Abstract
Peritoneal B1 cells are typified by spontaneous, constitutive secretion of IgM natural antibody, detected by ELISPOT assay, among other means. Recently, this key characteristic has been called into question, a reason for which we evaluated the integrity of IgM+ ELISPOT spots. We found that fixed B1 cells fail to produce ELISPOT spots, that interference with Golgi function inhibits ELISPOT spot formation, and that B1 cell-derived immunoglobulin in supernatant samples is EndoH-resistant. These findings indicate that spots produced by B1 cells on ELISPOT assay reflect secretory IgM actively exported by viable B1 cells. Current paradigms propose that interferon response factor 4 (IRF4) is required for plasma cell differentiation and immunoglobulin secretion. However, we found that IgM secretion by peritoneal B1 cells is not altered in IRF4-null mice. In contrast, spontaneous IgM secretion by splenic B1 cells, which amounts to much more IgM secreted per cell, is dramatically reduced in the absence of IRF4. These results indicate that peritoneal B1 cells spontaneously secrete low levels of IgM via an IRF4-independent non-classical pathway, and, considering the low level of serum IgM in IRF-null mice, further suggest that accumulation of serum immunoglobulin depends on IRF4-dependent secretion by splenic B1 cells.
Keywords: Antibodies, B cells, Cell differentiation, Spleen, Transcription factors
Introduction
B1 cells are defined by expression of the surface antigen CD5, in the context of characteristic B-cell markers, and play a key role in early protection against, and clearance of, bacterial and viral infection via constitutive production of non-immune serum IgM, termed “natural antibody” [1–4]. Natural antibody approximates the germline state due to relative absence of nontemplated N-region addition and somatic mutation, is both low affinity and broadly reactive, and includes autoreactive specificities. This germline-like immunoglobulin is repertoire-skewed, which is readily appreciated from the overrepresentation among B1 cells of VH11 and VH12 heavy chain variable gene segments that encode phosphatidyl choline binding, in comparison with the virtually undetectable levels found in B2 cells [5–7]. B1 cell-derived immunoglobulin also recognizes discrete microbial cell wall determinants, such as phosphorylcholine from S. pneumoniae [8–10]. B1 cells have been further shown to play a role in adaptive immune responses in models of contact sensitivity and sepsis; in addition, B1 cells present antigen efficiently to T cells and have the capacity to steer naïve CD4+ T-cell development toward Th17-cell differentiation [11–13]. The recent identification of a B1-cell progenitor [14] suggests that these and other distinctive phenotypic, transcriptomic, proteomic, and functional features displayed by B1 cells (reviewed in references [15–17]) derive from a separate B-cell lineage.
Natural antibody produced by B1 cells is referred to as non-immune because it is secreted spontaneously by naïve unstimulated B1 cells [18–21]. The mechanism of spontaneous immunoglobulin production by B1 cells has been investigated yet is still not fully understood. In conventional B2 cells immunoglobulin secretion has been shown to be controlled by several transcription factors: B-cell leukemia/lymphoma-6 (BCL-6), B lymphocyte inducer of maturation program 1 (BLIMP-1), paired box gene 5 (PAX-5), X-box binding protein-1 (XBP-1), and interferon response factor 4 (IRF4). In naïve B2 cells, BCL-6 represses BLIMP-1 expression, which allows PAX-5 to suppress XBP-1 [22–25]. When B2 cells are stimulated to differentiate, NF-κB is activated leading to expression of IRF4, which down-regulates BCL-6 and stimulates BLIMP-1 levels to increase [26, 27]. BLIMP-1 then inhibits PAX-5 permitting XBP-1 levels to rise and immunoglobulin secretion to proceed [25, 28, 29]. In sum, IRF4 acts to initiate a cascade of transcription factors that results in plasma cell differentiation and immunoglobulin secretion.
We recently reported that spontaneous IgM secretion by peritoneal B1 cells operates under a different paradigm than that characteristic of LPS-stimulated B2 cells [21]. In this study we found that, unlike immunoglobulin-secreting B2 cells, immunoglobulin-secreting B1 cells expressed negligible levels of BLIMP-1 and XBP-1 mRNA or protein (as well as negligible levels of BCL-6 and PAX-5), strongly suggesting that the pathway for IgM secretion in B1 cells is atypical and distinct.
These results sparked controversy regarding B1 cell biology. On the one hand, Nutt, Tarlinton and colleagues contended that B1 cells do not spontaneously or constitutively secrete IgM [30], regardless of BLIMP-1 status. On the other hand, despite earlier insistence that B cells, including B1 cells, fail to secrete immunoglobulin in the absence of BLIMP-1 [30, 31], this group, in a subsequent report, went on to describe a BLIMP-1-independent stage of antibody secreting cell differentiation, and concluded that BLIMP-1 is not required for some forms of immunoglobulin secretion but is indispensable for high-level antibody production [32], essentially reprising the findings we originally described for naïve B1 cells [21], and confirming that B cells can secrete immunoglobulin in the absence of BLIMP-1.
While the intent of these previous studies was to elucidate the molecular mechanisms regulating antibody secretion, their contradictory results and conclusions in fact confuse the issue as to whether or not B1 cells spontaneously secrete IgM, and, whether the mechanism regulating spontaneous immunoglobulin secretion in B1 cells is distinct from that characteristic of stimulated B2 cells. The present study was undertaken to resolve these issues and to extend the work in order to determine the role of the key transcription factor IRF4.
Results
Naïve peritoneal B1 cells secrete IgM that is readily detectable by ELISPOT assay, as previously reported [21, 33, 34], despite the fact that B1 cell spots are typically smaller in size and intensity as compared to those produced by LPS-stimulated B2 cells. Controversy regarding the validity of B1 cell-generated spots in the ELISPOT assay may have resulted from difficulty in visualizing secretion events of smaller magnitude or from attribution of secretion events to cell lysis and/or artifact. To clarify this situation and to confirm that the B1 cell-derived immunoglobulin detected by ELISPOT assay reflects a naturally occurring process, we evaluated production of spots by other B-cell populations and by fixed B1 cells; we determined the sensitivity of B1 cell spot production to Golgi blockade by Brefeldin A (BFA), and we assessed the condition of N-linked oligosaccharides in B1 cell immunoglobulin by examining Endo-H sensitivity.
