Abstract
Intolerance of uncertainty (IU) has been proposed to be an important maintaining factor in several anxiety disorders, including generalized anxiety disorder, obsessive-compulsive disorder, and social phobia. While IU has been shown to predict subjective ratings and decision-making during uncertain/ambiguous situations, few studies have examined whether IU also predicts emotional responding to uncertain threat. The present study examined whether IU predicted aversive responding (startle and subjective ratings) during the anticipation of temporally uncertain shocks. Sixty-nine participants completed three experimental conditions during which they received: no shocks, temporally certain/predictable shocks, and temporally uncertain shocks. Results indicated that IU was negatively associated with startle during the uncertain threat condition in that those with higher IU had a smaller startle response. IU was also only related to startle during the uncertain (and not the certain/predictable) threat condition, suggesting that it was not predictive of general aversive responding, but specific to responses to uncertain aversiveness. Perceived control over anxiety-related events mediated the relation between IU and startle to uncertain threat, such that high IU led to lowered perceived control, which in turn led to a smaller startle response. We discuss several potential explanations for these findings, including the inhibitory qualities of IU. Overall, our results suggest IU is associated with attenuated aversive responding to uncertain threat.
Keywords: intolerance of uncertainty, controllability, startle, emotion, anxiety
1. Introduction
Intolerance of uncertainty (IU) has been defined as the tendency to respond with negative emotional, cognitive, and behavioral reactions to uncertain situations and events (Dugas et al., 2004). Those who are intolerant of uncertainty report finding ambiguous situations stressful (particularly those in which a negative outcome is possible) and report having difficulty functioning under such circumstances. Individual differences in IU have been proposed to be a causal risk factor for worrying (Dugas et al., 2004), which subsequently can lead to the development and maintenance of several anxiety disorders, including generalized anxiety disorder (GAD; Dugas et al., 1998; Dugas and Ladouceur, 2000; Ladouceur et al., 1999), obsessive-compulsive disorder (OCD; Holaway et al., 2006; Tolin et al., 2003) and social anxiety (Boelen and Reijntjes, 2009). Therapeutic interventions aimed at increasing tolerance to uncertainty have also been shown to reduce worry, with changes in IU generally preceding changes in worry (Dugas and Ladouceur, 2000; Ladouceur et al., 2000a). These along with other studies (e.g., Ladouceur et al., 2000b) suggest a causal role between IU and pathological anxiety.
In numerous laboratory studies, individual differences in IU have been associated with subjective ratings and decision-making about uncertain and ambiguous situations. For example, individual differences in IU have been shown to predict negative appraisals of ambiguous situations (Koerner and Dugas, 2008), perceptions of threat (Bredemeier and Berenbaum, 2008; de Bruin et al., 2006), and the amount of information required to make a decision during an ambiguous situation (Ladouceur et al., 1997). Other studies have examined the relation between IU and emotional state (using physiological measures) during uncertain situations; however, these results are somewhat mixed. For example, Greco and Rodger (2001) found that high IU was associated with a smaller change in blood pressure and heart rate while engaging in a stress-inducing task. However, in a subsequent study, Greco and Rodger (2003) found that compared to low IU individuals, high IU individuals exhibited increased blood pressure while anticipating unpleasant pictures; though, this finding was for both predictable and uncertain unpleasant pictures (suggesting a general sensitivity to aversiveness, and not just uncertain aversiveness). These findings indicate that the relation between IU and emotional state during uncertain situations remains unclear.
The startle reflex is another psychophysiological tool that can be used for measuring emotional states during uncertain situations. Startle is a cross-species response to an abrupt intense stimulus, and is a well-documented indicator of defensive system activation (Lang, 1995). Startle is a particularly advantageous measurement in this context as it provides an online assessment of emotional states. Most importantly, unlike other psychophysiological measures (e.g., skin conductance) it has been shown to be sensitive to the valence of the emotion (Lang et al., 1993). Specifically, startle is potentiated (increased) during aversive emotional states, such as anxiety (Lang et al., 1998), and attenuated during positive emotional states (Giargiari et al., 2005; Lang et al., 1990). The neurobiology of startle has also been studied extensively (Koch, 1999). Basic research in animals has indicated that startle reflex to predictable and uncertain threat are mediated by overlapping but distinct neural systems, specifically the central nucleus of the amygdala for predictable threat, and the bed nucleus of the stria terminalis for uncertain threat (Davis, 2006; Gray and McNaughton, 2000).
Of particular relevance to the present study, startle has also been examined during situations in which the timing of aversive stimuli was uncertain. Grillon and colleagues developed a task where startle was measured while participants anticipated an aversive stimulus (e.g., shock) that was temporally predictable or uncertain (Grillon et al., 2004, 2006, 2008). Using this task, Grillon and colleagues found that, compared to healthy controls, individuals with particular anxiety disorders (i.e., panic disorder [PD] and posttraumatic stress disorder [PTSD]) exhibited abnormally elevated startle when the aversive stimuli were temporally uncertain, but not when the aversive stimuli were predictable (Grillon et al., 2008, 2009). This suggests that these anxiety disorders are associated with a hyper-responsiveness to uncertain threatening situations, but normal responsiveness to predictable threatening situations.
In the present study, we examined the association between individual differences in IU and startle to uncertain threat using Grillon and colleagues’ paradigm. We hypothesized that there would be an association between individual differences in IU and startle to uncertain threat. However, it was somewhat unclear whether the predicted direction of this association would be positive or negative. In many conceptualizations of IU, individuals who are intolerant of uncertainty exhibit an elevated negative response (self-report and behavior) during uncertain situations (although no studies have specifically examined this association using the startle reflex). Therefore, one possibility is that individuals who are highly intolerant of uncertainty will exhibit greater startle potentiation while anticipating uncertain threat, similar to the aforementioned findings for PD and PTSD (Grillon et al., 2008, 2009).
However, IU has been shown to have differential associations with various anxiety disorders – with stronger associations with measures of GAD (Dugas et al., 1998; Dugas and Ladouceur, 2000; Ladouceur et al., 1999) than with measures of PD (Dugas et al., 2001). This suggests that IU may not demonstrate the same pattern of association with startle responding to uncertain threat that has been shown with PD and PTSD. Moreover, studies have suggested that the ‘psychophysiological profile’ for GAD is different than that of other anxiety disorders (e.g., Hoehn-Saric et al., 1989; Lyonfields et al., 1995; Thayer et al., 1996).
An alternative possibility is that IU may be associated with smaller startle potentiation while anticipating uncertain threat, as IU may inhibit aversive responding similar to other measures of physiological responding that have been observed in GAD. For example, Hoehn-Saric et al. (1989) found that patients with GAD showed a weaker skin conductance response (a physiological index of arousal) relative to controls during a psychological stress task. Lyonfields et al. (1995) and Thayer et al. (1996) also found that GAD was associated with reduced autonomic flexibility due to lower cardiac vagal tone, suggesting impaired parasympathetic activity. In terms of startle studies, Lang, McTeague, and Cuthbert (2007) found that, relative to other anxiety disorders, GAD was associated with the weakest fear-potentiated startle response during a fear-imagery task, and also appeared attenuated compared to controls (although they never directly directly compare GAD participants to controls). In addition, a recent review on startle responding in anxiety disorders found that the literature on startle responding in GAD has yielded more inconsistent results than most other anxiety disorders (Vaidyanathan et al., 2009).
