Abstract
Background
Obesity is considered a risk factor for breast cancer. Modifying life-styles that reduce obesity offers the potential for prevention and improvement of outcomes from cancer. The effects of obesity and breast cancer in African-American women and Hispanic women have been explored in a limited number of studies. The aim of this study is to investigate the association of obesity with breast cancer in our minority cohort.
Methods
This is a cross-sectional study of 471 African-American and Hispanic women with and without breast cancer in South Los Angeles. Information regarding BMI and clinical factors were obtained by medical record abstraction. Logistic regression with multivariate analysis was used for analysis. Kaplan Meier survival analysis was used to assess disease-free survival.
Results
Women with breast cancer were more likely to be obese (BMI>30) than women without breast cancer (OR=2.0; P-value=0.01). There was a significant association of being overweight or obese and breast cancer in postmenopausal women (OR=2.3; P-value=0.03 and OR=2.9; P-value<0.01, respectively). We found that an association of obesity with breast cancer was significant only in African-American women (OR=2.70; P-value<0.01), especially postmenopausal African American women (OR=4.8; P-value<0.01). There was a borderline significant association of obesity with later stage of diagnosis (P-value = 0.06). Furthermore, there was an association of higher BMI (in both cut-off points of 30 and 28 kg/m^2) with poorer disease-free survival (P-value = 0.045 and 0.019, respectively).
Conclusion
Our data suggest an association of obesity with breast cancer, especially in postmenopausal women and most significantly in the African-American cohort.
Keywords: Obesity, Body Mass Index, African Americans, Hispanic Americans, Breast Cancer, Minority Health, Survival
Introduction
Obesity has become a significant health challenge in the U.S. in the recent decades with more than two-thirds of adults being overweight or obese which puts them at risk for chronic diseases including cancer (1–3). In specific relation to breast cancer, obesity has been of increasing importance as more studies reveal a significant association of obesity and breast cancer risk in postmenopausal women (4–6). Furthermore, obesity has been associated with later stage of diagnosis and poorer prognosis (4, 7). It should be noted that a large number of studies have been performed on predominantly Caucasian cohorts with fewer studies focused on ethnic minorities such as African-American and Hispanic women. From the limited number of studies on cohorts of African-American and Hispanic women, there is data to suggest that the risks and outcomes from increased Body Mass Index (BMI) in minority women may be different than non-minority women (8). Most of the studies do report that adiposity and BMI are modifiers of breast cancer risk and outcomes in African-American women and Hispanic women, though the findings from the studies appreciably differ in significance, ages at which BMI was assessed in relation to cancer, and degree of association between measures (9–15). Additional studies also find that minority women, especially African-American women, have more aggressive breast cancer subtypes (16, 17) and this may contribute to the disparate breast cancer survival observed in this cohort of women.
Thus, this study aims to assess the relationship between BMI, menopausal status, breast cancer, and clinicopathological breast cancer features in a hospital-based setting within a community comprised mostly of self-identified African-American and Hispanic individuals.
Methods and Materials
The study population was recruited from SPA 6 region of South Los Angeles County in California. The cohort comprised of women examined in the Mammography Clinic or the Hematology/Oncology Clinic at the Martin Luther King Ambulatory Care Center (MACC, formerly known as King-Drew Medical Center) between 1995 and 2007. Women were consented for an ongoing breast cancer study conducted in the Division of Cancer Research and Training at Charles R. Drew University of Medicine and Science and MACC. The study was approved by the Institutional Review Board. For follow up data, we conducted post-hoc medical records abstraction.
Figure 1 summarizes the process of selecting the subset of subjects for this study from total number of women (n= 1400). The inclusion/exclusion criteria were the following: a) Self-identified race/ethnicity: From self-report, 30% were African-American, 65% were Hispanic, and the remaining 5% were Caucasian or Asian subjects. When the African-American and Hispanic ethnicity criteria were applied, n=1330 women met the criteria. b) Confirmation of breast cancer: For cases, breast cancer status was determined by biopsy/pathology confirmed neoplasm of the breast, and only subjects who had documentation of this information were included in the study (n=368). For controls, normal was determined by normal mammogram results, and benign was determined by mammogram and biopsy confirmed non-cancer (i.e. benign breast disease). Only subjects who had documentation of either disease-free mammography or biopsy with benign results were included in the study (n = 962). c) Baseline documentation of Body weight and Body Mass Index (BMI). d) Medical records confirming the presence of at least one or more comorbidity: Since some comorbidities such as diabetes and/or hypertension may influence body weight and outcomes, we selected those with clear documentation on the “past known clinical diseases”. e) Over 30 years of age: In order to match with the cases which were usually older, we adjusted the inclusion criteria for the age in controls to be greater than 30 years old. After adjusting for criteria c, d, and e, there were a total of 555 subjects (237 cases and 318 controls) that fulfilled those criteria. In the 237 cases, the numbers of African-American and Hispanic women were present in similar numbers, with 47.5% African-American and 52.3% Hispanic. However, in the controls, there were more Hispanic women than African-American women. Therefore to have meaningful analysis, we performed one more selection tier. f) Matching controls by age and ethnicity to cases: The numbers of African-American and Hispanic controls were matched with cases. Specifically, we matched individual ages between case and controls within a 10 year range for both African-American and Hispanic women. Women who were within the range of the 10 year matched age categories were included in the study and those that were outside the age range were excluded. In the end, a total of 471 subjects (237 cases and 234 controls) were selected for the study.