B1-cell spontaneous IgM secretion is not reproduced by B2 cells or by fixed B1 cells
To demonstrate that the spots detected by short-term ELISPOT assay are a function of B1 cells alone, we compared naïve, sort-purified peritoneal B1 and splenic B2-cell populations. Results are shown in Fig. 1. Only B1 cells, but not B2 cells, produced substantial numbers of spots on ELISPOT assay, and this was true whether B cells were positively (Fig. 1) or negatively selected (data not shown). Like mature B2 cells, transitional B2 cells failed to produce spots on ELISPOT assay (data not shown). To confirm that spots produced by B1 cells require viable cell function and are not artifacts resulting from simple adherence of non-secreting B1 cells to ELISPOT membranes, we fixed B1 cells with paraformaldehyde or fixed and permeabilized B1 cells with methanol after sorting and before assay. Fixed and fixed-permeabilized B1 cells failed to produce ELISPOT spots, in contrast to sort-purified, viable B1 cells that yielded multiple spots on ELISPOT assay.
Figure 1.
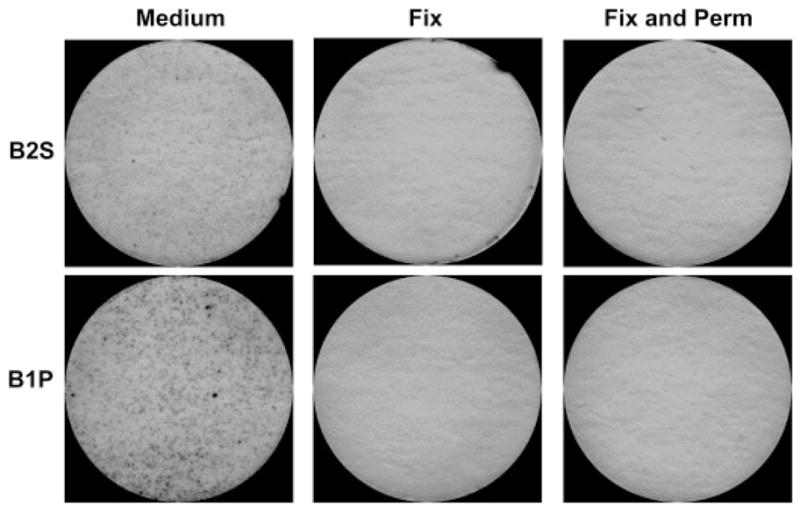
B1-cell spontaneous IgM secretion is not reproduced by fixed B1 cells. Freshly isolated sort-purified peritoneal B1 cells (B1P) and splenic B2 cells (B2S) were treated with medium alone, 16% paraformaldehyde for 10 min (Fix), or 16% paraformaldehyde for 10 min followed by 100% methanol for 10 min (Fix and Perm), after which all three B1 cell populations were washed and placed on anti-Ig-coated ELISPOT plates in culture medium and incubated for 4 h at 37°C. Plates were treated with alkaline phosphatase-conjugated goat anti-mouse IgM and developed with 5-bromo-4-chloro-3-indolyl phosphate/p-NBT chloride substrate. Representative ELISPOT membranes from one of two independent experiments are shown.
B1-cell spontaneous IgM secretion is inhibited by BFA
To further demonstrate that B1-cell production of IgM, as detected by ELISPOT assay, is due to protein secretion we treated naïve peritoneal B1 cells, and splenic B2 cells stimulated by LPS for 48 h, with BFA for 6 h prior to ELISPOT assay. BFA blocks protein secretion by disassembling the Golgi apparatus thereby causing accumulation of proteins in the ER [35]. Control B cells were either untreated or were treated with DMSO, the diluent for BFA, for 6 h. Results are shown in Fig. 2. In control cultures, naïve B1 cells produced substantial numbers of spots, as did LPS-stimulated B2 cells, although spots produced by B2 cells were larger and more intense than those produced by B1 cells, reflecting greater amounts of immunoglobulin. ELISPOT results for B1 cells were little affected by DMSO, and the same was true for B2 cells. However, B1-cell production of IgM was greatly reduced by BFA, as evidenced by the lower number of spots detected by ELISPOT assay (p = 0.005, DMSO versus BFA). Along the same lines, LPS-stimulated production of IgM by B2 cells was also significantly reduced by BFA (p = 0.02). Membranes from a representative experiment are displayed in Fig. 2A and the mean enumerated results from three independent experiments are displayed in Fig. 2B. Notably, B1 cells remained viable following exposure to BFA (greater than 90% trypan blue-excluding). These results further confirm that IgM produced by B1 cells and detected by ELISPOT assay is generated by a Golgi-dependent pathway, as is IgM produced by LPS-stimulated B2 cells.
Figure 2.
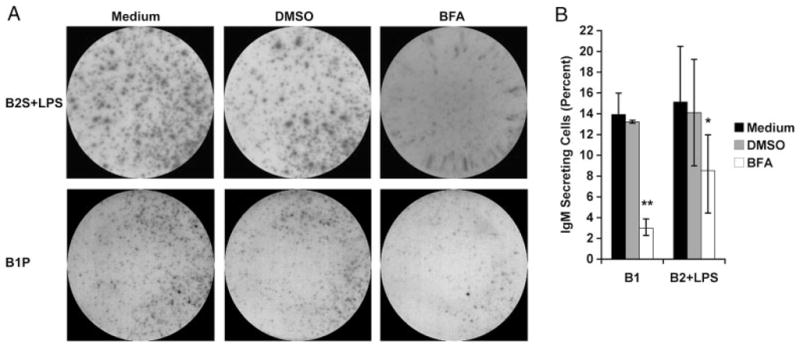
B1-cell spontaneous IgM secretion is inhibited by BFA. Freshly isolated peritoneal B1 cells (B1P), or splenic B2 cells (B2S) treated with LPS for 48 h, were incubated in the presence of 20 μg/mL BFA or diluent (DMSO) for 6 h. B cells were placed on ELISPOT plates for 4 h to assess IgM secretion. (A) Representative ELISPOT membranes from three independent experiments showing cells alone, with solvent DMSO, or with BFA. (B) Average of three experiments with ELISPOT data presented as percent of cells secreting IgM, and with lines indicating standard errors of the means. A paired, one-tailed Student’s t-test was used to determine the significance of differences between the solvent DMSO and BFA treatment in B1 cells (**p = 0.005) and stimulated B2 cells (*p = 0.02).
IgM spontaneously secreted by B1 cells is Endo H resistant
To further demonstrate that B1-cell production of IgM is due to protein secretion we examined the Endo H sensitivity of IgM immunoprecipitated from culture supernatants of naïve peritoneal B1 cells, and IgM immunoprecipitated from culture supernatants of splenic B2 cells stimulated by LPS. As a positive control, B2 cell lysates were also tested with Endo H. Endo H differentiates membrane and secretory IgM as a result of differences in N-linked oligosaccharides, with the former sensitive to and cleaved by Endo H, and the latter relatively resistant to Endo H digestion (as a result of oligosaccharide trimming) [36]. Results are shown in Fig. 3. After 72 h, IgM collected and immunoprecipitated from naïve B1 cell cultures was resistant to Endo-H glycosidase activity (Fig. 3A), as expected for secreted IgM. This was also true of supernatant IgM obtained from B2 cells stimulated by LPS for 72 h. In contrast, and as a control for the activity of Endo H, IgM immunoprecipitated from lysates of LPS-stimulated B2 cells was sensitive to Endo H cleavage.