Therefore, the hypothesized direction of an association between IU and aversive responding may depend on the type of emotional/behavioral response that high IU individuals exhibit when they are confronted with an uncertain situation. Put simply, if intolerance leads to a negative arousing response, one would predict a positive correlation between IU and aversive responding. If intolerance leads to an inhibitory response, one would predict a negative correlation between IU and aversive responding. Several factor analytic studies of measures of IU have found separable (though correlated) dimensions for IU mapping onto these facets – one labeled prospective anxiety and the other inhibitory anxiety (Carleton et al., 2007; McEnvoy and Mahoney, 2011). Thus, as a secondary aim of the study we will examine which subscales of IU appear to be driving the overall IU-aversive responding association.
Depending on the direction of the results, there are several potential mechanisms that could explain the hypothesized results. In the present study, we will specifically examine one mechanism - perceived controllability. Animal studies have long shown that the controllability of aversive stimuli predicts affective, cognitive, and physiological reactions to the stimuli (e.g., Glass and Singer, 1972; Weiss, 1971). Controllability also overlaps significantly with predictability, as controllable events are also, in turn, predictable (Mineka and Kihlstrom, 1978). Given the relation between predictability and controllability, one possibility is that IU may lead to changes in the perception about controllability over aversive events. To test this, we administered the Anxiety Control Questionnaire (ACQ; Rapee et al., 1996), a measure specifically designed to assess individual differences in perceived control over anxiety-related events. Similar to IU, research has indicated that a lack of perceived control can lead to negative subjective, behavioral, and physiological responding (Feldner and Hekmat, 2001; Sanderson et al., 1989; Zvolensky et al., 2001). In addition, perceived control over anxiety-related events is a proposed mediator of emotional/motivational tendencies and aversive responding (Barlow, 1988; Rapee et al., 1996). Therefore, as a third aim, we will examine whether perceived control over anxiety-related events mediates the relationship between IU and response to uncertain threat. We are specifically hypothesizing perceived control as the mediator and IU as the independent variable (and not the other way around) because aversive stimuli that are temporally uncertain are also, by nature, uncontrollable, but the opposite is not necessarily true (i.e., events that are uncontrollable may or may not be unpredictable; Mineka and Kihlstrom, 1978).
2. Material and methods
2.1 Participants
Sixty-nine (N = 69) introductory psychology students participated for course credit. Participants were excluded from the study if they reported any loss of hearing (including the startle probes in the baseline/habituation task [see below]), were visibly intoxicated, or were unable to read or write in English. Participant demographics are presented in Table 1. Participants in the present sample reflected the population of introductory psychology students at our institution and were predominately female and ethnically diverse. Informed consent was obtained prior to participation and the protocol was approved by the local Institutional Review Board.
Table 1.
Participant demographics and descriptive statistics for self-report questionnaires
| Age | M = 20.11 (SD = 2.79) | |||||
| % Female | 76.8% | |||||
| Ethnicity | ||||||
| Caucasian | 37.7% | |||||
| African-American | 11.6% | |||||
| Hispanic | 14.5% | |||||
| Asian | 30.4% | |||||
| Asian-Indian | 14.0% | |||||
| Other | 4.3% | |||||
| M | SD | SE | Skewness | Kurtosis | Range | |
| IUS 27-item | 58.23 | 19.47 | 2.43 | 0.89 | 0.44 | 31–114 |
| IUS 12-item | 28.48 | 9.16 | 1.15 | 0.58 | −0.43 | 16–50 |
| Prospective Anxiety | 18.56 | 6.26 | 0.78 | 0.59 | −0.31 | 8–35 |
| Inhibitory Anxiety | 9.92 | 3.74 | 0.47 | 0.53 | −0.47 | 5–19 |
| PSWQ | 48.86 | 12.01 | 1.59 | 0.59 | −0.03 | 27–77 |
| ACQ | 89.03 | 20.75 | 2.59 | 0.20 | −0.77 | 54–132 |
Note: IUS = Intolerance of Uncertainty Scale; PSWQ = Penn State Worry Questionnaire; ACQ = Anxiety Control Questionnaire.
2.2 Stimuli and Physiological Responses
Experiment stimulation was presented using PSYLAB (Contact Precision Instruments, London, UK) and EMG was recorded with Neuroscan 4.3 (Compumedics, Charlotte, NC). The acoustic startle stimulus was a 40-ms duration, 95 dB burst of white noise with near instantaneous rise time presented binaurally through headphones. The startle blink reflex was measured from two 4-mm Ag/AgCl electrodes placed over the orbicularis oculi muscle below the right eye, and was collected with a bandpass filter of 10–200 Hz at a sampling-rate of 1000 Hz. As per published guidelines (Blumenthal et al., 2005), one electrode was placed 1 cm directly below the pupil and the other approximately 1 cm lateral. The ground electrode was placed in the center of the forehead along the midline. A fourth electrode was placed on the back of the neck along the midline as part the 60-cycle noise cancellation procedures employed by our Neuroscan data acquisition system. This was not an active electrode, and did not contaminate EMG responses by any activity of the neck musculature.
The electric shocks were 40-ms in duration, and were administered to the wrist of the participant’s non-dominant hand. Shock intensity was determined using a work-up procedure for each subject (see section 2.3). The maximum shock level a participant could achieve was 5 µA. Within our sample, the mean shock level was 2.44 µA (SD = 1.25).
2.3 Procedure
After electrode placement, participants were seated in an electrically-shielded, sound-attenuated booth approximately 3.5 feet from a 19-inch computer monitor where visual stimuli were presented. Participants first completed a 2.5 minute baseline task where they were presented with 12 acoustic startle stimuli. This allowed participants to habituate to the acoustic noise probes to prevent initial exaggerated startle responses. Next, shock intensity was determined using a work-up procedure where participants received increasing levels of shock, until they reached a level they described as “highly annoying but not painful.” We determined shock level ideographically to be consistent with prior studies (Grillon et al., 2004) and to ensure that subjects experienced the shocks as aversive.
The experiment was a within-subjects design containing three conditions: no shock (N), predictable shock (P), and uncertain shock (U) (see Figure 1). Text at the bottom of the computer monitor informed participants of the current threat condition by displaying the following information: “no shock” (N), “shock at 1” (P), or “shock at anytime” (U). Each condition lasted 90-s, during which a 6-s visual countdown was presented four times. Prior investigations using this paradigm (i.e., Grillon et al., 2004, Grillon et al., 2006; Moberg and Curtin, 2009) utilized various 8-s geometric shapes as threat cues. For the present investigation, we instead chose to use a countdown. The reason for this change was to make shocks during the P condition completely predictable. That is, whereas an 8-s geometric cue provides more predictability compared to no cue, it does not provide complete predictability as participants do not know exactly when the shock will occur while the cue is on the screen. By using a countdown as the threat cue, shock timing was either completely predictable (as the countdown signaled exactly when the shock would occur) or completely uncertain (as the countdown did not signal when the shock would occur). The intertrial intervals (ITIs; i.e., time between countdowns) ranged from 13–20-s (M = 16.75-s) during which only the text describing the condition was on the screen. In the N condition, no shocks were delivered. In the P condition, participants received a shock when the countdown reached 1. In the U condition, shocks were administered at anytime (i.e., during the countdown or ITI). Startle probes were presented both during the countdown (1–5-s following countdown onset) and ITI (4–15-s following ITI onset). The time interval between a shock and the following startle probe was always greater than 10-s, which ensured that the subsequent startle response was never affected by an immediately preceding shock.
Figure 1.
Depiction of the neutral, predictable/certain, and uncertain study design.