Figure 1.
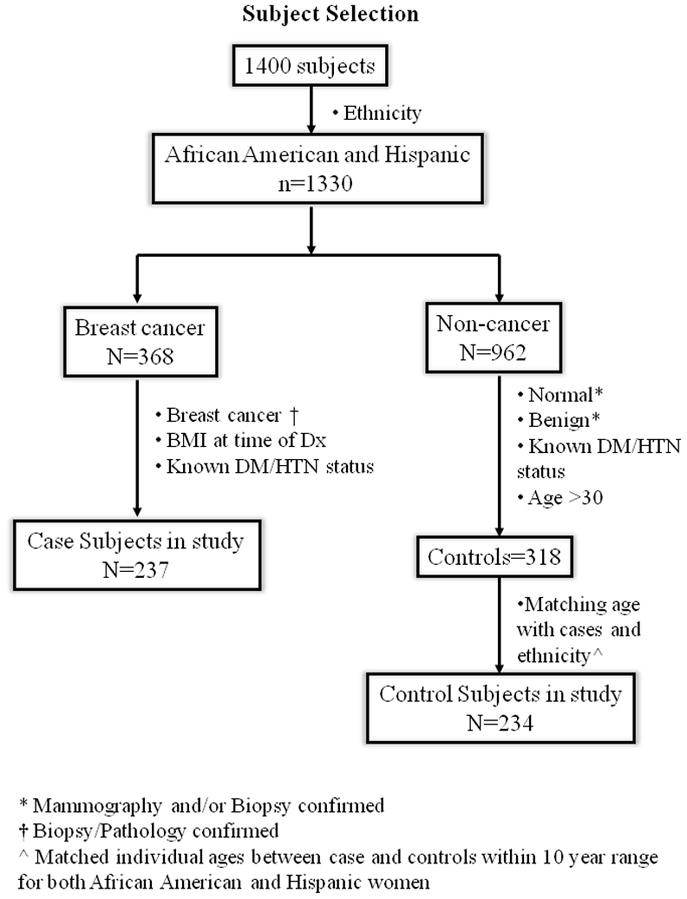
BMI was correlated with the following parameters: Age; ethnicity; clinicopathology; menopausal status; comorbidity; and disease free survival (DFS). BMI is defined as <18.5 Underweight, 18.5–24.9 Normal Weight, 25–29.9 Overweight, ≥30 Obese. It should be noted that no Underweight women (BMI<18.5) were identified in the study cohort, thus this category is omitted from analyses. Receptor status was defined as: ER+/PR+/HER2−; ER+/PR+/HER2+; ER−/PR−/HER2+; and Triple negative (ER−/PR−/HER2−). In addition, ER/PR status was considered “Positive” if >1% of tumor cell nuclei immunoreactive, or “Negative” if otherwise. HER2 status was considered “Positive” if HER2= 3+, “Negative” if HER2 = 0, 1+, 2+; (ii) Tumor Size (according to AJCC definitions); (iii) Lymph Node status (“Negative” if N = N0, “Positive” if N = N1, N2, N3); and (iv) Staging TNM (according to AJCC definitions).
Menopausal status
When the menopausal status was available from the records, that menopausal age was used to define the subject. For subjects for whom the menopausal status was not available from the medical information, the age of menopause was extrapolated based on the average age of menopause in the entire study cohort. The age range for menopause in African-American women in our study was from 40–56 years old (mean age = 48.9), and for Hispanic women was from 39–54 years old (mean age = 48.8). The average menopausal age was 48 for both ethnic groups. Therefore, for the subjects for whom the age at menopause was lacking, we used the mean age (48 years) as the age cut-off for menopausal status (premenopausal ≤48; peri-postmenopausal > 48).
Comorbidity
The metabolic characteristics of subjects with comorbidity have been strongly associated with high BMI, therefore comorbidity was taken into consideration in analysis. The term comorbidity is used to refer to breast subjects with diabetes and/or hypertension. Only these two comorbidities were assessed. A review of the literature revealed high prevalence of these specific comorbidities in minority populations, some of which were significantly associated with cancer prognosis (18, 19). Furthermore, a survey on our study cohort revealed the majority of the women with chronic disease had either diabetes type II, hypertension, or both. A very small and negligible number had other chronic diseases such as asthma, arthritis, etc; therefore, these subjects were omitted.
Disease free survival (DFS)
Survival and disease outcome in the study were assessed by 5 year DFS. DFS was assessed based on screening tests such as mammography, CT scan, Ultrasounds, Bone-scans that the patient underwent after treatment and resolution of the primary cancer. DFS for a patient was defined as not having any of the following: 1.) Reoccurrence; 2.) Metastatic disease; 3.) New primary tumor formation at another organ site. The DFS was utilized in the Kaplan Meier curve to assess the association between outcome and high body weight. Multiple models exploring the association of BMI and breast cancer DFS were explored using both traditional BMI classification (BMI ≥ 30 kg/m^2) as well as classification permutations in order to identify if there were any unique, clinically applicable and relevant results. Only one other BMI cut-off, (28 kg/m^2), was identified as being statistically and clinically relevant and therefore included as a parameter for assessing DFS in this study.
