed] [Google Scholar]
- 156.Avishai-Eliner S, Eghbal-Ahmadi M, Tabatchnik E, Brunson KL, Baram TZ. Downregulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid precedes early-life hippocampal glucocorticoid receptor-mRNA changes. Endocrinology. 2001;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fenoglio KA, Brunson KL, Avishai-Eliner S, Stone BA, Kapadia BJ, Baram TZ. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology. 2005;146:4090–6. doi: 10.1210/en.2004-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biological Psychiatry. 2004;55:367–75. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 159.Milde AM, Enger O, Murison R. The effects of postnatal maternal separation on stress responsivity and experimentally induced colitis in adult rats. Physiology and Behavior. 2004;81:71–84. doi: 10.1016/j.physbeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 160.Gareau MG, Jury J, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatric Research. 2006;59:83–8. doi: 10.1203/01.pdr.0000190577.62426.45. [DOI] [PubMed] [Google Scholar]
- 161.Welting O, Wijngaard RMVD, Jonge WJD, Holman R, Boeckxstaens GE. Assessment of visceral sensitivity using radio telemetry in a rat model of maternal separation. Neurogastroenterology and Motility. 2005;17:838–45. doi: 10.1111/j.1365-2982.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- 162.Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, et al. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointestinal Liver Physiology. 2002;282:G307–G16. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 163.Kinkead R, Gulemetova R, Bairam A. Neonatal maternal separation enhances phrenic responses to hypoxia and carotid sinus nerve stimulation in the adult anesthetized rat. Journal of Applied Physiology. 2005;99:189–96. doi: 10.1152/japplphysiol.00070.2005. [DOI] [PubMed] [Google Scholar]
- 164.Kinkead R, Joseph V, Lajeunesse Y, Bairam A. Neonatal maternal separation enhances dopamine D2-receptor and tyrosine hydroxylase mRNA expression levels in carotid body of rats. Canadian Journal of Physiology and Pharmacology. 2005;83:76–84. doi: 10.1139/y04-106. [DOI] [PubMed] [Google Scholar]
- 165.Genest SE, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation and sex-specific plasticity of the hypoxic ventilatory response in awake rat. The Journal of Physiology. 2004;554:543–57. doi: 10.1113/jphysiol.2003.052894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ábrahám IM, Kovacs KJ. Postnatal handling alters the activation of stress-related neuronal circuits. European Journal of Neuroscience. 2000;12:3003–14. doi: 10.1046/j.1460-9568.2000.00176.x. [DOI] [PubMed] [Google Scholar]
- 167.Card JP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. The Journal of Neuroscience. 2005;25:9102–11. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Banihashemi L, Rinaman L. Repeated brief postnatal maternal separation enhances hypothalamic gastric autonomic circuits in juvenile rats. Neuroscience. 2010:165. doi: 10.1016/j.neuroscience.2009.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2003;285:R479–R89. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Martinez-Peña-y-Valenzuela I, Rogers RC, Hermann GE, Travagli RA. Norepinephrine effects on identified neurons of the rat dorsal motor nucleus of the vagus. American Journal of Physiology Gastrointestinal Liver Physiology. 2004;286:G333–G9. doi: 10.1152/ajpgi.00289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pearson RJ, Gatti PJ, Sahibzada N, Massari VJ, Gillis RA. Ultrastructural evidence for selective noradrenergic innervation of CNS vagal projections to the fundus of the rat. Autonomic Neuroscience. 2007;136:31–42. doi: 10.1016/j.autneu.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Herman MA, Niedringhaus M, Alayan A, Verbalis JG, Sahibzada N, Gillis RA. Characterization of noradrenergic transmission at the dorsal motor nucleus of the vagus involved in reflex control of fundus tone. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2008;294:R720–R9. doi: 10.1152/ajpregu.00630.2007. [DOI] [PubMed] [Google Scholar]