The experiment consisted of two recording blocks, with a 5-minute rest period between blocks. Each block consisted of one presentation of each 90-s condition, during which the countdown appeared four times, in the following orders (counterbalanced): PNU or UNP. All participants received 16 electric shocks (8 during P and 8 during U), and 36 startle probes (12 during N, 12 during P, and 12 during U) during the countdown and ITI (with an equal number of startle probes occurring during the countdown and ITI).
After each block of trials, participants rated their level of anxiousness during the countdown and ITI for each condition. The self-report response scales ranged from 1 (Not at all) to 7 (Extremely). Participants also rated how intense, annoying, and anxiety provoking, on a scale ranging from 1 (Not at all) to 7 (Extremely), and the degree to which they would avoid the shocks, on a scale ranging from 1 (Would definitely not avoid) to 7 (Would definitely avoid).
2.4 Questionnaires
Participants completed the following questionnaires after completing the experimental task.
The Intolerance of Uncertainty Scale (IUS; Freeston et al., 1994) contains 27 items relating to the idea that uncertainty is unacceptable, reflects poorly on a person, and leads to frustration, stress, and the inability to take action. The IUS contains items such as, ‘Unforeseen events upset me greatly’ and ‘Uncertainty makes me uneasy, anxious, or stressed’. The IUS is designed to assess trait intolerance of uncertainty, as the directions instruct the responder to describe to what extent each item is characteristic of them. Items are rated on a five-point Likert scale ranging from 1 = ‘not at all characteristic of me’ to 5 = ‘entirely characteristic of me’, with higher scores representing a greater intolerance of uncertainty. A recent factor analysis of the IUS (Carleton et al., 2007) found a more parsimonious 12-item version that was highly correlated with the 27-item version, but had better psychometric properties. Carleton et al. also conducted a confirmatory factor analysis on the 12-item version of the IUS. This analysis indicated that a two-factor model provided the best fit, with the first 7-item factor being named Prospective Anxiety (IUS-PA; i.e., fear and anxiety based on future events) and the second 5-item factor being named Inhibitory Anxiety (IUS-IA; i.e., uncertainty inhibits action or experience). In further support of the Carleton et al. two-factor solution, McEnvoy and Mahoney (2011) recently compared six different factor structures of the IUS that have been identified in the literature, and found that the Carleton et al. 12-item two-factor model provided the best fit to the data. In the present study our primary analyses involved the 12-item total score, but similar analyses with the full 27-item scale yielded nearly identical results. We also conducted supplementary analyses using the IUS-PA and IUS-IA subscales identified by Carleton et al. An example item from the IUS-PA subscale was, ‘I can’t stand being taken by surprise’, and an example item from the IUS-IA scale was, ‘The smallest doubt can stop me from acting.’
The Penn State Worry Questionnaire (PSWQ; Meyer et al., 1990) includes 16 items that measure the tendency to engage in excessive, uncontrollable, and generalized worry. Given the overlap between IU and worry (Dugas et al., 2004), the PSWQ was included to examine whether the association between IU and aversive responding was specific to IU or whether general worry tendencies produced similar associations. The PSWQ contains items such as, ‘My worries overwhelm me’ and ‘I am always worrying about something.’ The PSWQ is designed to measure trait worry, as the directions instruct the responder to, ‘Choose the number that best describes how typical or characteristic each item is of you.’ Items are rated on a five-point Likert scale ranging from 1 = ‘not at all typical’ to 5 = ‘very typical’. Seven participants did not complete the PSWQ; therefore, the N’s for all PSWQ analyses slightly differed from the IUS analyses.
The Anxiety Control Questionnaire (ACQ; Rapee et al., 1996) is a 30-item self-report questionnaire designed to measure perceived control over anxiety-related events. The ACQ was included to examine whether perceived control over anxiety-related events mediated the relationship between IU and response to uncertain threat. The ACQ contains items such as, ‘I am able to control my level of anxiety’ and ‘I am usually able to avoid threat quite easily’. The ACQ is designed to measure trait perceived control over anxiety-related events, as the directions instruct the responder to, ‘Indicate how much you think each statement is typical of you.’ Participants rate their degree of agreement/disagreement on each item using a six-point Likert scale ranging from 0 = ‘strongly disagree to 5 = ‘strongly agree’, with higher scores indicating greater perceived control.
Descriptive statistics for the IUS, PSWQ, and ACQ are presented in Table 1. The means and standard deviations were consistent with previous investigations using the same scales (Buhr and Dugas, 2002, 2006; Carleton et al., 2007; Dugas et al., 2001; Meyer et al., 1990; Rapee et al., 1996; Zebb and Moore, 1999). There was a significant positive correlation between the IUS and PSWQ, r(57) = .64, p < .001, and significant negative correlations between the ACQ and IUS, r(64) = −.53, p < .001, and PSWQ, r(57) = −.59, p < .001. To simplify interpretation of the results, we reverse coded the ACQ, thus making the direction of the three scales comparable (i.e., higher scores on ACQ indicated they perceived their environment as uncontrollable).
2.5 Data Analysis
Blinks were scored according to guidelines provided by Blumenthal et al. (2005). Startle EMG was rectified and then smoothed using an FIR filter (low pass cutoff of 40 Hz). Peak amplitude of the blink reflex was determined in the 20–100 ms time frame following the startle probe onset relative to baseline (average baseline EMG level for the 50-ms preceding the startle probe onset). Blinks were scored as non-responses if EMG activity during the 20–100-ms post-stimulus time frame did not produce a blink peak that was visually differentiated from baseline activity. Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous or voluntary blink began before minimal onset latency and thus interfered with the startle probe-elicited blink response. Five participants were classified as non-responders and were excluded from analyses because they did not produce at least 2 blinks during each condition, leaving a total N = 64. We chose to analyze blink magnitude (i.e., condition averages which include values of 0 for non-response trials), as this is a more conservative estimate of blink response (Blumenthal et al., 2005). Blink magnitudes were also standardized within-subjects using t scores, which reduced the influence of outlier blink responses. T scoring blinks also provided for better comparison between-subjects, as changes in blink magnitude amongst ‘big blinkers’ can be better compared to changes in blink magnitude amongst ‘small blinkers.’ Of note, however, is that similar results were obtained using amplitude and raw scores.
Data for both startle and subjective anxiety were analyzed in two sets of analysis of variance (ANOVA) – one with IUS and one with PSWQ. Each ANOVA was a Condition (N, P, U) X Cue (Countdown vs. ITI) X Questionnaire repeated measures ANOVA with questionnaire (mean centered) entered as a continuous between-subjects factor and Condition and Cue as within-subjects factors. Three-way Questionnaire X Condition X Cue interactions were followed-up by first conducting separate Questionnaire X Condition ANOVAs for each level of Cue (countdown and ITI). These 2-way interactions were subsequently followed-up two ways. First, we conducted bivariate correlations between the individual difference variable and the dependent measure for each level of condition (i.e., N, P, U). Second, if the individual difference variable was not correlated with the dependent measure during the N condition1, change scores were computed for both the P and U conditions that represented response potentiation from the N condition. The change scores were then correlated with the individual difference variable. This method is superior to conducting median splits on the questionnaires as median splits reduce power (Maxwell and Delaney, 1993). Significant levels were adjusted for multiple comparisons using a Bonferroni correction.