Statistical analysis was performed using SPSS software (SPSS, Inc., Chicago, IL). The subject’s ethnic, menopausal, BMI and clinicopathological characteristic associations were assessed throughout using the χ2-test. Univariate logistic regression analysis was used to determine the association between BMI and breast cancer, as well as menopausal status and breast cancer in the total and ethnically sub-categorized cohort. Multivariate logistic regression analysis was also performed to assess the association between breast cancer and BMI in the total cohort and by the ethnically stratified cohort. The multivariate analysis for the association between BMI and breast cancer in the total cohort was adjusted for age, ethnicity, comorbidity, and menopausal status. The multivariate analysis for the association between menopausal status and breast cancer was adjusted for BMI and comorbidity. Survival analysis was assessed using Kaplan Meier survival curves with log rank test. Throughout all analyses, only the two-sided P-value which was less than 0.05 was considered statistically significant.
Results
There were a total of n = 471 women included in our study. The number of women with breast cancer were n = 237 and women without breast cancer were n = 234.
Ethnicity and menopausal status
The self-identified ethnic distribution of the subjects is shown in Table 1. A total of n = 224 African-American women were in the study, with n = 113 (50.5%) as cases and n = 111 (49.5%) as controls. A total of n = 247 Hispanic women were in the study with n = 124 (50.5%) cases and n = 123 (49.5%) controls. There were no statistically significant differences observed when stratifying according to the menopausal status between cases and controls, or by number of African-American vs. number of Hispanic women included in this study.
Table 1.
Characteristics of Subject (n = 471)
| Cases | Control | P-value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Total Subjects | 237 (50.3) | 234 (49.7) | |
| Ethnicity | |||
| African American | 113 (47.5) | 111 (47.4) | 1.000 |
| Hispanic | 124 (52.3) | 123 (52.6) | |
| Premenopausal | 96 | 78 | |
| African-American | 35 (36.5) | 19 (24.4) | 0.101 |
| Hispanic | 61 (63.5) | 59 (75.6) | |
| Perimenopausal/Postmenopausal | 141 | 154 | |
| African-American | 78 (55.3) | 90 (58.4) | 0.638 |
| Hispanic | 63 (44.7) | 64 (41.6) | |
| Body mass index (BMI) | |||
| Obesity (BMI>30) | 144 (60.8) | 119 (50.9) | 0.009 |
| Over weight (BMI 25–30) | 66 (27.8) | 70 (29.9) | |
| Normal (BMI 18.5–24.9) | 27 (11.4) | 45 (19.2) | |
BMI status
The distribution of BMI in the study subjects is also shown in Table 1. Overall, the majority of the women in the study cohort are in the obese and overweight BMI range. However, there is a significant difference (P =0.009) in the distribution of BMI between cases and controls. Cases have a higher percentage (60.8%) of women who have a BMI in the obese range (BMI > 30) compared to controls (50.9%). Controls were more likely to have normal BMI (19.2%) than cases (11.4%).
Age
The average age of breast cancer diagnosis for African-American and Hispanic women is shown in Table 2. The average age of diagnosis for African-American women in this study is 51.97 years old and 48.69 for Hispanic women. This difference is statistically significant (P = 0.01).
Table 2.
Age at the time of diagnosis of breast cancer patients (n = 237)
| Ethnicity | N | Mean±SD | P-Value |
|---|---|---|---|
| African American | 113 | 51.97±8.4 | |
| Hispanic | 124 | 48.69±10.8 | 0.01 |
Univariate analysis on BMI, Ethnicity, Menopausal Status and Breast Cancer
Univariate analysis, as shown in Table 3, reveals that body weight in the obese range is significantly associated with breast cancer (OR=2.0; 95% CI = 1.2–3.4; P-value = 0.010). When stratifying according to ethnicity (Table 4), the association between obesity and breast cancer was statistically significant in only African-American women (OR=2.7; 95% CI = 1.3–5.6; P-value = 0.008). Furthermore, when assessing the association between menopausal status and breast cancer, only premenopausal status was independently and significantly associated with breast cancer in African-American women (OR= 2.1; 95% CI= 1.1–4.0; P-value= 0.02). There was no association found between obesity and breast cancer, or menopausal status and breast cancer in Hispanic women.
Table 3.
Odds Ratios and 95% CI’s for the Association between Breast Cancer, BMI, and Menopausal Status (Univariate analysis)
| Cases | Control | OR (95% CI) | P-Value | |
|---|---|---|---|---|
| N | N | |||
| BMI Category (n=471) | ||||
| Normal | 27 | 45 | 1.0 (ref) | |
| Over weight | 66 | 70 | 1.6 (0.9– 2.8) | 0.129 |
| Obesity | 144 | 119 | 2.0 (1.2– 3.4) | 0.010 |
| Menopausal status (n=469) | ||||
| Premenopausal | 96 | 78 | 1.0 (ref) | |
| Perimenopausal/Postmenopausal | 141 | 154 | 1.3 (0.9 – 1.95) | 0.123 |
Table 4.