Figure 3.
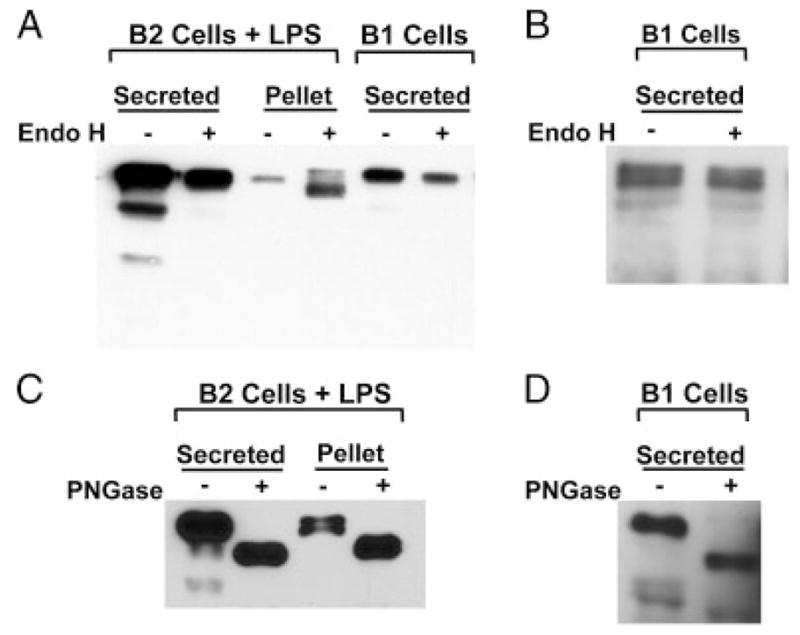
IgM spontaneously secreted by B1 cells is Endo H-resistant. (A) B2 cells were stimulated with LPS (25 μg/mL) for 72 h or B1 cells were cultured in medium alone for 72 h. Cell pellets were lysed in NP-40 lysis buffer for 30 min and then cleared by centrifugation. Supernatants and cell pellets were collected and pre-cleared with protein G. Secreted and cellular IgM was immunoprecipitated with rabbit anti-mouse IgM, eluted from protein G, and then treated with or without Endo H at 37°C overnight. Samples were run on an SDS-PAGE gel, transferred to nitrocellulose, blocked, and probed with goat anti-mouse IgM-HRP. (B) B1 cells were cultured for 5 h. Supernatants were harvested, and IgM was immunoprecipitated and subjected to Endo H treatment as described in (A). (C) B2 cells were stimulated with LPS (25 μg/mL) for 72 h. Supernatants and lysed cell pellets were harvested and IgM was immunoprecipitated as described in (A). Secreted and cellular IgM was eluted from protein G, and then treated with or without PNGase at 37°C overnight. Samples were run on an SDS-PAGE gel, transferred to nitrocellulose, blocked, and probed with goat anti-mouse IgM-HRP. (D) B1 cells were cultured for 5 h. Supernatants were harvested and IgM was immunoprecipitated and subjected to PNGase treatment as described in (C). For each of A–D, one of two comparable experiments is shown.
To recapitulate conditions utilized during ELISPOT analysis of spontaneous Ig secretion, we tested the Endo H sensitivity of supernatant IgM obtained from B1 cells cultured for only 5 h in medium alone. This shorter time period not only more closely replicates our typical B1 cell ELISPOT conditions but also minimizes the potentially confounding effects of putative B1 cell activation and/or cell death that might occur during the culture period. In agreement with our previous finding at 72 h, IgM obtained from 5 h B1 cell cultures was resistant to Endo H cleavage (Fig. 3B). These results demonstrate that IgM produced by B1 cells expresses the Endo H resistance characteristic of secreted immunoglobulin. As a positive control for IgM glycosylation that resists Endo H cleavage, stimulated B2 cell (Fig. 3C) and naïve B1 cell (Fig. 3D) secreted IgM was treated with PNGase, resulting in a much faster migrating species, which confirms the glycosylated status of secreted IgM and demonstrates the mobility of IgM lacking all N-linked glycosylation. Notably IgM secreted by stimulated B2 cells (Fig. 3A) and by unstimulated B1 cells (Fig. 3B) contained faster migrating species in addition to the predominant protein; these have been noted by other investigators but their origin as differentially glycosylated or degradation products has never been addressed.
All together, these results demonstrate that IgM produced by B1 cells is unique to viable B1 cells, depends on intact Golgi function, and manifests the characteristics of secreted immunoglobulin. We conclude that the peritoneal B1 cell-generated spots detected by ELISPOT assay reflect secreted IgM.
B1 cells spontaneously secrete IgM independently of IRF4
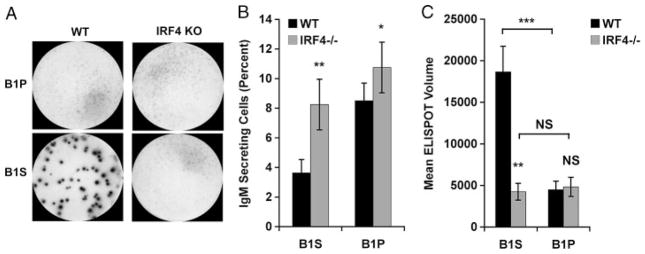
To extend our previous work that described unique transcriptional mechanisms governing spontaneous IgM secretion by B1 cells, we examined the requirement for the transcription factor IRF4 in promoting this process, by analyzing IRF4-null animals. Naïve peritoneal B1 cells were obtained from IRF4 KO and WT littermate control mice and secretion of IgM was evaluated by ELISPOT assay. Results are shown in Fig. 4. IRF4-null B1 cells spontaneously secreted IgM with the same frequency as littermate control B1 cells (p = NS). ELISPOT membranes from a representative experiment are displayed in Fig. 4A and enumerated results from three independent experiments are displayed in Fig. 4B. These results demonstrate that IRF4, which has been shown to be necessary for induction of B2-cell plasmacytic differentiation through suppression of BCL-6 expression and stimulation of BLIMP-1 expression, is not necessary for spontaneous IgM secretion by naïve peritoneal B1 cells. We conclude that B1 cells utilize a distinct, IRF4-independent mechanism in the service of spontaneous, constitutive immunoglobulin secretion.