- 173.Rinaman L, Dzmura V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2007;293:R1495–R503. doi: 10.1152/ajpregu.00393.2007. [DOI] [PubMed] [Google Scholar]
- 174.Rinaman L. Hindbrain contributions to anorexia. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2004;287:R1035–R6. doi: 10.1152/ajpregu.00437.2004. [DOI] [PubMed] [Google Scholar]
- 175.Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology. 2003;144:1357–67. doi: 10.1210/en.2002-221076. [DOI] [PubMed] [Google Scholar]
- 176.Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria teminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. The Journal of Neuroscience. 2006;26:11442–53. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rinaman L, Card JP, Schwaber JS, Miselis RR. Ultrastructural demonstration of a gastric monosynaptic vagal circuit in the nucleus of the solitary tract in rat. Journal of Neuroscience. 1989;9:1985–96. doi: 10.1523/JNEUROSCI.09-06-01985.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Balcita-Pedicino JJ, Rinaman L. Noradrenergic axon terminals contact gastric pre-autonomic neurons in the paraventricular nucleus of the hypothalamus in rats. Journal of Comparative Neurology. 2007;501:608–18. doi: 10.1002/cne.21267. [DOI] [PubMed] [Google Scholar]
- 179.Koehnle TJ, Rinaman L. Early experience alters limbic forebrain Fos responses to a stressful interoceptive stimulus in young adult rats. Physiology and Behavior. 2010;100:105–15. doi: 10.1016/j.physbeh.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Banihashemi L, Rinaman L. Central neural responses to restraint stress are altered in rats with an early life history of repeated brief maternal separation. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kuhn CM, Pauk J, Schanberg SM. Endocrine responses to mother-infant separation in developing rats. Developmental Psychobiology. 1990;23:395–410. doi: 10.1002/dev.420230503. [DOI] [PubMed] [Google Scholar]
- 182.Levine S, Huchton DM, Weiner SG, Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Developmental Psychobiology. 1991;24:547–58. doi: 10.1002/dev.420240803. [DOI] [PubMed] [Google Scholar]
- 183.Friedman MI. Some determinants of milk ingestion in suckling rats. Journal of Comparative and Physiological Psychology. 1975;89:636–47. [Google Scholar]
- 184.Kuhn CM, Schanberg SM. Responses to maternal separation: mechanisms and mediators. International Journal of Developmental Neuroscience. 1998;16:261–70. doi: 10.1016/s0736-5748(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 185.McCain GC, Ludington-Hoe SM, Swinth JY, Hadeed AJ. Heart rate variability responses of a preterm infant to kangaroo care. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2005;34:689–94. doi: 10.1177/0884217505281857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dodd VL. Implications of kangaroo care for growth and development in preterm infants. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2005;34:218–32. doi: 10.1177/0884217505274698. [DOI] [PubMed] [Google Scholar]
- 187.Lorenz DN. Gastric emptying of milk in rat pups. American Journal of Physiology. 1985;248:R732–R8. doi: 10.1152/ajpregu.1985.248.6.R732. [DOI] [PubMed] [Google Scholar]
- 188.Blass EM. Behavioral and physiological consequences of suckling in rat and human newborns. Acta Paediatrica Supplement. 1994;397:71–6. doi: 10.1111/j.1651-2227.1994.tb13268.x. [DOI] [PubMed] [Google Scholar]
- 189.van Oers HJ, Kloet ERd, Levine S. Persistent effects of maternal deprivation on HPA regulation can be reversed by feeding and stroking, but not by dexamethasone. Journal Of Neuroendocrinology. 1999;11:581–8. doi: 10.1046/j.1365-2826.1999.00329.x. [DOI] [PubMed] [Google Scholar]