To conduct mediational analyses we followed MacKinnon, Lockwood, and Williams’s (2004) recommendations and used a nonparametric bootstrapping method. This approach has been shown to be statistically more powerful than other tests of mediation (MacKinnon et al., 2002). Specifically, we tested the proposed mediational model using the SPSS macro provided by Preacher and Hayes (2004), which provides a bootstrap estimate of the indirect effect between the independent variable and dependent variable, an estimated standard error, and 95% confidence intervals for the population value of the indirect effect. Confidence intervals for the indirect effect that do not include zero indicate a significant indirect effect at the p < .05 significance level. Direct and indirect effects of IUS on ACQ and startle magnitude were tested using 5,000 bootstrap samples.
3. Results
3.1 Manipulation check of the aversiveness of the shocks
Participants rated the shocks as moderate to extremely intense (M = 4.97, SD = 0.83), annoying (M = 5.98, SD = 0.84), and anxiety provoking (M = 5.37, SD = 1.14). Participants also rated that they would avoid receiving the shocks again to a high degree (M = 5.59, SD = 1.26). These results thus validate the aversiveness of the shocks. Scores on the IUS, PSWQ, and ACQ were not associated with shock ratings (all p’s > .19).
3.2 Startle magnitude during task not including IUS in model
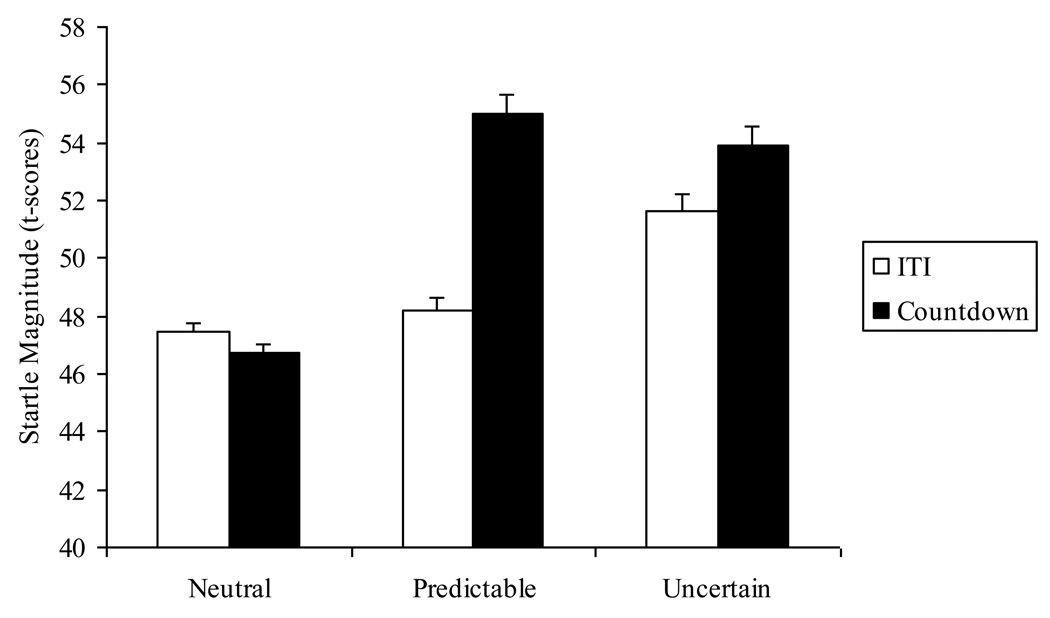
Figure 2 displays means (and standard errors) for startle magnitude across conditions and cues. Results indicated main effects of Condition, F(2, 126) = 49.05, p < .001, ηp2 = .44, and Cue, F(1, 63) = 44.27, p < .001, ηp2 = .41, and a Condition X Cue interaction, F(2, 126) = 36.40, p < .001, ηp2 = .37. We examined the Condition X Cue interaction by conducting separate repeated measures ANOVAs (N vs. P vs. U) for each level of Cue (i.e., countdown and ITI). During the countdown, startle magnitude differed among the conditions, F(2, 126) = 71.22, p < .001, ηp2 = .53, due to greater startle during the P and U conditions, compared to the N condition, F(1, 63) = 159.89, p < .001, ηp2 = .72; F(1, 63) = 97.46, p < .001, ηp2 = .61, respectively, while the P and U conditions did not differ, F(1, 63) = 1.64, ns, ηp2 = .03. Startle magnitude during the ITI also differed among the conditions, F(2, 126) = 17.48, p < .001, ηp2 = .22, due to greater startle during the U condition compared to the N and P conditions, F(1, 63) = 27.26, p < .001, ηp2 = .30; F(1, 63) = 15.09, p < .001, ηp2 = .19, respectively, while the N and P conditions did not differ, F(1, 63) = 1.88, ns, ηp2 = .03.
Figure 2.
Startle magnitude (t-scores) at different levels of condition and cue. Error bars represent standard error.
3.3 Startle magnitude during task including IUS in model
Next, we examined whether IUS and/or PSWQ was associated with startle magnitude during the different threat conditions2. Results indicated a three-way IUS X Condition X Cue interaction, F(2, 124) = 4.28, p < .05, ηp2 = .07, but no PSWQ X Condition X Cue interaction, F(2, 110) = 1.29, ns, ηp2 = .02, suggesting that the results were specific to IUS. To follow-up the IUS X Condition X Cue interaction, we conducted separate IUS X Condition repeated measures ANOVAs for each level of Cue (i.e., ITI and countdown separately). As predicted, results indicated an IUS X Condition interaction for startle magnitude during the ITI, F(2, 124) = 3.73, p < .05, ηp2 = .06, but not during the countdown, F(2, 124) = 0.71, ns, ηp2 = .01.
IUS scores were next correlated with startle magnitude during the ITI for the different threat conditions (see Table 2). Results indicated that IUS scores were negatively correlated with startle magnitude during the ITI of the U condition but not with the ITI of the N or P conditions. In addition, comparison of the correlation coefficients indicated that the association between IUS and startle magnitude during the ITI in the U condition was significantly greater than that during the P condition, Fischer’s z = 1.97, p < .05 and the N condition at a trend level, Fischer’s z = 1.80, p = .07. In other words, intolerance of uncertainty was associated with smaller startle magnitude during the anticipation of temporally uncertain threat but not temporally certain threat.
Table 2.
Correlation coefficient (r) between IUS scores and startle magnitude for responses during the ITI only (top row) and potentiation from the neutral (N) condition ITI (bottom row)
| Neutral | Predictable | Uncertain | |
|---|---|---|---|
| Startle response during ITI only | .07 | .08 | −.30* |
| Startle potentiation from N condition ITI |
.04 | .−.26* |
Note: IUS = Intolerance of Uncertainty Scale; ITI = intertrial interval;
p < .05.
As IUS was not correlated with startle during the N condition, we also conducted change scores from N (see Table 2) to examine whether responses on IUS were associated with potentiated startle to the U condition. These results showed that IUS scores were also negatively correlated with startle potentiation from the N to U condition, but not from the N to P condition.
3.4 Subscales of IUS
We examined whether the two subscales of the IUS identified by Carleton et al. (2007; i.e., Inhibitory Anxiety [IUS-IA] and Prospective Anxiety [IUS-PA)]) produced similar results as the 12-item IUS or whether a particular subscale of IUS was driving the results. Results indicated an IUS-IA X Condition interaction, F(2, 124) = 5.81, p < .01, ηp2 = .09, but no IUS-PA X Condition interaction, F(2, 124) = 1.98, p = .15, ηp2 = .03. Similar to the 12-item IUS, IUS-IA was negatively associated with startle magnitude during the ITI of the U condition, r(64) = −.39, p = .001, but not during the N, r(64) = .01, ns, or P conditions, r(64) = .07, ns. In addition, IUS-IA remained significantly associated with startle magnitude during the ITI of the U condition after controlling for IUS-PA, pr(61) = −36, p < .01. In sum, these results suggest that the inhibitory anxiety subscale of the IUS was largely driving the IU effects on startle.