Figure 4.
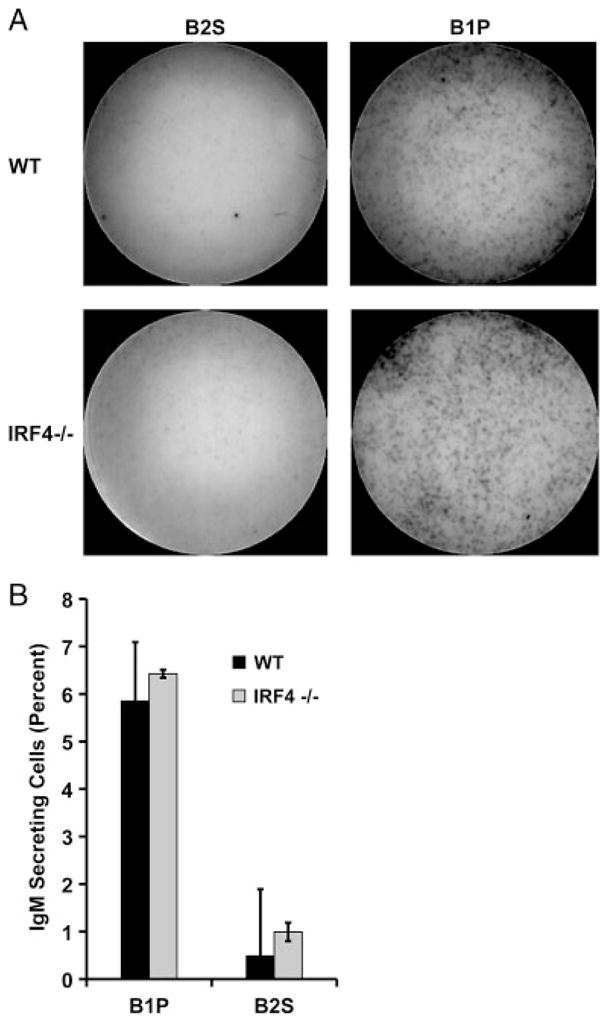
B1 cells spontaneously secrete IgM independently of IRF4. Freshly isolated peritoneal B1 cells (B1P) or splenic B2 cells (B2S) from WT or IRF4 KO mice were immediately placed on pre-coated ELISPOT plates for 4 h. (A) Representative ELISPOT membranes from one of three independent experiments showing naïve B1P and B2S cells. (B) Mean number of IgM-secreting B cells in three experiments with lines indicating standard errors of the means. A paired, two-tailed Student’s t-test was used to determine the significance of differences between WT and IRF4 KO B1P or B2S cell secretion. No significant differences were found.
Serum IgM is low in IRF4-null animals
B1 cells are responsible for natural immunoglobulin, which comprises the majority of resting serum IgM [4, 19, 20, 37]. However, serum levels of IgM are very low in IRF4-null animals [38], despite continued spontaneous secretion of IgM by B1 cells. To verify this apparent contradiction in the mice studied here, we examined serum samples from seven IRF4 KO and seven littermate control mice for IgM content by ELISA assay. Results are shown in Fig. 5. The mean level of serum IgM for IRF4 KO mice was essentially at baseline and thus represented a minimal fraction of the level of WT littermate control mice (p = 0.0001). These results suggest that spontaneous IgM secretion by peritoneal B1 cells, here shown to be IRF4-independent, is not directly responsible for natural serum IgM, despite the well-described connection between B1 cells and natural immunoglobulin.
Figure 5.
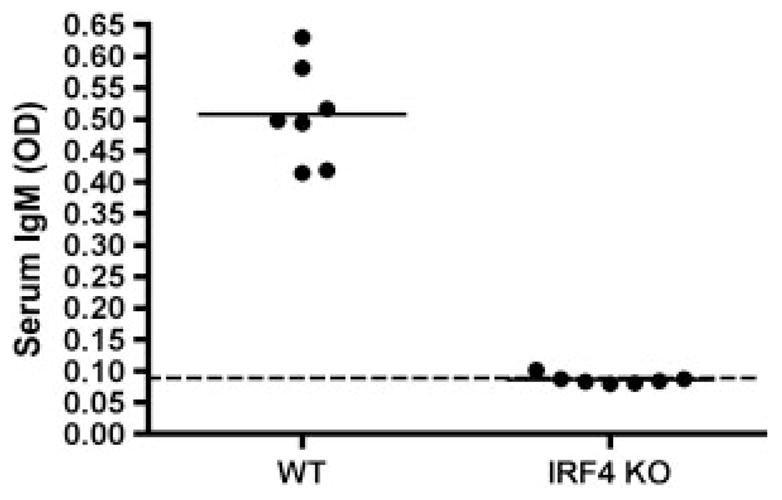
Serum IgM is low in IRF4-null animals. Serum was collected from seven WT and seven IRF4 KO mice. The samples were evaluated for IgM by ELISA as described in Materials and methods. The dotted line represents baseline OD in the absence of serum.
Splenic B1 cells spontaneously secrete large amounts of IgM in an IRF4-dependent fashion
The results described above suggest the possibility that a non-peritoneal pool of B1 cells might produce amounts of IgM more substantial than the amounts produced by peritoneal B1 cells, and in so doing give rise to serum IgM. B1 cells are known to reside in the spleen as well as the peritoneal cavity, and it has been reported that peritoneal B1 cells can migrate to the spleen following stimulation and upregulate immunoglobulin secretion there [39, 40]. To determine the relative capacity of splenic B1 cells to secrete IgM, sort-purified B1 cells were obtained from the spleens and peritoneal cavities of normal and IRF4 KO animals, and were examined by ELISPOT assay. Results are shown in Fig. 6. Naïve splenic B1 (B1S) cells secreted IgM, and, although the fraction actively secreting was less than for peritoneal B1 cells, the amount secreted per cell, as judged by spot volume (area × intensity) was much greater (p = 0.0007) than for peritoneal B1 cells (Fig. 6C). This difference was not due to a putative decline in B1P cell “activation” in the absence of the peritoneal environment, because exposure to LPS throughout the incubation period had no effect on the number or volume of ELISPOT spots produced by peritoneal B1 cells (data not shown). Loss of IRF4 greatly affected B1S cell IgM secretion by markedly reducing (p = 0.009) mean spot volume to the range characteristic of peritoneal B1 cells. In contrast, as noted previously, loss of IRF4 had little effect on B1P cell IgM secretion (Fig. 6B and C). ELISPOT membranes from a representative experiment are displayed in Fig. 6A and the mean enumerated results from three independent experiments are displayed in Fig. 6B and C. Interestingly, in the absence of IRF4 the proportion of B1S cells secreting any IgM increased (p = 0.007), even as the amount of IgM secreted per cell dramatically decreased. The increase in secreting B1S cells could mean that additional low-intensity spots become apparent when overlying or interfering high-intensity spots are eliminated by IRF4 deficiency. Alternatively, and considering that in the absence of IRF4 the number of peritoneal B1 cells that secreted small amounts of IgM per cell also increased (p = 0.02), it may be that IRF4 inhibits low-level secretion. Regardless of the explanation, these results all together indicate that naïve splenic B1 cells secrete substantial amounts of IgM, exceeding on a per cell basis that secreted by naïve peritoneal B1 cells, and that the increased level of IgM secretion by B1S cells, but not the lower level of IgM secretion by B1P cells, is IRF4-dependent.