- 190.Kuhn CM, Evoniuk G, Schanberg SM. Loss of tissue sensitivity to growth hormone during maternal deprivation in rats. Life Science. 1979;25:2090–7. doi: 10.1016/0024-3205(79)90202-9. [DOI] [PubMed] [Google Scholar]
- 191.Weller A, Corp ES, Tyrka A, Ritter RC, Brenner L, Gibbs J, et al. Trypsin inhibitor and maternal reunion increase plasma cholecystokinin in neonatal rats. Peptides. 1992;13:939–41. doi: 10.1016/0196-9781(92)90052-5. [DOI] [PubMed] [Google Scholar]
- 192.Hofer MA. The role of nutrition in the physiological and behavioral effects of early maternal separation on infant rats. Psychosomatic Medicine. 1973;35:350–9. doi: 10.1097/00006842-197307000-00009. [DOI] [PubMed] [Google Scholar]
- 193.Uvnäs-Moberg K. Gastrointestinal hormones in mother and infant. Acta Paediatrica Supplement. 1989;351:88–93. doi: 10.1111/j.1651-2227.1989.tb11216.x. [DOI] [PubMed] [Google Scholar]
- 194.Uvnäs-Moberg K, Widström AM, Marchini G, Winberg J. Release of GI hormones in mother and infant by sensory stimulation. Acta Paediatrica Scandinavia. 1987:76. doi: 10.1111/j.1651-2227.1987.tb17254.x. [DOI] [PubMed] [Google Scholar]
- 195.Weller A, Feldman R. Emotion regulation and touch in infants: the role of cholecystokinin and opioids. Peptides. 2003;24:779–88. doi: 10.1016/s0196-9781(03)00118-9. [DOI] [PubMed] [Google Scholar]
- 196.Lewis LD, Williams JA. Regulation of cholecystokinin secretion by food, hormones, and neural pathways in the rat. Am J Physiol Gastrointest Liver Physiol. 1990;258:G512–G8. doi: 10.1152/ajpgi.1990.258.4.G512. [DOI] [PubMed] [Google Scholar]
- 197.Liddle RA. Regulation of cholecystokinin secretion by intraluminal releasing factors. American Journal of Physiology. 1995;269:G319–G27. doi: 10.1152/ajpgi.1995.269.3.G319. [DOI] [PubMed] [Google Scholar]
- 198.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. American Journal of Physiology. 1997;272:R1245–R51. doi: 10.1152/ajpregu.1997.272.4.R1245. [DOI] [PubMed] [Google Scholar]
- 199.Schwartz GJ, Moran TH. Integrative gastrointestinal actions of the brain-gut peptide cholecystokinin in satiety. Progress in Psychobiology and Physiological Psychology. 1998;17:1–34. [Google Scholar]
- 200.Robinson PH, Moran TH, Goldrich M, McHugh PR. Development of cholecystokinin binding sites in the rat upper gastrointestinal tract. American Journal of Physiology. 1987;252:G529–G34. doi: 10.1152/ajpgi.1987.252.4.G529. [DOI] [PubMed] [Google Scholar]
- 201.Robinson PH, Moran TH, McHugh PR. Gastric cholecystokinin receptors and the effect of cholecystokinin on feeding and gastric emptying in the neonatal rat. Annals of the New York Academy of Science. 1985;448:627–9. [Google Scholar]
- 202.Weller A, Blass EM. Behavioral evidence for cholecystokin-opiate interactions in neonatal rats. American Journal of Physiology. 1988;255:R901–R7. doi: 10.1152/ajpregu.1988.255.6.R901. [DOI] [PubMed] [Google Scholar]
- 203.Blass EM, Shide DJ. Endogenous cholecystokinin reduces vocalization in isolated 10-day-old rats. Behavioral Neuroscience. 1993;107:488–92. doi: 10.1037//0735-7044.107.3.488. [DOI] [PubMed] [Google Scholar]
- 204.Weller A, Dubson L. A CCK(A)-receptor antagonist administered to the neonate alters mother-infant interactions in the rat. Pharmacology Biochemistry and Behavior. 1998;59:843–51. doi: 10.1016/s0091-3057(97)00530-3. [DOI] [PubMed] [Google Scholar]
- 205.Weber B, Manfredo HN, Rinaman L. A potential gastointestinal link between enhanced postnatal maternal care and reduced anxiety-like behavior in adolescent rats. Behavioral Neuroscience. 2009;123:1178–84. doi: 10.1037/a0017659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Woltman TA, Hulce M, Reidelberger RD. Relative blood-brain barrier permeabilities of the cholecystokinin receptor antagonists devazepide and A-65186 in rats. The Journal of Pharmacy and Pharmacology. 1999;51:917–20. doi: 10.1211/0022357991773348. [DOI] [PubMed] [Google Scholar]