Odds Ratios and 95% CI’s for the Association between Breast Cancer, BMI, and Menopausal Status- Stratified by Ethnicity (Univariate analysis)
| African American | P-value | Hispanic | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Cases | Control | OR (95% CI) | Cases | Control | OR (95% CI) | |||
| N | N | N | N | |||||
| BMI Category | ||||||||
| Normal | 14 | 27 | 1.0 (ref) | 13 | 18 | 1.0 (ref) | ||
| Over weight | 22 | 29 | 1.5 (0.6 – 3.4) | 0.381 | 44 | 41 | 1.5 (0.6–3.4) | 0.350 |
| Obesity | 77 | 55 | 2.7 (1.3 – 5.6) | 0.008 | 67 | 64 | 1.5 (0.7–3.2) | 0.358 |
| Menopause status | ||||||||
| Menopausal/Postmenopausal | 78 | 90 | 1.0 (ref) | 63 | 64 | 1.0 | ||
| Premenopausal | 35 | 19 | 2.1 (1.1 – 4.0) | 0.02 | 61 | 59 | 1.1 (0.6–1.7) | 0.847 |
Table 5 shows that by univariate analysis, there is a statistically significant association in the total cohort with postmenopausal cases and the overweight and obese BMI categories (OR = 2.3; 95% CI = 1.1–5.1; P-value = 0.032 and OR = 2.9; 95% CI = 1.4–5.8; P-value = 0.004, respectively). Furthermore, when evaluating the association between variables by ethnic breakdown, there is a statistically significant association of obesity with breast cancer in postmenopausal African-American women (OR = 4.8; 95% CI = 1.8–12.7; P-value = 0.002). There was no significant association found between BMI status and premenopausal women in any category, and no significant association found in Hispanic women.
Table 5.
Odds Ratios and 95% CI’s for the Association between Breast Cancer, BMI, and Menopausal Status (Univariate analysis)
| Premenopausal | P-Value | Postmenopausal | P-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Cases | Control | OR (95% CI) | Cases | Control | OR (95% CI) | |||
| N | N | N | N | |||||
| BMI status | ||||||||
| Total | ||||||||
| Normal | 14 | 12 | 1.0 | 13 | 33 | 1.0 | ||
| Over weight | 29 | 29 | 1.1 (0.5 – 2.3) | 0.825 | 37 | 40 | 2.3 (1.1 – 5.1) | 0.032 |
| Obesity | 53 | 37 | 1.7 (0.8 – 3.4) | 0.174 | 91 | 81 | 2.9 (1.4 – 5.8) | 0.004 |
| African-American | ||||||||
| Normal | 8 | 4 | 1.0 | 6 | 23 | 1.0 | ||
| Over weight | 6 | 6 | 0.5 (0.1 – 2.6) | 0.410 | 16 | 22 | 2.8 (0.9 – 8.4) | 0.069 |
| Obesity | 21 | 9 | 1.2 (0.3 – 4.9) | 0.833 | 56 | 45 | 4.8 (1.8 – 12.7) | 0.002 |
| Hispanic | ||||||||
| Normal | 6 | 8 | 1.0 | 7 | 10 | 1.0 | ||
| Over weight | 23 | 23 | 1.3 (0.4 – 3.6) | 0.640 | 21 | 18 | 1.7 (0.5 – 5.3) | 0.385 |
| Obesity | 32 | 28 | 1.5 (0.5 – 4.9) | 0.482 | 35 | 36 | 1.4 (0.5 – 4.1) | 0.548 |
Multivariate analysis on BMI, Ethnicity, Menopausal Status and Breast Cancer
The results from multivariate analysis were also performed with each variable and controlled for comorbidity, BMI, and/or Menopausal status (Table 6.). The multivariate analysis confirms the association of obesity with breast cancer (OR = 2.0; 95% CI = 1.1–3.6; P-value = 0.022). Furthermore, an association between obesity and breast cancer was confirmed by multivariate analysis in African-American women (OR=2.3; 95% CI = 1.0–5.0; P-value = 0.039). Premenopausal status in African-American women was still significantly associated with breast cancer, independent of BMI status (OR= 2.5; 95% CI = 1.2–5.3; P-value = 0.013). No association was found between obesity, menopausal status, and breast cancer in Hispanic women.
Table 6.