Figure 6.
Splenic B1 cells spontaneously secrete large amounts of IgM in an IRF4-dependent fashion. Sort-purified peritoneal B1 cells (B1P) and splenic B1 cells (B1S) were isolated from WT and IFR4 KO mice and placed on ELISPOT plates for 4 h to assess IgM secretion. (A) Representative ELISPOT membranes from one of three independent experiments. (B) Mean percent IgM-secreting B cells in eight WT and eight IRF4 KO mice with lines indicating standard errors of the means. (C) Mean of average spot volumes for eight WT and eight IRF4 KO mice with lines indicating standard errors of the means. A paired, two-tailed Student’s t-test was used to determine the significance of differences between WT and IRF4 KO B1S cells in terms of proportion of B1S IgM secretors (**p = 0.007), in terms of proportion of B1P IgM secretor (*p = 0.02), in terms of mean spot volume per B1S secreting cell (**p = 0.009), in terms of mean spot volume per B1P secreting cell (NS), and between WT B1S versus B1P cells in terms of mean spot volume per secreting B1 cell (***p = 0.0007). NS: not significant.
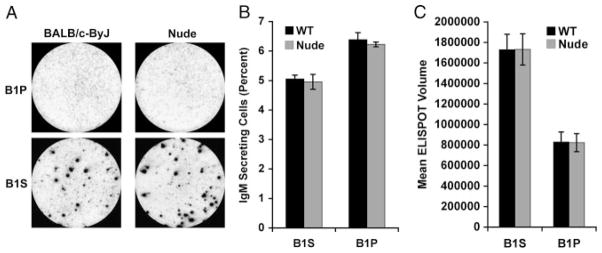
Spontaneous IgM secretion by splenic B1 cells is T-cell-independent
To determine whether T cells are required for high-level IgM secretion by B1S cells we carried out the same ELISPOT comparisons of sorted B-cell populations using WT and nude BALB/c mice. Results are shown in Fig. 7. As noted above, fewer WT B1S cells secreted IgM, and the level of secretion amounted to more IgM per cell, in comparison to B1P cells, and this was the same (p = NS) when B cells were obtained from normal and from T-cell-deficient mice. These results suggest that T cells are not required for high-level IgM secretion by B1S cells.
Figure 7.
Spontaneous IgM secretion by splenic B1 cells is T-cell-independent. Sort-purified peritoneal B1 cells (B1P) and splenic B1 cells (B1S) were isolated from WT and nude mice and placed on ELISPOT plates for 4 h to assess IgM secretion. (A) Representative ELISPOT membranes from one of two independent experiments. (B) Mean percent IgM-secreting B cells in six WT and six nude mice with lines indicating standard errors of the means. (C) Mean of average spot volumes for six WT and six nude mice with lines indicating standard errors of the means. A paired, two-tailed Student’s t-test was used to determine the significance of differences between WT and nude mice in terms of proportion of B1P or B1S cells secreting IgM. No significant differences were found.
Discussion
Spontaneous, constitutive secretion of IgM is a key distinguishing characteristic of B1 cells that accounts in large measure for circulating protective natural antibody in both mouse and human. Secretion of IgM by unstimulated B1 cells has been verified in vivo on the basis of adoptive transfer experiments and has been demonstrated in vitro on the basis of ELISA analyses of culture supernatants and ELISPOT assays of purified populations. Recently, we reported that spontaneous IgM secretion by B1 cells violates the accepted transcription factor paradigm for plasma cell differentiation because secretion occurs in B1 cells that express negligible levels of BLIMP-1 and PAX-5 [21]. Thus, natural IgM-secreting B1 cells are BLIMP-1−/loPAX-5−/lo, unlike plasma cells and in vitro stimulated B2 cells. As part of this study we reconfirmed previous ELISPOT results that demonstrated spontaneous IgM secretion by naïve, unmanipulated B1 cells [33]. Although a subsequent study by Savitsky and Calame corroborated our results by showing that peritoneal B1 cells do indeed spontaneously secrete IgM as judged by ELISA [31], in later work Nutt, Tarlinton, and colleagues reported no antibody-secreting cells could be detected by ELISPOT among B1 cells in their experiments [30].
The controversy so generated regarding the spontaneous secretion of IgM by B1 cells led to the present study designed to probe the degree to which the output of ELISPOT assays, in terms of spots, reflects B1 cell immunoglobulin secretion. By several different criteria, we demonstrated that the spots generated during short-term ELISPOT culture of B1 cells immediately following isolation ex vivo truly reflect IgM secretion. Thus, generation of ELISPOT spots requires viable B1 cells. Further, addition of BFA prior to ELISPOT culture, which blocks protein transport from the endoplasmic reticulum to the Golgi, showed that production of spots depends on the Golgi apparatus, which is intimately involved in processing immunoglobulin for export [41, 42]; because IgM secretion is an “assembly line” process and secretory IgM is, presumably, in the “pipeline” at the time BFA is added, BFA-mediated inhibition cannot be complete, though it was substantial. Finally, resistance to Endo H digestion showed that immunoglobulin accumulating in unstimulated B1 cell culture supernatants is secretory in nature. From these results, all together, we conclude that peritoneal B1 cell-generated spots detected by our ELISPOT assays do indeed reflect IgM spontaneously secreted in the absence of stimulation, consistent with earlier reports, and that the failure of some other investigators to identify antibody-secreting B1 cells most likely reflects insufficient sensitivity or analysis of ELISPOT membranes. Although there was variability in the proportion of peritoneal B1 cells that spontaneously secreted IgM in different experiments ranging from approximately 6 to 14% during the course of this study, each individual assay was well-controlled and differences between B1 and B2 cells were always significant (Figs. 1 and 4), resulting in a high degree of confidence in our conclusions.