3.5 Does ACQ mediate the IU-uncertain threat association?
Since our results indicated a negative association between IU and startle to uncertain threat, we examined whether the ACQ mediated this association. Mediational analyses were only conducted for startle magnitude during the ITI of the U condition, as it was only during this condition that startle was significantly associated with IUS. Before conducting meditational analyses, all three variables (i.e., IUS, ACQ, and startle magnitude during ITI of U condition) were z-scored to produce standardized β weights. Results indicated that the mediational model (see Figure 3) was predictive of significant variance in startle magnitude, R2 = .17, F = 6.08, p < .01. IUS was significantly predictive of scores on the ACQ, β = 0.53, t(63) = 4.88, p < .001, and the ACQ, in turn, was significantly predictive of startle magnitude, β = −0.33, t(63) = −2.41, p < .05. IUS was also directly predictive of startle magnitude, β = −0.30, t(63) = −2.43, p < .05. Most importantly, as predicted, analyses indicated that there was a significant indirect effect of IUS, mediated through ACQ, on startle magnitude, β = −0.12 (95% confidence interval [CI]: −0.35 to −0.06). In addition, even though the hypothesized model had IUS as the predictor and ACQ as the mediator, when these two variables were switched (ACQ as the predictor; IUS as the mediator), the results indicated no indirect effect of ACQ, mediated through IUS, on startle magnitude.
Figure 3.
Results of mediational analysis. Coefficients are standardized regression weights. The mediating effect of ACQ (i.e., perceived control over anxiety-related events) was significant (p < .05; see section 3.5 for details). After controlling for the mediator effect, the direct association between IUS and startle magnitude was no longer significant (p = .38). ACQ = anxiety control questionnaire, IUS = intolerance of uncertainty scale; ***p < .001, ** p < .01, * p < .05.
3.6 Subjective anxiety
Table 3 provides means and standard deviations of subjective anxiety across conditions and cues. Results indicated a main effect of condition, F(2, 126) = 232.25, p < .001, ηp2 = .79, and cue, F(1, 63) = 21.89, p < .001, ηp2 = .26; as well as a Condition X Cue interaction, F(2, 124) = 5.47, p < .01, ηp2 = .08. During the countdown, subjective anxiety differed among the conditions, F(2, 126) = 205.07, p < .001, ηp2 = .77, due to greater anxiety during the P and U conditions compared to the N condition, F(1, 63) = 195.12, p < .001, ηp2 = .76; F(1, 63) = 249.65, p < .001, ηp2 = .80, respectively, and greater anxiety during the U compared to the P condition, F(1, 63) = 48.88, p < .001, ηp2 = .44. During the ITI, subjective anxiety also differed among the conditions, F(2, 126) = 184.64, p < .001, ηp2 = .75, due to greater subjective anxiety during the U condition compared to the N and P conditions, F(1, 63) = 242.86, p < .001, ηp2 = .79; F(1, 63) = 63.81, p < .001, ηp2 = .50, respectively, and greater anxiety during the P compared to N condition, F(1, 63) = 172.60, p < .001, ηp2 = .73.
Table 3.
Means and standard deviations of subjective anxiety in arbitrary units (au) across cues and conditions
| Neutral | Predictable | Uncertain | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| ITI | 1.78 a | 1.08 | 4.28 b | 1.36 | 5.52 c | 1.34 |
| Countdown | 2.16a | 1.20 | 4.85 b | 1.30 | 5.59 c | 1.30 |
Note: Means with different subscripts across rows were significantly different from each other (p < .05).
Results indicated that there was a main effect of IUS for subjective anxiety, F(1, 62) = 4.23, p < .05, but no IUS X Condition, F(2, 124) = 0.89, ns, IUS X Cue, F(2, 124) = 0.09, ns, or IUS X Condition X Cue interaction, F(2, 124) = 0.63, ns, ηp2 = .01. Additionally, there was no PSWQ X Condition X Cue interaction for subjective anxiety, F(2, 110) = 0.27, ns, ηp2 < .01.
4. Discussion
To the best of our knowledge, the present study is the first to examine whether individual differences in IU are associated with startle response during a laboratory paradigm that manipulated the certainty of aversive events (i.e., shocks). Relative to other physiological measures, such as skin conductance, startle response is a relatively specific physiological indicator of aversive and anxious responding (Grillon and Baas, 2003), and thus the present results add significantly to the growing literature on the relation between IU and aversive responding (Bredemeier and Berenbaum, 2008; Greco and Roger, 2001, 2003; Koener and Dugas, 2008; Ladouceur et al., 1997).
As predicted, IU was associated with startle magnitude during the uncertain, but not predictable, threat condition. Specifically, individual differences in IU were negatively correlated with startle magnitude during the ITI in the U threat condition, but not in the N or P threat conditions. Change scores (i.e., U-N), which adjust for baseline startle responses, further supported this finding, showing that IU was negatively correlated with startle potentiation during the ITI from the N to U threat condition. Moreover, as IU was not associated with startle potentiation from the N to P threat condition, IU does not appear to impact response to threat more generally, but rather may only be specific to uncertain threatening situations.
In addition, the Carleton et al. (2007) inhibitory anxiety (IUS-IA) subscale appeared to be driving this negative association. Specifically, individual differences in the IUS-IA, but not the prospective anxiety (IUS-PA) subscale were negatively correlated with startle magnitude during the ITI in the U threat condition. Thus, our results suggest that when encountered with uncertain threatening events; individuals who are highly intolerant of uncertainty are likely to demonstrate inhibited aversive responding (as indexed by smaller startle responding).
One way to interpret the IU-IA subscale is that it taps defensive freezing (Blanchard et al., 2001) as some items indicate behavioral freezing in response to uncertain situation (e.g., ‘When it’s time to act, uncertainty paralyses me’). On the other hand, other items do not seem to tap freezing (e.g., ‘I must get away from all uncertain situations’). Given that animal studies suggest defensive freezing is commonly associated with an increase in startle response (e.g., Blanchard et al., 1986); if the IU-IA subscale was largely tapping defensive freezing, one would expect a positive association between IU-IA and startle during the U threat condition. However, this is not what we found, and based on our results (a negative association between IU-IA and startle during U threat condition) one can infer that the IU-IA subscale does not exclusively tap defensive freezing in the face of uncertainty. Future studies, however, are still needed to clarify the behavioral correlates of the different IU subscales.
There are several potential mechanisms through which IU may affect aversive responding to uncertain threat. One possibility is that, given the strong association between IU and worry (Dugas et al., 2004) and GAD (Dugas et al., 1998; Dugas and Ladouceur, 2000; Ladouceur et al., 1999), individuals high in IU may engage in excessive worry during uncertain situations, and this may subsequently reduce their anxiety response. However, our findings do not support this explanation as even though we found a high correlation between IU and PSWQ (as others have; Buhr and Dugas, 2002; Carleton et al., 2007; Schienle et al., 2010), individual differences on the PSWQ were not associated with startle during the uncertain threat condition. This finding corresponds with a previous investigation in which Grillon et al. (2009) found that individuals with GAD did not exhibit abnormally elevated startle during the uncertain threat condition, but rather were comparable to controls.