Odds Ratios and 95% CI’s for the Association between Breast Cancer, BMI, and Menopausal Status (Multivariate analysis)†
| Cases | Control | OR (95% CI) | P-Value | |
|---|---|---|---|---|
| N | N | |||
| Total | ||||
| BMI status†† | ||||
| Normal | 21 | 43 | 1.0 | |
| Over weight | 53 | 65 | 1.6 (0.9–3.1) | 0.138 |
| Obesity | 116 | 117 | 2.0 (1.1–3.6) | 0.022 |
| Menopausal status‡ | ||||
| Perimenopausal/Postmenopausal | 117 | 151 | 1.0 | |
| Premenopausal | 73 | 74 | 1.4 (0.9–2.2) | 0.132 |
| African American | ||||
| BMI status | ||||
| Normal | 13 | 25 | 1.0 | |
| Over weight | 20 | 26 | 1.6 (0.6–3.9) | 0.318 |
| Obesity | 62 | 54 | 2.3 (1.0–5.0) | 0.039 |
| Menopausal status | ||||
| Perimenopausal/Postmenopausal | 68 | 88 | 1.0 | |
| Premenopausal | 27 | 17 | 2.5 (1.2–5.3) | 0.013 |
| Hispanic | ||||
| BMI status | ||||
| Normal | 8 | 18 | 1.0 | |
| Over weight | 33 | 39 | 1.9 (0.7–5.1) | 0.187 |
| Obesity | 54 | 63 | 2.0 (0.8–5.0) | 0.147 |
| Menopausal status | ||||
| Perimenopausal/Postmenopausal | 49 | 63 | 1.0 | |
| Premenopausal | 46 | 57 | 1.0 (0.6–1.9) | 0.990 |
All analysis was adjusted for comorbidity.
BMI analysis was controlled for menopausal status in the Total, African-American, and Hispanic cohort.
Menopausal status analysis was controlled for BMI in the Total, African-American, and Hispanic cohort.
BMI association with breast tumor characteristics
In Table 7, the clinicopathological features of breast cancer were assessed for an association with BMI categories. Our data suggest that breast tumor subtypes, hormonal receptor status, tumor size, and lymph node involvement were not associated with any specific BMI category. There was borderline significance (P = 0.062) for an association of breast cancer with stage of diagnosis and obesity. The distribution demonstrated that 37.2% of obese women in our study were diagnosed at Stage III/IV compared to 27.1% of overweight women, and 20.8% of women in the normal BMI range.
Table 7.
Association between BMI and Cancer Clincopathological Features of Breast Cancer
| Obesity | Over weight | Normal | P-value | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Subtype | ||||
| ER+/PR+/HER2− (n=76) | 47 (61.8) | 18 (23.7) | 11 (14.5) | 0.876 |
| ER+/PR+/HER2+ (n=18) | 13 (72.2) | 4 (22.2) | 1 (5.6) | |
| ER−/PR−/HER2+ (n=30) | 17 (56.7) | 9 (30.0) | 4 (13.3) | |
| ER−/PR−/HER2− (n=61) | 36 (59.0) | 18 (29.5) | 7 (11.5) | |
| ER/PR status | ||||
| ER/PR positive (n=116) | 73 (62.9) | 29 (25.0) | 14 (12.1) | 0.595 |
| ER/PR negative (n=92) | 53 (57.6) | 28 (30.4) | 11 (12.0) | |
| HER2 status | ||||
| HER2 negative (n=137) | 83 (60.6) | 36 (26.3) | 18 (13.1) | 0.696 |
| HER2 positive (n=48) | 30 (62.5) | 13 (27.1) | 5 (10.4) | |
| Tumor size | ||||
| T0/Tis/T1 (n=51) | 30 (23.4) | 15 (25.9) | 6 (25.0) | 0.538 |
| T2 (n=95) | 58 (45.3) | 24 (41.4) | 13 (54.2) | |
| T3/T4 (n=64) | 40 (31.3) | 19 (32.8) | 5 (20.8) | |
| Lymph node | ||||
| Negative (n=81) | 50 (40.3) | 26 (44.8) | 5 (21.7) | 0.292 |
| Positive (n=124) | 74 (59.7) | 32 (55.2) | 18 (78.3) | |
| Stage | ||||
| 0–II (n=143) | 81 (62.8) | 43 (72.9) | 19 (79.2) | 0.062 |
| III–IV (n=69) | 48 (37.2) | 16 (27.1) | 5 (20.8) |
BMI and five year disease free survival (DFS)
Figure 2 shows the 5 year disease -free survival in breast cancer patients classified by two different BMI groupings. As shown in Figure 2A, the DFS in women with BMI ≥ 30 is significantly poorer than in women with BMI <30 kg/m^2 (P-value=0.045). When assessing the DFS in women grouped by the BMI cut-off point of 28 kg/m^2 as in Figure 2B, there is an even more significant association found with the higher BMI≥28 and poor DFS (P-value=0.019).
Figure 2. The 5-year Disease-Free Survival in breast cancer patients.
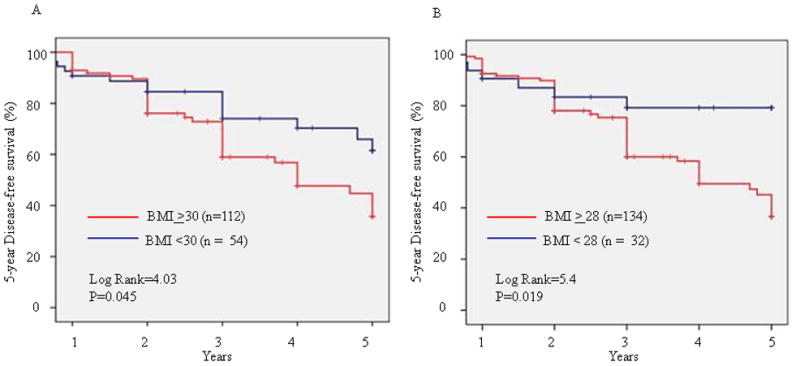
Breast cancer patients were categorized into two groups according to BMI. 5-year disease-free survival was analyzed by Kaplan-Meier survival analysis with log rank test.