Although B1 cells spontaneously and constitutively secrete IgM, we previously reported that they express negligible levels of BLIMP-1 or PAX-5, and thus would appear to export immunoglobulin through a non-classical pathway [21]. More recently, Fairfax et al. reported that B1 cells contain a very low level of BLIMP-1 (amounting to 1/70th the level present in antibody secreting B2 cells) [30], which in keeping with our previous work, supports the notion that peritoneal B1 cells are BLIMP-1−/lo. With regard to PAX-5, Nutt, Tarlinton, and colleagues at one point reported that they detected PAX-5 mRNA and protein in peritoneal B1 cells (although at levels much reduced in comparison to B2 cells) [30], but later showed that the PAX-5-suppressed gene, Embigin, is expressed in B1 cells and that PAX-5-activated genes, Cd79a and Blnk, are downregulated in B1 cells [32]. This later work indicates that PAX-5 is functionally inactive in B1 cells, regardless of the exact level of expression in relation to naïve B2 cells, which we previously reported to be negligible. Thus, aside from disagreement regarding the capacity of B1 cells to spontaneously secrete IgM, which is addressed by the present study, previous reports generally support our earlier finding that peritoneal B1 cells express negligible levels of BLIMP-1 and PAX-5. In other words, as we reported before, naive B1 cells are BLIMP-1−/loPAX-5−/lo and thus spontaneously secrete IgM via a non-classical pathway.
This would seem to violate the paradigm that a rise in BLIMP-1 is necessary to repress PAX-5. However, Nutt, Tarlinton, and colleagues demonstrated that loss of PAX-5 is not dependent on increased BLIMP-1, in the course of identifying BLIMP-1-independent differentiation to an immunoglobulin-secreting preplasmablast stage of B-cell development that is BLIMP-1nullPAX-5lo [32]. In this work preplasmablasts produced only small spots on ELISPOT assay, much like peritoneal B1 cells [32]. Thus, our earlier finding that spontaneously IgM-secreting (secrIg) B1 cells are secrIgloBLIMP-1−/loPAX-5−/lo antedates the separate identification of secrIgloBLIMP-1nullPAX-5lo preplasmablast cells described by Nutt, Tarlinton, and colleagues. In consideration of the layered immune system as proposed by Kantor and Herzenberg [43], it may be that secrIgloBLIMP-1−/loPAX-5−/lo immunoglobulin secretion by B1 cells represents a phylogenetically and ontologically early immune mechanism that is recapitulated by B2 cells on the way to “switching” to full antibody-secreting cell differentiation. Alternatively, it may be that secrIgloBLIMP-1−/loPAX-5−/lo immunoglobulin secretion is simply one point in a continuum of antibody-secreting cell differentiation, and that in B1 cells (and in B2 cells in which a rise in BLIMP-1 is genetically precluded) further differentiation is blocked, in the case of B1 cells through an as yet unknown mechanism.
To further examine differences in the regulation of immunoglobulin secretion between B1 cells and B2 cells, we determined the role of the key transcription factor, IRF4, finding that IRF4 deficiency had no effect on spontaneous IgM secretion by peritoneal B1 cells (secrIgloBLIMP-1−/loPAX-5−/loIRF4null). It is well known, however, that circulating IgM is markedly diminished in IRF4 KO mice [38], which we reconfirmed in the very same mice that were used for ELISPOT analyses. This raises the possibility that low level spontaneous IgM secretion by peritoneal B1 cells might not be the origin of B1 cell-dependent natural serum immunoglobulin. It has been suggested that peritoneal B1 cells migrate to the spleen and either initiate or expand high level immunoglobulin secretion there [39, 40, 44, 45]. For this reason we assessed splenic B1 cells in comparison to peritoneal B1 cells and found that indeed, splenic B1 cells secrete much more IgM per cell than do peritoneal B1 cells; most importantly, high level splenic B1-cell immunoglobulin secretion is completely IRF4-dependent (and, in contrast to some reports, references [44, 46], is T-cell-independent as well). In the absence of IRF4, splenic B1 cells secrete only a small, peritoneal B1 cell-like, amounts of IgM, which is resistant to the loss of IRF4. Thus, spontaneous IgM secretion by B1 cells is of two types: low-level secretion by peritoneal B1 cells (and splenic B1 cells) that is IRF4-independent and high-level secretion only by splenic B1 cells that is IRF4-dependent.
These results may clarify previously published studies in which loss of IRF4 produced markedly diminished natural serum IgM, even though, as shown here, spontaneous IgM secretion by peritoneal B1 cells was not affected. It is now clear that there are two distinct IgM-secreting pathways associated with B1 cell populations and operating spontaneously, one in which IgM secretion proceeds at a low level through a non-classical (IRF4-independent, BLIMP-1−/loPAX-5−/lo) pathway that characterizes both peritoneal and splenic B1 cells, and the other in which IgM secretion proceeds at a high level that is classically IRF4-dependent and that characterizes splenic B1 cells and not peritoneal B1 cells. Our work indicates that the latter pathway is responsible for the serum accumulation of natural IgM. However, it is important to note that our identification of IRF4-dependent, high-secreting B1 cells in the spleen does not rule out the possibility that such cells exist elsewhere, although in mice few B1 cells have been identified beyond coelemic cavities and splenic tissue. It may be speculated that low-level IgM secretion by peritoneal B1 cells serves a separate, local function.
In previous work we have identified many differences between peritoneal and splenic B1 cells [47, 48], of which the intensity of, and pathway for, IgM secretion now appears to be yet another. Although other investigators have reported that activated peritoneal B1 cells migrate to the spleen, it remains to be determined whether this is the origin of splenic B1 cells and if so what role, if any, local and antigenic factors play in this process. Thus, it is impossible to say at the present time whether high-secreting splenic B1 cells represent a stable, distinct B1a subset or a transient stage of B1a cell differentiation, activity and/or function. Most importantly, the genesis of natural serum IgM is here shown to lie with splenic B1 cells rather than peritoneal B1 cells.
Materials and methods
Mice
Male BALB/cByJ mice of 6–8 wk age were obtained from The Jackson Laboratory. Germline deleted IRF4 null mice were kindly provided by Dr. Ulf Klein (Columbia University, New York, NY) and were produced by crossing previously described mice in which the IRF4 locus is flanked by loxP and frt sites with Flp-recombinase expressing mice to eliminate IRF4 in all embryonic cells. The phenotype of these mice matches the known characteristics of IRF4 KO mice [38]. Male nude BALB/c (C.Dg/AnNTc-Foxn1nuNE9) and control BALB/cAnN mice of 6–8 wk age were obtained from Taconic. Mice were cared for and handled in accordance with National Institutes of Health and institutional guidelines.