Another potential mechanism that may account for IU’s effect on unpredictable threat responsivity is a lowered perception of controllability. Consistent with this hypothesis, we found that individual differences on the ACQ, a measure of perceived control over anxiety-related events, mediated the relationship between IU and startle during uncertain threat. Specifically, higher IU led to lowered perceived control over anxiety-related events, which in turn led to a smaller increase in startle response during the uncertain threat condition. In addition, this was not just due to the high correlation between the ACQ and IUS, as IUS did not mediate the relationship between ACQ and startle to uncertain threat. Several researchers have posited that perceived control over anxiety-related events may mediate the relationship between emotional/motivational tendencies and aversive responding (Barlow, 1988; Rapee et al., 1996). The present results therefore suggest that one of those emotional/motivational tendencies may be IU.
There are several explanations as to why low perceived control over anxiety-related events was associated with a smaller increase in startle response while anticipating uncertain threat. One explanation is that, in an attempt to regain control over anxiety-related events, individuals with low perceived control implement some kind of emotion regulation and/or avoidance strategy (Gould and Edelstein, 2010). Indeed, prior studies have shown that employing an emotion regulation strategy can effectively reduce affective startle responding (e.g., Dillon and LaBar, 2005; Jackson et al., 2000; Lissek et al., 2007). While the specific emotion regulation strategy employed in those studies may be different than the one used by individuals high in IU, it is nonetheless suggestive than our result for IU may be due to abnormal regulatory mechanisms. Another possibility for how low perceived control may have an effect is that a reduced sense of control may have led to an overall lack of responsivity – a finding shown by several researchers (Alloy et al., 1990; Chorpita and Barlow, 1998).
Several theorists have identified predictability/certainty as one feature of aversive events that differentiates the aversive emotional states of fear and anxiety (Barlow, 2000; Grillon et al., 2008; Hamm and Weike, 2005). Fear is important for survival, as it prepares an organism for more immediate fight, flight, or immobilization responses, and is associated with predictable danger. Anxiety is elicited when the perceived danger is less certain (or present) and requires a sustained state of vigilance and defensive preparedness. While both fear and anxiety are aversive emotions, animal studies have suggested that they are mediated by overlapping but separable neuroanatomical systems (Davis, 1998; 2006). Within the present task, predictable aversive events may have elicited a phasic fear response during the threat cue, while uncertain aversive events may have elicited an anxiety response during the ITI (see Grillon et al., 2004; Grillon, 2008). Regarding our results for IU, this would suggest that IU moderated participants’ anxiety, but not fear response.
Similar to prior investigations using the NPU task (Grillon et al., 2004, 2006; Lissek et al., 2005; Moberg and Curtin, 2009), our results yielded an overall Condition X Cue interaction (i.e., collapsed across differences in IU). During the countdown, startle was potentiated during the predictable and uncertain conditions relative to the neutral condition. However, during the ITI, startle was only potentiated during the uncertain condition relative to the neutral condition and was not potentiated during the predictable condition. That is, unlike prior investigations using the NPU task, we did not find elevated startle during the ITI of the predictable relative to the ITI of the neutral condition. For example, in one study, during the ITI of the predictable condition participants still exhibited slightly potentiated startle relative to the ITI of the neutral condition even though they were completely safe from shocks during both conditions (P – N = 3.9 [t-score difference]; Grillon et al., 2009). In the present study, the analogous comparison was markedly reduced (P - N = 0.7 [t-score difference]), indicating that startle during the predictable and neutral ITI were more equivalent.
A possible reason for this difference is that previous studies using the NPU paradigm (e.g., Grillon et al., 2004; Moberg and Curtin, 2009) utilized geometric shapes as threat cues, while the present study used a countdown. While a geometric cue provides more predictability compared to no cue, the threat is not completely predictable as participants do not know exactly when it will occur while the cue is present. By using a countdown as the threat cue, our P condition became completely predictable, thus reducing any physiological anxiety that may be present during the ITI of the P condition.
4.1 Subjective Anxiety
Overall, the experimental task was largely effective in changing participants’ self-reported anxiety. During the countdown, participants reported greater anxiety during the P and U compared to the N condition. During the ITI, participants reported greater anxiety during the U compared to P and N condition. Importantly though, individual differences in IU did not moderate subjective anxiety during any of the conditions. One explanation for the lack of association between IU and subjective anxiety is that the experimental task was a ‘strong situation.’ Situational strength has long been identified as an important factor that moderates the relationship between individual differences and behavior (Caspi and Moffitt, 1993; Cooper and Withey, 2009; Mischel, 1977). Strong situations provide unambiguous stimuli that generally yield uniform reactions across individuals. In contrast, weak situations are more ambiguous events that attenuate the influence of the situation and increase the contribution of individual differences on subsequent responses. Within our study, the P and U conditions may have been so effective in eliciting subjective aversive responses that they produced a restricted range of subjective responses. Indeed, during the U condition, even though the scale ranged from 1 to 7, 75.0% of participants rated their anxiety between 5 and 7 during the ITI and 78.1% made ratings in this range during the countdown.
4.2 Gender
We did not find sex differences for both startle and subjective responses. In a previous study Grillon (2008) reported greater startle potentiation during the ITI of the U condition in female relative to male participants. However, our lack of replication of this finding may have been due to low power as our sample was predominately female (76.8%). Future investigations should include a larger sample of male participants in order to examine any potential sex differences in the relation between IU and aversive responding to uncertain threat.
4.3. Limitations
The present study had several limitations. First, subjective anxiety ratings during each experimental condition were obtained at the end of each experimental block rather than during the experiment, and thus may have been susceptible to memory bias. However, even emotions ratings obtained ‘online’ during the experiment may have been subject to demand effects. Second, despite being relatively stable over time (Dugas et al., 1997; Meyer et al., 1990; Rapee et al., 1996), questionnaires were given after the experimental task, and state influences from the laboratory session may have influenced responding to the trait measures (i.e., IUS, PSQW, ACQ). Third, our startle probes were 95dB while most investigations with human subjects use startle probes that are 100dB or greater. This likely had minimal effects on our results as Blumenthal and Goode (1991) have demonstrated that acoustic stimuli as low as 50dB can effectively elicit startle responses. Fourth, the bandpass filter used to record startle data (10–200 Hz) was narrow and cut off potentially important high-frequency data in the 200–500 Hz range. However, prior studies have suggested though that high-frequency components provide a negligible contribution to the EMG signal (van Boxtel et al., 1998). Finally, our sample was predominately female (76.8%) and restricted to college-aged students, which may limit generalizability to the general population.
4.4 Summary
In summary, we found that individual differences in IU were associated with smaller startle response while anticipating temporally uncertain shocks and this association was largely driven by the inhibitory aspects of IU. In addition, this association was mediated by individual differences in perceived control over anxiety-related events. We also found that individual differences in worry were not associated with startle during uncertain threat, suggesting that IU’s inhibitory effects on aversive responding were likely not achieved via excessive worrying. Given the novelty of our results, we hope to replicate our findings in future studies, as well as further elucidate the mechanisms underlying the relationship between IU and startle to uncertain threat. Overall, our results argue for the importance of the IU construct as a potential target for treatment of anxiety disorders, including GAD (Dugas and Ladouceur, 2000; Ladouceur et al., 1999), OCD (Holaway et al., 2006; Tolin et al., 2003), and social phobia (Boelen and Reijntjes, 2009).
Research highlights.