Discussion
This study examined the association between obesity and breast cancer in African-American and Hispanic women attending the breast and/or hematology-oncology clinic at our Medical Center in South Los Angeles, California. There were similar number of women from each ethnic cohort and similar number of women in cases and control categories. It was interesting to note that the average age of cancer diagnosis for both self-identified ethnic groups in our study is much younger than the national average of 61 years old for Caucasians (20). However, some of our findings are similar to those reported for mostly Caucasian women who showed positive association between high BMI and breast cancer (21–23). Our results show that the association of obesity with breast cancer is significant in postmenopausal but not premenopausal women (5, 21, 22). The increased risk of breast cancer in postmenopausal obese women has been well established by multiple studies (5, 21), reviews (2, 8, 24, 25), and meta-analyses (5–7).
The specific mechanism whereby postmenopausal but not premenopausal women have obesity-related risk with breast cancer is not yet clearly known. However, some studies cite the potential involvement of estrogens. It is well documented that the production of estrogen between premenopausal and postmenopausal women is significantly different. The main source of estrogen production in premenopausal women is the ovaries whereas for postmenopausal women it is from peripheral adipose tissue (2, 8, 26). In postmenopausal women, fat cells aromatize ovarian and adrenal androgens into estrogen. Elevated circulating levels of free estrogen as well as estrone and androgens, have been shown to be associated with breast cancer (2, 8, 27).
The association of breast cancer and obesity in postmenopausal women may also be influenced by decreased physical activity observed in older women (> 50 years of age) (28). The most recent studies suggest exercise may be beneficial for attenuating cancer risk (29). Postmenopausal women who exercised moderately had lower insulin, glucose, and insulin-like growth factor-1 (IGF-I) levels (2, 30, 31). Insulin and IGF-I are capable of functioning in a mitogenic capacity by promoting cell growth, therefore, decreasing levels of insulin and IGF-I are beneficial protective measures against cancer (32). Earlier studies from our group showed that high circulating levels of IGF1 and low IGFBP3 levels were significantly associated with increased incidence of breast cancer as well as poor survival in cancer patients (33).
The association of obesity in postmenopausal African-American women in our study is consistent with some (9, 10, 11) but not all reports (12, 13) in the literature. Significant differences between the study designs of the aforementioned studies such as cohort size, age distribution, breast tumor subtype diagnosis distribution, classification of BMI, definition of obesity, and socioeconomic status of subjects examined may have contributed to the differences found between various studies. Though the results from our present study are consistent with the overall national trends and some ethnic-specific findings, the modest sample size should be noted as a limitation of this study. Furthermore, the assumption that high BMI is from high adiposity also merits caution when assessing body weight in African-American women. BMI is a variable calculated from total weight and height. It does not take into consideration “fat-free mass” such as high muscle mass or high bone density (34, 35). It should be noted, however, that even if the weight in the BMI is contributed from increased muscle mass as opposed to fat mass, muscle content has been correlated with increased circulatory levels of IGF-I. IGF-I as mentioned before is strongly associated with breast cancer (32, 33, 36).
It is interesting to note that we did not observe any significant trends between BMI and breast cancer in self-identified Hispanic women. A recent large study by Slattery et al (14) on Hispanic women from the Southwest also did not identify an association between high BMI and breast cancer. In fact, that study, as well as others (37, 38), found trends that weight gain and higher BMI was protective against breast cancer thus suggesting a different metabolic consequence of obesity on breast cancer in Hispanic women. Similarly, our study did not identify an association between high BMI and breast cancer in Hispanic women; though the limitation of our smaller sample size should be noted.
Recent large studies, such as by Hines et al (39) and Sweeny et al (40) within the scope of the 4-Corners Breast Study, have suggested multiple potential reasons for the lower association with breast cancer observed even in obese Hispanic women. Hines and colleagues suggested that many of the risk factors associated with increased risk for breast cancer in Non-Hispanic Whites (NHW) do not apply to Hispanic women. Examples include recent use of estrogen plus progestin therapy, younger age at menarche, and increased alcohol consumption. Sweeny et al (40) also found a strong correlate with risk-reducing reproductive factors, such as younger age at first birth, higher parity, and longer time since last pregnancy, which were more prevalent in Hispanic women. In addition to the above factors, there may be genetic components in metabolism which may contribute to the differences observed for risk in Hispanic women. For example, the study by Slattery (14) assessed the association of BMI and breast cancer risk in the Hispanic and NHW women based on genetic admixture. Slattery observed that Hispanic women who had a high degree of genetically-defined American Indian ancestry had decreased risk of breast cancer associated with high weight/obesity and fat distribution.