Cell purification and flow cytometry
Peritoneal washout cells and splenocytes were obtained from 8–14 wk old mice and stained with fluorescence-labeled antibodies to B220 and CD5 (peritoneal washout cells) or B220 (splenocytes). B-cell populations were sort-purified (BD Biosciences) as follows: splenic B2 cells, B220hi/CD5−; peritoneal B1 cells, B220lo/CD5+; splenic B1 cells, CD23−B220loCD5+. Post-sort analysis of the peritoneal B1 cell and splenic B2 cell populations showed each to be ≥98% pure.
ELISPOT assay
ELISPOT assay was carried out as previously described [21]. In brief, sort-purified, naive B cells were distributed onto Multi-Screen*-IP Plates (Millipore) precoated with goat anti-mouse Ig (H+L) and then incubated in RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, 100 U/mL penicillin and 100 μg/mL streptomycin for 4 h at 37°C and 5% CO2. Plates were treated with alkaline phosphatase-conjugated goat anti-mouse IgM (Southern Biotechnology Associates) and developed with 5-bromo-4-chloro-3-indolyl phosphate/p-NBT chloride substrate (KPL). IgM-secreting B cells were enumerated using Phoretix Expression software (NonLinear Dynamics) with filtering for circularity, peak height, volume and area. Significance was determined by paired Student’s t-test.
Endo H and PNGase digestion
Purified B cells were cultured in the presence or absence of LPS (Sigma) for either 5 or for 72 h. Cells and supernatant fluids were collected. Cells were lysed in NP-40 lysis buffer for 30 min and then cleared by centrifugation at 13 000 × g for 15 min at 4°C. Cell lysates and supernatant fluids were then pre-cleared with protein G (Santa Cruz Biotechnology). IgM was immunoprecipitated with rabbit anti-mouse IgM (MP Biochemicals) plus protein G beads, and then eluted from protein G in 50 mM sodium citrate (pH 3.5), pH adjusted with 1.5 M sodium citrate, and then treated with or without 250 units Endo H (New England Biolabs) at 37°C overnight. For PNGase treatment, IgM was immunoprecipitated with rabbit anti-mouse IgM plus protein G beads, and then eluted from protein G by boiling in 0.5% SDS with 0.1 M 2-ME at 100°C for 5 min, then diluted 1:7.5 in 0.2 M sodium phosphate (pH 8.6) with 0.5% NP-40, and then treated with or without 1 unit/mL PNGase (New England Biolabs) at 37°C overnight. Samples were run on a 10% SDS-PAGE gel and transferred to Hybond nitrocellulose membranes (Amersham). The membranes were then blocked with 5% non-fat dry milk in TBST for 1 h at room temperature and probed with goat anti-mouse IgM-HRP (Southern Biotech). Detection was performed with enhanced chemiluminescence (Pierce) for B2+LPS samples and femto ECL (Pierce) for B1a cell 5 h samples.
ELISA assay
To measure serum IgM, ELISA assay was carried out as previously described [49]. In brief, 50 μL of diluted serum was incubated for 2 h in wells of 96-well plates (NUNC Maxisorp) pre-coated with goat anti-mouse Ig (H+L) (Southern Biotechnology Associates), along with IgM standards. Bound IgM was detected using anti-mouse IgM conjugated to horseradish peroxidase (Sigma), followed by development with 2,2-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (Sigma) and determination of absorbance at 405 nm.
Acknowledgments
The authors thank Dr. Ulf Klein for helpful discussions and for generously providing IRF4-null mice. This work was supported by United States Public Health Service grants AI029690 and AI060896 awarded to TLR by the National Institutes of Health.
Abbreviations
- BCL-6
B-cell leukemia/lymphoma-6
- BFA
Brefeldin A
- BLIMP-1
B lymphocyte inducer of maturation program 1
- IRF4
interferon response factor 4
- PAX-5
paired box gene 5
- XBP-1
X-box binding protein-1
Footnotes
Conflict of interest: The authors have declared no financial or commercial conflict of interest.
References
- 1.Su SD, Ward MM, Apicella MA, Ward RE. The primary B cell response to the O/core region of bacterial lipopolysaccharide is restricted to the Ly-1 lineage. J Immunol. 1991;146:327–331. [PubMed] [Google Scholar]
- 2.Briles DE, Forman C, Hudak S, Claflin JL. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982;156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel RM. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 4.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercolino TJ, Locke AL, Afshari A, Sasser D, Travis WW, Arnold LW, Haughton G. Restricted immunoglobulin variable region gene usage by normal Ly-1 (CD5+) B cells that recognize phosphatidyl choline. J Exp Med. 1989;169:1869–1877. doi: 10.1084/jem.169.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardy RR, Carmack CE, Shinton SA, Riblet RJ, Hayakawa K. A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J Immunol. 1989;142:3643–3651. [PubMed] [Google Scholar]
- 7.Wang H, Clarke SH. Positive selection focuses the VH12 B-cell repertoire towards a single B1 specificity with survival function. Immunol Rev. 2004;197:51–59. doi: 10.1111/j.0105-2896.2004.0098.x. [DOI] [PubMed] [Google Scholar]
- 8.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, Leong JM. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol. 2003;170:3819–3827. doi: 10.4049/jimmunol.170.7.3819. [DOI] [PubMed] [Google Scholar]
- 10.Mi QS, Zhou L, Schulze DH, Fischer RT, Lustig A, Rezanka LJ, Donovan DM, et al. Highly reduced protection against Streptococcus pneumoniae after deletion of a single heavy chain gene in mouse. Proc Natl Acad Sci USA. 2000;97:6031–6036. doi: 10.1073/pnas.110039497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Zhong X, Gao W, Degauque N, Bai C, Lu Y, Kenny J, Oukka M, et al. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur J Immunol. 2007;37:2400–2404. doi: 10.1002/eji.200737296. [DOI] [PubMed] [Google Scholar]
- 14.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 15.Herzenberg LA. B-1 cells: the lineage question revisited. Immunol Rev. 2000;175:9–22. [PubMed] [Google Scholar]
- 16.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 17.Rothstein TL. Cutting edge commentary: two B-1 or not to be one. J Immunol. 2002;168:4257–4261. doi: 10.4049/jimmunol.168.9.4257. [DOI] [PubMed] [Google Scholar]
- 18.Sidman CL, Shultz LD, Hardy RR, Hayakawa K, Herzenberg LA. Production of immunoglobulin isotypes by Ly-1+B cells in viable motheaten and normal mice. Science. 1986;232:1423–1425. doi: 10.1126/science.3487115. [DOI] [PubMed] [Google Scholar]