Intolerance of uncertainty (IU) is related to several anxiety disorders
IU was negatively correlated with startle while anticipating uncertain threat
This association was mediated by perceived control over anxiety-related events
Findings suggest a relationship between IU and defensive responding to uncertainty
Acknowledgements
This project was funded by National Institute of Mental Health Grant R21MH080689 awarded to Stewart A. Shankman. We would like to thank Alicia Oplustil for her outstanding contributions in collecting data and Evelyn Behar for her assistance in this project. We would also like to thank Christian Grillon and Shmuel Lissek, whose work inspired the idea for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Calculating change scores can be problematic when there are group (or individual) differences during the comparison condition (Nelson et al., in press). Therefore, change scores (i.e., P-N, U-N) were only calculated if the individual difference variable (i.e., IUS) was not correlated with the response during the N condition (i.e., the comparison condition). We chose this strategy to prevent calculating potentially misleading correlations. For example, if individual differences in IUS scores had been correlated with startle during the N and U conditions, it would be difficult to determine whether a significant correlation between IUS scores and startle potentiation (i.e., U-N) was due to startle responding during the N or U condition.
Given the large percentage of women in the sample, we also tested for gender differences by conducting four-way Gender X IUS X Condition X Cue ANOVAs with gender entered as a between-subjects factor. Overall, there was no main effect of Gender for both startle, F(1, 61) = 0.97, ns, or subjective anxiety, F(1, 61) = 1.71, ns. Results also indicated there was no Gender X IUS X Condition X Cue interaction for both startle, F(2, 122) = 1.44, ns, ηp2 = .02, and subjective anxiety, F(2, 122) = 0.38, ns, ηp2 < .01. Additionally, all aforementioned main effects and interactions for startle and subjective anxiety remained significant when gender was included as a between-subjects factor in the model.
Contributor Information
Brady D. Nelson, University of Illinois – Chicago, 1007 West Harrison (M/C 285), Chicago, IL 60657, USA, bnelso7@uic.edu
Stewart A. Shankman, University of Illinois - Chicago, 1007 West Harrison (M/C 285), Chicago, IL 60657, USA, stewarts@uic.edu
References
- Alloy LB, Kelly KA, Mineka S, Clements CM. In: Comorbidity of anxiety and depressive disorders: A helplessness-hopelessness perspective. Maser JD, Cloninger CR, editors. Washington, DC: American Psychiatric Association; 1990. pp. 499–543. [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am. Psychol. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Anxiety and its Disorders: The Nature and Treatment of Anxiety and Panic. New York, NY: Guilford Press; 1988. [Google Scholar]
- Blanchard DC, Hynd AL, Minke KA, Minemoto T, Blanchard RJ. Human defensive behaviors to threat scenarios show parallels to fear- and anxiety-related patterns of non-human mammals. Neurosci. Biobehav. R. 2001;25:761–770. doi: 10.1016/s0149-7634(01)00056-2. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Flannelly KJ, Blanchard DC. Defensive reactions of laboratory and wild Rattus norvegicus. J. Comp. Physiol. Psychol. 1986;100:101–107. [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Goode CT. The startle eyeblink response to low intensity acoustic stimuli. Psychophysiology. 1991;28:296–306. doi: 10.1111/j.1469-8986.1991.tb02198.x. [DOI] [PubMed] [Google Scholar]
- Boelen PA, Reijntjes A. Intolerance of uncertainty and social anxiety. J. Anxiety Disord. 2009;23:130–135. doi: 10.1016/j.janxdis.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Bredemeier K, Berenbaum H. Intolerance of uncertainty and perceived threat. Behav. Res. Ther. 2008;46:28–38. doi: 10.1016/j.brat.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Buhr K, Dugas MJ. The intolerance of uncertainty scale: Psychometric properties of the english version. Behav. Res. Ther. 2002;40:931–946. doi: 10.1016/s0005-7967(01)00092-4. [DOI] [PubMed] [Google Scholar]
- Buhr K, Dugas MJ. Investigating the construct validity of intolerance of uncertainty and its unique relationship with worry. J. Anxiety Disord. 2006;20:222–236. doi: 10.1016/j.janxdis.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Norton MAPJ, Asmundson GJG. Fearing the unknown: A short version of the intolerance of uncertainty scale. J. Anxiety Disord. 2007;21:105–117. doi: 10.1016/j.janxdis.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. When do individual differences matter? A paradoxical theory of personality coherence. Psychol. Inq. 1993;4:247–271. [Google Scholar]
- Chorpita BF, Barlow DH. The development of anxiety: The role of control in the early environment. Psychol. Bull. 1998;124:3–21. doi: 10.1037/0033-2909.124.1.3. [DOI] [PubMed] [Google Scholar]
- Cooper WH, Withey MJ. The strong situation hypothesis. Pers. Soc. Psychol. Rev. 2009;13:62–72. doi: 10.1177/1088868308329378. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am. Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol. Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- de Bruin GO, Rassin E, Muris P. Worrying in the lab: Does intolerance of uncertainty have predictive value? Behav. Change. 2006;23:138–147. [Google Scholar]
- Dillon DG, LaBar KS. Startle modulation during conscious emotion regulation is arousal-dependent. Behav. Neurosci. 2005;119:1118–1124. doi: 10.1037/0735-7044.119.4.1118. [DOI] [PubMed] [Google Scholar]
- Dugas MJ, Buhr K, Ladouceur R. The role of intolerance of uncertainty in etiology and maintenance. In: Heimberg RG, Turk CL, Mennin DS, editors. Generalized Anxiety Disorder: Advances in Research and Practice. New York, NY: Guilford Press; 2004. pp. 143–163. [Google Scholar]
- Dugas MJ, Freeston MH, Ladouceur R. Intolerance of uncertainty and problem orientation in worry. Cognitive Ther. Res. 1997;21:593–606. [Google Scholar]
- Dugas MJ, Gagnon F, Ladouceur R, Freeston MH. Generalized anxiety disorder: A preliminary test of a conceptual model. Behav. Res. Ther. 1998;36:215–226. doi: 10.1016/s0005-7967(97)00070-3. [DOI] [PubMed] [Google Scholar]
- Dugas MJ, Gosselin P, Ladouceur R. Intolerance of uncertainty and worry: Investigating specificity in a nonclinical sample. Cognitive Ther. Res. 2001;25:551–558. [Google Scholar]
- Dugas MJ, Ladouceur R. Treatment of GAD: Targeting intolerance of uncertainty in two types of worry. Behav. Modif. 2000;24:635–657. doi: 10.1177/0145445500245002. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Hekmat H. Perceived control over anxiety-related events as a predictor of pain behaviors in a cold pressor task. J. Behav. Ther. Exp. Psychiatry. 2001;32:191–202. doi: 10.1016/s0005-7916(01)00034-9. [DOI] [PubMed] [Google Scholar]
- Freeston MH, Rhéaume J, Letarte H, Dugas MJ. Why do people worry? Pers. Indiv. Differ. 1994;17:791–802. [Google Scholar]
- Giargiari TD, Mahaffey AL, Craighead WE, Hutchison KE. Appetitive responses to sexual stimuli are attenuated in individuals with low levels of sexual desire. Arch. Sex. Behav. 2005;34:547–556. doi: 10.1007/s10508-005-6280-y. [DOI] [PubMed] [Google Scholar]
- Glass DC, Singer JE. Urban Stress: Experiments on Noise and Social Stressors. New York, NY: Academic Press; 1972. [Google Scholar]
- Gould CE, Edelstein BA. Worry, emotion control, and anxiety control in older and young adults. J. Anxiety Disord. 2010;24:759–766. doi: 10.1016/j.janxdis.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. 2nd edn. Oxford: Oxford University Press; 2000. [Google Scholar]
- Greco V, Roger D. Coping with uncertainty: The construction and validation of a new measure. Pers. Indiv. Differ. 2001;31:519–534. [Google Scholar]