In our study, we also observed a borderline significant trend (P=0.06) between the association of obesity and later stage of diagnosis. This is consistent with most studies which have reported an association between higher BMI and later stage of diagnosis (41, 42). Some studies have suggested that obese women may be less likely to seek mammograms and treatment due to psychosocial factors such as feelings of embarrassment as well as conflicted feelings of trust towards healthcare providers (43, 44). The significance of our present study is of further importance when considering it in the context of additional studies which have implicated obesity in survival from breast cancer. A recent meta-analyses by Protani et al. which assessed the results from over forty-three studies from 1963–2005, found that that there was a significantly poor survival in obese women with breast cancer compared to non-obese women with breast cancer (7). Our data confirm this trend in our minority patients who are obese with a BMI ≥30 (Figure 2A). Furthermore, when assessing the lower BMI cut-off point of 28 kg/m^2, we observe an even greater significant association of poorer DFS with high BMI (Figure 2B). These observations suggest that in the case of African American and Hispanic breast cancer patients, it may be potentially useful to consider BMI is ≥28 in addition to the traditional BMI ≥30 for risk assessment and design intervention studies that lower the BMI.
In sum, our findings demonstrate that high BMI is associated with breast cancer in African-American women but not Hispanic women. These data suggest that additional studies are warranted to understand ethnic differences in assessing factors that may contribute to the development of breast cancer among different ethnic groups. Obesity is risk factor that contributes to poorer health and risk for disease through multifactorial pathways Identifying and overcoming these mechanisms may help alleviate health disparities in this population, especially in African-American women, who have high risk-associated factors and high health inequalities. The health risks associated with obesity may be addressed through lifestyle interventions such as promotion of a healthier diet and improved exercise habits. Ultimately, it is clear that there is a need for strategic, community-oriented and culturally appropriate public health interventions to reduce obesity which would improve the lives of women.
Acknowledgments
This work was supported in part by NIH/NCI Grants: 1U54CA14393, R25DK067015, BC043180 to J.V. Vadgama
Footnotes
Financial Disclosure: The Authors have no financial interests to disclose.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, Thung MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U. S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson GD, Rose DP. Breast cancer and obesity: an update. Nutr Cancer. 2003;45(1):1–16. doi: 10.1207/S15327914NC4501_1. [DOI] [PubMed] [Google Scholar]
- 5.van den Brandt PA, Spiegelmann D, Yuan SS, et al. Pooled Analysis of Prospective Cohort Studies on Height, Weight, and Breast Cancer Risk. Am J Epidemiol. 2000;152(6):514–27. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 6.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 7.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123(3):627–35. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 8.McTiernan A. Associations between Energy Balance and Body Mass Index and Risk of Breast Carcinoma in Women from Diverse Racial and Ethnic Backgrounds in the U.S. Cancer. 2000;88(5 Suppl):1248–55. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1248::aid-cncr12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Schatzkin A, Palmer JR, Rosenberg L, et al. Risk factors for breast cancer in black women. J Natl Cancer Inst. 1987;78:213–7. [PubMed] [Google Scholar]
- 10.Zhu K, Caulfield J, Hunter S, Roland CL, Payne-Wilks K, Texter L. Ann Epidemiol. 2005;15:123–8. doi: 10.1016/j.annepidem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 11.McCullough ML, Feigelson HS, Diver WR, Patel AV, Thun MJ, Calle EE. Risk factors for fatal breast cancer in African-American women and White women in a large US prospective cohort. Am J Epidemiol. 2005;162:734–42. doi: 10.1093/aje/kwi278. [DOI] [PubMed] [Google Scholar]
- 12.Palmer JR, Adams-Campbell LL, Boggs DA, et al. A Prospective Study of Body Size and Breast Cancer in Black Women. Cancer Epidemiol Biomarkers Prev. 2007;16:1795–1802. doi: 10.1158/1055-9965.EPI-07-0336. [DOI] [PubMed] [Google Scholar]
- 13.Hall IJ, Newman B, Millikan RC, Moorman PG. Body size and breast cancer risk in black women and white women: the Carolina Breast Cancer Study. Am J Epidemiol. 2000;151:754–64. doi: 10.1093/oxfordjournals.aje.a010275. [DOI] [PubMed] [Google Scholar]
- 14.Slattery ML, Sweeney C, Edwards S, Herrick J, et al. Body size, weight change, fat distribution and breast cancer risk in Hispanic and non-Hispanic white women. Breast Cancer Res Treat. 2007;102(1):85–101. doi: 10.1007/s10549-006-9292-y. [DOI] [PubMed] [Google Scholar]
- 15.Gilliland FD, Hunt WC, Baumgartner KB, et al. Reproductive factors for breast cancer in Hispanic and non-Hispanic white women: the New Mexico Women’s Health Study. Am J Epidemiol. 1998;148:683–92. doi: 10.1093/aje/148.7.683. [DOI] [PubMed] [Google Scholar]