- 19.Forster I, Rajewsky K. Expansion and functional activity of Ly-1+B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol. 1987;17:521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- 20.Ishida H, Hastings R, Kearney J, Howard M. Continuous anti-interleukin 10 antibody administration depletes mice of Ly-1 B cells but not conventional B cells. J Exp Med. 1992;175:1213–1220. doi: 10.1084/jem.175.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tumang JR, Frances R, Yeo SG, Rothstein TL. Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J Immunol. 2005;174:3173–3177. doi: 10.4049/jimmunol.174.6.3173. [DOI] [PubMed] [Google Scholar]
- 22.Allman D, Jain A, Dent A, Maile RR, Selvaggi T, Kehry MR, Staudt LM. BCL-6 expression during B-cell activation. Blood. 1996;87:5257–5268. [PubMed] [Google Scholar]
- 23.Reljic R, Wagner SD, Peakman LJ, Fearon DT. Suppression of signal transducer and activator of transcription 3-dependent B lymphocyte terminal differentiation by BCL-6. J Exp Med. 2000;192:1841–1848. doi: 10.1084/jem.192.12.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usui T, Wakatsuki Y, Matsunaga Y, Kaneko S, Koseki H, Kita T. Overexpression of B cell-specific activator protein (BSAP/Pax-5) in a late B cell is sufficient to suppress differentiation to an Ig high producer cell with plasma cell phenotype. J Immunol. 1997;158:3197–3204. [PubMed] [Google Scholar]
- 25.Reimold AM, Ponath PD, Li YS, Hardy RR, David CS, Strominger JL, Glimcher LH. Transcription factor B cell lineage-specific activator protein regulates the gene for human X-box binding protein 1. J Exp Med. 1996;183:393–401. doi: 10.1084/jem.183.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito M, Gao J, Basso K, Kitagawa Y, Smith PM, Bhagat G, Pernis A, et al. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell. 2007;12:280–292. doi: 10.1016/j.ccr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25:225–236. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Lin KI, Angelin-Duclos C, Kuo TC, Calame K. Blimp-1-dependent repression of Pax-5 is required for differentiation of B cells to immunoglobulin M-secreting plasma cells. Mol Cell Biol. 2002;22:4771–4780. doi: 10.1128/MCB.22.13.4771-4780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 30.Fairfax KA, Corcoran LM, Pridans C, Huntington ND, Kallies A, Nutt SL, Tarlinton DM. Different kinetics of blimp-1 induction in B cell subsets revealed by reporter gene. J Immunol. 2007;178:4104–4111. doi: 10.4049/jimmunol.178.7.4104. [DOI] [PubMed] [Google Scholar]
- 31.Savitsky D, Calame K. B-1 B lymphocytes require Blimp-1 for immunoglobulin secretion. J Exp Med. 2006;203:2305–2314. doi: 10.1084/jem.20060411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kallies A, Hasbold J, Fairfax K, Pridans C, Emslie D, McKenzie BS, Lew AM, et al. Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity. 2007;26:555–566. doi: 10.1016/j.immuni.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Klinman DM, Holmes KL. Differences in the repertoire expressed by peritoneal and splenic Ly-1 (CD5)+B cells. J Immunol. 1990;144:4520–4525. [PubMed] [Google Scholar]
- 34.Scholz JL, Crowley JE, Tomayko MM, Steinel N, O’Neill PJ, Quinn WJ, 3rd, Goenka R, et al. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci USA. 2008;105:15517–15522. doi: 10.1073/pnas.0807841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988;263:18545–18552. [PubMed] [Google Scholar]
- 36.Brewer JW, Randall TD, Parkhouse RM, Corley RB. Mechanism and subcellular localization of secretory IgM polymer assembly. J Biol Chem. 1994;269:17338–17348. [PubMed] [Google Scholar]
- 37.Hayakawa K, Hardy RR, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittrucker HW, Matsuyama T, Grossman A, Kundig TM, Potter J, Shahinian A, Wakeham A, et al. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science. 1997;275:540–543. [Google Scholar]
- 39.Itakura A, Szczepanik M, Campos RA, Paliwal V, Majewska M, Matsuda H, Takatsu K, Askenase PW. An hour after immunization peritoneal B-1 cells are activated to migrate to lymphoid organs where within 1 day they produce IgM antibodies that initiate elicitation of contact sensitivity. J Immunol. 2005;175:7170–7178. doi: 10.4049/jimmunol.175.11.7170. [DOI] [PubMed] [Google Scholar]
- 40.Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, Fagarasan S. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sousa M, Parodi AJ. The molecular basis for the recognition of misfolded glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. EMBO J. 1995;14:4196–4203. doi: 10.1002/j.1460-2075.1995.tb00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 43.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe N, Ikuta K, Fagarasan S, Yazumi S, Chiba T, Honjo T. Migration and differentiation of autoreactive B-1 cells induced by activated gamma/delta T cells in antierythrocyte immunoglobulin transgenic mice [In Process Citation] J Exp Med. 2000;192:1577–1586. doi: 10.1084/jem.192.11.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawahara T, Ohdan H, Zhao G, Yang YG, Sykes Mm. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J Immunol. 2003;171:5406–5414. doi: 10.4049/jimmunol.171.10.5406. [DOI] [PubMed] [Google Scholar]
- 46.Campos RA, Szczepanik M, Lisbonne M, Itakura A, Leite-de-Moraes M, Askenase PW. Invariant NKT cells rapidly activated via immunization with diverse contact antigens collaborate in vitro with B-1 cells to initiate contact sensitivity. J Immunol. 2006;177:3686–3694. doi: 10.4049/jimmunol.177.6.3686. [DOI] [PubMed] [Google Scholar]
- 47.Fischer GM, Solt LA, Hastings WD, Yang K, Gerstein RM, Nikolajczyk BS, Clarke SH, Rothstein TL. Splenic and peritoneal B-1 cells differ in terms of transcriptional and proliferative features that separate peritoneal B-1 from splenic B-2 cells. Cell Immunol. 2001;213:62–71. doi: 10.1006/cimm.2001.1860. [DOI] [PubMed] [Google Scholar]
- 48.Tumang JR, Hastings WD, Bai C, Rothstein TL. Peritoneal and splenic B-1 cells are separable by phenotypic, functional, and transcriptomic characteristics. Eur J Immunol. 2004;34:2158–2167. doi: 10.1002/eji.200424819. [DOI] [PubMed] [Google Scholar]
- 49.Hastings WD, Tumang JR, Behrens TW, Rothstein TL. Peritoneal B-2 cells comprise a distinct B-2 cell population with B-1b-like characteristics. Eur J Immunol. 2006;36:1114–1123. doi: 10.1002/eji.200535142. [DOI] [PubMed] [Google Scholar]