- Greco V, Roger D. Uncertainty, stress, and health. Pers. Indiv. Differ. 2003;34:1057–1068. [Google Scholar]
- Grillon C. Greater sustained anxiety but not phasic fear in women compared to men. Emotion. 2008;8:410–413. doi: 10.1037/1528-3542.8.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin. Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V, et al. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol. Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav. Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am. J. Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol. Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm AO, Weike AI. The neuropsychology of fear learning and fear regulation. Int. J. Psychophysiol. Special Issue: Neurobiology of Fear and Disgust. 2005;57:5–14. doi: 10.1016/j.ijpsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, McLeod DR, Zimmerli WD. Somatic manifestations in women with generalized anxiety disorder - psychophysiological responses to psychological stress. Arch. Gen. Psychiatry. 1989;46:1113–1119. doi: 10.1001/archpsyc.1989.01810120055009. [DOI] [PubMed] [Google Scholar]
- Holaway RM, Heimberg RG, Coles ME. A comparison of intolerance of uncertainty in analogue obsessive-compulsive disorder and generalized anxiety disorder. J. Anxiety Disord. 2006;20:158–174. doi: 10.1016/j.janxdis.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog. Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Koerner N, Dugas MJ. An investigation of appraisals in individuals vulnerable to excessive worry: The role of intolerance of uncertainty. Cognitive Ther. Res. 2008;32:619–638. [Google Scholar]
- Ladouceur R, Dugas MJ, Freeston MH, Léger E, Gagnon F, Thibodeau N. Efficacy of a cognitive-behavioral treatment for generalized anxiety disorder: Evaluation in a controlled clinical trial. J. Consult. Clin. Psychol. 2000a;68:957–964. [PubMed] [Google Scholar]
- Ladouceur R, Dugas MJ, Freeston MH, Rhéaume J, Blais F, Boisvert J, et al. Specificity of generalized anxiety disorder symptoms and processes. Behav. Ther. 1999;30:191–207. [Google Scholar]
- Ladouceur R, Gosselin P, Dugas MJ. Experimental manipulation of intolerance of uncertainty: A study of a theoretical model of worry. Behav. Res. Ther. 2000b;38:933–941. doi: 10.1016/s0005-7967(99)00133-3. [DOI] [PubMed] [Google Scholar]
- Ladouceur R, Talbot F, Dugas MJ. Behavioral expressions of intolerance of uncertainty in worry. Behav. Modif. 1997;21:355–371. doi: 10.1177/01454455970213006. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. Am. Psychol. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biol. Psychiatry. 1998;44:1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychol. Rev. 1990;97:377–395. [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, McTeague LM, Cuthbert BN. In: Fear, anxiety, depression, and the anxiety disorder spectrum: A psychophysiological analysis. Treat TA, Bootzin RR, Baker TB, editors. New York, NY: Psychology Press; 2007. pp. 167–195. [Google Scholar]
- Lissek S, Baas JMP, Pine DS, Orme K, Dvir S, Rosenberger E, et al. Sensation seeking and the aversive motivational system. Emotion. 2005;5:396–407. doi: 10.1037/1528-3542.5.4.396. [DOI] [PubMed] [Google Scholar]
- Lissek S, Orme K, McDowell DJ, Johnson LL, Grillon C. Effects of emotional regulation on startle potentiation to the treat of electric shocks. Poster presented at the 44th Annual Meeting of the Society for Psychophysiological Research; Santa Fe, NM. 2004. [Google Scholar]
- Lissek S, Orme K, McDowell DJ, Johnson LL, Luckenbaugh DA, Baas JM, et al. Emotion regulation and potentiated startle across affective picture and threat-of-shock paradigms. Biol. Psychol. 2007;76:124–133. doi: 10.1016/j.biopsycho.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyonfields JD, Borkovec TD, Thayer JF. Vagal tone in generalized anxiety disorder and the effects of aversive imagery and worrisome thinking. Behav. Ther. 1995;26:457–466. [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivar. Behav. Res. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol. Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD. Bivariate median splits and spurious statistical significance. Psychol. Bull. 1993;113:181–190. [Google Scholar]
- McEvoy PM, Mahoney AEJ. Achieving certainty about the structure of intolerance of uncertainty in a treatment-seeking sample with anxiety and depression. J. Anxiety Disord. 2011;25:112–122. doi: 10.1016/j.janxdis.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the penn state worry questionnaire. Behav. Res. Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Mineka S, Kihlstrom JF. Unpredictable and uncontrollable events: A new perspective on experimental neurosis. J. Abnorm. Psychol. 1978;87:256–271. doi: 10.1037//0021-843x.87.2.256. [DOI] [PubMed] [Google Scholar]
- Mischel W. The interaction of person and situation. In: Magnusson D, Endler NS, editors. Personality at the Crossroads: Current Issues in Interactional Psychology. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1977. pp. 333–352. [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: Startle response during unpredictable versus predictable threat. J. Abnorm. Psychol. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Shankman SA, Olino TM, Klein DN. Defining reactivity: How several methodological decisions can affect conclusions about emotional reactivity in depression. Cognition Emotion. doi: 10.1080/02699931.2010.551185. in press. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments & Computers. Special Issue: Web-based archive of norms, stimuli, and data: Part 2. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Craske MG, Brown TA, Barlow DH. Measurement of perceived control over anxiety-related events. Behav. Ther. 1996;27:279–293. [Google Scholar]
- Sanderson WC, Rapee RM, Barlow DH. The influence of an illusion of control on panic attacks induced via inhalation of 5.5% carbon dioxide-enriched air. Arch. Gen. Psychiatry. 1989;46:157–162. doi: 10.1001/archpsyc.1989.01810020059010. [DOI] [PubMed] [Google Scholar]
- Schienele A, Köchel A, Ebner F, Reishofer G, Schäfer A. Neural correlates of intolerance of uncertainty. Neurosci. Lett. 2010;479:272–276. doi: 10.1016/j.neulet.2010.05.078. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol. Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Abramowitz JS, Brigidi BD, Foa EB. Intolerance of uncertainty in obsessive-compulsive disorder. J. Anxiety Disord. 2003;17:233–242. doi: 10.1016/s0887-6185(02)00182-2. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, Cuthbert BN. Linking dimensional models of internalizing psychopathology to neurobiological systems: Affect-modulated startle as an indicator of fear and distress disorders and affiliated traits. Psychol. Bull. 2009;135:909–942. doi: 10.1037/a0017222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boxtel A, Boelhouwer AJW, Bos AR. Optimal EMG signal bandwidth and interelectrode distance for the recording of acoustic, electrocutaneous, and photic blink reflexes. Psychophysiology. 1998;35:690–697. [PubMed] [Google Scholar]
- Weiss JM. Effects of coping behavior in different warning signal conditions on stress pathology in rats. J. Comp. Physiol. Psych. 1971;77:1–13. doi: 10.1037/h0031583. [DOI] [PubMed] [Google Scholar]
- Zebb BJ, Moore MC. Another look at the psychometric properties of the anxiety control questionnaire. Behav. Res. Ther. 1999;37:1091–1103. doi: 10.1016/s0005-7967(98)00206-x. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Heffner M, Eifert GH, Spira AP, Feldner MT. Incremental validity of perceived control dimensions in the differential prediction of interpretive biases for threat. J. Psychopathol. Behav. 2001;23:75–83. [Google Scholar]