- 16.Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;16;97(6):439–48. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 17.Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169(10):1251–9. doi: 10.1093/aje/kwp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braithwaite D, Tammemagi CM, Moore DH, et al. Hypertension is an independent predictor of survival disparity between African-American and white breast cancer patients. Int J Cancer. 2009;124(5):1213–9. doi: 10.1002/ijc.24054. [DOI] [PubMed] [Google Scholar]
- 19.Rollison DE, Giuliano AR, Sellers TA, et al. Population-based case-control study of diabetes and breast cancer risk in Hispanic and non-Hispanic White women living in US southwestern states. Am J Epidemiol. 2008;167(4):447–56. doi: 10.1093/aje/kwm322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altekruse SF, Kosary CL, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2007. National Cancer Institute; Bethesda, MD: 2010. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site. [Google Scholar]
- 21.Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States) Cancer Causes and Control. 2002;13:741–751. doi: 10.1023/a:1020239211145. [DOI] [PubMed] [Google Scholar]
- 22.Lahmann PH, Hoffmann K, Allen N, et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer And Nutrition (EPIC) Int J Cancer. 2004;111(5):762–71. doi: 10.1002/ijc.20315. [DOI] [PubMed] [Google Scholar]
- 23.Velie EM, Schairer C, Flood A, He JP, Khattree R, Schatzkin A. Empirically derived dietary patterns and risk of postmenopausal breast cancer in a large prospective cohort study. Am J Clin Nutr. 2005;82(6):1308–19. doi: 10.1093/ajcn/82.6.1308. [DOI] [PubMed] [Google Scholar]
- 24.Carmichael AR, Bates T. Obesity and breast cancer: a review of the literature. The Breast. 2004;13:85–92. doi: 10.1016/j.breast.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Cleary MP, Grossmann ME. Obesity and Breast Cancer: The Estrogen Connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr. 1987;45(1 Suppl):277–82. doi: 10.1093/ajcn/45.1.277. [DOI] [PubMed] [Google Scholar]
- 27.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–26. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 28.Carlson SA, Densmore D, Fulton JE. Differences in physical activity prevalence and trends from 3 U.S. surveillance systems: NHIS, NHANES, and BRFSS. J Phys Act Health. 2009;6 (Suppl 1):S18–27. doi: 10.1123/jpah.6.s1.s18. [DOI] [PubMed] [Google Scholar]
- 29.Monninkhof EM, Elias SG, Vlems FA, et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18(1):137–57. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 30.Irwin ML, Varma K, Alvarez-Reeves M, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):306–13. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiology, Biomarkers & Prevention. 2003;12:721–7. [PubMed] [Google Scholar]
- 32.LeRoith D, Raizada MK, editors. Adv Exp Med Biol; Proceedings of the 4th International Symposium on Insulin, IGFs, and their Receptors; Woods Hole, Massachusetts. April 20–23, 1993; 1993. pp. 1–417. [PubMed] [Google Scholar]
- 33.Vadgama JV, Wu Y, Datta G, Khan H, Chillar R. Plasma insulin-like growth factor-I and serum IGF-binding protein 3 can be associated with the progression of breast cancer, and predict the risk of recurrence and the probability of survival in African-American and Hispanic women. Oncology. 1999;57(4):330–40. doi: 10.1159/000012052. [DOI] [PubMed] [Google Scholar]
- 34.Hull HR, Thornton J, Wang J, et al. Fat-free mass index: changes and race/ethnic differences in adulthood. Int J Obes (Lond) 2010 Jun 8; doi: 10.1038/ijo.2010.111. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52(6):953–9. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 36.Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 37.Slattery ML, Edwards S, Murtaugh MA, et al. Physical activity and breast cancer risk among women in the southwestern United States. Ann Epidemiol. 2007;17(5):342–53. doi: 10.1016/j.annepidem.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murtaugh MA, Sweeney C, Giuliano AR, et al. Diet patterns and breast cancer risk in Hispanic and non-Hispanic white women: the Four-Corners Breast Cancer Study. Am J Clin Nutr. 2008;87(4):978–84. doi: 10.1093/ajcn/87.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hines LM, Risendal B, Slattery M, Baumgartner KB, et al. Comparative analysis of breast cancer risk among Hispanic and non-Hispanic White women. Cancer. 2010;116:3215–23. doi: 10.1002/cncr.25154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sweeney C, Baumgartner KB, Byers T, et al. Reproductive history in relation to breast cancer risk among Hispanic and non-Hispanic white women. Cancer Causes Control. 2008;19(4):391–401. doi: 10.1007/s10552-007-9098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones BA, Kasi SV, Curnen MG, Owens PH, Dubrow R. Severe obesity as an explanatory factor for the black/white difference in stage at diagnosis of breast cancer. Am J Epidemiol. 1997;146(5):394–404. doi: 10.1093/oxfordjournals.aje.a009292. [DOI] [PubMed] [Google Scholar]
- 42.Cui Y, Whiteman MK, Flaws JA, Langenberg P, Tkaczuk KH, Bush TL. Body mass and stage of breast cancer at diagnosis. Int J Cancer. 2002;98(2):279–83. doi: 10.1002/ijc.10209. [DOI] [PubMed] [Google Scholar]
- 43.Amy NK, Aalborg A, Lyons P, Keranen L. Barriers to routine gynecological cancer screening for White and African-American obese women. Int J Obes (Lond) 2006;30(1):147–55. doi: 10.1038/sj.ijo.0803105. [DOI] [PubMed] [Google Scholar]
- 44.Maruthur NM, Bolen S, Brancati FL, Clark JM. Obesity and mammography: a systematic review and meta-analysis. J Gen Intern Med. 2009;24(5):665–77. doi: 10.1007/s11606-009-0939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


